Abstract
Context
Recent randomized trials among patients with pre-existing cardiovascular disease (CVD) have failed to support benefits of B-vitamin supplementation on cardiovascular risk. Observational data suggest benefits may be greater among women, who have been underrepresented in published randomized trials.
Objective
To test whether a combination of folic acid, vitamin B6, and vitamin B12 lowers risk of CVD among high-risk women with and without CVD.
Design, Setting, and Participants
Within an ongoing randomized trial of antioxidant vitamins, 5,442 female US health professionals 42 years of age or older, with either a history of CVD or three or more coronary risk factors were randomized to a combination pill containing folic acid, vitamin B6, and vitamin B12 or a matching placebo and were followed for 7.3 years from April, 1998 until July, 2006.
Intervention
2.5 mg of folic acid, 50 mg of vitamin B6, and 1 mg vitamin B12
Main Outcome Measures
A composite outcome of myocardial infarction (MI), stroke, coronary revascularization, or CVD mortality.
Results
Compared to placebo, active treatment with the combination pill did not decrease the risk of the primary combined endpoint (226.9 per 10,000 person-years versus 219.2 per 10,000 person-years; relative risk =1.03; 95 percent confidence interval, 0.90–1.19, P=0.65), or any of the secondary outcomes including MI (34.5 per 10,000 person-years versus 39.5 per 10,000 person-years; relative risk =0.87; 95 percent confidence interval, 0.63–1.22), stroke (41.9 per 10,000 person-years versus 36.8 per 10,000 person-years in placebo; relative risk =1.14; 95 percent confidence interval, 0.82–1.57), and CVD mortality (50.3 per 10,000 person-years versus 49.6 per 10,000 person-years; relative risk =1.01; 95 percent confidence interval, 0.76–1.35). In a blood sub-study, geometric mean plasma homocysteine level was decreased by 18.5% (95% CI, 12.5–24.1; P<0.001) in the active arm (n=150) over that observed in the placebo arm (n=150) for a difference of 2.27 μmol per liter (95% CI, 1.54 –2.96).
CONCLUSION
Over the longest follow-up recorded thus far, a combination of folic acid/vitamin B6/vitamin B12 did not reduce a combined endpoint of total cardiovascular events among high-risk women despite significant homocysteine lowering.
Trial Registration
clinicaltrials.gov Identifier: NCT00000541
INTRODUCTION
Homocysteine levels have been directly associated with cardiovascular risk in observational studies1; and daily supplementation with folic acid, vitamin B6, and/or vitamin B12 have been shown to reduce homocysteine levels to varying degrees in intervention studies2. Based upon these data, several randomized trials were designed to test the hypothesis that supplementation with folic acid and/or B-vitamins would prevent cardiovascular disease (CVD). However, currently published trials among patients with pre-existing vascular disease have not demonstrated a benefit of folic acid or B-vitamins on CVD risk3. Participants in observational studies were followed for longer durations then participants in randomized trials 1; and therefore, it is plausible that homocysteine lowering may have had a greater impact if participants were treated and followed for longer periods of time. Meta-analyses of randomized trials do suggest that benefits may be greater with longer treatment durations4, but the majority of published trials have two or less years of follow-up3, with only one trial having five years of follow-up.5
Women have been underrepresented both in observational studies and in randomized trials of homocysteine lowering, and limited data available from meta-analysis of observational studies suggest that women may benefit from homocysteine lowering to a greater extent. In the most recent meta-analysis of these observational studies, a 25% lower homocysteine level was associated with a 32% (95% CI, 15%–45%) lower risk of CHD among women as compared to a 15% lower risk (95% CI, 8%–21%) among men 1. Although women have been included in meta-analyses of randomized trials3, 6, the relative risks for women have not been separately estimated. Given the paucity of data among women and the known influences of estrogen on homocysteine levels7, 8, adequately powered randomized trials of homocysteine lowering among women are still needed9.
In the present study, the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS), a randomized double-blind, placebo-controlled trial, we tested whether a combination of folic acid, vitamin B6, and vitamin B12 would reduce total cardiovascular events among women at high risk for the development of CVD over the longest reported treatment duration and follow-up. Also, we present data that begins to explore remaining questions regarding the likelihood of potential benefits in the setting of folic acid fortification, and the role dietary folate intake plays in modifying the effectiveness of these agents.
METHODS
Study Design
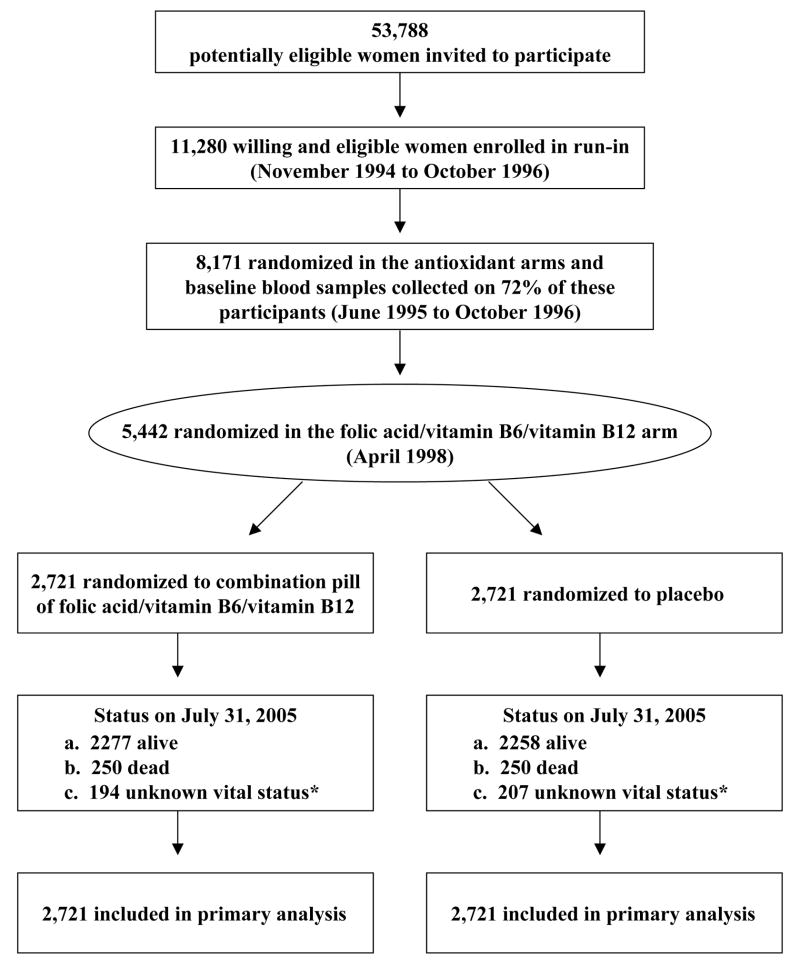
WAFACS is a randomized, double-blind, placebo-controlled trial evaluating whether a combination pill of folic acid (2.5 mg daily), vitamin B6 (50 mg daily), and vitamin B12 (1 mg daily) reduces the risk of important vascular events among high risk women with either a history of CVD or at least three cardiovascular risk factors. The WAFACS trial began in 1998, when the folic acid/vitamin B6/B12 component was added to an ongoing 2×2×2 factorial trial of three antioxidant vitamins (vitamin C, E, and beta-carotene), the Women’s Antioxidant Cardiovascular Study (WACS), expanding it to a four-arm factorial trial. The factorial design allows an examination not only of the main effects of each agent but also of potential interactions between agents. Interactions between antioxidant vitamins and folic acid were plausible, as homocysteine lowering might have antioxidant effects10. Details of the overall trial design11 and the results for the antioxidant arm have been reported previously12. This report describes the CVD results of the folic acid/vitamin B6/B12combination pill treatment arm (Figure 1).
