Abstract
Following the ideas introduced by Huxley (Huxley 1957, Prog. Biophys. Biophys. Chem. 7, 255–318), it is generally supposed that muscle contraction is produced by temporary links, called crossbridges, between myosin and actin filaments, which form and break in a cyclic process driven by ATP splitting. Here we consider the interaction of the energy in the crossbridge, in its various states, and the force exerted. We discuss experiments in which the mechanical state of the crossbridge is changed by imposed movement and the energetic consequence observed as heat output and the converse experiments in which the energy content is changed by altering temperature and the mechanical consequences are observed. The thermodynamic relationship between the experiments is explained and, at the first sight, the relationship between the results of these two types of experiment appears paradoxical. However, we describe here how both of them can be explained by a model in which mechanical and energetic changes in the crossbridges occur in separate steps in a branching cycle.
Keywords: muscle contraction, temperature dependence, thermodynamics, muscle force
1. Introduction
Many muscles will work between 0 and 40°C, and temperature greatly influences how they perform. These changes in performance with temperature are related by thermodynamic principles to certain heat changes that have been observed in muscle. By considering together both these types of experiment, we can obtain information about the mechanism of contraction.
The specific process we will consider is the crossbridge cycle that powers muscle contraction. We assume that the force and work that muscle produces during stimulation is the result of forces developed by many individual ‘crossbridges’, each of which is an attachment of a single myosin head to a thin filament. We assume also that these interactions split ATP in a cyclic process, in general one molecule for each cycle of attachment and detachment, although cycles may occur without ATP splitting during the stretch of active muscle (Curtin & Davies 1973; Linari et al. 2003). Thus the ATPase cycles are the energy transducers that convert chemical energy into work and heat during contraction. Many different experimental approaches (mechanical, structural and biochemical) have provided results that can be interpreted within this crossbridge framework by detailing the kinetics of steps within a cycle such as that shown in model 1 (figure 1). It is not obvious, however, how to map the steps observed by one technique (e.g. mechanical) onto those suggested by a different technique (e.g. biochemical). Partly for want of this mapping, there is no quantitative standard theory of muscle contraction, which can model the results of many different types of experiment (Smith et al. 2008). Because certain steps in the cycle are strongly temperature dependent, the application of the methods described below can help with the mapping problem.
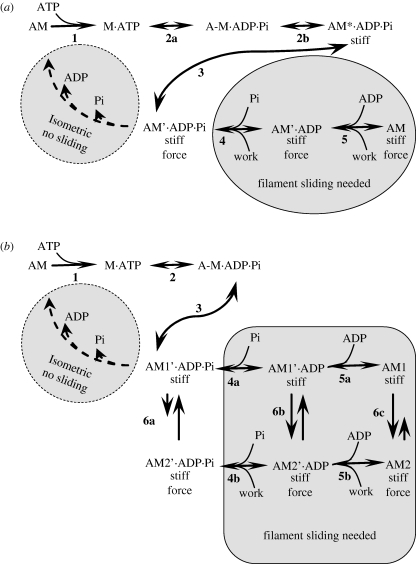
Figure 1.
(a) Model 1. A kinetic scheme for ATP splitting by a crossbridge. Adapted, in part, from Coupland et al. (2001), Sleep et al. (2005) and Caremani et al. (2008). Reaction 3 is the force-generating event. State AM′.ADP.Pi is the branch point after which the reactions that complete the ATPase cycle differ according to whether filament sliding in the shortening direction occurs or not. (b) Model 2. A modification to model 1 (see text §§6 and 7). The force-generating events are reactions 6a, b, c.
Since we shall be using a model framed in terms of the behaviour of a single crossbridge, we will express the forces and fluxes in appropriately scaled units. Results of thermodynamic measures (ΔH or ΔG) made on ensembles of myosin molecules, which would conventionally be expressed in kJ mol−1, will here be scaled by Avogadro's number and given as zJ per myosin molecule. Work done by one crossbridge is obtained, in zJ, as the product of the force on it (pN) and the distance it moves (nm). The conventional scaling for expressing observations on muscle fibres are: heat or work production per unit volume; force per unit cross-sectional area; and length change per unit fibre length. To convert these to the desired units, we need to know (i) how many crossbridges there are in a volume of muscle fibre of length one-half sarcomere and of a given cross section and (ii) how much relative motion of actin and myosin molecules occurs for a given length change in the fibre. The relevant values follow. (i) From the spacing of the thick filaments, their architecture (Squire 1981) and the proportion of the cross-section containing filaments (85%, Mobley & Eisenberg 1975), we find that a volume of muscle of length 1.05 μm (the length of one-half sarcomere in frog muscle at its optimum length) and of cross section 1 μm2 contains 167 000 myosin heads, each of which is a potential crossbridge site. Since only a proportion of these sites will be joined to actin, we need also to estimate the proportion attached so that mechanical measurements can also be expressed per attached crossbridge. (ii) The regular sarcomere spacing in a muscle fibre makes it possible to express length changes per half sarcomere, which gives the relative movement of the thick and thin filaments. However, the filaments themselves are compliant and the actual displacement of actin relative to the myosin sites can be found only after taking account of filament compliance. Any change in fibre length consists of two components: a change in the filaments and a change in the crossbridges. In this review, for experiments where imposed length changes have been used to deduce crossbridge properties (such as compliance), the filament length changes have been subtracted to leave only the length changes experienced by the crossbridges. To do this, we have used the thin filament compliance, 0.82 nm pN−1, observed by Linari et al. (1998) who found the shortening of the thin filaments to be 1.12 nm when isometric force was dropped to zero from 1.36 pN per crossbridge site. The thick filament compliance we used, 1.23 nm pN−1, is based on the data of Reconditi et al. (2004), and is expressed in a similar way to that for the thin filament. The conclusions we reach are quite sensitive to the values of these filament compliances.
