Abstract
Protein kinases control the flow of information through cell-signaling pathways. A detailed analysis of their behavior enhances our ability to understand normal cellular states and to devise therapeutic interventions for diseases. The design and application of “Environmentally-Sensitive”, “Deep-Quench” and “Self-Reporting” sensor systems for studying protein kinase activity are described. These sensors allow real-time activity measurements in a continuous manner for a wide variety of kinases. As these sensors can be adapted from an in vitro screen to imaging kinase activity in living cells, they support both preliminary and later stages of drug discovery.
Keywords: Signal Transduction, Sensors, Fluorescent peptides, Assays, Inhibitors, Drug Discovery
1. Introduction
Cells exhibit a wide range of exceptionally versatile responses upon exposure to chemical or environmental signals. The list of such responses includes growth, differentiation, changes in metabolism, alterations in ion fluxes, modifications in gene expression, and death, among others. Between these two end-points, namely the cell’s exposure to a signal and its subsequent response, lies a modular communication system consisting of a series of convergent and divergent interaction-networks. The formation of a majority of these networks and the flow of information through them is exquisitely controlled and precisely timed by the action of protein kinases. All protein kinases act to catalyze the transfer of the γ-phosphoryl group of ATP to the side chain oxygen atom of amino acid residues in peptides and proteins. Generally, protein kinases are divided into two subfamilies based on their ability to phosphorylate the aromatic side chain of tyrosine or the aliphatic side chain of serine or threonine. In addition kinases differ in their cellular expression, their catalytic regulation, and their preferences for specific amino acid sequences that encompass the phosphoryatable residue [1].
Just as the role played by protein kinases appears crucial for normal cellular communication and physiology, not surprisingly, aberrant protein kinase action is associated with numerous disease states [2]. This makes misbehaving members of the protein kinase family potential drug targets for numerous diseases such as, cardiovascular disease, central nervous system disorders, inflammation, endocrinological disorders and cancer. Indeed, inhibitors of protein kinases have been recently developed as anti-cancer agents [3]. The clinical success of these agents underscores the need to focus research efforts on understanding the roles played by these enzymes and to discover more modulators of their action. Such a focus will increase our understanding of the differences between normal and diseased cellular states and may also lead to the development of novel therapeutic interventions. The later may prevent the progression of, or even reverse, disease states associated with aberrant protein kinases.
The current gold standard for the measurement of protein kinase activity is the radioactive-ATP assay, which measures the amount of 32P, or 33P, incorporated into a peptide or protein substrate. In principle, this assay can be applied to the entire protein kinase family. However, as an in vitro assay, it cannot reproduce elements of the intracellular milieu (e.g. [ATP] > 1 mM). In addition, radioactive-ATP assay is discontinuous, necessitating that the kinase reaction be stopped and then subjected to a series of post-reaction processing steps. Furthermore, the method is difficult to automate, labor intensive, and of questionable utility when adapted to whole-cell or animal tests. Clearly, the use of radioactivity requires special handling and disposal techniques.
Other phosphorylation detection methods have been developed to reduce or completely eliminate radioactivity use. For example, certain assays use antibodies to differentiate between phosphorylated and non-phosphorylated forms of protein kinase substrates. Methods that employ radiolabeled antibodies use less radioactivity than the radioactive-ATP assay, while radioactivity use is completely eliminated when antibodies are labeled with fluorescent molecules. These methods make use of western-blots or immuno-precipitations to detect the phosphorylated substrate after cell-lysis in a discontinuous format. Assays that use a labeled-antibody format can also be adapted to fluorescence microscopy techniques to reveal a protein’s phosphorylation state within the cell just prior to being fixed. Unfortunately, these assays require time-consuming washes and are dependent on selective antibodies. In addition, generating antibodies that selectively target specific phosphorylated protein substrates can be problematic. Antibody use is eliminated by certain proteomic methods such as 2D-gel electrophoresis followed by mass-spectrometry. However, the disadvantages of assay discontinuity remain. Typically, an assay with a cell-destructive format shows no spatial resolution and provides poor temporal resolution with respect to cellular behavior.
