Abstract
Large herbivorous vertebrates have strong interactions with vegetation, affecting the structure, composition and dynamics of plant communities in many ways. Living large herbivores are a small remnant of the assemblages of giants that existed in most terrestrial ecosystems 50 000 years ago. The extinction of so many large herbivores may well have triggered large changes in plant communities. In several parts of the world, palaeoecological studies suggest that extinct megafauna once maintained vegetation openness, and in wooded landscapes created mosaics of different structural types of vegetation with high habitat and species diversity. Following megafaunal extinction, these habitats reverted to more dense and uniform formations. Megafaunal extinction also led to changes in fire regimes and increased fire frequency due to accumulation of uncropped plant material, but there is a great deal of variation in post-extinction changes in fire. Plant communities that once interacted with extinct large herbivores still contain many species with obsolete defences against browsing and non-functional adaptations for seed dispersal. Such plants may be in decline, and, as a result, many plant communities may be in various stages of a process of relaxation from megafauna-conditioned to megafauna-naive states. Understanding the past role of giant herbivores provides fundamental insight into the history, dynamics and conservation of contemporary plant communities.
Keywords: trophic cascade, ecosystem engineer, plant anachronisms, herbivory, seed dispersal, plant–animal interactions
1. Introduction
We live in a zoologically impoverished world, from which all the hugest, and fiercest, and strangest forms have recently disappeared… (Wallace 1876, p. 150)
Before the global spread of the modern Homo sapiens, all continents and most islands had rich faunas of very large vertebrates: mammoths; ground sloths; giant kangaroos; moa; and many others. These mostly vanished following human arrival (Martin & Steadman 1999; figure 1). Why these extinctions happened has been debated since Owen (1861) argued that human hunting was the cause. Owen's view remains controversial, but much recent evidence has pointed to a major role for hunting in driving the extinctions (e.g. Surovell et al. 2005; Johnson 2006; Koch & Barnosky 2006). This review examines the impact of prehistoric megafaunal extinctions on terrestrial ecosystems, without addressing the cause of those extinctions. Nonetheless, the question of cause is crucial, because if one believes that large animals went extinct because their environments changed and could no longer support them, as some have argued (Martin & Klein 1984), there is no strong reason to look for environmental changes that might have followed from those extinctions. On the other hand, the removal by people of large animals from environments that were otherwise suitable for them may well have had large ecological repercussions. That is the view I take here.
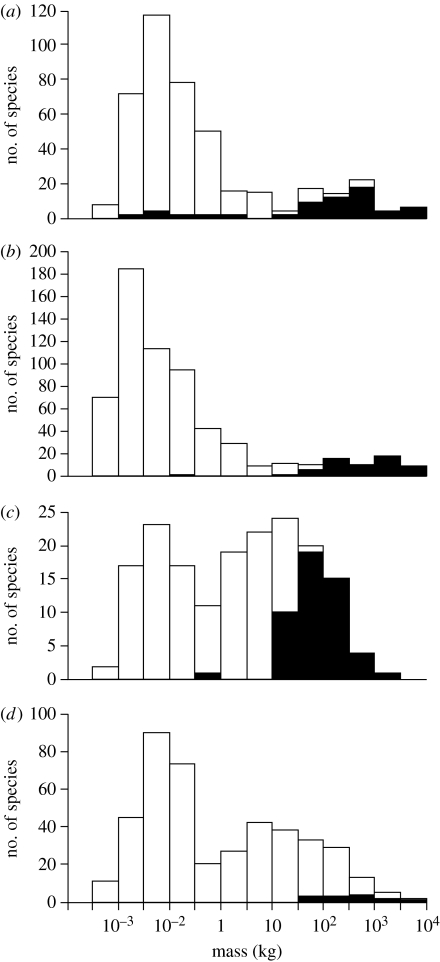
Figure 1.
Body mass distributions of herbivorous mammals in (a) North America, (b) South America, (c) Australia and (d) Africa, distinguishing species that were present in the Late Pleistocene but went extinct before the historical period (filled bars) from survivors into the historical period (open bars). Data adapted from Smith et al. (2003) except for extinct Australian mammals, which are from Johnson (2006).
Most of the extinct megafauna were herbivores. I focus specifically on changes in the structure, composition and dynamics of plant communities that can be attributed to the loss of those large herbivores that were present in terrestrial ecosystems at the beginning of the Last Glacial cycle ca 130 ka (i.e. thousands of years ago), but which went extinct before the historical period. This evidence comes mainly from Europe, Australia, Northern Eurasia and Beringia, North America, Madagascar and New Zealand. For a summary of the timing of extinction and human arrival in those places, against the background of changing climates through the Last Glacial cycle, see figure 2.
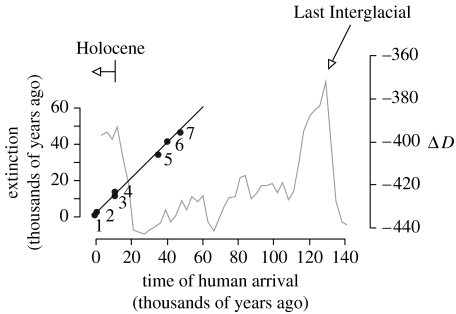
Figure 2.
Time of arrival of modern Homo sapiens in different parts of the world in relation to the time of megafaunal extinction, against the background of changes in global temperature through the Last Glacial cycle. The line shows the relationship expected if human arrival and megafauna extinction were simultaneous. Regions are (1) New Zealand (Worthy & Holdaway 2002; Wilmshurst et al. 2008), (2) Madagascar (Burney et al. 2003, 2004), (3) northeast Siberia (Pitul'ko 2001; Stuart et al. 2004), (4) North America (Solow et al. 2006), (5) southern Europe (Stuart 2005; Roebroeks 2008), (6) Tasmania (Turney et al. 2008) and (7) mainland Australia (Gillespie et al. 2006; Johnson 2006). Temperature is indicated by deuterium ratios in Antarctic ice, from EPICA (2004); high values indicate high temperatures.
2. Large herbivores and vegetation
Big herbivores have big effects on plants. Beyond the direct impacts of herbivory on the physiology, form and growth of individual plants, herbivores shape plant communities in many ways: by reducing vegetation density and creating gaps; facilitating species coexistence; dispersing seeds; suppressing sensitive species; reducing fire potential by preventing accumulation of dry plant tissue; and accelerating nutrient recycling via urine and faeces (Knapp et al. 1999; Gill 2006; Hester et al. 2006). The strength of these effects varies across space, especially where foraging intensity is affected by abiotic factors such as availability of water. Large herbivores therefore often promote spatial heterogeneity in vegetation formations. Herbivory by large vertebrates also drives the evolution of plant defences, such as spines and densely branched growth architecture (Cooper & Owen-Smith 1986; Janzen 1986; Grubb 1992; Bond & Silander 2007).
Owen-Smith (1988) argued that the impact of herbivory on vegetation increases with herbivore body size, such that ‘megaherbivores’ (defined as species weighing more than 1000 kg) can have overwhelming effects. This is because (i) larger species have greater capacity to use high-fibre material, and so consume a higher proportion of plant tissue, (ii) they are more general in their habitat use and maintain higher total biomass over large areas, and (iii) very large size confers invulnerability to predation, allowing megaherbivore populations to escape predator regulation and increase to the point where they have strong interactions with vegetation. Among African large herbivores, megaherbivores contribute 40–70 per cent of total mammalian large-herbivore biomass in savannahs while making up only a small proportion of the total number of species (Owen-Smith 1988). Studies of living large herbivores demonstrate their power to shape vegetation formations. African elephants (Loxodonta africana) maintain open conditions in savannah by suppressing woody regeneration (Dublin et al. 1990), and create grassy openings in forests and thickets (Owen-Smith 1999; R. M. Cowling 2009, personal communication); intense localized grazing by white rhinos (Ceratotherium simum) maintains short-grass lawns within mosaics of tussock grass and thickets, which impede the spread of fire and thereby protect wooded patches from conflagrations (Owen-Smith 1988; Waldram et al. 2008); shifting patchy grazing by bison (Bison bisou) maintains high species diversity in tallgrass prairie (Knapp et al. 1999).