(a). Crossbridge mechanics
In this section, we discuss experiments using single intact frog muscle fibres. The techniques used have allowed the effects of compliance in the attachments of these fibres to be eliminated and the effects of filament compliance to be separated from those of crossbridge compliance. A range of length changes has been imposed, probing the interesting nonlinearities in the responses to length perturbations. The conclusions we reach are different from those derived from consideration of mechanical experiments made with permeabilized rabbit fibres reviewed for example by Davis & Epstein (2007). In most of the experiments discussed therein, only a single amplitude of length change has been used, with the aim of restricting the analysis to the narrow range of length change within which the muscle responds in a linear manner.
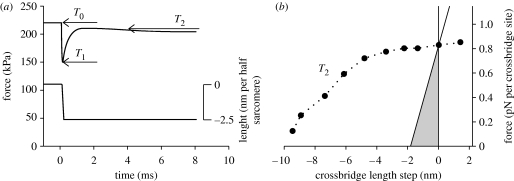
During isometric contraction, force is exerted by a proportion, probably less than half, of the myosin heads attaching to the actin filaments. The average force exerted by each attached head is approximately 3 pN. In length-step experiments, the length of the muscle fibre is changed suddenly (within approx. 100 μs) so that the myosin filaments move relative to the actin filaments. The force changes during these movements (figure 2a) show that the crossbridges have a linear stress–strain curve (figure 2b) and a compliance that is independent of the speed of the movement. They behave as undamped elastic elements. A shortening of approximately 2 nm is sufficient to reduce the isometric force in the crossbridges to 0 (figure 2b), so the energy elastically stored is no more than 3 zJ per attached crossbridge (=3 pN per attached crossbridge×2 nm×0.5), which is small compared with the free energy provided by splitting one ATP molecule (approx. 90 zJ). This elastically stored energy is the immediate source of the mechanical work done by muscle when shortening under steady load. Therefore, to explain the observation that up to 40 per cent of the energy from ATP splitting can be converted into work (Smith et al. 2005), it must be supposed that the crossbridge is recharged with work a number of times within each ATPase cycle. This is envisioned as the crossbridge passing through a number of mechanically distinct states while remaining attached, and that the equilibrium between these states is dependent on the length of the crossbridge.
Figure 2.
(a) An example of the force recorded during and after a rapid length step. Experimental data for a frog single muscle fibre redrawn from Piazzesi et al. (2003). Force: upper trace, left-hand scale. Length: lower trace, right-hand scale. (b) The relationship of T2 (circles) to the size of the length step. The length-step values represent the extent of length change within the crossbridges themselves after taking account of filament compliance. Plotted from the data of Piazzesi et al. (2003) for 2°C. The solid lines represent the stress–strain relationship of the crossbridges found by plotting force versus length during rapid length steps (fig. 3 of Piazzesi et al. 2003). The shaded area represents the work stored elastically in the crossbridges during isometric contraction.
This conclusion was also reached by Huxley & Simmons (1971) from the observation that, following a length step, there is a rapid recovery of force towards the isometric value (figure 2a). This recovery is too quick to be due to the turnover (detachment and reattachment) of crossbridges. Correspondingly, the stress–strain curve of an attached crossbridge studied on a time scale that allows these transitions to occur (the T2 curve, figure 2b) is quite different from that on the sub-millisecond time scale of the length steps. In particular, the energy ‘stored’ in the crossbridge is much greater on the slower time scale. The number of these mechanically distinct states, the equilibria among them and the kinetics of the transitions between them would be expected to be major determinants of an emerging standard model.
(b). Actomyosin ATPase cycle
In addition to these mechanically distinct crossbridge states, there are multiple biochemically distinct states in the actomyosin (AM) ATPase cycle (figure 1, model 1). Experiments with isolated proteins, with isolated myofibrils and with skinned fibres (in which concentrations of reactants and products can be manipulated), have shown that the attachment of ATP to AM causes rapid dissociation of the crossbridge and ATP is then split on myosin while detached from actin. The resultant myosin product complex is stable and can attach to actin, first in a weakly bound state (A-M.ADP.Pi) then in a strongly bound state (AM*.ADP.Pi). The products dissociate, first phosphate (Pi) then ADP, to complete the cycle. Thus the attached crossbridge (strongly bound myosin) can potentially exist in a number of states (AM*.ADP.Pi, AM′.ADP and AM). At least some of the transitions between these states are reversible because, for example, when the Pi level is suddenly increased (Dantzig et al. 1992), the force changes on a time scale much faster than the turnover of crossbridges. Detailed kinetic analyses of these and other perturbation experiments (Fortune et al. 1991) suggest that there may be more than three kinetically distinct AM states.
How do these biochemical states, defined either by the bound products or by their kinetic properties, relate to the different states revealed by rapid mechanics experiments described above? In the simplest case, there might be a one to one mapping in which mechanical and biochemical steps occur simultaneously. Alternatively, they might occur sequentially with a particular mechanical change having to precede a particular biochemical step on a fixed pathway. Or, more complex, the mechanical and biochemical changes might be able to occur independently on a branched pathway in which each type of change influences the other. The task at hand is to test, starting with the simplest case, which models can successfully explain the results of a variety of experiments.
2. Three complementary thermal experiments
(a). Effect of temperature on equilibria
If a process produces heat (is exothermic), then raising the temperature will reduce the equilibrium constant and shift the equilibrium composition of a reactant/product mixture towards reactants. Conversely, heat-absorbing processes have their equilibria shifted towards products by an increase in temperature. The quantitative expression of the relationship between heat of reaction and temperature sensitivity of the equilibrium constant is the van't Hoff equation (box S1 in the electronic supplementary material). If we can measure the composition of a system and suspect it is close to equilibrium, we can test this by comparing its temperature sensitivity to the predictions of the van't Hoff equation. If the equation fits the data well, we obtain the reaction heat (ΔH value) and entropy change (ΔS value) both per molecule of reaction and from these the free energy change (ΔG value) can be found for each temperature. A ΔH value is useful in conjunction with calorimetric experiments (§2c below). Also, where an independent measure of the free energy change of an experimental equilibrium system (J) can be made at two temperatures, the ΔG value (J per molecule) can be used to find how many active molecules are in the experimental system. We give an example below in §4.