2. Sensor Development Strategies
Phosphorylated peptide products of a variety of kinases bind modules of cellular communication networks, namely protein interaction domains. These domains can be used for designing sensors of a wide variety of protein kinases. Fluorescent molecules (fluorophores) can be incorporated either into protein interaction domains, using protein labeling methods, or into peptides which bind to these domains, via solid-phase peptide synthesis [4]. Incorporating the flurophores synthetically into peptides provides access to a diverse array of unnatural substituents. It also affords the ability to construct and screen peptide libraries and perform large-scale synthesis. Fluorescence-based measurements eliminate radioactivity and are more sensitive than other spectroscopic methods such as absorbance. In addition, fluorophores possess other intrinsic properties that can be used for the design of kinase sensors, including polarization, FRET, quenching, metal-ion enhancement, and environment sensitivity. Peptide sensors can be adapted to in vitro screens as long as large changes are exhibited upon phosphorylation. Furthermore, these sensors can be adapted to imaging kinase activity in living cells if the fluorophores are bright, excited well into the visible range, and are photostable (i.e. “cell-friendly”). Keeping these parameters in mind, we developed a library-based approach to identify fluorescently-labeled peptides which report a change in phosphorylation status. In our approach, peptide libraries are constructed from consensus sequences using different fluorophores at different positions. They are screened to identify those species that fluorescently respond to phosphorylation. We have constructed an array of fluorophore-labeled peptide sensors, applicable to a wide range of kinases, with large dynamic ranges, and having “cell-friendly” photophysical properties.
3. “Environmentally-Sensitive” Protein Kinase Sensors
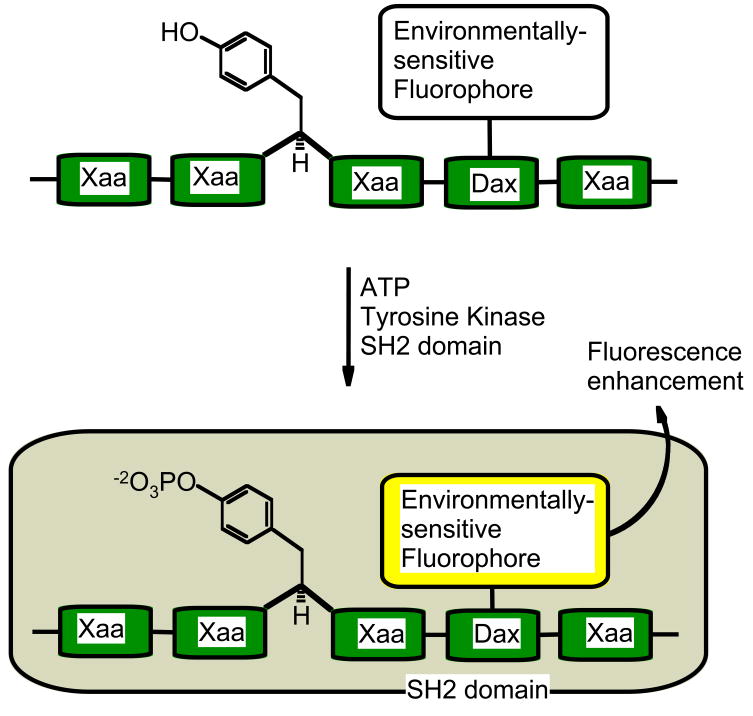
Changes in solvent polarity cause environmentally-sensitive fluorophores to display changes in their emission wavelength and fluorescence quantum yields. A kinase sensor can be devised by appending an environmentally-sensitive fluorophore anywhere on a peptide provided the peptide experiences some form of phosphorylation dependent solvent change. Due to a large range of available environmentally-sensitive fluorophores, it may be possible to simultaneously assess the activity of two or more kinases using such a strategy. As protein interaction domains are capable of binding phosphorylated peptides, they can provide the required regions of solvent-change (Figure 1). For example, the SH2 domains and 14-3-3 domains bind peptides containing phospho-tyrosine and phospho-serine residues, respectively.
Figure 1.
Environmentally-Sensitive system: Phosphorylation of a fluorophore-substituted tyrosine peptide results in its association with an SH2 domain, and the fluorophore experiences a change in environment and an increase in quantum yield. Dax represents either a Dap or Dab residue (see Figure 2 for structures) and Xaa an amino acid of the consensus sequence.