Many large herbivores are partly frugivorous and disperse the seeds of large-fruited woody plants (Janzen & Martin 1982; Donatti et al. 2007). Large herbivores are also seed dispersers for herbaceous plants in which small fruits are intermingled with leaves and are incidentally consumed along with foliage (Janzen 1984). Larger herbivores can consume greater volumes of fruit and move seeds over longer distances (Guimaraes et al. 2008). Germination rates of seeds swallowed and defecated by mammals increase with body size, probably because seeds spend more time in the gut of large mammals and are more thoroughly scarified (Bodmer & Ward 2006). Herbs that rely for seed dispersal on herbivores that incidentally consume small fruit are likely to be best served by species that take big, undiscriminating mouthfuls of foliage, and, in this respect, the very largest grazers are most valuable (Janzen 1984).
It is therefore reasonable to expect that the recent extinction of so many of the world's largest herbivores might have caused major transformations of vegetation over much of the world. North America, for example, had a Pleistocene megaherbivore assemblage considerably richer than that of present-day Africa (Martin 2005; figure 1). Given what we know of the impact of the African elephant alone as a habitat engineer, continental North America's four (or more) species of proboscideans must have had even greater ecological effects over a wider range of environments; ground sloths might have been just as powerful again. The ecological consequences of their mass extinction, along with large bovids, cervids, camels, horses, glyptodonts, peccaries and so on, should have been immense (Martin 2005).
Such extinctions might have triggered the following three general types of change to vegetation.
Loss of open vegetation and habitat mosaics. Vegetation that had been kept open by herbivory should have been replaced by dense or closed formations, and mosaics that had been maintained by herbivore pressure should have given way to uniform or zonal vegetation patterns.
Increased fire. With accumulation of uncropped plant material and increased vegetation density, fire may have increased (Flaunery 1990). This could have affected vegetation in two distinct ways. Fire might have replaced animals as a ‘herbivore’ and maintained vegetation in a form similar to that created by animals; even so, fire is a more aggressive and less discriminating consumer of plant matter than true herbivores, so some vegetation pattern may have been lost in this transition and species with traits that allowed survival of fire or post-fire regeneration would have been strongly favoured (Bond & Keeley 2005). Alternatively, raised fire potential might have shifted vegetation to new states, such as extensive uniform grasslands in which recurrent fire prevents succession to woodland or forest.
Decline of coevolved plants. Traits that had been adaptive in a world with large herbivores may have become maladaptive in a post-megafauna world, leading to decline and extinction of plants burdened with them.
3. Effects on vegetation structure and diversity
(a) Europe
Vera (2000) argued that large herbivores in lowland temperate Europe once maintained shifting mosaics of grassland, thicket and tall forest. Herbivory converted stands of tall forest into open parkland or grassland by suppressing woody regeneration; these open areas would be invaded by thickets of thorny scrub resistant to browsing, which, in turn, provided patchy refuges where seedlings of palatable trees could re-establish; emergent trees then shaded out understorey scrubs and grew into forest stands destined to repeat the cycle. The patchiness maintained by this herbivore-driven cyclic succession benefited three groups of light-demanding plants in particular: herbs; scrub species with structural defences against large browsers; and trees such as oaks Quercus and hazel Corylus that can regenerate neither in the deep shade of a forest canopy nor under heavy browsing in the open (Vera et al. 2006). Without large herbivores, these mosaics reverted to uniform closed forest with lower species diversity. Effects of large herbivores on vegetation would have been especially powerful in ecological zones favourable to large mammalian herbivores, such as lowlands or flood plains in relatively dry climates with high soil nutrient availability. These may have been hotspots for biodiversity under the influence of large mammals, but now ‘only a few remnants of this rich system remain scattered over northwestern Europe, mostly in small nature reserves’ (Bakker et al. 2004).
This hypothesis sparked a controversy over the character of primeval wooded landscapes in lowland temperate Europe, in which two somewhat idealized images were contrasted: Vera's ‘wood–pasture’ mosaic of groves and glades, versus the tall, structurally uniform, closed-canopy ‘high forest’ imagined by most botanists as the original vegetation over most of Europe (Birks 2005). Most tests of Vera's hypothesis have used the Early Holocene (10−6 ka) as a reference period, for three reasons: the climate of the Early Holocene was similar to today's; direct human impact on forest cover and structure was not yet significant; and the large mammal herbivores that were on hand to shape vegetation then (aurochs, bison, deer, wild horses, etc.) are still available to managers now, albeit in some cases only as domesticated descendants.
Palaeoecological tests tend to support the high forest over the wood–pasture picture for the Early Holocene, although not always exclusively. In Early Holocene pollen records from Wales, percentages of non-arboreal pollen (NAP) at most sites are low enough to be consistent with closed high forest, but a few sites have high NAP counts, indicating open vegetation possibly maintained by herbivory (Fyfe 2007). Soepboer & Lotter (2009) modelled pollen accumulation from contemporary vegetation formations in Switzerland, representing high forest and wood–pasture types, and compared predicted pollen profiles from these types with actual profiles from 6 ka lake sediments. These were more consistent with high forest than wood pasture, but with a higher than expected representation of open habitats indicating a fairly high frequency of disturbed or naturally open habitats. Mitchell (2005) tested the effects of large herbivores on vegetation structure by comparing Early Holocene pollen records from Ireland and Europe. The only large herbivores in Ireland in the Early Holocene were wild boar (Sus scrofa) and red deer (Cervus elaphus) (thought to have been rare or absent over most of Ireland), whereas Europe had a richer community consisting of deer, aurochs, tarpan, bison, beaver and wild boar. Pollen frequencies for oak, hazel and herbs in Ireland fell within the range of European data, indicating no large effect of mammal herbivores on forest structure and suggesting predominantly high forest vegetation in both places.
However, these studies measured the effects on vegetation of a severely weakened large-herbivore assemblage, which had already lost mammoths, rhinos, hippos and others (Stuart 1999). To test the effects on vegetation of the full Pleistocene suite of large herbivores, Svenning (2002) compared the representation of NAP in pollen assemblages from the Last Interglacial, when they were extant (figure 2), with the Early Holocene after they had gone. The two periods were climatically similar, so differences in vegetation can be attributed to the effects of herbivores, at least tentatively. This comparison suggested that, in uplands, forests were largely closed at the Last Interglacial just as in the Early Holocene, except on infertile sites where there was open woodland or heath in both periods. On flood plains, however, many sites show evidence of open vegetation at the Last Interglacial, while pollen records from the Early Holocene indicate closed forest. High densities of large herbivores on flood plains at the Last Interglacial are evidenced by pollen of herb species characteristic of disturbed ground, and a high abundance and diversity of dung beetles. Early Holocene beetle assemblages have comparatively few dung or open-ground beetles (Svenning 2002).
A specific example of an extinct large herbivore creating open vegetation may be provided by giant deer Megaloceros giganteus in Ireland (Bradshaw & Mitchell 1999). Following retreating ice sheets, giant deer recolonized Ireland at the end of the Last Glacial cycle (Stuart et al. 2004), and increased in abundance between 16 and 13 ka. Initially, the dominant vegetation in their range was willow Salix scrubland, which was progressively replaced by juniper Juniperus and crowberry Empetrum scrub, and then transformed to open grassland. The deer would have eaten all these plants, but shrubs were probably favoured because of their high phosphorous content (Bradshaw & Mitchell 1999). Heavy browsing can therefore account for the succession of scrub to grassland, which does not have a climatic explanation. This succession was interrupted by Younger Dryas (YD) cooling, when giant deer went extinct in Ireland. When warming resumed, the vegetation that re-formed in the absence of giant deer was mixed juniper scrub and birch Betula woodland (Bradshaw & Mitchell 1999).