(b). Use of temperature change as a perturbation
If an equilibrium process is temperature sensitive then suddenly changing the temperature puts the system out of equilibrium, and the speed with which it returns to equilibrium can be observed. It is also generally possible to perturb the system in other ways, such as mechanically or chemically. In the simplest system (a single reaction), all perturbations will give the same speed of recovery; when they do not, this is evidence that a more complex model is required to describe the system (see examples in §§4 and 6).
(c). Calorimetric measurement
If a reaction with a large heat change is occurring at a specific time, for example after a perturbation, in principle, the resultant enthalpy change can be observed. If we know the heat of the reaction (ΔH) and the extent of the reaction from an independently monitored signal after the perturbation, we can perform an energy balance test. If we have correctly defined the system, the heat produced will equal the product of ΔH and the extent of reaction. This provides a stringent test for whether the system really has been adequately specified (see example in §5).
3. Temperature dependence of isometric force
Isometric muscle force increases with temperature, but the slope of the relationship is not constant. Typically, the slope decreases and may even become negative as normal body temperature is approached (Rall & Woledge 1990). The fact that force increases with temperature suggests that an endothermic process is involved in force generation. Piazzesi et al. (2003) studied the force–temperature relationship in single intact frog muscle fibres using rapid mechanical perturbations to measure stiffness and an analysis based on thermodynamic principles (box S2 in the electronic supplementary material). They showed that the stiffness (slope of solid curve in figure 2b) was independent of temperature, indicating that altering temperature did not alter the fraction of attached crossbridges (q). Therefore, they were able to interpret the temperature dependence of force output in terms of the equilibrium between two attached crossbridge states of equal stiffness: a non-force-producing state, A1, and a force-producing state, A2, which produces force Fo.
| 3.1 |
Piazzesi et al. assumed that both ΔH and ΔS were temperature independent, allowing the unknowns, q.Fo, ΔH and ΔS, to be found by fitting the equation [S12] in the electronic supplementary material to the observed relationship between force and temperature. See box S3 in the electronic supplementary material for a consideration of the possibility that ΔH and ΔS are temperature dependent.
As shown in figure 3, the equation fits the data well giving these best-fit values (±s.e.) q.Fo=1.71±0.03 pN per site, ΔH=+114±7 zJ per site and ΔS=+0.414±0.024 zJ per site K−1. Using these values, the free energy difference between the states (ΔG) can be calculated at each temperature (equation [S3] in the electronic supplementary material). At 2°C ΔG is close to zero (+0.1 zJ per site) and at 10°C it is −3.3 zJ site (the negative free energy indicates that the transition from A1 to A2 is spontaneous). Thus, at 2°C, the attached crossbridges are about equally distributed between the A1 and A2 states, whereas at 10°C 70 per cent are in the A2 state. At 10°C, the transition from A1 to A2 can do 3.4 zJ more work than it can at 2°C.
Figure 3.
The dependence of force on temperature. Circles, experimental data from Piazzesi et al. (2003); solid curve, fitted to these data with ΔH and ΔS constant (equation [S12] in the electronic supplementary material); dashed curve, fitted with ΔH and ΔS as functions of temperature (equation [S13] in the electronic supplementary material); dotted curve, prediction of the model described in figure 6.
A natural question at this point is whether the A1 and A2 states we have been discussing correspond to the AM*.ADP.Pi and AM′.ADP.Pi states shown in model 1. We address this question in §7.
(a). Using the interpretation of temperature dependence of force to define a crossbridge model
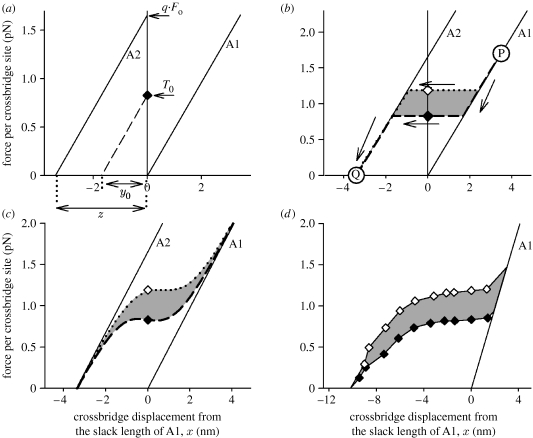
(i). Finding z, the displacement for one state transition
As both the crossbridge states (A1 and A2) hypothesized to explain the force–temperature relationship have the same stiffness, their force–length relationships are two parallel lines (figure 4a) separated by a horizontal distance z. As explained and illustrated in figure 4a, the value of z is given by y0.q.Fo/T0. Since y0 and T0 have been measured at each temperature and the value of q.Fo is given by the curve fitted to the force–temperature data (figure 3a), z can be found. Using the values at 2, 5, 10 and 17°C measured by Piazzesi et al. (2003) gives z values of 3.7, 3.4, 3.3 and 3.7 nm at these temperatures. On the basis of this evidence, the value of z is independent of temperature. The average of these values and four similar values from Decostre et al. (2005) is 3.4 nm, which we will use in further interpretation.
Figure 4.
(a) Relationship of force and crossbridge length (x). At x=0, the A1 state has zero force and the A2 state has force q.Fo, where q is the proportion of crossbridges that are attached. During isometric contraction, all the crossbridges are near x=0 and the equilibrium mixture exerts force T0 (diamond). The forces of these two states are shown as functions of x by the solid lines and z is the horizontal displacement between them. The dashed line shows the force during a quick release from T0. y0 is the intercept of this line on the x-axis. It can be seen from the similarity of the right triangles with bases y0 and z that y0/z=T0/q.Fo. Thus: z=y0.q.Fo/T0. (b) An illustration of a ‘thought experiment’ to measure the effect of temperature on the work done by the A1–A2 transition. A group of crossbridges is shortened from P to Q at a speed sufficiently slow for them to remain in equilibrium between the two states. During the movement, the composition of the equilibrium mixture changes from all A1 at P to all A2 at Q, and passes through the observed isometric points (filled diamond, open diamond) at x=0. The diagram shows the forces exerted during the movement, using the horizontal lines as simple approximations for the forces of the equilibrium mixtures. At the lower temperature, the path followed is the dashed line, and at the higher temperature the dotted line. The extra work done at the higher temperature is the shaded area. (c) A refinement of figure 3b using the conclusion that the proportion of attached bridges is 35%. This allows the calculation of the actual force of the equilibrium mixture. Again the shaded area represents the extra work done at the higher temperature. (d) The A1 line is the same as in the other sections of this figure. The points are observations of T2 (figure 2) by Piazzesi et al. (2003) for frog muscle fibres at 2°C (filled diamonds) and 10°C (open diamonds). Lines join the experimental points. The line to the right of the points was drawn to meet the A1 line and to mimic the trend shown in (c). The shaded area enclosed between the two curves is the extra work done at the higher temperature during crossbridge displacement from +3 to −10.2 nm.