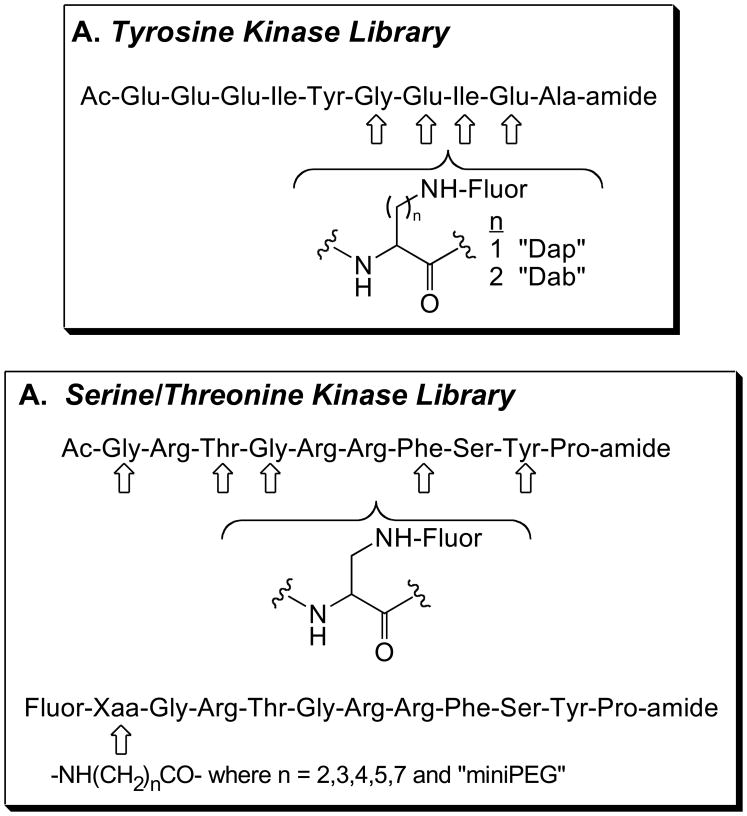
A 24-member peptide library was constructed via Fmoc solid phase peptide synthesis based on a Src tyrosine kinase substrate sequence. The amino acid residue at four different positions was replaced by side chain protected Dap or Dab residue, which provide an attachment site for fluorophores (see Figure 2A and Ref. [5]). Following the synthesis of the desired amino acid sequence, the amine handle of the side chain was selectively de-protected and various fluorophores (Dapoxy, NBD, Cascade Yellow) were attached. The library was screened in the presence of the Src Kinase and Lck-SH2 interaction domain (Src kinase/SH2 system) and sensors that display up to a 7-fold phosphorylation-induced enhancement in fluorescence were identified [5]. A dapoxyl fluorophore attached to Dab residue positioned four residues away from the site of phosphorylation (Y+4), displays the largest fluorescent enhancement. Although not as dramatic, Src phosphorylation-dependent changes were observed with other fluorophores as well. It appears a hydrophobic subsite in the SH2 domain, proximal to the Y+3 or Y+4 positions is responsible for these enhancements in fluorescence. For example, an NBD fluorophore (smallest fluorophore in the series) attached to a Dab residue at the Y+3 position and a Cascade Yellow fluorophore (largest flurophore in the series) appended to the same residue and position both fluorescently report changes in phosphorylation status.
Figure 2.
Substrate library design used to generate protein kinase sensors: (A) The tyrosine kinase substrate library developed for the Src kinase and (B) the serine/threonine kinase substrate library developed for the cAMP-dependent protein kinase. Fluor = pyrene, dapoxyl, NBD, and cascade yellow.
By contrast, an analogous library devised for the cAMP-dependent protein kinase (PKA) provided leads that display only modest fluorescent responses when used in conjunction with a posphoserine-binding (14-3-3) domain. In this particular case, the PKA substrate library was constructed using amine-handles at seven different positions along the consensus sequence, to which various fluorophores were coupled (see Figure 2B). The lead pyrene-based species displays a 1.6-fold phosphorylation-induced enhancement in fluorescence [6]. The modest fluorescent response in the PKA/14-3-3 system may be a consequence of the fluorophore’s inability to access a hydrophobic subsite.