Figure 1.
Flow Diagram of the Folic Acid/Vitamin B6/Vitamin B12 Component of the Women’s Antioxidant and Folic Acid Cardiovascular Study.
* Mortality information was complete for over 99% of person-years of follow-up.
The study was sponsored by the National Heart, Lung, and Blood Institute of the National Institutes of Health. The study vitamins and matching placebo were provided by BASF Corporation, Mount Olive, NJ. The trial was approved by the institutional review board of the Brigham and Women’s Hospital, Boston, MA, and all patients provided written informed consent. An external independent data and safety monitoring board monitored the safety of the participants and the overall quality and scientific integrity of the study.
Study Population
In the parent trial, WACS, 8171 female health professionals throughout the United States were randomized in a 2 × 2 × 2 factorial design between June 1995 and October 1996 to vitamin C (500 mg/day), vitamin E (600 IU every other day), and beta-carotene (50 mg every other day) versus respective matching placebos, yielding eight treatment groups. Of these women, 5,922 (72.5%) returned a blood sample at the beginning of the trial, prior to the initiation of folic acid fortification; and 98.8% completed a semi-quantitative food-frequency questionnaire, which was utilized to assess baseline dietary intake, including folate.
Women were eligible for WACS if they were 40 years or older, postmenopausal or had no intention of becoming pregnant, and had a reported history of CVD or had at least three cardiac risk factors. CVD was defined as a reported history of myocardial infarction (MI), stroke, coronary or peripheral revascularization, angina pectoris, or transient ischemic attack. Qualifying cardiac risk factors were diagnosed hypertension, high cholesterol, diabetes mellitus, parental history of premature MI (i.e., before age 60), obesity (body mass index ≥ 30 kg/m2), and current cigarette use 12. Women were excluded if they had a history of cancer (excluding nonmelanoma skin cancer) within the past ten years, any serious non-CVD illness, or were currently using warfarin or other anticoagulants.
To be eligible for the folic acid/vitamin B6/B12 component, potential participants in the ongoing eight arm trial had to be additionally willing to forgo individual supplements of folic acid, vitamin B6, and vitamin B12 at levels beyond the U.S. recommended daily allowance (RDA) of 400 mcg of folic acid, 2 mg of vitamin B6, or 6 mg of vitamin B12 2 during the trial. Multivitamin use at or below these RDA levels was allowed. In April 1998, 5,442 of these women who were willing were additionally randomized to receive a combination pill containing 2.5 mg of folic acid, 50 mg of vitamin B6, and 1 mg vitamin B12 (active treatment) or matching placebo daily, thus creating a total of 16 distinct treatment groups. All study investigators, personnel, and participants were unaware of the participants’ treatment assignments.
Follow-up procedures
Following randomization, and annually thereafter, women were mailed monthly calendar packs containing active agents or placebos. Participants were followed annually with questionnaires on compliance, use of non-study supplements, and occurrence of major illnesses or adverse effects. Written permission for medical records was sought from participants who reported cardiovascular endpoints or from the next of kin in case of death. Death certificates were also obtained. An endpoints committee of physicians who were blinded to randomized treatment assignment adjudicated all primary and secondary cardiovascular outcome events.
Study medications and end point ascertainment were continued in a blinded fashion until the scheduled end of the trial, July 31, 2005. Follow-up and validation of reported end-points were completed in July, 2006 for a follow-up duration of 7.3 years. At the scheduled end of the trial in 7/31/2005, morbidity and mortality follow-up was 92.6% complete. If counted in terms of person-time, mortality information was complete for over 99% of person-years of follow-up.
Blood Substudy
Women in WAFACS who donated a baseline blood sample in 1996 prior to the initiation of background dietary folic acid fortification in the US food supply in 1998 were eligible for this preplanned sub-study. From the 2596 eligible women, 300 (150 in the active treatment and 150 in the placebo arm) women provided a blood sample at the end of randomized treatment. These women were randomly selected from among those who were compliant with study medications. Plasma levels of folate (Roche chemiluminescence method on the 2010 Elecsys auto-immunoanalyzer, Roche Diagnostics) and homocysteine (Roche enzymatic assay on the Hitachi 917 analyzer, Roche Diagnostics) were measured in baseline and follow-up samples in a blinded fashion in the same analytical run.
Study outcomes
The primary outcome was a combined end point of cardiovascular morbidity and mortality, which included incident MI, stroke, coronary revascularization procedures (coronary artery bypass grafting or percutaneous coronary intervention), and cardiovascular mortality. The individual components of total MI, total stroke, and total coronary heart disease events (MI, coronary revascularization, and CHD death) were pre-specified secondary end points.