(b) Australia
Johnson (2006) reviewed long pollen records extending over the period 45–50 ka when mainland Australia's Pleistocene megafauna went extinct, and noted some evidence of post-extinction expansion of shrubland. This is expected, because many of the extinct large herbivores were browsers, but loose chronological control over events in the middle of the Last Glacial cycle means that the evidence is equivocal.
Miller et al. (2005) found evidence of a major reorganization of vegetation coincident with megafauna extinction across the semi-arid southern mainland of Australia. Stable isotope analysis showed that before 50 ka, emus and wombats had broad diets consisting of a mixture of C4 (subtropical and arid grasses) and C3 plants (shrubs, trees and temperate grasses), with a wide range of values among individual samples suggesting heterogeneous foraging environments. By 45 kyr, their diets had contracted to C3 plants only. Miller et al. (2005) argued that this reflected a habitat change from a mosaic of trees, shrubs and grassland to a more uniform shrub steppe. Climate cannot account for this change.
A similar change is implied by Prideaux et al.'s (2007) analysis of a Middle Pleistocene (400−230 kyr ago) fauna from a cave site on the Nullarbor Plain. This site preserves an exceptionally rich community of large mammalian herbivores, consisting of a giant wombat and 18 extinct large kangaroos. The wide range of body sizes, morphologies (including two arboreal kangaroos) and feeding modes in this assemblage indicates a more diverse vegetation than now, probably a mosaic of woodland, shrubland and grassland, with a high proportion of plants with palatable leaves and fleshy fruits. The present-day vegetation is a uniform chenopod shrub steppe (in which the existence of tree kangaroos is unimaginable). The climate experienced by the Middle Pleistocene community was evidently similarly arid to today, so climate change cannot explain the gross simplification of the ecosystem (Prideaux et al. 2007). Both Miller et al. (2005) and Prideaux et al. (2007) suggested that landscape burning by Aboriginal people caused these vegetation changes—the problems with this interpretation are noted below.
Western Tasmania in the Last Glacial was covered by open herbfields with scattered trees and shrubs, and this vegetation appears not to have changed with Tasmania's megafauna extinction (Turney et al. 2008). Why this difference from mainland ecosystems? Turney et al. (2008) reported only broad structural categories of vegetation from pollen analysis, which may have been too coarse to resolve changes in vegetation pattern. Also, the extinct large-herbivore community of Tasmania was relatively simple, consisting of only five medium–large species and not including the 2–3 tonne Diprotodon optatum, the largest Pleistocene herbivore of Australia. If very large species have disproportionate effects as habitat engineers, the impact of Pleistocene herbivores on vegetation may have been less powerful in Tasmania than on much of the mainland where Diprotodon was widespread.
(c) Extinction of the mammoth steppe
During most of the Pleistocene, the unglaciated expanses of northern Eurasia and North America were covered by a vast, low steppe of grasses, forbs and sedges (Zimov et al. 1995; Goetcheus & Birks 2001; Guthrie 2001; Yurtsev 2001; van Geel et al. 2007). This ‘mammoth steppe’ was dry but botanically diverse, and it supported a high biomass of woolly mammoths, bison, horses and other large herbivores. From ca 13 ka, it was replaced by waterlogged habitats of wet mossy tundra, shrub tundra and taiga or deciduous forest, with reduced plant diversity (Guthrie 2001; Kienast et al. 2001; Willerslev et al. 2003, 2008; Edwards et al. 2005).
Zimov et al. (1995) argued that the mammoth steppe had been created by the mammoths themselves (with help from other large herbivores). Heavy cropping by large herbivores suppressed woody regrowth and prevented accumulation of litter, but stimulated above-ground grass production, especially of deep-rooted species resilient to grazing. This led to high rates of transpiration of soil moisture, so that topsoil remained dry and favourable to grass growth. Rapid nutrient recycling via herbivore dung helped maintain productivity (Zimov et al. 1995), while exposure of soil led to more rapid thawing and warmer soils in summer (Walker et al. 2001). Megafaunal extinction sounded the doom of the mammoth steppe. In the absence of heavy grazing, water tables rose, suppressing herbaceous plants but favouring mosses. Mosses have low rates of evapotranspiration and form a continuous insulating layer, so moss-covered soils remained cool and waterlogged. Breakdown and recycling of nutrients slowed down, organic matter accumulated on the soil surface and soil fertility declined. With freedom from browsing, low-growing shrubs and trees regenerated while grasses and other productive herbs declined further.
The transformation of mammoth steppe to tundra is usually explained by the change to a warmer, wetter and less continental climate in the transition to the Holocene (Guthrie 2001). If so, the mammoth steppe should also have disappeared during previous interglacials; by contrast, Zimov's hypothesis predicts that it should have persisted through previous interglacials under the influence of large herbivores, and disappeared only in their absence at the end of the Pleistocene. Kienast et al. (2008) used plant macrofossils to reconstruct in detail plant communities from the Last Interglacial at a site in northeast Siberia. They found a species-rich mosaic of dry steppe vegetation (composed of forbs, sedges and grasses) interspersed with patches of shrub tundra and thickets, arctic herbfields, wetlands and pioneer communities composed of species typical of dry, disturbed sites. A high abundance in the ancient community of grazing-tolerant plants, as well as spores of dung fungi, indicates a strong impact of large herbivores. The current vegetation is a relatively species-poor, wet, mossy tundra. Kienast et al. (2008) interpreted their results as indicating a more continental climate with higher mean temperatures at the Last Interglacial, suggesting that sea levels around northeast Siberia did not rise as high then as they have done in the Holocene.
Well-resolved vegetation histories might also reveal an abrupt change in steppe/tundra vegetation immediately following mega herbivore extinction. Distinguishing this against the background of climate instability of this time is difficult, but Hu et al.'s (2002) detailed vegetation history from Nimgun Lake in southwestern Alaska may be a case where this is possible. This record shows evidence of warming effects on arctic vegetation from 15 ka to 13 ka, but with an especially sharp transition at 13.6 ka consisting of a sudden rise in birch Betula and decline of sedges, along with other indicators of increased moisture and vegetation cover. Hu et al. (2002) interpreted this change as being due to a sharp increase in temperature that is recorded in the oxygen isotope record from the Greenland ice sheet, but that took place 1000 years earlier. The change coincides more closely with mammoth extinction at 13.6 kyr (ago) (Guthrie 2006; Solow et al. 2006). Perhaps the effects of climate warming on vegetation were initially damped by mammoths and other large herbivores, and were only fully realized with their extinction (Hu et al. suggested that the main effect of the temperature increase on local climate and vegetation was via raised sea level, which lagged temperature).
The most powerful test of Zimov et al.'s (1995) hypothesis will be by direct observation of the effects on tundra vegetation of restoration of large herbivores. This is being tested in the bold ‘Pleistocene Park’ experiment in Yakutia, where as many as possible of the original large mammals or their close analogues are being restored to a Siberian tundra environment in hope of recreating a functioning Pleistocene steppe ecosystem (Zimov 2005).
(d) North America
Guthrie (1984) argued that many Pleistocene environments of North America were composed of complex mosaics of steppe, woodland/thicket and closed forest, which maintained high regional diversity in many groups of organisms. In the transition to the Holocene, these mosaics were replaced by large-scale zonal patterns of vegetation. Spatial analysis of mammal communities in the Late Pleistocene shows greater differentiation over scales of several hundred kilometres than in Holocene and current communities, indicating a shift from a fine- to coarse-grained pattern of environmental variation (Graham et al. 1996). This environmental change coincided with North American megafaunal extinction, and Guthrie (1984) argued that it was the cause of that extinction. However, the pace of change in plant communities peaked between 13 and 10 ka (Williams et al. 2004), in the immediate aftermath of megafaunal extinction. During this period, the widespread ‘spruce parkland’ and ‘mixed parkland’ biomes declined to near-extinction. These vegetation types are thought to have consisted of scattered stands of trees among fields with abundant sedges, grasses and other forbs, and may have been kept open by large herbivores (Williams et al. 2001).