4. “Deep-Quench” Protein Kinase Sensors
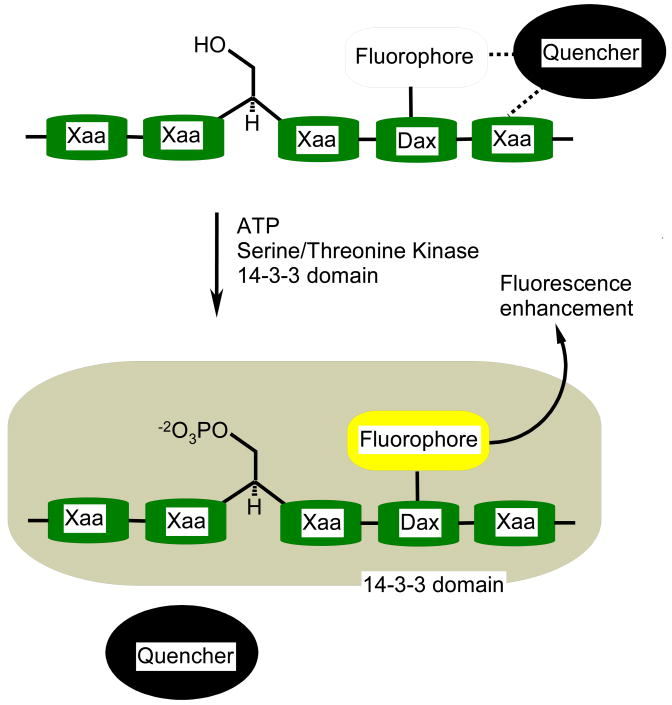
Collisions of fluorophores with other molecules results in the loss of excitation energy as heat instead of emitted light. Due to factors such as self-quenching, negative deviations from linearity in fluorescence emissivity versus concentration curves are seen at high concentrations of fluorophores. Indeed, quenching interactions can result in a nearly complete loss in fluorescence. Is it possible to construct a kinase sensor whose fluorescence is quenched and then subsequently recovered upon phosphorylation? In the presence of a quencher in solution, the sequestration of a kinase phosphorylable peptide by a binding domain has the potential to reduce quenching interactions. However, ideal fluorophore-quencher pairs need to be identified.
A screening-based approach was used with 47 potential quenchers in conjunction with the pyrene flurophore, that was covalently appended to ten different sites on PKA consensus peptides [6]. We employed the 14-3-3 domain as a phosphopeptide-capture motif whose presence is designed to relieve pyrene fluorescence quenching by sequestering the phosphorylated peptide (see Figure 3). Phosphorylation-induced fluorescence responses vary as a function of both quencher and fluorophore position on the consensus peptide sequence. Lead peptide/quencher pairs furnished fluorescence enhancements of 21-fold (Ponceau S), 55-fold (Aniline Blue WS), and 64-fold (Rose Bengal). All of the quencher molecules are negatively charged, which suggests that these species may form complexes with the positively charged peptide substrates. The mechanism responsible for the observed fluorescence change is likely the kinase-mediated phosphorylation of the peptide substrate followed by its subsequent binding to the 14-3-3 domain. The latter presumably disrupts the peptide/quencher complex, resulting in a dramatic fluorescent change. The “Deep-Quench” method furnishes the most dramatic kinase-dependent fluorescent responses reported to date.
Figure 3.
Deep Quench system: Phosphorylation of the peptide sensor results in its sequestration from the quencher in solution, by the 14-3-3 domain. Dax represents either a Dap or Dab residue (see Figure 2 for structures) and Xaa an amino acid of the consensus sequence.