A MI was confirmed if symptoms met World Health Organization criteria and if the event was associated with either diagnostic ECG changes or elevated cardiac enzymes. Coronary revascularization was confirmed by medical record review. Confirmed stroke was defined as a new neurologic deficit of sudden onset that persisted for more than 24 hours or until death within 24 hours. Clinical information, computed tomographic scans, and magnetic resonance images were used to distinguish hemorrhagic from ischemic events. Coronary revascularization was confirmed if a coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) was documented in the medical record. Death due to cardiovascular cause was confirmed by examination of autopsy reports, death certificates, medical records, and information obtained from the next of kin or other family members. Death from any cause was confirmed by the end points committee on the basis of a death certificate. Only confirmed end points were included in these analyses, except for total mortality, which included an additional 43 reported deaths.
Power calculations
Power calculations were performed under the assumption that all four agents would have beneficial effects on CVD which would be additive (on the log scale) when used in combination. This affects the power calculations by reducing the incidence in all exposed groups and provides a conservative estimate of the study’s power to detect effects of each agent. We assumed additive 10% risk reductions for each of the antioxidant vitamins and the folic acid/vitamin B6/vitamin B12 combination pill. We estimated that the 5442 women who were randomized would provide 82% power to detect an observed 20% reduction in the primary endpoint.
Statistical Analysis
Baseline characteristics were compared by randomized groups using t-tests, chi-square tests for proportions, and tests for trend for ordinal categories. Primary analyses were performed on an intent-to-treat basis, including all 2721 randomized women in each treatment group, as randomized. For both the primary and secondary analyses, person time was calculated until the first confirmed endpoint specified by the analysis or to the end of the trial if no endpoint occurred. Kaplan-Meier curves were used to estimate cumulative incidence over time by randomized treatment group, and the log-rank test was used to compare survival curves. Cox proportional hazards models were used to calculate relative risks, expressed as a hazards ratio, and 95% confidence intervals after adjustment for age and other randomized treatment assignments (vitamin E, vitamin C, and beta-carotene). The proportionality assumption was tested using an interaction term for treatment with log time, and was met for each of the primary and secondary analyses. To examine the impact of noncompliance, a post-hoc sensitivity analysis censored women when they stopped taking at least two-thirds of their study medications, reported taking outside supplements, or were missing compliance information.
Pre-specified subgroup analyses according to antioxidant treatment assignment(s), presence or absence of prior CVD, dietary folic acid intake, smoking, diabetes, aspirin, hormone therapy, and multivitamin use were performed using stratified Cox proportional hazards models. These analyses utilized baseline exposure assessments and were restricted to those participants with non-missing subgroup data at baseline. Additional exploratory subgroup analyses were conducted to evaluate the consistency of the results. Tests for effect modification by subgroup used interaction terms between subgroup indicators and randomized assignment, with a test for trend for ordinal subgroup categories. The raw distributions and median values of plasma homocysteine and folate levels in the blood substudy were compared using the nonparametric Wilcoxon Rank Sum test. For homocysteine, geometric means were compared after natural logarithmic transformation to compare differences between treatment groups. Analyses were conducted using SAS version 9 (SAS Institute, Cary, NC), using two-sided tests with a significance level of 0.05.
RESULTS
Characteristics of the Patients
In April 1998, 2721 female health professionals participating in the WACS trial were randomly assigned to active treatment with folic acid, vitamin B6, and vitamin B12 and 2721 were assigned to placebo. Baseline characteristics at the time of randomization in 1998 and responses to the dietary questionnaire administered prior to randomization in 1996 are displayed in table 1. The mean age of the population was 62.8 years and 64.2 % of women had a history of CVD. There were no statistically significant differences in baseline characteristics or in dietary intake of study vitamins between the randomized groups (Table 1). The median dietary intake of folic acid including supplements was 432 ug, of which approximately 15% (63 ug) was estimated to be derived from dietary intake of folic acid fortified grains. The use of permitted multivitamins with less than the RDA of folic acid, vitamin B6, and vitamin B12 at least 4 days per month ranged from 19 percent in the beginning to 31 percent at the end of the trial.
Table 1.
Baseline Characteristics of the Entire WAFACS Participants and the Blood Substudy
| Characteristic | Active Group N=2721 | Placebo Group N=2721 |
|---|---|---|
| Age (years), mean ± standard deviation | 62.8 ± 8.8 | 62.8 ± 8.8 |
| Age (years), no (%) 40–54 |
582 (21.4) | 584 (21.5) |
| 55–64 | 990 (36.4) | 970 (35.6) |
| 65+ | 1149 (42.2) | 1167 (42.9) |
| Prior Cardiovascular Disease*, no (%) | 1764 (64.8) | 1728 (63.5) |
| Risk Factors, no (%) Hypertension† |
2360 (86.7) | 2335 (85.8) |
| Elevated Cholesterol‡ | 2118 (77.8) | 2150 (79.0) |
| Diabetes | 570 (21.0) | 574 (21.1) |
| Current Smoking | 311 (11.4) | 334 (12.3) |
| Parental History of MI§ | 1056 (38.9) | 1097 (40.5) |
| BMI ≥ 30kg/m2 | 1341 (49.3) | 1349 (49.6) |
| Alcohol Intake (at least weekly) | 897 (33.0) | 889 (32.7) |
| Current Medication Use, no (%) Aspirin || |
1446 (51.1) | 1385 (48.9) |
| Beta-Blockers | 684 (26.6) | 697 (27.0) |
| Lipid-lowering drugs | 914 (33.6) | 938 (34.5) |
| ACE inhibitors | 627 (24.3) | 668 (25.8) |
| Hormone-replacement therapy | 1320 (48.5) | 1329 (48.8) |
| Multivitamins | 616 (22.6) | 631 (23.2) |
| Median Dietary Intake (Range 25th–75thpercentiles)** Folic acid, ug/d |
424.4 (308.6– 664.4) | 438.7 (309.7– 666.8) |
| Vitamin B6, ug/d | 2.48 (1.81–3.81) | 2.54 (1.81–3.87) |
| Vitamin B12, ug/d | 7.10 (4.62–10.9) | 6.98 (4.69–11.0) |
Reported history of myocardial infarction, stroke, coronary revascularization, angina pectoris, transient ischemic attack, carotid endarterectomy, or peripheral artery surgery
Self-reported systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg; self-reported physician-diagnosed hypertension; or reported treatment with medication for hypertension.
Self reported high cholesterol, cholesterol level ≥ 240 mg/dl; self-reported physician diagnosed, high cholesterol levels; or reported treatment with cholesterol lowering medication.
Parental history of myocardial infarction in father prior to age 60 or mother prior to age 65.
