Abstract
Classically, a high-power association relates the neurotransmitter release probability to the concentration of presynaptic Ca2+. Activated by the action potential waveform, voltage-gated Ca2+ channels mediate Ca2+ entry into presynaptic terminals. Inside the terminal, Ca2+ ions rapidly bind to endogenous intracellular buffers and could trigger Ca2+ release from internal Ca2+ stores. The resulting space-time profile of free Ca2+ determines the time course and probability of neurotransmitter release through the interaction with molecular release triggers strategically located in the vicinity of release sites. Following a rapid concentration transient, excess Ca2+ has to be removed from the cytosol through the process involving Ca2+ uptake by the endoplasmatic reticulum stores, sequestration by mitochondria and/or extrusion into the extracellular medium. The ongoing synaptic activity could affect any of the multiple factors that shape presynaptic Ca2+ dynamics, thus arbitrating use-dependent modification of the neurotransmitter release probability. Here we present an overview of major players involved in Ca2+ dependent presynaptic regulation of neurotransmitter release and discuss the relationships arising between their actions.
Keywords: synaptic transmission, presynaptic mechanisms, plasticity, calcium signaling
Introduction
The Ca2+ dependence of neurotransmitter release is a fundamental property of chemical synapses (Katz and Miledi 1968). Classically, the release rate at the neuromuscular junction is described as increasing with the fourth power of external Ca2+ concentration (Dodge and Rahamimoff 1967). Whether a similar, high-power association relates the release probability at central synapses to the intra-terminal Ca2+ level, resting or transient, remains incompletely understood. Increases in the residual Ca2+ concentration following a single or multiple action potentials (APs) have been found to parallel an increase in the release probability (Zucker and Regehr 2002). In the calyx of Held, however, fast Ca2+ transients generated by rapid photolytic Ca2+ uncaging inside the terminal trigger neurotransmitter release with a high degree of co-operativity (Schneggenburger and Neher 2000). When APs arrive at the terminal, the spatio-temporal profile of internal Ca2+ is formed by the kinetics and distribution of Ca2+ channels interacting with endogenous Ca2+ buffers and pump systems distributed within the terminal. The resulting wave of free Ca2+ acts upon the synaptic release triggers strategically located near the release sites. This action determines the time course and probability of neurotransmitter release (Meinrenken and others 2003). Following a rapid concentration transient, excess Ca2+ is removed from the cytosol through the process involving uptake by the endoplasmic reticulum (ER) stores, sequestration by mitochondria and/or extrusion into the extracellular medium.
The ongoing synaptic activity could in principle influence any of the multiple factors that shape the AP-driven presynaptic Ca2+ dynamics (Figure 1). The physiological consequence of these effects lies in use-dependent modification of the neurotransmitter release probability, such as short-term or long-term facilitation or depression. The molecular machinery of presynaptic Ca2+ regulation thus provides a multitude of potential targets for the external, neural-activity mediated signal which could trigger changes in the synaptic strength. The roles of these target mechanisms and their relative contribution to the shaping of the presynaptic Ca2+ profile and remain a subject of intense investigation. Here we present an overview of major players involved in Ca2+ dependent regulation of neurotransmitter release and discuss the relationships arising between their actions.
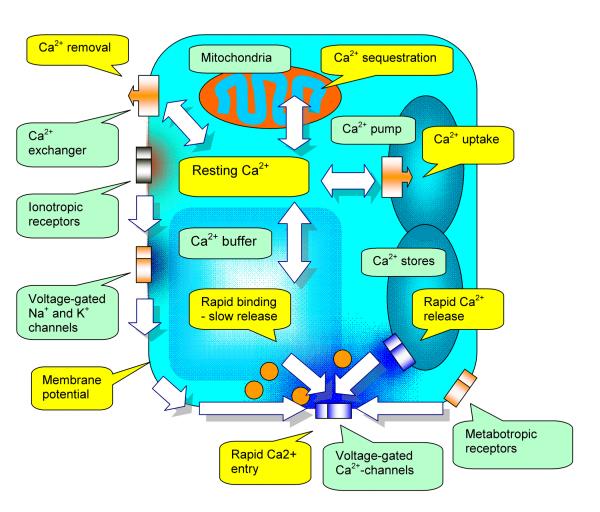
Figure 1.