Robinson et al. (2005) described changes in vegetation and abundance of large herbivorous mammals at four sites in New York state from ca 19 ka, when ice sheets that covered the area during the last glaciation retreated, to the last few millennia. They used spore counts of the dung fungus Sporormiella as a sensitive proxy measure of biomass of large herbivores. The Early Postglacial vegetation of the region was conifer forest dominated by pine Pinus and spruce Picea, along with less abundant broadleaf tree taxa; sedges were quite abundant, and pollen of grasses and other herbs were also present, indicating a reasonably open and diverse understorey. Fossils of two species of extinct megafauna were recorded at these sites: mastodon Mammut americanum, a browser typically associated with conifer forest, and the stag elk Cervalces, also a browser (but thought to have preferred open forest habitats; Breda 2008). Populations of large herbivores collapsed at ca 14.5 ka, 1500 years before the onset of YD cooling. In the aftermath of the herbivore collapse, the vegetation remained as conifer forest, with some increase in broadleaved taxa and a decline in herbs. Large-herbivore collapse was associated with a change in the nature of sediments, from clays to darker organic muds and peats. This presumably represents increased input of organic matter to sediments, and could reflect an increase in vegetation density and accumulation of uncropped plant material, perhaps along with raised water tables creating consistently wetter conditions.
Other sedimentary profiles across North America show a similar but more short-lived change at close to the same time, represented by the ‘black mats’. The black mats are thin layers of dark sediment, with higher organic matter content than layers immediately above and below them, which were laid down in many places just before the YD (Haynes 2008). They are typically in low-lying sites where marshes, wet meadows or ponds could form and are generally thought to indicate a brief interval of raised water tables leading to saturation of soils, ponding and increased incorporation of organic matter in muds and peats (Quade et al. 1998; Haynes 2008). The charcoal content of black mats is often also high (Firestone et al. 2007). Haynes (2008) suggested that the black mats were due to reduced evaporation, and therefore more effective recharge of groundwater, under cooler temperatures in the approach to the YD but before the development of dry conditions characteristic of the YD itself. It remains puzzling, however, that black mats are such a distinctive feature of this time, but not of other similar climate events such as the Oldest Dryas. Observations of mammoth bones blanketed by black mat deposits show that they were laid down almost immediately after megaherbivore extinction (Haynes 2008).
Most interpretations of the association of black mats and megafaunal extinction assume that this link tells us something about the cause of the extinction. The black mats themselves cannot diagnose that cause, because it is so difficult to fathom how a short-lived increase in water availability could have wiped out mammoths, mastodons and the rest. But their unusual presence at this time hints at something very strange, some unprecedented ‘environmental and biotic disturbance’ (Haynes 2008). Firestone et al. (2007) interpret the black mats as evidence of a catastrophic impact of an extraterrestrial object that caused megafauna extinction, the synchronous termination of the Clovis culture and the YD, but this radical proposal is not widely accepted (Kerr 2007; Gillespie 2008; Haynes 2008).
Perhaps this mystery could be resolved if we considered that rather than being somehow bound up with the cause of megafauna extinction, the black mats formed as a consequence of that extinction. This could have happened by similar mechanisms as proposed by Zimov et al. (1995) for the steppe/tundra transition, and reflected in Robinson et al.'s (2005) data: cessation of cropping by large herbivores leading to thickening of vegetation, accumulation of organic matter, raised water tables, and reduced recycling of nutrients.
(e) New Zealand
New Zealand's prehuman large terrestrial herbivores were the 10 species of moa, together with large terrestrial geese and ducks (Worthy & Holdaway 2002). The diversity and abundance of these herbivores was highest in relatively dry environments on the eastern side of New Zealand, especially on the South Island (Worthy & Holdaway 2002; Rogers et al. 2005). The prehuman vegetation of the southeastern drylands was a complex of dry low forests, woodlands and shrublands, dominated by confers and small-leaved angiosperms (Rogers et al. 2005). Rich assemblages of herb and grass species indicate high light availability in the understorey, and perhaps a patchwork of small glades in a forest/woodland/shrub mosaic.
Rogers et al. (2005) and Lee et al. (in press) suggest that herbivory by moa maintained high species diversity and created habitat mosaics in this environment. Heavy cropping of small trees and shrubs may have kept much of the understorey open, well-lit and suitable for shade-intolerant herbs. Shrubs with divaricate growth were prominent mid-storey plants in wooded habitats; their dense tangles of stems, with small leaves allowing light to reach the ground, might have created ‘nurse thickets’ that protected light-requiring seedlings of other species and promoted patchy regeneration of trees sensitive to browsing (Rogers et al. 2005). In other places, heavy and persistent browsing might have maintained open light-filled glades. Lee et al. (in press) interpret a mid-Holocene pollen diagram from a southeast dryland site (McGlone & Moar 1998) as having ‘…high pollen frequencies of several diminutive herbs unprecedented in contemporary dryland communities or pollen rains’, indicating a decline in herb diversity following herbivore extinction. Rogers et al.'s (2005) review of charcoal diagrams in the south eastern dryland eco-region suggests that not only were fires rare in the prehuman period, but they were also patchy and small-scaled. Why, in this dry and flammable environment, did small fires not rapidly spread and consume large areas? Perhaps heavy cropping of plant matter by large herbivores prevented accumulation of dry fuel, and the vegetation patchiness created by them impeded the spread of fires.
An impact of large herbivores is less obvious in the cool-temperate rainforest that covered most of the rest of low- and mid-altitude New Zealand, except perhaps in one respect. Lee et al. (in press) note that New Zealand forests on productive sites are often rich in conifers. Elsewhere, conifers tend to be restricted to unproductive sites under pressure of competition from fast-growing angiosperms on productive sites (Coomes et al. 2005). A key factor that currently prevents conifer regeneration on productive sites is heavy shading of the forest floor by understorey canopies of tall ferns. Possibly, conifer regeneration once depended on terrestrial herbivores to keep the forest floor open, and a thickening of understorey vegetation in the last few centuries has initiated a long-term conifer decline (Lee et al. in press).
4. Flammable new worlds
The prediction that fire should have increased after megafaunal extinction holds true in most cases where it can be tested, but with much variation in the way in which fire subsequently affected vegetation dynamics. In the northeastern USA, Robinson et al.'s (2005) data are consistent with a pure form of the ‘herbivore replacement’ hypothesis. Burning increased several hundred years after megafaunal extinction, suggesting that plant biomass that had been consumed by herbivores before the extinctions was consumed by fire afterwards, after an interval of increased accumulation of fuel. This did not induce a change in the dominant vegetation type, as would have been the case had fire consumed more biomass than herbivores had formerly done, or selected for plants with very different forms or life strategies.
In Madagascar, Burney et al.'s (2003) investigation using Sporormiella showed that before human arrival, large-herbivore biomass was the highest in the dry southwest of the island, where the vegetation was a semi-arid mosaic of dry forest, palm savannah and bushlands. Fire was rare in this environment while large-herbivore biomass was high. Herbivore biomass collapsed at 2 ka, very soon after human arrival (Burney et al. 2004), and approximately 200 years later there was a sudden rise in charcoal, followed by the conversion of the original wooded vegetation mosaic to grassland (Burney 1993). Elsewhere in Madagascar, the central highlands were covered by mesic wooded savannahs, with low to moderate Sporormiella counts and high charcoal indicating a vegetation shaped more by fire than herbivory, while in the humid rainforests of the north both Sporormiella and charcoal were low. In neither the central highlands nor the north was there a major change in fire and vegetation at 2 ka. Charcoal increased approximately 1000 years later when cattle were introduced and, it seems, fire was used by pastoralists to create grassland.
In the southeastern drylands of New Zealand, fire had been rare before the arrival of people 720 years ago (Wilmshurst et al. 2008), because, although the eastern lowlands are dry and have a naturally flammable vegetation, there was a lack of natural ignition by lightning (Ogden et al. 1998; Rogers et al. 2005). The plants of this region are therefore almost completely lacking in adaptations to survive fire or regenerate in its wake. Most of this vegetation was destroyed by fire after human arrival and replaced by grassland, evidently as a result of intentional burning to remove forest cover (McGlone & Basher 1995; McGlone & Wilmshurst 1999; McGlone 2001). This happened so quickly that it is impossible to tell what effect the removal of herbivores might have had on natural fire regimes.