Aspirin use at least 4 times per month.
Any multivitamin use in the past month.
Estimated from the semiquantitative food frequency questionnaire
Adherence and Adverse Event
Compliance was assessed through self-report on annual study questionnaires and was defined as taking at least two-thirds of the study pills. Average compliance over the course of follow-up was approximately 83% for active and placebo agents with no significant difference between active and placebo groups. Use of open label folic acid supplements, B6, or B12 supplements containing above the RDA for at least 4 days per month ranged from 2 to 11 percent in the active group to 2 to 13 percent in the placebo group over the course of the study. There were no serious adverse events reported that were conclusively related to study interventions.
Primary Analysis
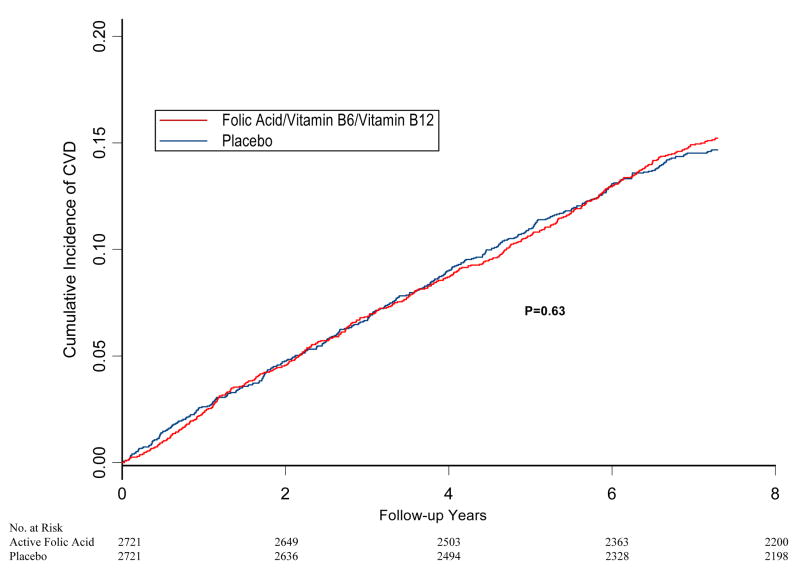
During the 7.3 years of follow-up, 796 women (14.6%) experienced a confirmed CVD event included in the primary endpoint, with some experiencing more than one event. Overall, 139 MIs, 148 strokes, 508 coronary revascularization procedures, and 190 cardiovascular deaths occurred in the population over the course of the study. There was no difference in the cumulative incidence of the primary combined endpoint in the active versus placebo treatment groups at any time during study follow-up (Figure 2). A total of 406 women (14.9%) in the active treatment arm and 390 (14.3%) in the placebo arm experienced at least one cardiovascular event included in the primary endpoint (226.9 per 10,000 person-years versus 219.2 per 10,000 person-years for active versus placebo). This corresponded to an overall relative risk of 1.03 (95% confidence interval, 0.90–1.19, p=0.65) after controlling for age and antioxidant treatment assignment (Table 2). There remained no evidence for a treatment effect in sensitivity analysis censoring at non-compliance (relative risk=1.05; 95% confidence interval 0.90–1.23, p=0.53) or if coronary revascularization procedures were excluded from the primary endpoint (relative risk=0.96; 95% confidence interval 0.80–1.17, p=0.72).
Figure 2.
Cumulative incidence of major vascular disease (myocardial infarction, stroke, coronary revascularization, or cardiovascular death), by randomized folic acid and B-vitamin intervention in the Women’s Antioxidant and Folic Acid Study (WAFACS). P-value is from log-rank test.
Table 2.
Relative Risks Of Clinical Outcomes According To Treatment Assignment with Folic acid/Vitamin B6/Vitamin B12 versus Placebo.
| Outcome | Active N=2721 | Placebo N=2721 | Relative risk* (95% CI) | P value |
|---|---|---|---|---|
| No of Patients (%) | ||||
| Combined Major Cardiovascular Disease† | 406 (14.9) | 390 (14.3) | 1.03 (0.90–1.19) | 0.65 |
| Myocardial Infarction | 65 (2.4) | 74 (2.7) | 0.87 (0.63–1.22) | 0.42 |
| Stroke | 79 (2.9) | 69 (2.5) | 1.14 (0.82–1.57) | 0.44 |
| Ischemic‡ | 69 (2.5) | 62 (2.3) | 1.10 (0.78–1.56) | 0.57 |
| Hemorrhagic‡ | 10 (0.4) | 6 (0.2) | 1.65 (0.60–4.53) | 0.33 |
| Coronary Revascularization§ | 253 (9.3) | 255 (9.4) | 0.99 (0.83–1.17) | 0.87 |
| Coronary Artery Bypass Grafting|| | 87 (3.2) | 98 (3.6) | 0.88 (0.66–1.17) | 0.38 |
| Percutaneous Coronary Intervention|| | 192 (7.1) | 177 (6.5) | 1.08 (0.88–1.33) | 0.46 |
| Cardiovascular death | 96 (3.5) | 94 (3.5) | 1.01 (0.76–1.35) | 0.93 |
| Myocardial infarction, stroke, and cardiovascular death | 205 (7.5) | 211 (7.8) | 0.96 (0.80–1.17) | 0.72 |
| Total coronary heart disease#| | 283 (10.4) | 280 (10.3) | 1.00 (0.85–1.18) | 0.96 |
| Total mortality | 250 (9.2) | 256 (9.4) | 0.97(0.81–1.15) | 0.73 |
Estimated from Cox proportional hazards models that adjusted for age and randomized treatment assignment to vitamin E., vitamin C, and beta-carotene.
The primary outcome is defined as a composite endpoint comprising the first of any of these events: non-fatal myocardial infarction, stroke, coronary revascularization procedures (coronary artery bypass grafting or percutaneous coronary intervention), and cardiovascular mortality.
Stroke type was unknown for 1 woman in the placebo group.
Composite endpoint comprised of the first coronary artery bypass grafting or percutaneous coronary intervention.
Includes all incident coronary artery bypass grafting operations and percutaneous coronary intervention respectively.
Composite endpoint comprised of the first of any of these events: non-fatal myocardial infarction, coronary revascularization procedures (coronary artery bypass grafting or percutaneous coronary intervention), and coronary heart disease death.