(ii). Finding the proportion of attached sites (q)
As described above, the explanation of the temperature dependence of force led to the conclusion that at 10°C the transition from A1 to A2 can do 3.4 zJ more work per attached crossbridge than it can at 2°C. Figure 4b shows how we can use a ‘thought experiment’ to estimate from Piazzesi's data the temperature-induced increase in the maximum work per crossbridge site (attached plus detached). To obtain maximum work from the transition, we start with all bridges in A1, which can be achieved by starting at a sufficient crossbridge displacement (at P in the diagram). We then shorten the bridges, sufficiently slowly that an equilibrium between A1 and A2 is maintained, and continue the shortening until the force reaches zero (at Q). In this simplified diagram, the force of the equilibrium mixture is represented by a horizontal line drawn through the isometric point (filled diamond at 2°C, open diamond at 10°C); thus the path followed at the lower temperature is the dashed curve and at the higher temperature is the dotted curve. The extra work done at the higher temperature is the shaded area. It is equal to z multiplied by the difference between the isometric forces at the two temperatures. The numerical value for this extra work for Piazzesi's experiment is 1.23 zJ per crossbridge site.
Comparing this to the thermodynamically derived value from the same experiment, 3.4 zJ per attached crossbridge, it can be seen that approximately 35 per cent (1.23/3.4=0.36) of the crossbridges are attached (q=∼0.35). A more sophisticated version of the argument taking account of the actual shape of the force curve for the equilibrium mixture (illustrated in figure 4c) is also compatible with an assumption of 35 per cent attachment. Decostre et al. (2005), however, concluded that the proportion of attached crossbridges is only 20 per cent. The difference from our estimate arises because they estimated the extra work at the higher temperature in a different way, using the change with temperature in the elastically stored energy, the area under the instantaneous force–length curve. But this seems wrong to us, because the quick length changes used to measure this curve do not allow the A1–A2 transition to occur.
(iii). Finding the number of state transitions
The observation of the T2 curve at two different temperatures arises from a real experiment similar to the thought experiment above. The difference is that in the actual experiment the crossbridge makes all the possible transitions, not just the A1–A2 transition considered in figure 4b. Figure 4d shows the two T2 curves. The extra area under the T2 curve at the higher temperature is shaded and amounts to 3.7 zJ per crossbridge site. This represents the extra work done by all the transitions between states. The value is approximately three times that obtained from consideration of the single transition in figure 4b. This suggests that, if all transitions are equivalent, there are three of them, and therefore that there are four attached states.
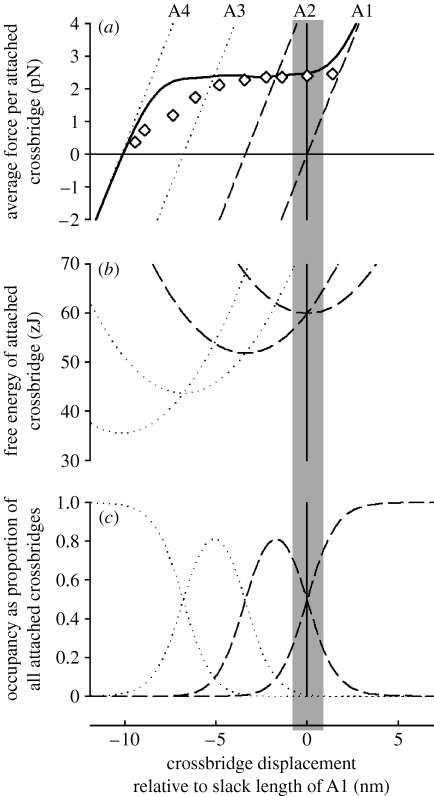
(iv). A model to explain the T2 curve
This information about isometric force, the displacement distance (z), proportion of crossbridges attached (q) and the number of attached states, is sufficient without further assumptions to build a model of the type used by Huxley & Simmons (1971) and Piazzesi et al. (2003) to explain the shape of the T2 curve. Such a model is shown and explained in figure 5. Comparing the thick curve in figure 5a with the experimental observation of the T2 curve (open symbols) shows the model to be successful in predicting the width of the curve, but the shape matches only approximately. The likely reason for the poor match in shape is that the constraints on the attachment of crossbridges owing to (i) different longitudinal spacing of actin and myosin sites (Huxley & Tideswell 1996; Smith et al. 2008) and (ii) effects of filament compliance on the proximity of actin and myosin sites (Smith et al. 2005) have not been included here.
Figure 5.
(a) Force, (b) free energy, (c) occupancy for each of the four states of the attached crossbridges at 2°C. States A1 and A2 are shown by dashed curves and the other states by dotted curves. The shaded area is the region within which crossbridges can attach in isometric contraction. The thick curve in (a) is the force of the equilibrium mixture of the four crossbridge states. Diamonds show observations of T2 from Piazzesi et al. (2003). This diagram is built up as follows. From the proportion of crossbridges attached (35%), the stiffness of the attached crossbridge states is found by dividing the observed stiffness (solid line, figure 2b) by 0.35. This allows the dashed force curve for A1 to be drawn. The value of z is then used to draw the curve for A2 offset by 3.4 nm. The two dotted curves (A3, A4) are added with the same offsets. From the slope of these curves, the shape of the curves relating free energy to length is found. Since it is observed that A1 and A2 have equal free energy at x=0 (at 2°C), these two curves are offset vertically to cross at x=0, as shown in (b). The other free energy curves are added in a similar way. The occupancy of each state is calculated from the free energy (ΔG) curves using the Boltzmann distribution (see box S6 in the electronic supplementary material) The force of the equilibrium mixture of attached states (solid curve in (a)) is the weighted mean of the forces of the individual states, using the occupancy as the weighting factors.