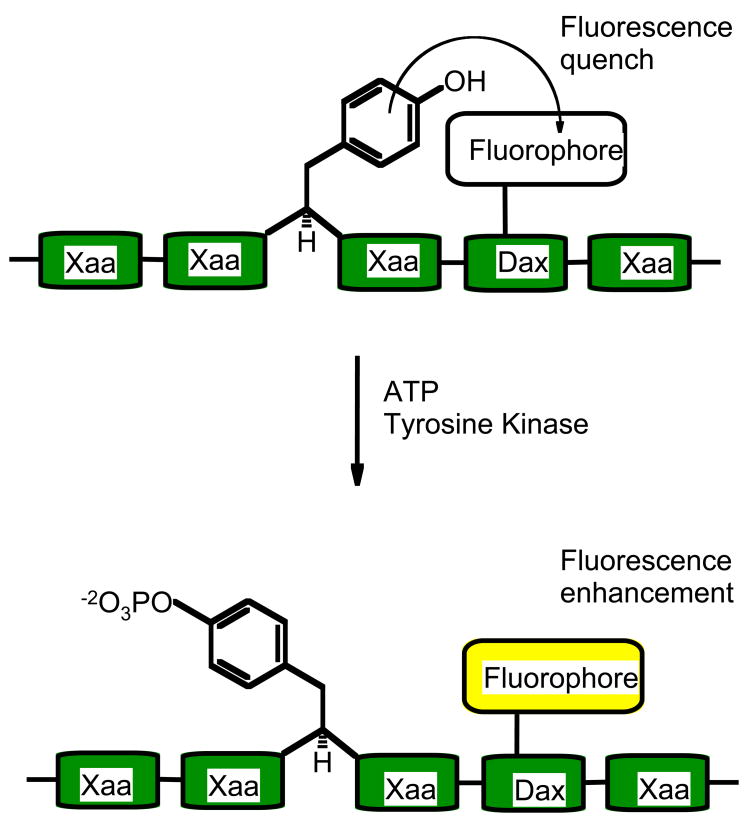
5. “Self-Reporting” Protein Kinase Sensors
Aromatic amino acids such as tryptophan, phenylalanine and tyrosine are known to engage other aromatic species, including fluorophores such as pyrene, in π-π stacking interactions which reduce fluorescence quantum yields. As the tyrosine residue is known to engage in such interactions, its phosphorylation by a tyrosine kinase could potentially provide an increase in quantum yields. Thus, a domain-free sensor system can be devised that will not need the addition of quencher species (see Figure 4). However, the position of the fluorescent molecule on a peptide needs to be adjusted so that the kinase phosphorylation event produces a relief in quenching.
Figure 4.
Self-Reporting system: Phosphorylation of tyrosine reduces its ability to quench the fluorescence of a nearby fluorophore. Dax represents either a Dap or Dab residue (see Figure 2 for structures) and Xaa an amino acid of the consensus sequence.
A library of peptides was constructed in which pyrene was appended to various sites relative to the phosphorylatable tyrosine. Leads display up to a 5-fold enhancement in fluorescence in response to phosphorylation [7]. An examination of the non-phosphorylated sensor and its phosphorylated counterpart by 2D NOESY NMR spectroscopy revealed dramatic differences in nuclear Overhauser enhancements (nOes). In particular, nOes between the tyrosine and pyrene protons, highly suggestive of π-π interactions, are present in the non-phosphorylated state but sharply reduced, or completely absent, in the phosphorylated state. The size of phosphate group and/or its charge may disrupt the π-π interactions between tyrosine and pyrene, which in turn, is likely responsible for producing a liberated and highly fluorescent phosphorylated peptide. In addition to pyrene, we have found that a variety of fluorophores can be successfully employed in the “Self-Reporting” sensor system [8]. The photophysical properties associated with these fluorophores may ultimately prove useful for simultaneously monitoring multiple protein kinases (cascade yellow: λex = 400 nm, λem = 550 nm; cascade blue: λex = 400 nm, λem = 422 nm; Oregon green: λex = 495 nm, λem = 520 nm).
6. Applications
We have obtained a wide variety of protein kinase sensors that display dramatic phosphorylation-dependent changes in fluorescence. These sensors function in a continuous manner with a wide variety of fluorophores. They are applicable to an equally wide variety of protein kinases. The “Environmentally-Sensitive” and “Deep-Quench” sensors have been employed to analyze both tyrosine and serine/threonine protein kinases, while the “Self-Reporting” sensors have been used to analyze numerous tyrosine kinases. These analyses include determinations of steady state kinetic parameters, assessments of inhibitor IC50 values and, real-time observations of kinase activity in living cells.