Secondary and Other Outcomes
Among the pre-specified secondary cardiovascular outcomes, total CHD events occurred in 283 women (156.5 per 10,000 person-years) in the active-treatment group and in 280 (155.8 per 10,000 person-years) in the placebo group (relative risk, 1.00; 95% confidence interval: 0.85–1.18; P=0.96) (Table 2). When analyzed separately, there were again no significant differences for each of the components of the primary outcome including MI (34.5 per 10,000 person-years versus 39.5 per 10,000 person-years; relative risk =0.87; 95 percent confidence interval, 0.63–1.22), stroke (41.9 per 10,000 person-years versus 36.8 per 10,000 person-years; relative risk =1.14; 95 percent confidence interval, 0.82–1.57), and CVD mortality (50.3 per 10,000 person-years versus 49.6 per 10,000 person-years; relative risk =1.01; 95 percent confidence interval, 0.76–1.35) between the active treatment and placebo groups. Also, the risk of death from any cause was similar between treatment groups (relative risk, 0.97; 95% confidence interval: 0.81–1.15; P=0.73).
Subgroup Analyses
There were no significant treatment effects with respect to the primary outcome in any of the prespecified or exploratory sub-groups evaluated (Table 3). Of particular interest, there was a similar lack of benefit among women without prior CVD as compared to those with prior CVD (P for interaction=0.93), although the trial was not powered to detect a specific benefit in this sub-group. Also, there was no evidence that either dietary folate intake or multivitamin use modified the treatment effect, although power was limited for these subgroups as well. The test for interaction was significant for treatment with angiotensin converting enzyme inhibitors (P= 0.03); however, this was not a pre-specified subgroup analysis.
Table 3.
Effect of Randomized Treatment Assignment on the Primary Outcome in Prespecified and Exploratory Subgroups.
| No. of Patients* | No. of Events (%)* | |||||
|---|---|---|---|---|---|---|
| Characteristic | Active | Placebo | Active | Placebo | RR (95% CI) | P-value for Interaction |
| Overall | 2721 | 2721 | 406 (14.9) | 390 (14.3) | 1.03 (0.90–1.19) | |
| Age (years) 40–54 |
582 | 584 | 50 (8.6) | 43 (7.4) | 1.17 (0.78–1.76) | |
| 55–64 | 990 | 970 | 119 (12.0) | 133 (13.7) | 0.85 (0.67–1.09) | 0.55 |
| 65+ | 1149 | 1167 | 237 (20.6) | 214 (18.3) | 1.12 (0.93–1.34) | |
| Prior Cardiovascular Disease† Yes |
1764 | 1728 | 329 (18.7) | 314 (18.2) | 1.03 (0.89–1.21) | 0.93 |
| No | 957 | 993 | 77 (8.1) | 76 (7.65) | 1.01 (0.73–1.39) | |
| Diabetes Yes |
570 | 574 | 142 (24.9) | 143 (24.9) | 0.99(0.78–1.24) | 0.67 |
| No | 2151 | 2147 | 264 (12.3) | 247 (11.5) | 1.06(0.89–1.26) | |
| Hypertension‡ Yes |
2360 | 2335 | 373 (15.8) | 363 (15.6) | 1.01 (0.87–1.16) | 0.30 |
| No | 361 | 386 | 33 (9.1) | 27 (7.0) | 1.32 (0.80–2.20) | |
| Elevated Cholesterol§ Yes |
2118 | 2150 | 340 (16.1) | 331 (15.4) | 1.03 (0.88–1.19) | 0.77 |
| No | 603 | 571 | 66 (11.0) | 59 (10.3) | 1.07 (0.76–1.52) | |
| Current Smoking Yes |
311 | 334 | 56 (18.0) | 70 (21.0) | 0.87 (0.61–1.24) | 0.34 |
| No | 2410 | 2387 | 350 (14.5) | 320 (13.4) | 1.07 (0.92–1.25) | |
| Parental History of MI|| Yes |
1056 | 1097 | 165 (15.6) | 167 (15.2) | 1.03(0.83–1.27) | 0.90 |
| No | 1656 | 1610 | 238 (14.4) | 219 (13.6) | 1.04 (0.87–1.26) | |
| BMI < 25kg/m2 |
616 | 566 | 100 (16.2) | 82 (14.5) | 1.12 (0.84–1.50) | |
| 25-< 30kg/m2 | 764 | 806 | 126 (16.5) | 127 (15.8) | 1.07 (0.84–1.37) | 0.41 |
| 30± kg/m2 | 1341 | 1349 | 180 (13.4) | 181 (13.4) | 0.97 (0.79–1.20) | |
| Alcohol Intake# < 1/month |
1494 | 1499 | 247 (16.5) | 234 (15.6) | 1.05 (0.88–1.26) | 0.73 |
| ≥ 1/month | 1227 | 1222 | 159 (13.0) | 156 (12.8) | 1.00 (0.80–1.25) | |
| Folate Intake# Below median (<= 432 ug/day) |
1322 | 1263 | 207 (15.7) | 183 (14.5) | 1.07 (0.87–1.30) | 0.88 |
| Above median (> 432 ug/day) | 1263 | 1323 | 181 (14.3) | 183 (13.8) | 1.04 (0.85–1.28) | |
| Aspirin** Yes |
1446 | 1385 | 270 (18.7) | 244 (17.6) | 1.07(0.90–1.28) | 0.37 |
| No | 1274 | 1336 | 136 (10.7) | 146 (10.9) | 0.93(0.73–1.17) | |
| Beta-Blockers Yes |
684 | 697 | 125 (18.3) | 123 (17.7) | 1.04 (0.81–1.34) | 0.93 |
| No | 1887 | 1883 | 263 (13.9) | 249 (13.2) | 1.03 (8.7–1.23) | |
| Lipid-lowering drugs Yes |
914 | 938 | 178 (19.5) | 171 (18.2) | 1.05 (0.85–1.30) | 0.83 |
| No | 1807 | 1783 | 228 (12.6) | 219 (12.3) | 1.02 (0.85–1.23) | |
| ACE inhibitors Yes |
627 | 668 | 102 (16.3) | 125 (18.7) | 0.81 (0.63–1.06) | 0.03 |
| No | 1957 | 1920 | 287 (14.7) | 247 (12.9) | 1.15 (0.97–1.36) | |
| Hormone-replacement therapy Yes |
1320 | 1329 | 172 (13.0) | 191 (14.4) | 0.89(0.72–1.09) | 0.05 |
| No | 1401 | 1392 | 234 (16.7) | 199 (14.3) | 1.18 (0.97–1.42) | |
| Multivitamins*** Yes |
616 | 631 | 94 (15.3) | 87 (13.8) | 1.09 (0.81–1.46) | 0.67 |
| No | 2105 | 2088 | 312 (14.8) | 302 (14.5) | 1.02 (0.87–1.19) | |
| Vitamin C Active |
1349 | 1356 | 213 (15.8) | 177 (13.1) | 1.21(0.99–1.48) | 0.03 |
| Placebo | 1372 | 1365 | 193 (14.1) | 213 (15.6) | 0.89(0.73–1.08) | |
| Vitamin E Active |
1364 | 1356 | 190(13.9) | 198 (14.6) | 0.94(0.77–1.15) | 0.22 |
| Placebo | 1357 | 1365 | 216(15.9) | 192 (14.1) | 1.12(0.93–1.37) | |
| Beta-Carotene Active |
1358 | 1349 | 199(14.7) | 203 (15.1) | 0.96(0.79–1.17) | 0.32 |
| Placebo | 1363 | 1372 | 207(15.2) | 187 (13.6) | 1.11(0.91–1.35) | |
Numbers do not always some to group totals due to missing information for some of the subgroup variables
Reported history of myocardial infarction, stroke, coronary revascularization, angina pectoris, transient ischemic attack, carotid endarterectomy, or peripheral artery surgery
Self-reported systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg; self-reported physician-diagnosed hypertension; or reported treatment with medication for hypertension.