(v). Summary and further steps
A surprisingly complete and self-consistent description of isometric force and the force transients can be derived from the temperature dependence of force and of force response to length steps. The following are ways to further investigate the model.
Measure the force in the A1 state at x=0. Is it actually zero as has so far been assumed? It is not clear what experiment will test this point.
Test whether ΔH is temperature independent as has been assumed. This is discussed in the electronic supplementary material; the conclusion is that there is some, but not conclusive, evidence that ΔH falls as temperature rises.
Measure the force after a rapid temperature change. The model predicts a rapid readjustment to a new equilibrium distribution between A1 and A2 states, similar to that caused by a quick length step. Observations on this point are discussed in §4.
It is a feature of the model that during the T1–T2 transition, heat (enthalpy) is absorbed. Is this observed? We describe experiments in §5.
The model has implications for muscle efficiency. Are these compatible with observations? We shall discuss this question elsewhere.
4. Temperature jump: adding heat
The conclusion from the experiments discussed in §3 was that the force-producing process (equation (3.1)) is endothermic and so force would be expected to increase when temperature is suddenly increased (a temperature jump). This is indeed what is found (Bershitsky & Tsaturyan 1989, 1992; Coupland & Ranatunga 2003). Force increases after a temperature jump without an increase in the number of attached crossbridges (Bershitsky & Tsaturyan 2002). If the shift in the A1–A2 equilibrium were the only consequence of a temperature jump or a length step, then the time course of force change after the perturbation would be similar in both cases because the rate constants of the same reactions would be the determining factor for each. However, the evidence shows that the time course of force change after a temperature jump is considerably slower than the time course of force recovery after a length step (figure 6a). In particular, recovery after temperature jump lacks the rapid phase of force recovery, which is thought to be due to re-equilibration of the different attached states of the crossbridges. Davis & Harrington (1993) and Davis & Epstein (2007) have also observed this difference of time course between force responses to length steps and temperature jumps. Their analysis, based on fitting the responses with multiple exponential components, concludes that a component within the length-step response represents the same process as a component in the temperature-step response. This conclusion is challenged by Bershitsky & Tsaturyan (2002) who showed that there is no interaction between the two types of perturbation response when they are applied together, and conclude that the two types of response represent different processes.
Figure 6.
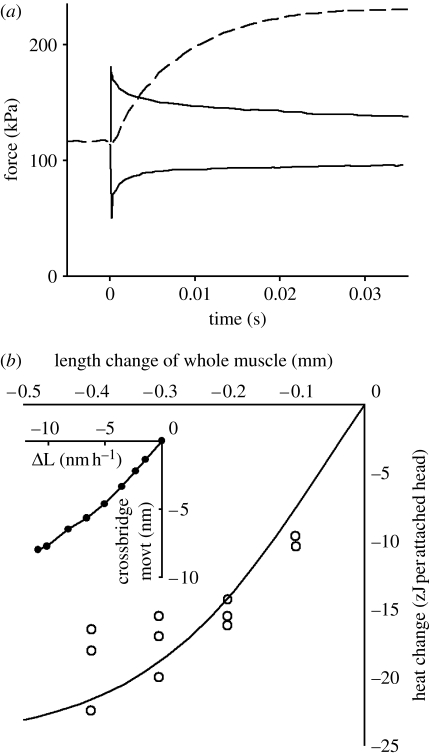
(a) Observations of force responses to temperature jump (dashed curve) and to length steps (solid curves). Redrawn from Bershitsky & Tsaturyan (2002). (b) Heat change during the rapid tension recovery after a quick release. Open circles, observations of Gilbert & Ford (1988). It has been assumed here that 35% of the bridges were attached and the heat change per attached bridge is plotted. The curve is calculated from simulated length steps using the reaction scheme shown in figure 7a. To compare these results with the observations it was assumed that a length change of the whole muscle of 0.5 mm corresponded to a crossbridge displacement of 3 nm. The inset figure shows the relationship between the imposed length change and the length taken up by the T1–T2 transition. Data from Piazzesi et al. (2003).
We propose a new explanation of the different time courses of the force response to length step and to temperature jump (§6, below). Our explanation also appertains to the experimental results discussed in §5, which will therefore be described next.
5. Calorimetry gives a paradoxical result
We can get information about the enthalpy changes for transitions between the crossbridge states from experiments on muscle. Gilbert & Ford (1988) measured the heat change during the fast increase in force from T1 to T2 that follows a length step. The experiments were done with whole sartorius muscle from frog. Although their fastest releases (complete in 1 ms) were rather slow by today's standards, the force records clearly show both T1 and T2. The heat records were corrected for heat diffusion delay, for heat produced due to work done against the friction between the moving muscle and the thermopile, and for thermoelastic heat (box S4 in the electronic supplementary material). Figure 6b shows their result. The T1–T2 transition absorbed an amount of heat that depended on the size of the release, but was not proportional to the drop in force from the isometric level, T0–T2. Instead, heat absorption increased strongly with step size for small to moderate steps, and became relatively constant for large releases, which is similar to the length change that occurs in the crossbridges during the T1–T2 transition (see inset to figure 6b). This characteristic was taken as evidence that the heat absorption they had measured came from the transitions that are responsible for the increase in force from T1 to T2. The maximum heat absorption they detected is equivalent to heat absorption during the increase in force from T1 to T2 of 6.9 zJ per crossbridge site. If only 35 per cent of the heads are attached, the heat absorption is equivalent to 20 zJ per attached head. This confirms that the transition is endothermic, but the ΔH value is much less than that for the A1–A2 transition described above, 114 zJ per attached head, obtained from the temperature dependence of isometric force.