In Australia, there is little evidence for increased fire linked to megafaunal extinction. Charcoal histories show complex patterns through time, with no strong or general increase in charcoal around human arrival and megafaunal extinction (Lynch et al. 2007; Turney et al. 2008). If anything, the period from 50 to 40 ka had fewer changes in fire pattern than other times (Kershaw et al. 2002; Johnson 2006). This could be because the vegetation changes that followed megafaunal extinction were predominantly expansions of shrublands, which, under the conditions of low CO2, temperature and rainfall in the middle of the Last Glacial cycle, were too sparse to sustain frequent fire (Johnson 2006).
5. The fate of anachronistic plants
The extinction of megafauna left many plant species stranded as anachronisms in a post-megafauna world, investing in defences against non-existent browsers and producing fruit that few or no animals eat, with seeds that go undispersed. Anachronistic plants can be found in all the parts of the world that lost megafauna in recent prehistory.
In Australia, for example, very few native mammals eat Acacia foliage, but many Australian Acacias have defensive spines and growth forms typical of species subject to large-mammal browsing. Some of these Acacias switch from defended to undefended forms at the browse height of the largest extinct marsupials (Johnson 2006). This is one of a series of examples in which defensive growth forms are strongly expressed in younger plants that have foliage within reach of extinct terrestrial browsers, while the same plants assume undefended growth forms above this ghost browse height; others are the genus Cyanea from Hawaii, once browsed by giant geese (Givnish et al. 1994); many plants from the Mascarene Islands that coevolved with giant tortoises (Eskildsen et al. 2004); and New Zealand's divaricate plants.
The divaricate form is characterized by wide-angled branching of tough narrow stems, creating a densely interlaced architecture, and associated with small leaves and reduced leaf number on outer branches. It is thought to have evolved to limit the foraging efficiency of moa (Atkinson & Greenwood 1989; Worthy & Holdaway 2002), as it does living analogues of moa (ostriches and emus; Bond et al. 2004). In the dry thicket vegetation of southwest Madagascar, many plants have thin, wiry divaricating stems and zigzag branch growth (Bond & Silander 2007). These traits make the branches spring-like: when a branch is pulled, it extends a long distance, and immediately retracts when released. Bond & Silander (2007) argued that these ‘wire plants’ had evolved under browsing pressure from elephant birds, which fed by clamping and pulling on stems. Their springiness would have increased handling time for foraging birds, making wire plants less attractive than other plants not defended in this way.
Plants with obsolete defences may well have gone into decline following megafauna extinctions, for three reasons. First, investment in growth forms that helped reduce tissue loss from browsing may have compromised other physiological functions, such as photosynthetic capacity. While such traits conferred fitness benefits in environments with large browsers, they would put the same species at a disadvantage in resource competition with undefended plants once the large browsers were gone. Janzen (1986) reviewed plant traits in the nopaleras, a vegetation formation of the Chihuahuan Desert dominated by large perennial plants with defensive growth forms that presumably evolved under pressure of megafaunal browsers. He suggested that after megafauna extinction, these plants would contract to monospecific stands, each species surviving only where habitat conditions are ideal for it and where it is not exposed to resource competition from others.
Second, many of these plants were originally most successful in ecological zones that were heavily used by large herbivores, which maintained dry and open conditions and suppressed fire. If these conditions changed as a result of herbivore extinction, and especially if fire became a more significant control of vegetation, ‘megafauna plants’ might have declined as a result. Third, in many parts of the world, the original large-herbivore guild has been partially replaced by introduced species with different foraging equipment, against which the ancient defences are useless. For example, divaricate plants in New Zealand are well defended against large browsing birds, but are more susceptible to the browsing ungulates that are the birds' modern replacements (Bond et al. 2004).
Plants species that had depended wholly or partly on large herbivores for seed dispersal should have suffered declines in distribution and genetic variance following megafauna extinction, potentially leading to extinction. Guimaraes et al. (2008) tested for the existence of obsolete ‘megafauna fruit’ in Brazil, by identifying fruit traits of African species currently dispersed by elephants, then mapping those traits onto a comprehensive database of fruit traits in the Brazilian flora. This method identified 103 species matching elephant-dispersed African fruit, and which presumably were once dispersed by gomphotheres in Brazil. Such species are well represented in some plant communities, such as the Pantanal, a region of seasonally wet and dry flood plains in which 30 per cent of fleshy-fruited tree species have fruit with megafaunal characteristics. However, most have restricted distributions, reflecting the fact that few or no living animals disperse their seeds. Molecular genetic analysis of several species demonstrates lack of gene flow, by showing moderate levels of genetic variability within populations but high differentiation among populations. Many show high capacity for vegetative propagation or vigorous resprouting, or are long-lived, or are able to establish beneath the parent plant (Donatti et al. 2007). Probably, local populations persist largely because of these traits, while other megafauna-dispersed species that lacked them may have gone extinct.
Plants with megafauna fruit in Central and North America typically have restricted distributions in lowlands and flood plains, reflecting their current reliance on gravity and water to move large seeds (Janzen & Martin 1982; Barlow 2000). There are also indications of extinction of large-fruited species that were widespread in the Pleistocene, such as in the genus Maclura, which declined from several species to a single narrowly distributed survivor, the Osage orange Maclura pomifera (Barlow 2000). Lee et al. (in press) argue that many New Zealand herbs fit Janzen's (1984) syndrome of small-fruited plants dependent for seed dispersal on incidental consumption of seeds by large folivores, and note that a high proportion of these are now threatened with extinction.
These observations suggest that, in many parts of the world, vegetation communities are in various stages in a process of long-term relaxation from a megafauna-conditioned to a megafauna-naive state, due to initial decline and ultimate extinction of plants that had formerly interacted strongly with extinct large herbivores. Possibly, this process could be reconstructed by comparisons of the representation in plant communities of traits such as structural defences against large terrestrial browsers and megafaunal seed dispersal syndromes, in relation to time since megafauna extinction.
6. Conclusion
There is a vast literature on vegetation changes in the Quaternary, but surprisingly little of it has considered the effects of recently extinct large herbivores on vegetation, or investigated the consequences for vegetation dynamics of megafaunal extinction. There are probably several reasons for this. The argument over whether the megafaunal extinctions had a human or environmental cause has confused the issue. Some extinctions happened at times of rapid climate change. Any changes in vegetation that coincided with extinction are perhaps too readily attributed to changes in temperature, rainfall or atmospheric CO2. This thinking has often gone further, to conclude that extinction of megafauna was a consequence of vegetation change, as if even powerful creatures such as mammoths were sensitively and helplessly subject to climate-driven shifts in vegetation.
There is, however, evidence of significant changes in the structure, composition and pattern of plant communities following megafaunal extinctions, and reason to think that the ecological aftershocks of those extinctions are still with us. Megafaunal extinction was apparently not associated with vegetation change in all places (Barnosky et al. 2004), but it seems clear that in many parts of the world, biodiverse and complex habitat mosaics in dry, lowland wooded landscapes, which had been maintained by large herbivores, were simplified and impoverished as a result of herbivore extinction. Many other vegetation communities contain assemblages of anachronistic plants that may be in long-term decline. To understand living plant communities, we need to re-imagine them with their full complement of Pleistocene megafauna. This insight should also provide the foundation for ecological restoration, which should aim to reinstate interactions between large herbivores and vegetation where that is still possible (Galetti 2004; Donlan et al. 2006).
Acknowledgements
I thank Mauro Galetti, Richard Gillespie, Bill Lee and Euan Ritchie and Guy Robinson for their comments on the manuscript and for providing references, and Richard Cowling and Vance Haynes helpful discussion.