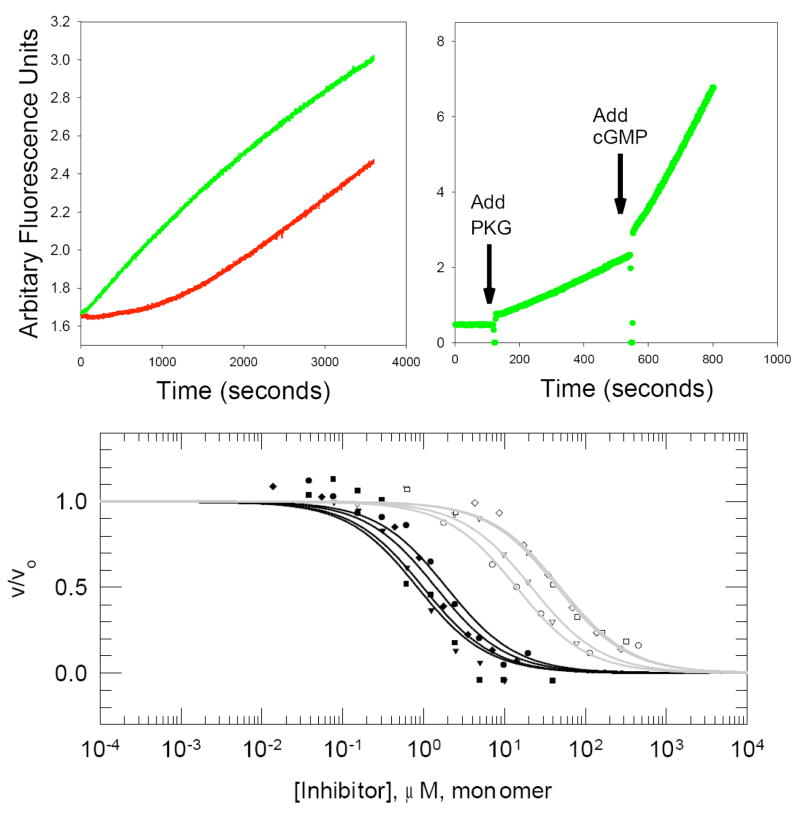
The continuous fluorescent readout of these sensors furnishes clean temporal analysis of an entire kinase-catalyzed reaction. By contrast, a discontinuous assay provides only a single data point over the same time period. A full time course assay not only leads to increased precision in the determination of kinetic parameters, but can provide additional insight concerning the behavior of the enzyme. For example, we have employed a “Self-Reporting” sensor to examine the phosphorylation-dependent activation of the Brk tyrosine kinase [7]. Unactivated Brk kinase displays a bi-phasic reaction progress curve with an initial lag in product-formation followed by a sharp rise (see Figure 5A, red curve). Pre-incubation of the kinase with ATP, allows for Brk activation via autophosphorylation. This leads to the elimination of the lag-phase (see Figure 5A, green curve). In short, the activation state of a kinase, regulated by autophosphorylation, is revealed by a simple examination of the shape of its progress curve. The continuous format also allows one to observe, in real-time, the effect of small molecules on kinase activity. For example, in a single reaction cuvette, a continuous “Deep-Quench” sensor was employed to report the cGMP-dependent activation of the cGMP dependent protein kinase (see Figure 5B and Ref. [6]). In addition, these fluorescent sensing systems can be used to examine both peptide-based and ATP-site-directed inhibitors (Figure 5C) under conditions that mimic the intracellular milieu (i.e. physiological concentrations of ATP) [6]. For example, the “Self Reporting” sensor proved to be exceptionally useful in demonstrating the striking selectivity displayed by an active-site directed bivalent inhibitor between a subset of kinases [9].
Figure 5.