6. Paradox resolution
Muscle is a machine for energy conversion that couples the splitting of ATP to the performance of work by filament sliding. The thermodynamic definition of coupling, due to Kedem & Caplan (1965), is explained in the electronic supplementary material, box S5. According to their definition of a tightly coupled system, neither of the coupled processes can occur without the other and such a system has an efficiency of 1.0. Clearly, muscle is not tightly coupled because it fails to meet either of these criteria. During isometric contraction, ATP splitting by myofibrils occurs without sliding of the filaments, and during contractions with filament sliding, the maximum efficiency is much less than 1.0, approximately 0.4 (Smith et al. 2005). It follows that the coupling in muscle is not tight. Consequently, there must be a branch (or branches) in the pathway represented in the model so that the two processes can occur somewhat independently. It is an open question where these branches are to be found, but it is generally understood that branches are needed. Unfortunately, branches are not obvious in the usual representations of models of the crossbridge cycle.
Model 1 (figure 1) illustrates this point. The solid curve shows a ‘typical’ representation of the crossbridge cycle; it is an unbranched pathway that includes a sliding step or steps (the working stroke). However, this representation is not adequate for isometric conditions in which crossbridge detachment occurs without the working stroke. The dashed curve has therefore been added to represent the isometric route in which the working stroke is not completed, but Pi and ADP are released sequentially to arrive at AM. So to accommodate both isometric conditions and contractions with sliding (and indeed sliding at different velocities), there is a (usually implied) branch point before or within the sliding step. A closer definition of the branching structure is required for an understanding of efficiency and how it varies with velocity of sliding. Although an in-depth discussion of efficiency is beyond our remit here, we give an example of how a branched model can be used to reconcile the following two paradoxes, which we have encountered above.
Length-step perturbations produce much faster transient responses than temperature jumps (§4). This is particularly notable for shortening steps. The model described below allows two types of change, mechanical and biochemical, to occur independently of each other.
Much less heat is absorbed in the T1–T2 transition than expected from the ΔH obtained from temperature dependence of isometric force (§5).
(a). A crossbridge model that can reconcile length-step and temperature-jump results
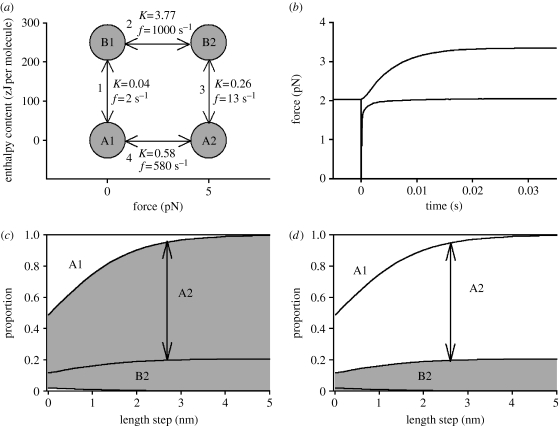
This kinetic scheme has four attached crossbridge states having the enthalpy content and force-exerting capacities shown in figure 7a. The application of heat promotes the shifts A1→B1 and A2→B2. The transitions A1→A2 and B1→B2 are promoted by a shortening step and occur as force recovers from T1 to T2 after the step. The thermodynamic and kinetic specifications of the model are shown in figure 7a. Figure 7c,d shows the occupancy of each state before (length step=0) and after length steps of different sizes. The proportion in each state is the vertical distance between the curves; for example, the arrow shows the proportion in state A2 after a step of 2.6 nm. The shading in figure 7c shows the high force states, and in figure 7d the high enthalpy content states. For a length step of 2.6 nm, there is a considerable increase in the occupancy of high force states from 0.41 to 0.94, but the increase in occupancy of high enthalpy content states is modest (0.11–0.19). The heat absorption is therefore also modest (250×0.08=20 zJ per molecule). Only if all the bridges made this transition from low to high enthalpy content states would the heat absorption be the full molecular enthalpy change of 250 zJ.
Figure 7.
(a) The enthalpy content and force-producing capacity of four attached crossbridge states. States A1 and A2 are the same as in figure 5; the forces shown here are those at x=0 in figure 5a. States B1 and B2 are mechanically the same as states A1 and A2, respectively, but have different enthalpy contents. For simplicity, states A3 and A4 in figure 5, and the corresponding states B3 and B4, are not included here although they would be needed for a full simulation of the length-step and temperature-jump experiments. K values shown are the equilibrium constants assumed; f values are rate constants for the ‘forward’ reaction (i.e. upwards or rightwards on this diagram). (b) Simulated force responses to a temperature jump (upper curve) and to a length step (lower curve). (c,d) Distribution of attached bridges among states A1, B1, B2 and A2 as a function of length steps of various sizes. The proportion in each state is the vertical distance between the curves; for example, the arrow shows the proportion in state A2 after a step of 2.6 nm. In (c) the high force states (A2 and B2) are shaded, and the low force states (A1 and B1) are unshaded. In (d) the high enthalpy states (B1 and B2) are shaded, and low enthalpy states (A1 and A2) are unshaded.
Using this reaction scheme, we have simulated the redistribution of states after a temperature jump and after a length step. We also calculated the amount and time course of the force increase and the enthalpy change that follows each perturbation. Comparison of the force simulations (figure 7b) with the experiments shown in figure 6a shows approximate agreement. Thus paradox 1 above can be resolved; the scheme accounts for the slower force increase after a temperature jump than after a length step.
The second paradox is addressed in figure 6 b that shows that the model's prediction of heat absorption following length steps agrees with the observations of Gilbert & Ford (1988). The predicted temperature dependence of isometric force is shown in figure 3 (dotted curve). The scheme gives reasonable explanations of both sets of observations and thus resolves the paradox that arises if the two-state model (equation (3.1)) is used.