References
- Atkinson I.A.E., Greenwood R.M. Relationships between moas and plants. N. Z. J. Ecol. 1989;12:67–96. [Google Scholar]
- Bakker E.S., Olff H., Vandenberghe C., Maeyer K. de, Smit R., Gleichman J.M., Vera F.W.M. Ecological anachronisms in the recruitment of temperate light-demanding tree species in wooded pastures. J. App. Ecol. 2004;41:571–582. doi:10.1111/j.0021-8901.2004.00908.x [Google Scholar]
- Barlow C. Basic Books; New York, NY: 2000. The ghosts of evolution. [Google Scholar]
- Barnosky A.D., Koch P.L., Feranec R.S., Wing S.L., Shabel A.B. Assessing the causes of Late Pleistocene extinctions on the continents. Science. 2004;306:70–75. doi: 10.1126/science.1101476. doi:10.1126/science.1101476 [DOI] [PubMed] [Google Scholar]
- Birks H.J.B. Mind the gap: how open were European primeval forests? Trends Ecol. Evol. 2005;20:154–156. doi: 10.1016/j.tree.2005.02.001. doi:10.1016/j.tree.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Bodmer R., Ward D. Frugivory in large mammalian herbivores. In: Danell K., Duncan P., Bergstrom R., Pastor J., editors. Large herbivore ecology, ecosystem dynamics and conservation. Cambridge University Press; Cambridge, UK: 2006. pp. 232–260. [Google Scholar]
- Bond W.J., Keeley J.E. Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 2005;20:387–394. doi: 10.1016/j.tree.2005.04.025. doi:10.1016/j.tree.2005.04.025 [DOI] [PubMed] [Google Scholar]
- Bond W.J., Silander J.A. Springs and wire plants: anachronistic defences against Madagascar's extinct elephant birds. Proc. R. Soc. B. 2007;274:1985–1992. doi: 10.1098/rspb.2007.0414. doi:10.1098/rspb.2007.0414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond W.J., Lee W.G., Craine J.M. Plant structural defences against browsing birds: a legacy of New Zealand's extinct moas. Oikos. 2004;104:500–508. doi:10.1111/j.0030-1299.2004.12720.x [Google Scholar]
- Bradshaw R., Mitchell F.J.G. The palaeoecological approach to reconstructing former grazing–vegetation interactions. For. Ecol. Manage. 1999;120:3–12. doi:10.1016/S0378-1127(98)00538-6 [Google Scholar]
- Breda M. Palaeoecology and palaeoethology of the Plio-Pleistocene genus cervalces (Cervidae, Mammalia) in Eurasia. J. Vertebr. Paleontol. 2008;28:886–899. doi:10.1671/0272-4634(2008)28[886:PAPOTP]2.0.CO;2 [Google Scholar]
- Burney D.A. Late Holocene environmental changes in arid southwestern Madagascar. Quat. Res. 1993;40:98–106. doi:10.1006/qres.1993.1060 [Google Scholar]
- Burney D.A., Robinson G.S., Burney L.P. Sporormiella and the Late Holocene extinctions in Madagascar. Proc. Natl Acad. Sci. USA. 2003;100:10800–10805. doi: 10.1073/pnas.1534700100. doi:10.1073/pnas.1534700100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney D.A., Burney L.P., Godfrey L.R., Jungers W.L., Goodman S.M., Wright H.T., Jull A.J.T. A chronology for late prehistoric Madagascar. J. Hum. Evol. 2004;47:25–63. doi: 10.1016/j.jhevol.2004.05.005. doi:10.1016/j.jhevol.2004.05.005 [DOI] [PubMed] [Google Scholar]
- Coomes D.A., et al. The hare, the tortoise and the crocodile: the ecology of angiosperm dominance, conifer persistence and fern filtering. J. Ecol. 2005;93:918–935. doi:10.1111/j.1365-2745.2005.01012.x [Google Scholar]
- Cooper S.M., Owen-Smith N. Effects of plant spinescence on large mammalian herbivores. Oecologia. 1986;68:446–455. doi: 10.1007/BF01036753. doi:10.1007/BF01036753 [DOI] [PubMed] [Google Scholar]
- Donatti C.I., Galetti M., Pizo M.A., Guimaraes P.R.J., Jordano P. Living in the land of ghosts: fruit traits and the importance of large mammals as seed dispersers in the Pantanal, Brazil. In: Dennis A.J., Green R.J., Schupp E.W., Westcott D.A., editors. Seed dispersal: theory and its application in a changing world. CAB International; Wallingford, CT: 2007. pp. 104–123. [Google Scholar]
- Donlan C.J., et al. Pleistocene rewilding: an optimistic agenda for twenty-first century conservation. Am. Nat. 2006;168:660–681. doi: 10.1086/508027. doi:10.1086/508027 [DOI] [PubMed] [Google Scholar]
- Dublin H.T., Sinclair A.R.E., McGlade J. Elephants and fire as causes of multiple stable states in the Serengeti–Mara woodlands. J. Anim. Ecol. 1990;59:1147–1164. doi:10.2307/5037 [Google Scholar]
- Edwards M.E., Brubaker L.B., Lozhkin A.V., Anderson P.M. Structurally novel biomes: a response to past warming in Beringia. Ecology. 2005;86:1696–1703. doi:10.1890/03-0787 [Google Scholar]
- EPICA. Eight glacial cycles from an Antarctic ice core. Nature. 2004;429:623–628. doi: 10.1038/nature02599. doi:10.1038/nature02599 [DOI] [PubMed] [Google Scholar]
- Eskildsen L.I., Olesen J.M., Jones C.G. Feeding response of the Aldabra giant tortoise (Geochelone gigantea) to island plants showing heterophylly. J. Biogeogr. 2004;31:1785–1790. doi:10.1111/j.1365-2699.2004.01092.x [Google Scholar]
- Firestone R.B., et al. Evidence for an extraterrestrial impact 12 900 years ago that contributed to the megafaunal extinctions and the Younger Dryas cooling. Proc. Natl Acad. Sci. USA. 2007;104:16016–16021. doi: 10.1073/pnas.0706977104. doi:10.1073/pnas.0706977104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery T.F. Pleistocene faunal loss: implications of the after-shock for Australia's past and future. Archaeology in Oceania. 1990;25:45–67. [Google Scholar]
- Fyfe R. The importance of local-scale openness within regions dominated by closed woodland. J. Quat. Sci. 2007;22:571–578. doi:10.1002/jqs.1078 [Google Scholar]
- Galetti M. Parks of the Pleistocene: recreating the Cerrado and the Pantanal with megafauna. Natureza Conservacao. 2004;2:93–100. [Google Scholar]
- Gill R. The influence of large herbivores on tree recruitment and forest dynamics. In: Danell K., Bergstrom R., Duncan P., Pastor J., editors. Large herbivore ecology, ecosystem dynamics and conservation. Cambridge University Press; Cambridge, UK: 2006. pp. 170–202. [Google Scholar]
- Gillespie R. Updating Martin's glogal extinction model. Quat. Sci. Rev. 2008;27:2522–2529. doi:10.1016/j.quascirev.2008.09.007 [Google Scholar]
- Gillespie R., Brook B.W., Baynes A. Short overlap of humans and megafauna in Pleistocene Australia. Alcheringa. 2006;1(Special issue):163–186. doi:10.1080/03115510608619580 [Google Scholar]
- Givnish T.J., Sytsma K.J., Smith J.F., Hahn W.J. Thorn-like prickles and heterophylly in Cyanea: adaptations to extinct avian browsers on Hawaii? Proc. Natl Acad. Sci. USA. 1994;91:2810–2814. doi: 10.1073/pnas.91.7.2810. doi:10.1073/pnas.91.7.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetcheus V.G., Birks H.H. Full-glacial upland tundra vegetation preserved under tephra in the Beringia National Park, Seward Peninsula, Alaska. Quat. Sci. Rev. 2001;20:135–147. doi:10.1016/S0277-3791(00)00127-X [Google Scholar]
- Graham R.W., et al. Spatial response of mammals to late quaternary environmental fluctuations. Science. 1996;272:1601–1606. doi: 10.1126/science.272.5268.1601. doi:10.1126/science.272.5268.1601 [DOI] [PubMed] [Google Scholar]
- Grubb P.J. A positive distrust of simplicity—lessons from plant defences and from competition among plants and among animals. J. Ecol. 1992;80:585–610. doi:10.2307/2260852 [Google Scholar]
- Guimaraes P.R., Jr, Galetti M., Jordano P. Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate. PLoS ONE. 2008;3:e1745. doi: 10.1371/journal.pone.0001745. doi:10.1371/journal.pone.0001745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie R.D. Mosaics, allelochemicals, and nutrients: an ecological theory of Late Pleistocene megafaunal extinctions. In: Martin P.S., Klein R.G., editors. Quaternary extinctions: a prehistoric revolution. University of Arizona Press; Tucson, AZ: 1984. pp. 259–298. [Google Scholar]