Sensor applications. (A) Autophosphorylation of Blk kinase: The initial lag-period seen with Blk-catalyzed phosphorylation (red curve) was eliminated by pre-incubating the Blk enzyme with ATP (green curve). (B) Small molecule activation of PKG: Addition of enzyme to the reaction cuvette gives a rate of product formation, which is significantly enhanced upon the addition of cGMP. (C) The activity of a wide array of kinases can be examined using a single sensing system. The response of eight tyrosine kinases was assayed at different inhibitor concentrations. Fitted curves for Group A enzymes (Src, Yes, FynT and Fgr) are shown in black while fitted curves for Group B (Hck, Blk, LynA and Lck) enzymes are shown in gray. Src (closed circle), Yes (closed triangle), FynT (closed square), Fgr (closed diamond), Hck (open circle), Blk (open triangle), LynA (open square) and Lck (open diamond).
In addition to in vitro conditions, these kinase sensors have been adapted to cell-based formats. Indeed, the “Self-Reporting” sensors have been converted into photoactivatable derivatives that are impervious to phosphorylation until “switched on” using light of the appropriate wavelength (~360 nm). This allows the sensor to be preloaded into the cell and then activated with exquisite temporal control [8].
7. Conclusions and Perspectives
Both the ability to reliably detect the phosphorylation event and the manner in which this detection is made are of importance to research and development efforts. These “ends and means” establish the extent of what can be learned about the action of protein kinases, and what can effectively be leveraged in terms of moving drug-discovery efforts forward. In vitro assays allow for the determination of kinetic parameters, and the order of kinetic steps involved the formation and dissociation of enzyme-substrate and enzyme-product complexes. Additionally they also provide for the material-sensitive design of high-throughput screens used in the rapid testing of a large number of compounds. Hence, although deficient in certain aspects, in vitro assays are the method of choice in the initial stages of protein kinase research and inhibitor discovery efforts. Peptide-based fluorescent sensors enjoy the additional advantage that they be seamlessly converted from a continuous in vitro screen to living cell-based studies, which represent the later-stages of drug discovery. These in vitro and cell-based systems, when used in combination, could potentially produce dramatic benefits by reducing the late-stage failures that are common in drug company pipelines. They may even help in nurturing nascent fields of investigation such as live-cell enzymology.
Acknowledgments
The National Institutes of Health, Panomics Inc. and Sigma-Aldrich are gratefully acknowledged for generous financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawrence DS, Wang Q. Seeing is believing: peptide-based fluorescent sensors of protein tyrosine kinase activity. ChemBiochem. 2007;8:373–378. doi: 10.1002/cbic.200600473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortutay C, Valiaho J, Stenberg K, Vihinen M. KinMutBase: a registry of disease-causing mutations in protein kinase domains. Hum Mutat. 2005;25:435–442. doi: 10.1002/humu.20166. [DOI] [PubMed] [Google Scholar]
- 3.Mohindru M, Verma A. Kinase inhibitors translate lab discoveries into exciting new cures for cancers. Indian J Pediatr. 2004;71:713–718. doi: 10.1007/BF02730661. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence DS. Signaling protein inhibitors via the combinatorial modification of peptide scaffolds. Biochim Biophys Acta. 2005;1754:50–57. doi: 10.1016/j.bbapap.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 5.Wang Q, Lawrence DS. Phosphorylation-driven protein-protein interactions: a protein kinase sensing system. J Am Chem Soc. 2005;127:7684–7685. doi: 10.1021/ja050789j. [DOI] [PubMed] [Google Scholar]
- 6.Sharma V, Agnes RS, Lawrence DS. Deep quench: an expanded dynamic range for protein kinase sensors. J Am Chem Soc. 2007;129:2742–2743. doi: 10.1021/ja068280r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Q, Cahill SM, Blumenstein M, Lawrence DS. Self-reporting fluorescent substrates of protein tyrosine kinases. J Am Chem Soc. 2006;128:1808–1809. doi: 10.1021/ja0577692. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Dai Z, Cahill SM, Blumenstein M, Lawrence DS. Light-regulated sampling of protein tyrosine kinase activity. J Am Chem Soc. 2006;128:14016–14017. doi: 10.1021/ja065852z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hah JM, Sharma V, Li H, Lawrence DS. Acquisition of a “Group A”-selective Src kinase inhibitor via a global targeting strategy. J Am Chem Soc. 2006;128:5996–5997. doi: 10.1021/ja060136i. [DOI] [PMC free article] [PubMed] [Google Scholar]