7. Pi binding and its temperature dependence
Less force is produced by permeabilized muscle fibres when the Pi concentration is increased (Cooke & Pate 1985; Dantzig et al. 1992; Coupland et al. 2001; Tesi et al. 2002). This has been considered to be due to an increased rate of reaction 4 in model 1 (figure 1), increasing the level of the AM′.ADP.Pi state and also that of AM*.ADP.Pi. Since AM*.ADP.Pi produces no force the average force is reduced. Coupland et al. (2001) showed that the relative force depression caused by Pi is less at higher temperatures than at lower temperatures. They suggest that this is due to reaction 3 in model 1 being endothermic with a ΔH value of approximately +130 zJ per molecule.
When the change in Pi is made suddenly (Tesi et al. 2002), force falls at a rate that is not only clearly faster than the crossbridge turnover rate, but also clearly slower than the transient responses produced by a length step. An explanation for the relatively slow response in the phosphate-step experiments has been proposed by Sleep et al. (2005; Smith & Sleep 2004). They suggest that the rapid ‘force-generating event’ (reaction 3 in model 1) is followed by a slower phosphate release step (reaction 4). It is therefore natural to consider whether the endothermic reaction 3 is the same as the transition from A1 to A2, the equilibrium that is perturbed by temperature change and has also been shown to be endothermic with a similar ΔH.
Recent experiments have shown, however, that this mapping of states is unlikely to be correct. Caremani et al. (2008), using permeabilized rabbit psoas fibres at 12°C, have found that when force is reduced by Pi the sarcomere stiffness (which depends on both crossbridge stiffness and filament stiffness) also falls. When force is reduced by increasing Pi, the relationship between force and stiffness is the same as that found when force is reduced by using submaximal calcium activation (Linari et al. 2007). This is quite different from the effect of changing force by altering temperature, which leaves sarcomere stiffness unchanged. From these observations, it is apparent that the force per attached crossbridge is not altered by increasing Pi; the force is less at higher Pi solely because the number of attached crossbridges is reduced. As pointed out by Caremani et al., model 1 (figure 1) can be adapted to explain this result by omitting the state AM*.ADP.Pi, a state which is stiff but produces no force, as shown in model 2. The effect of Pi in this model is due to increasing the occupancy of the weakly bound state A-M.ADP.Pi. Since AM*.ADP.Pi does not exist in model 2, we no longer have a single candidate state to map against A1. Instead, the model 2 has a mixture of the mechanical states corresponding to A1 and A2 within each of the biochemical states AM′.ADP.Pi, AM′.ADP and AM. The proportions of A1 and A2 are altered by temperature and in this way temperature change can alter the force per attached crossbridge, although changing Pi concentration cannot do so.
The available description of the interactions of Pi and temperature with force and stiffness seems, however, to be incomplete. If Caremani's conclusion, that force per crossbridge is unchanged by Pi, were true at all temperatures, then the effects of Pi change and temperature change would be multiplicative. For example, if the Pi level is increased enough to halve the force, then the temperature sensitivity of force should also be halved, since only half as many crossbridges are there to produce force and respond to temperature change. This, however, is not the result seen by Coupland et al. (2001) when they investigated the effect on force of both temperature and Pi over a wide range. They found that, at temperatures between 10 and 20°C, the effect of Pi and temperature changes were additive, not multiplicative. In other words, the sensitivity of force to temperature is independent of Pi level. This suggests that Caremani's conclusion cannot apply across this whole temperature range and that, at some temperatures, the force per attached crossbridge is sensitive to temperature. Measurements of stiffness as a function of both Pi and temperature are needed to clarify the relationship between these variables and further test model 2.
8. Conclusion
Two independent lines of argument have led us to the idea that the different states of the working stroke A1, A2, etc., cannot be identified with individual biochemical states of model 1. Instead, a mixture of the working stroke states must be possible within each of several biochemical states. Model 2 (figure 1) shows such a scheme. The way in which the progression through the working stroke influences the progression although the biochemical cycle is represented in such schemes by the difference between the kinetic constants for the parallel reactions such as 1 and 3 in figure 7a. In this scheme, the working stroke (reaction 4) promotes the biochemical process (reaction 3) because the equilibrium constant for reaction 3 is larger than that for reaction 1.
In our first argument for parallel reaction pathways, the biochemical cycle was represented by a step from a low to a high enthalpy state and in the second argument by a transition between states with and without Pi. The simplest model would combine these two events into a single transition shown as 4 in model 2. Until recently, it seemed worthwhile to further explore whether a model similar to this could account for the way both Pi and temperature change force. However, it is now apparent that such a model in which Pi and temperature modulate the same reaction is inconsistent with the recent observations of Caremani et al. (2008) that the force depressions caused by Pi and by cooling work by different mechanisms. It is not apparent to us how to escape from this dilemma, but a more complete experimental description of the effects of Pi and temperature on force per attached crossbridge would certainly help to give a deeper understanding in this area.
We have tried in the above accounts of the interpretation of experimental data relating to skeletal muscle to avoid most of the detailed analysis that would be essential to a fully quantitative crossbridge model. The interested reader is directed to Smith et al. (2008) for consideration of some of these factors. Our aim has been to illustrate how combining the study of temperature dependence of equilibrium mixture of crossbridge states with calorimetry and with responses to rapid temperature jump can lead to new insights into the crossbridge cycle. In particular, we show that an explicitly branched pathway allowing mechanical transitions and biochemical transitions to occur independently can successfully predict results from several of these different approaches.