- Guthrie R.D. Origin and causes of the mammoth steppe: a story of cloud cover, woolly mammal tooth pits, buckles, and inside–out Beringia. Quat. Sci. Rev. 2001;20:549–574. doi:10.1016/S0277-3791(00)00099-8 [Google Scholar]
- Guthrie R.D. New carbon dates link climate change with human colonization and Pleistocene extinctions. Nature. 2006;441:207–209. doi: 10.1038/nature04604. doi:10.1038/nature04604 [DOI] [PubMed] [Google Scholar]
- Haynes C.V., Jr Younger Dryas ‘black mats’ and the Rancholabrean termination in North America. Proc. Natl Acad. Sci. USA. 2008;105:6520–6525. doi: 10.1073/pnas.0800560105. doi:10.1073/pnas.0800560105 0800560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester A., Bergman M., Iason G.R., Moen J. Impacts of large herbivores on plant community structure and dynamics. In: Danell K., Bergstrom R., Duncan P., Pastor J., editors. Large herbivore ecology, ecosystem dynamics and conservation. Cambridge University Press; Cambridge, UK: 2006. pp. 97–141. [Google Scholar]
- Hu F.S., Lee B.Y., Kaufman D.S., Yoneji S., Nelson D.M., Henne P.D. Response of tundra ecosystem in southwestern Alaska to Younger-Dryas climatic oscillation. Glob. Chang. Biol. 2002;8:1156–1163. doi:10.1046/j.1365-2486.2002.00550.x [Google Scholar]
- Janzen D.H. Dispersal of small seeds by big herbivores: foliage is the fruit. Am. Nat. 1984;123:338. doi:10.1086/284208 [Google Scholar]
- Janzen D.H. Chihuahuan desert nopaleras: defaunated big mammal vegetation. Annu. Rev. Ecol. Syst. 1986;17:595–636. doi:10.1146/annurev.es.17.110186.003115 [Google Scholar]
- Janzen D.H., Martin P.S. Neotropical anachronisms: the fruits the gomphotheres ate. Science. 1982;215:19–27. doi: 10.1126/science.215.4528.19. doi:10.1126/science.215.4528.19 [DOI] [PubMed] [Google Scholar]
- Johnson C.N. Cambridge University Press; Cambridge, UK: 2006. Australia's mammal extinctions: a 50 000 year history. [Google Scholar]
- Kerr R.A. Mammoth-killer impact gets mixed reception from earth scientists. Science. 2007;316:1264–1265. doi: 10.1126/science.316.5829.1264. doi:10.1126/science.316.5829.1264 [DOI] [PubMed] [Google Scholar]
- Kershaw A.P., Clark J.S., Gill A.M., D'Costa D.M. A history of fire in Australia. In: Bradstock R.A., Williams J.E., Gill A.M., editors. Flammable Australia: the fire regimes and biodiversity of a continent. Cambridge University Press; Cambridge, UK: 2002. pp. 3–25. [Google Scholar]
- Kienast F., Siegert C., Dereviagin A., Mai D.H. Climatic implications of late quaternary plant macrofossil assemblages from the Taymyr Peninsula, Siberia. Glob. Planet. Change. 2001;31:265–281. doi:10.1016/S0921-8181(01)00124-2 [Google Scholar]
- Kienast F., Tarasov P., Schirrmeister L., Grosse G., Andreev A.A. Continental climate in the east Siberian Arctic during the last interglacial: implications from palaeobotanical records. Glob. Planet. Change. 2008;60:535–562. doi:10.1016/j.gloplacha.2007.07.004 [Google Scholar]
- Knapp A.K., Blair J.M., Briggs J.M., Collins S.L., Hartnett D.C., Johnson L.C., Towne E.G. The keystone role of bison in north American tallgrass prairie—bison increase habitat heterogeneity and alter a broad array of plant, community, and ecosystem processes. Bioscience. 1999;49:39–50. doi:10.2307/1313492 [Google Scholar]
- Koch P.L., Barnosky A.D. Late quaternary extinctions: state of the debate. Annu. Rev. Ecol. Evol. Syst. 2006;37:215–250. doi:10.1146/annurev.ecolsys.34.011802.132415 [Google Scholar]
- Lee, W. G., Wood, J. R. & Rogers, G. M. In press. Legacy of avian-dominated plant–herbivore systems in New Zealand. N. Z. J. Ecol
- Lynch A.H., Beringer J., Kershaw P., Marshall A., Mooney S., Tapper N., Turney C., Van Der Kaars S. Using the paleorecord to evaluate climate and fire interactions in Australia. Annu. Rev. Earth Planet. Sci. 2007;35:215–239. doi:10.1146/annurev.earth.35.092006.145055 [Google Scholar]
- Martin P., Klein R., editors. Quaternary extinctions. University of Arizona Press; Tuscon, AZ: 1984. [Google Scholar]
- Martin P.S. University of California Press; Berkeley/Los Angeles, CA: 2005. Twilight of the mammoths. [Google Scholar]
- Martin P.S., Steadman D.W. Prehistoric extinctions on islands and continents. In: MacPhee R.D.E., editor. Extinctions in near time: causes, contexts and consequences. Kluwer Academic/Plenum Publishers; New York, NY: 1999. pp. 17–56. [Google Scholar]
- McGlone M.S. The origin of the indigenous grasslands of southeastern South Island in relation to pre-human woody ecosystems. N. Z. J. Ecol. 2001;25:1–15. [Google Scholar]
- McGlone M.S., Basher L.R. The deforestation of the upper awatere catchment, Inland Kaikoura Range, Marlborough, South Island, New Zealand. N. Z. J. Ecol. 1995;19:53–66. [Google Scholar]
- McGlone M.S., Moar N.T. Dryland Holocne vegetation history, Central Otago and the Mackenzie Basin, South Island, New Zealand. N. Z. J. Bot. 1998;36:91–111. [Google Scholar]
- McGlone M.S., Wilmshurst J.M. Dating initial Maori environmental impact in New Zealand. Quat. Int. 1999;59:5–16. doi:10.1016/S1040-6182(98)00067-6 [Google Scholar]
- Miller G.H., Fogel M.L., Magee J.W., Gagan M.K., Clarke S.J., Johnson B.J. Ecosystem collapse in Pleistocene Australia and a human role in megafaunal extinction. Science. 2005;309:287–290. doi: 10.1126/science.1111288. doi:10.1126/science.1111288 [DOI] [PubMed] [Google Scholar]
- Mitchell F.J.G. How open were European primeval forests? Hypothesis testing using palaeoecological data. J. Ecol. 2005;93:168–177. doi:10.1111/j.1365-2745.2004.00964.x [Google Scholar]
- Ogden J., Basher L.E.S., McGlone M. Fire, forest regeneration and links with early human habitation: evidence from New Zealand. Ann. Bot. 1998;81:687–696. doi:10.1006/anbo.1998.0637 [Google Scholar]
- Owen R. Adam and Charles Black; Edinburgh, UK: 1861. Palaeontology, or, a systematic study of extinct animals and their geological relations. [Google Scholar]
- Owen-Smith N. The interaction of humans, megaherbivores and habitats in the Late Pleistocene extinction event. In: MacPhee R.D.E., editor. Extinctions in near time: causes, contexts and consequences. Kluwer Academic/Plenum Publishers; New York, NY: 1999. pp. 57–70. [Google Scholar]
- Owen-Smith R.N. Cambridge University Press; Cambridge, UK: 1988. Megaherbivores: the influence of very large body size on ecology. [Google Scholar]
- Pitul'ko V. Terminal Pleistocene—Early Holocene occupation in northeast Asia and the Zhokhov assemblage. Quat. Sci. Rev. 2001;20:267–275. doi:10.1016/S0277-3791(00)00117-7 [Google Scholar]
- Prideaux G.J., et al. An arid-adapted Middle Pleistocene vertebrate fauna from south-central Australia. Nature. 2007;445:422–425. doi: 10.1038/nature05471. doi:10.1038/nature05471 [DOI] [PubMed] [Google Scholar]
- Quade J., Forester R.M., Pratt W.L., Carter C. Black mats, spring-fed streams, and late-glacial-age recharge in the southern great basin. Quat. Res. 1998;49:129–148. doi:10.1006/qres.1997.1959 [Google Scholar]
- Robinson G.S., Burney L.P., Burney D.A. Landscape paleoecology and megafaunal extinction in southeastern New York state. Ecol. Monogr. 2005;75:295–315. doi:10.1890/03-4064 [Google Scholar]
- Roebroeks W. Time for the Middle to Upper Paleolithic transition in Europe. J. Hum. Evol. 2008;55:918–926. doi: 10.1016/j.jhevol.2008.08.008. doi:10.1016/j.jhevol.2008.08.008 [DOI] [PubMed] [Google Scholar]
- Rogers, G., Walker, S. & Lee, W. G. 2005 The role of disturbance in dryland New Zealand: past and present. Department of Conservation, Wellington, New Zealand.