Self reported high cholesterol, cholesterol level ≥ 240 mg/dl; self-reported physician diagnosed, high cholesterol levels; or reported treatment with cholesterol lowering medication.
Parental history of myocardial infarction in father prior to age 60 or mother prior to age 65.
Estimated from the semiquantitative food frequency questionnaire
Aspirin use at least 4 times per month.
Any multivitamin use in the past month.
With respect to the antioxidant vitamins, there was evidence for an interaction between randomized treatment assignment to vitamin C and the combination therapy with folic acid, vitamin B6, and vitamin B12 on the primary endpoint (Table 3). As compared to those randomized to both placebos, the relative risk for folic acid was lower among those on placebo versus active vitamin C. (P-interaction = 0.03). There were no other significant two-way or three-way interactions among the agents for the primary endpoint.
Effect of Supplementation versus Fortification on Folic Acid and Homocysteine Levels
The 300 women in the blood sub-study were similar with respect to all the clinical characteristics outlined in table 1, except that smokers (8.0% in blood group versus 12.1% in non-blood group; P=0.003) and women with a history of diabetes (16.7% in blood group versus versus 21.3% in non-blood group; P=0.06) were underrepresented among the compliant women in the blood substudy. The distributions of baseline and follow-up folic acid and homocysteine levels among 150 participants in the placebo arm and 150 participants in the active arm are displayed in table 4. Prior to the initiation of fortification and randomization, median folate levels were similar in the active treatment (8.9 ng/mL; interquartile range, 6.0–13.4) and placebo arms (8.8 ng/mL; interquartile range, 6.4–12.8) (P= 0.94), with 34% of the study population having levels considered inadequate (<7 ng/mL). Median plasma homocysteine levels were also similar at baseline in the active treatment (12.1μmol/L; interquartile range, 10.2–15.0) as compared to the placebo group (12.5μmol/L; interquartile range, 9.6–15.5; P=0.96), with 27.7 % of the study population having a level > 15.0 μmol/L (Table 4).
Table 4.
Distribution of Plasma Levels of Folate and Homocysteine in 300 participants in the Blood Sub-Study at Baseline Prior to Randomization and at the End of Treatment and Follow-up.
| Baseline | Follow-up | |||
|---|---|---|---|---|
| Characteristic | Placebo (n=150) | Active (n=150) | Placebo (n=150) | Active (n=150) |
| Folate (ng/ml) | ||||
| <7 | 52 (34.7) | 49 (32.7) | 2 (1.33) | 0 (0.00) |
| 7-<15 | 71 (47.3) | 80 (53.3) | 69 (46.0) | 1 (0.67) |
| 15-<25 | 22 (14.7) | 19 (12.7) | 54 (36.0) | 21 (14.0) |
| 25-<40 | 4 (2.67) | 2 (1.33) | 18 (12.0) | 54 (36.0) |
| ≥40 | 1 (0.67) | 0 (0.00) | 7 (4.67) | 74 (49.3) |
| Homocysteine (μmol/liter) | ||||
| < 9 | 30 (20.0) | 21 (14.0) | 21 (14.0) | 64 (42.7) |
| 9-<12 | 40 (26.7) | 52 (34.7) | 57 (38.0) | 43 (28.7) |
| 12-<15 | 35 (23.3) | 39 (26.0) | 35 (23.3) | 28 (18.7) |
| ≥15 | 45 (30.0) | 38 (25.3) | 37(24.7) | 15 (10.0) |
At the end of study follow-up, the median folic acid level increased significantly in the placebo group to 15.4 (interquartile range, 11.5–22.6; P<0.001); however, the relative increase in folic acid level was greater in the active treatment arm, where 49.3% of subjects had a folic acid level greater than 40 ng per mL (the upper limit of the assay) as compared to 4.7% in the placebo group (Table 4). Despite significant increases in folic acid levels among the placebo group, there was no apparent reduction in homocysteine levels at the end of the study as compared to those measured at the beginning of the study in the placebo group (median=11.8 μmol/L; interquartile range, 9.8–14.9; P=0.99). In comparison, homocysteine levels were significantly reduced in the active treatment arm (median level; 9.8μmol/L; interquartile range, 7.9–12.4; P=0.001), and the number of women with significantly elevated homocysteine levels of greater than 15 μmol per liter was reduced to 10%.
In order to directly compare the degree of additional homocysteine lowering observed in the active over the placebo group, we computed the difference between treatment groups in the change in the natural logarithm of homocysteine level from baseline to follow up, adjusting for baseline levels. The geometric mean homocysteine level was decreased by 18.5% (95% CI, 12.5–24.1; P<0.001) in the active arm over that observed in the placebo arm for a difference of 2.27 μmol per liter (95% CI, 1.54 –2.96) from the placebo geometric mean homocysteine level of 12.28 μmol per liter.