References
- Bershitsky S.Y., Tsaturyan A.K.1989Effect of joule temperature jump on tension and stiffness of skinned rabbit muscle fibers. Biophys. J 56, 809–816 (doi:10.1016/S0006-3495(89)82727-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershitsky S.Y., Tsaturyan A.K.1992Tension responses to joule temperature jump in skinned rabbit muscle fibres. J. Physiol 447, 425–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershitsky S.Y., Tsaturyan A.K.2002The elementary force generation process probed by temperature and length perturbations in muscle fibres from the rabbit. J. Physiol 540, 971–988 (doi:10.1113/jphysiol.2001.013483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caremani M., Dantzig J., Goldman Y.E., Lombardi V., Linari M.2008Effect of inorganic phosphate on the force and number of myosin cross-bridges during the isometric contraction of permeabilized muscle fibres from rabbit psoas. Biophys. J 95, 5798–5808 (doi:10.1529/biophysj.108.130435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Pate E.1985The effects of ADP and phosphate on the contraction of muscle fibers. Biophys. J 48, 789–798 (doi:10.1016/S0006-3495(85)83837-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland M.E., Ranatunga K.W.2003Force generation induced by rapid temperature jumps in intact mammalian (rat) skeletal muscle fibres. J. Physiol 548, 439–449 (doi:10.1113/jphysiol.2002.037143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland M.E., Puchert E., Ranatunga K.W.2001Temperature dependence of active tension in mammalian (rabbit psoas) muscle fibres: effect of inorganic phosphate. J. Physiol 536, 879–891 (doi:10.1111/j.1469-7793.2001.00879.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N.A., Davies R.E.1973Chemical and mechanical changes during stretching of activated frog skeletal muscle. Cold Spring Harb. Symp. Quant. Biol 37, 619–626 [Google Scholar]
- Dantzig J.A., Goldman Y.E., Millar N.C., Lacktis J., Homsher E.1992Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J. Physiol 451, 247–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.S., Epstein N.D.2007Mechanism of tension generation in muscle: an analysis of the forward and reverse rate constants. Biophys. J 92, 2865–2874 (doi:10.1529/biophysj.106.101477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.S., Harrington W.F.1993A single order–disorder transition generated tension during the Huxley–Simmons phase 2 in muscle. Biophys. J 65, 1886–1898 (doi:10.1016/S0006-3495(93)81259-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decostre V., Bianco P., Lombardi V., Piazzesi G.2005Effect of temperature on the working stroke of muscle myosin. Proc. Natl Acad. Sci. USA 102, 13 927–13 932 (doi:10.1073/pnas.0506795102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune N.S., Geeves M.A., Ranatunga K.W.1991Tension responses to rapid pressure release in glycerinated rabbit muscle fibers. Proc. Natl Acad. Sci. USA 88, 7323–7327 (doi:10.1073/pnas.88.16.7323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S.H., Ford L.E.1988Heat changes during transient tension responses to small releases in active frog muscle. Biophys. J 54, 611–617 (doi:10.1016/S0006-3495(88)82996-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A.F., Simmons R.M.1971Proposed mechanism of force generation in striated muscle. Nature 233, 533–538 (doi:10.1038/233533a0) [DOI] [PubMed] [Google Scholar]
- Huxley A.F., Tideswell S.1996Filament compliance and tension transients in muscle. J. Muscle Res. Cell Motil 17, 507–511 (doi:10.1007/BF00123366) [DOI] [PubMed] [Google Scholar]
- Kedem O., Caplan S.R.1965Degree of coupling and its relation to efficiency of energy conversion. Trans. Farad. Soc 61, 1897–1911 (doi:10.1039/tf9656101897) [Google Scholar]
- Linari M., Dobbie I., Reconditi M., Koubassova N., Irving M., Piazzesi G., Lombardi V.1998The stiffness of skeletal muscle in isometric contraction and rigor: the fraction of myosin heads bound to actin. Biophys. J 74, 2459–2473 (doi:10.1016/S0006-3495(98)77954-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linari M., Woledge R.C., Curtin N.A.2003Energy storage during stretch of active single fibres from frog skeletal muscle. J. Physiol 548, 461–474 (doi:10.1113/jphysiol.2002.032185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linari M., Caremani M., Piperio C., Brandt P., Lombardi V.2007Stiffness and fraction of myosin motors responsible for active force in permeabilized muscle fibers from rabbit psoas. Biophys. J 92, 2476–2490 (doi:10.1529/biophysj.106.099549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley B.A., Eisenberg B.R.1975Sizes of components in frog skeletal muscle measured by methods of stereology. J. Gen. Physiol 66, 31–45 (doi:10.1085/jgp.66.1.31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzesi G., Reconditi M., Koubassova N., Decostre V., Linari M., Lucii L., Lombardi V.2003Temperature dependence of the force-generating process in single fibres from frog skeletal muscle. J. Physiol 549, 93–106 (doi:10.1113/jphysiol.2002.038703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall J.A., Woledge R.C.1990Influence of temperature on mechanics and energetics of muscle contraction. Am. J. Physiol 259, R197–R203 [DOI] [PubMed] [Google Scholar]
- Reconditi M., et al. 2004The myosin motor in muscle generates a smaller and slower working stroke at higher load. Nature 428, 578–581 (doi:10.1038/nature02380) [DOI] [PubMed] [Google Scholar]
- Sleep J., Irving M., Burton K.2005The ATP hydrolysis and phosphate release steps control the time course of force development in rabbit skeletal muscle. J. Physiol 563, 671–687 (doi:10.1113/jphysiol.2004.078873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.A., Sleep J.2004Mechanokinetics of rapid tension recovery in muscle: the myosin working stroke is followed by a slower release of phosphate. Biophys. J 87, 442–456 (doi:10.1529/biophysj.103.037788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.A., Geeves M.A., Sleep J., Mijailovich S.M.2008Towards a unified theory of muscle contraction. I: foundations. Ann. Biomed. Eng 36, 1624–1640 (doi:10.1007/s10439-008-9536-6) [DOI] [PubMed] [Google Scholar]
- Smith N.P., Barclay C.J., Loiselle D.S.2005The efficiency of muscle contraction. Prog. Biophys. Mol. Biol 88, 1–58 (doi:10.1016/j.pbiomolbio.2003.11.014) [DOI] [PubMed] [Google Scholar]
- Squire J.1981The structural basis of muscular contraction. New York, NY: Plenum Press [Google Scholar]
- Tesi C., Colomo F., Piroddi N., Poggesi C.2002Characterization of the cross-bridge force-generating step using inorganic phosphate and BDM in myofibrils from rabbit skeletal muscles. J. Physiol 541, 187–199 (doi:10.1113/jphysiol.2001.013418) [DOI] [PMC free article] [PubMed] [Google Scholar]