- Smith F.A., Lyons S.K., Ernest S.K.M., Jones K.E., Kaufman D.M., Dayan T., Marquet P.A., Brown J.H., Haskell J.P. Body mass of late quaternary mammals. Ecology. 2003;84:3403. doi:10.1890/02-9003 [Google Scholar]
- Soepboer W., Lotter A.F. Estimating past vegetation openness using pollen–vegetation relationships: a modelling approach. Rev. Palaeobot. Palynol. 2009;153:102–107. doi:10.1016/j.revpalbo.2008.07.004 [Google Scholar]
- Solow A.R., Roberts D.L., Robbirt K.M. On the Pleistocene extinctions of Alaskan mammoths and horses. Proc. Natl Acad. Sci. USA. 2006;103:7351–7353. doi: 10.1073/pnas.0509480103. doi:10.1073/pnas.0509480103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart A.J. Late Pleistocene megafaunal extinctions: a European perspective. In: MacPhee R.D.E., editor. Extinctions in near time: causes, contexts and consequences. Kluwer Academic/Plenum Publishers; New York, NY: 1999. pp. 257–270. [Google Scholar]
- Stuart A.J. The extinction of woolly mammoth (Mammuthus primigenius) and straight-tusked elephant (Palaeoloxodon antiquus) in Europe. Quat. Int. 2005;126–128:171–177. doi:10.1016/j.quaint.2004.04.021 [Google Scholar]
- Stuart A.J., Kosintsev P.A., Higham T.F.G., Lister A.M. Pleistocene to Holocene extinction dynamics in giant deer and woolly mammoth. Nature. 2004;431:684–689. doi: 10.1038/nature02890. doi:10.1038/nature02890 [DOI] [PubMed] [Google Scholar]
- Surovell T., Waguespack N., Brantingham P.J. Global archaeological evidence for proboscidean overkill. Proc. Natl Acad. Sci. USA. 2005;102:6231–6236. doi: 10.1073/pnas.0501947102. doi:10.1073/pnas.0501947102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenning J.-C. A review of natural vegetation openness in north-western Europe. Biol. Conserv. 2002;104:133–148. doi:10.1016/S0006-3207(01)00162-8 [Google Scholar]
- Turney C.S.M., et al. Late-surviving megafauna in Tasmania, Australia, implicate human involvement in their extinction. Proc. Natl Acad. Sci. USA. 2008;105:12150–12153. doi: 10.1073/pnas.0801360105. doi:10.1073/pnas.0801360105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geel B., Zazula G.D., Schwegerc C.E. Spores of coprophilous fungi from under the Dawson tephra (25,300 14C years BP), Yukon Territory, northwestern Canada. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007;252:481–485. doi:10.1016/j.palaeo.2007.04.017 [Google Scholar]
- Vera F.W.M. CABI Publishing; Oxford, UK: 2000. Grazing ecology and forest history. [Google Scholar]
- Vera F.W.M., Bakker E.S., Olff H. Large herbivores: missing partners of western European light-demanding tree and shrub species? In: Danell K., Duncan P., Bergstrom R., Pastor J., editors. Large herbivore ecology, ecosystem dynamics and conservation. Cambridge University Press; Cambridge, UK: 2006. pp. 203–231. [Google Scholar]
- Waldram M., Bond W., Stock W. Ecological engineering by a mega-grazer: white rhino impacts on a South African Savanna. Ecosystems. 2008;11:101–112. doi:10.1007/s10021-007-9109-9 [Google Scholar]
- Walker D.A., Bockheim J.G., Chapin Iii F.S., Eugster W., Nelson F.E., Ping C.L. Calcium-rich tundra, wildlife, and the ‘mammoth steppe’. Quat. Sci. Rev. 2001;20:149–163. doi:10.1016/S0277-3791(00)00126-8 [Google Scholar]
- Wallace A.R. The geographical distribution of animals, with a study of the relations of living and extinct faunas as elucidating past changes of the earth's surface. vol. 1. Harper and Brothers; New York, NY: 1876. [Google Scholar]
- Wetterich S., Kuzmina S., Andreev A.A., Kienast F., Meyer H., Schirrmeister L., Kuznetsova T., Sierralta M. Palaeoenvironmental dynamics inferred from late quaternary permafrost deposits on Kurungnakh Island, Lena Delta, northeast Siberia, Russia. Quat. Sci. Rev. 2008;27:1523–1540. doi:10.1016/j.quascirev.2008.04.007 [Google Scholar]
- Willerslev E., et al. Diverse plant and animal genetic records from Holocene and Pleistocene sediments. Science. 2003;300:791–795. doi: 10.1126/science.1084114. doi:10.1126/science.1084114 [DOI] [PubMed] [Google Scholar]
- Williams J.W., Shuman B.N., Webb T. Dissimilarity analyses of Late-Quaternary vegetation and climate in eastern North America. Ecology. 2001;82:3346–3362. [Google Scholar]
- Williams J.W., Shuman B.N., Webb T.I., Bartlein P.J., Leduc P.L. Late-quaternary vegetation dynamics in North America: scaling from taxa to biomes. Ecol. Monogr. 2004;74:309–334. doi:10.1890/02-4045 [Google Scholar]
- Wilmshurst J.M., Anderson A.J., Higham T.F.G., Worthy T.H. Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proc. Natl Acad. Sci. USA. 2008;105:7676–7680. doi: 10.1073/pnas.0801507105. doi:10.1073/pnas.0801507105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthy T.H., Holdaway R.N. Canterbury University Press; Christchurch, New Zealand: 2002. The lost world of the moa. [Google Scholar]
- Yurtsev B.A. The Pleistocene ‘tundra-steppe’ and the productivity paradox: the landscape approach. Quat. Sci. Rev. 2001;20:165–174. doi:10.1016/S0277-3791(00)00125-6 [Google Scholar]
- Zimov S.A. Pleistocene park: return of the mammoth's ecosystem. Science. 2005;308:796–798. doi: 10.1126/science.1113442. doi:10.1126/science.1113442 [DOI] [PubMed] [Google Scholar]
- Zimov S.A., Chuprynin V.I., Oreshko A.P., Iii F.S.C., Reynolds J.F., Chapin M.C. Steppe–tundra transition: a herbivore-driven biome shift at the end of the Pleistocene. Am. Nat. 1995;146:765–794. doi:10.1086/285824 [Google Scholar]