DISCUSSION
In this large-scale, placebo-controlled randomized trial among high-risk women, we found no overall effects of a combination of folic acid, vitamin B6, and vitamin B12 on the primary outcome of total CVD events over the largest number of person-years and the longest follow-up period reported thus far (7.3 years). In subgroup analyses, there was no heterogeneity of treatment effect among those above or below the median of folate intake and among women with or without prior vascular disease. A possible interaction with randomized vitamin C and with nonrandomized angiotensin converting enzyme inhibitor treatment on the primary outcome was observed; however, due to the large number of comparisons these results could have been due to chance.
These null results for women are consistent with those previously reported in randomized trials composed primarily of men with pre-existing vascular disease3. In the Heart Outcomes Prevention Evaluation Trial (HOPE) 25, the same regimen of folic acid, vitamin B6, and vitamin B12 failed to significantly lower the risk of a combined outcome of death from cardiovascular causes, MI, and stroke among 5522 patients with prior vascular disease over an average of five years. Although there was a significant reduction in the secondary end point of stroke in this trial (relative risk, 0.75; 95% confidence interval, 0.59 to 0.97), a reduction in stroke was not found with a similar B-vitamin regimen in the Vitamins Intervention for Stroke Prevention (VISP) trial13, where stroke was the primary endpoint. The HOPE-2 trial also reported an increased risk of hospitalization for unstable angina (relative risk, 1.24; 95% confidence interval, 1.04–1.49) among those randomized to active treatment.
In the Norwegian Vitamin (NORVIT) Trial14, a 2 × 2 factorial trial of vitamin B6 and a combination pill of folic acid/vitamin B12 among recent post MI patients, a marginally significant 22% (95% confidence interval, 0–50%; p=0.05) increased risk of recurrent MI, stroke, and sudden death was found among those assigned to the combination of folic acid/B12 and vitamin B6 over a median follow-up of 40 months. Smaller studies among patients post coronary intervention have found both decreased15 and increased16 rates of restenosis among patients treated with B-vitamin regimens. In the present study, we found no evidence for a benefit on stroke or any evidence for harm regarding the primary composite endpoint or any of the individual secondary endpoints including coronary revascularization.
Concerns have been raised regarding the power of this and other current trials to adequately test the homocysteine hypothesis17, 18; especially in countries where folic acid fortification of the food supply has taken place 19. Observational studies suggested that fortification of grain products with 140 mcg of folic acid per 100g, which began in 1996 and became mandatory in the United States and Canada by 1998, significantly reduced mean plasma homocysteine concentrations among middle-aged individuals, and decreased the prevalence of high homocysteine levels (>13 μmol per liter) from 29.8% to 18.7% 20. Based upon these data, it has been estimated that additional B-vitamin supplementation in such fortified population would only lower homocysteine by about 10%21. In our trial, we observed a greater but still somewhat modest lowering of homocysteine (18.5% reduction). Although, we observed almost complete elimination of low folic acid concentrations (<7 ng per milliliter) in the placebo group after fortification, there was no apparent reduction over time on homocysteine concentrations or on the prevalence of elevated homocysteine levels (15+ μmol per liter) in this population. Although homocysteine levels were unchanged in the placebo group, folic acid fortification likely prevented further elevations in homocysteine levels that would have otherwise taken place due to the aging of the population.
Initially, epidemiologic studies, which were primarily retrospective and cross-sectional, suggested that reducing plasma homocysteine by 5 μmol/L would decrease vascular risk by one third22. However, a more recent meta-analysis of prospective observational studies suggested that risk reductions associated with homocysteine lowering would be much more modest1. In these studies, a 25% lower homocysteine (approximately 3 μmol per liter) was associated with 11% reduction in coronary heart disease risk and a 19% lower stroke risk1. The expected reductions in cardiovascular events may have been even lower in our trial where the reduction in homocysteine levels was only 18.5% (2.3 μmol per liter) among compliant patients. Although our trial was not initially powered to detect such modest reductions in cardiovascular events, the 95% confidence intervals for the primary endpoint excludes with reasonable certainty reductions as low as a 10% in the combined endpoint of total cardiovascular events. However, such modest plausible reductions in the individual secondary endpoints of stroke, MI, and cardiovascular death cannot be excluded even in a trial of this size.
There are several caveats and/or limitations to this study, which warrant consideration. First, the study was conducted in a population of health professionals, who were at a relatively low risk of folate deficiency. Women were allowed to take the RDA of folic acid and B-vitamins and were also exposed to folic acid fortified grain products during the course of the trial. Although the blood study suggests that a significant proportion of the women were folate deficient at the beginning of the trial, this was virtually eliminated over the course of study. Therefore, we cannot rule out the possibility that this same regimen may have resulted in an even greater reduction in homocysteine levels in a more folate deficient population, which might have translated into an observable benefit on cardiovascular events. Alternatively, the optimal dose of these vitamins may actually be lower than that tested in this and other trials, and the potential for harm at higher doses has been raised by other studies14. Also, since homocysteine levels were only measured in 5% of the sample, we were unable to determine whether women with high homocysteine levels at baseline may have benefited to a greater extent both with respect to homocysteine lowering and cardiovascular events.
Although this trial was the first to include a significant number of participants without prior cardiovascular disease (n=1950), power is still insufficient to exclude moderate treatment effects in primary prevention. Also, morbidity and mortality follow-up rates in this high-risk population were lower than in other trials of primary prevention utilizing similar methodology23; and it is thus plausible that a larger primary prevention population with higher rates of follow-up might have demonstrated a benefit. However, since the lost to follow-up rates did not differ between the two treatment groups in our study; this is unlikely to account for the null findings observed. These remaining issues, along with the hypotheses regarding possible novel drug interactions with vitamin C and angiotensin enzyme converting inhibitors raised by our subgroup analyses, warrant further investigation in future studies.
In summary, in the WAFACS trial, a combination pill of 2.5 mg of folic acid, 50 mg of vitamin B6, and 1 mg vitamin B12 had no beneficial or adverse effects on a combined outcome of total major cardiovascular events in a high-risk population of women with prior cardiovascular disease or three or more coronary risk factors over 7.3 years of follow-up. Background folic acid fortification in the food supply, although not associated with homocysteine lowering over the long-term follow-up, may still have contributed to these null findings in this population, which was at low risk for folic acid deficiency. Our results are consistent with prior randomized trials performed primarily among men with established vascular disease3, and do not support the use of folic acid and B-vitamin supplements as preventative interventions for CVD in these high-risk fortified populations.
Acknowledgments
Funding/Support: This study was supported by investigator-initiated grant HL46959 from the National Heart, Lung, and Blood Institute. Vitamin E and its placebo were supplied by Cognis Corporation (LaGrange, IL). All other agents and their placebos were supplied by BASF Corporation (Mount Olive, NJ). Pill packaging was provided by Cognis and BASF.
Other Acknowledgements: We are indebted to the 5442 participants in the Women’s Antioxidant and Folic Acid Cardiovascular Study for their dedicated and conscientious collaboration; to the entire staff of the Women’s Antioxidant and Folic Acid Cardiovascular Study: including Marilyn Chown, Shamikhah Curry, Margarette Haubourg, Felicia Zangi, Tony Laurinaitis, Geneva McNair, Philomena Quinn, Harriet Samuelson, Ara Sarkissian, and Martin Van Denburgh; to Michelle Albert, Gavin Blake, Claudia Chae, Michael Fisher, Carlos Kase, Tobias Kurth, I-Min Lee, Aruna Pradhan, Paul Ridker, Jackie Suk, and James Taylor for their assistance in the conduct of the trial.
Footnotes
Author Contributions: Dr. Albert had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Albert, Gaziano, Manson
Acquisition of data: Albert, Gaziano, Zaharis, MacFadyen, Danielson, Buring, Manson
Analysis and interpretation of data: Cook, Albert, Manson
Drafting of the manuscript: Albert
Critical revision of the manuscript for important intellectual content: Cook, Albert, Gaziano, Zaharis, MacFadyen, Danielson, Buring, Manson
Statistical expertise: Cook
Obtained funding: Albert, Gaziano, Manson
Administrative, technical, or material support: Zaharis, MacFadyen, Danielson, Manson
Study supervision: Albert, MacFadyen, Danielson, Manson
Other Financial Disclosures: Dr Gaziano has received investigator initiated study support in the form of vitamin pills and packaging from Wyeth. Dr Buring has received investigator initiated study support in the form of vitamin pills and packaging from Natural Source Vitamin E Association. Dr. Manson has received investigator-initiated study support in the form of vitamin pills and packaging from Cognis and BASF.
Role of the Sponsors: Neither Cognis nor BASF provided any input into the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Data Safety and Monitoring Board: L. Cohen, R. Collins, T. Colton, D. DeMets, I.C. Henderson, A. La Croix, R. Prentice, and N. Wenger (chair) and M.F. Cotch, F. Ferris, L. Friedman, P. Greenwald., N. Kurinij, M. Perloff, E. Schron, A. Zonderman (ex officio members).
References
- 1.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288(16):2015–22. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 2.Homocysteine Lowering Trialists’ Collaboration. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. BMJ. 1998;316(7135):894–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006;296(22):2720–6. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson CM. Lowering homocysteine for stroke prevention. Lancet. 2007;369(9576):1841–2. doi: 10.1016/S0140-6736(07)60830-7. [DOI] [PubMed] [Google Scholar]
- 5.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Qin X, Demirtas H, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369(9576):1876–82. doi: 10.1016/S0140-6736(07)60854-X. [DOI] [PubMed] [Google Scholar]
- 7.Hak AE, Polderman KH, Westendorp IC, et al. Increased plasma homocysteine after menopause. Atherosclerosis. 2000;149(1):163–8. doi: 10.1016/s0021-9150(99)00321-4. [DOI] [PubMed] [Google Scholar]
- 8.Lobo RA. Homocysteine in women’s health. Menopause. 2003;10(4):271–3. doi: 10.1097/01.GME.0000072222.06570.94. [DOI] [PubMed] [Google Scholar]
- 9.Ioannidis JP, Cappelleri JC, Lau J. Issues in comparisons between meta-analyses and large trials. JAMA. 1998;279(14):1089–93. doi: 10.1001/jama.279.14.1089. [DOI] [PubMed] [Google Scholar]
- 10.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338(15):1042–50. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 11.Bassuk SS, Albert CM, Cook NR, et al. The Women’s Antioxidant Cardiovascular Study: design and baseline characteristics of participants. J Womens Health. 2004;13(1):99–117. doi: 10.1089/154099904322836519. [DOI] [PubMed] [Google Scholar]
- 12.Cook NR, Albert CM, Gaziano JM, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167(15):1610–8. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291(5):565–75. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 14.Bonaa KH, Njolstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–88. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 15.Schnyder G, Roffi M, Pin R, et al. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N Engl J Med. 2001;345(22):1593–600. doi: 10.1056/NEJMoa011364. [DOI] [PubMed] [Google Scholar]
- 16.Lange H, Suryapranata H, De Luca G, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med. 2004;350(26):2673–81. doi: 10.1056/NEJMoa032845. [DOI] [PubMed] [Google Scholar]
- 17.B-vitamin Treatment Trialists’ Collaboration. Homocysteine-lowering trials for prevention of cardiovascular events: a review of the design and power of the large randomized trials. Am Heart J. 2006;151(2):282–7. doi: 10.1016/j.ahj.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Wald DS, Wald NJ, Morris JK, Law M. Folic acid, homocysteine, and cardiovascular disease: judging causality in the face of inconclusive trial evidence. BMJ. 2006;333(7578):1114–7. doi: 10.1136/bmj.39000.486701.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bostom AG, Selhub J, Jacques PF, Rosenberg IH. Power Shortage: clinical trials testing the “homocysteine hypothesis” against a background of folic acid-fortified cereal grain flour. Ann Intern Med. 2001;135(2):133–7. doi: 10.7326/0003-4819-135-2-200107170-00014. [DOI] [PubMed] [Google Scholar]
- 20.Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340(19):1449–54. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 21.Bostom AG, Jacques PF, Liaugaudas G, Rogers G, Rosenberg IH, Selhub J. Total homocysteine lowering treatment among coronary artery disease patients in the era of folic acid-fortified cereal grain flour. Arterioscler Thromb Vasc Biol. 2002;22(3):488–91. doi: 10.1161/hq0302.105369. [DOI] [PubMed] [Google Scholar]
- 22.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274(13):1049–57. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 23.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the Primary Prevention of Cardiovascular Disease and Cancer. JAMA. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]