A diagram of the presynaptic terminal depicting cellular mechanisms which contribute to the dynamics of free Ca2+. Cellular devices are labeled by green callouts, dynamic processes are shown in yellow callouts, and block arrows indicate the direction of action.
Electrogenic presynaptic membrane mechanisms
Ca2+ enters presynaptic terminals mainly through the voltage-gated Ca2+ channels (VGCCs). According to their activation properties and pharmacological profiles, VGCCs are divided into P/Q, N, L, R (high-voltage activated) and T (low-voltage activated) types, which is reflected in the channel subunit composition (reviewed in (Budde and others 2002)). Individual neurons often express all types of VGCCs, which are however distributed differentially among cell compartments, in accordance with their adaptive purposes. In numerous experimental studies carried out in central synapses, presynaptic Ca2+ influx has been associated mainly with P/Q-type and/or N-type VGCCs. The involvement of other channel types, in particular R-type VGCCs has also been reported (Wu and others 1998).
Activation of VGCCs is driven by the membrane potential but also depends strongly on the channel current state. Insights into the molecular machinery which controls AP-driven Ca2+ influx and Ca2+-dependent release have come primarily from whole-terminal recordings in the giant terminals of the calyx of Held (Forsythe 1994) and, recently, in large hippocampal mossy fiber boutons (Geiger and Jonas 2000)). It has been universally accepted that the opening of presynaptic VGCCs is driven mainly by the AP waveform. The latter, however, could change during repetitive spiking or membrane depolarization because of inactivation of presynaptic Na+ and K+ channels: as a result, the AP amplitude decreases while its duration increases. In the calyx of Held, for instance, a high frequency train of APs leads to a smaller yet broader spike (Borst and others 1995). Although the effect of such changes on synaptic transmission is not always straightforward, broader APs often correspond to a greater total influx of Ca2+ which has long been considered as a critical determinant of synaptic release (Jackson and others 1991). Indeed, whole-terminal recordings from giant mossy fiber boutons in acute hippocampal slices have proposed that post-train inactivation of K+ channels could be primarily responsible for the AP broadening and therefore increased synaptic transmission at the granule cell – CA3 pyramidal cell synapses (Geiger and Jonas 2000).
This voltage-dependent regulation of VGCCs could provide a mechanism by which sub-threshold changes in the presynaptic membrane potential control rapid Ca2+ influx. It has been found that changing the holding presynaptic voltage from −60 to −100 mV in hippocampal granule cells has a biphasic effect on presynaptic Ca2+ signaling in the axon: hyperpolarization first facilitates and then inhibits AP-induced Ca2+ transients at proximal axonal boutons (Ruiz and others 2003). Similarly, it has been shown that moderate presynaptic depolarization could increase the resting presynaptic Ca2+ level at the calyx of Held terminals, which in turn augments the neurotransmitter release probability (Awatramani and others 2005). The latter phenomenon was attributed to the appearance of a residual Ca2+ conductance mediated by P/Q-type VGCCs. The resulting increase in the background Ca2+ levels force corresponds to a greater fraction of endogenous Ca2+ buffering molecules being Ca-bound inside the terminal. The increase of the Ca-bound buffer reduces the intra-terminal Ca2+ buffering capacity thus boosting the magnitude of free Ca2+ transients that follows AP-evoked Ca2+ entry (see below). This mechanism could in principle provide a causal link between sub-threshold tonic activation of presynaptic ionotropic receptors and the neurotransmitter release probability (reviewed in (Semyanov and others 2004)). Whether a similar effect could be achieved through remote electrotonic influences arising from somatic or dendritic depolarization depends on the effective electrotonic space constant of the soma-axon system. In hippocampal dentate granule cells, somatic voltage does affect presynaptic Ca2+ kinetics at axonal boutons occurring 100-200 μm from the soma (Ruiz and others 2003) but has no influence on remote boutons in area CA3 (Scott and Rusakov, unpublished).
Another electrogenic mechanism potentially suitable for presynaptic control of Ca2+-dependent signaling lies in the relationships between morphology and cable properties of the axon. Classically, the AP propagation through individual branches of the end axonal arborization at the neuromuscular junction could fail. In accordance with cable theory, this conduction failure occurs because of a jump in the effective cable properties near the point of bifurcation (Parnas 1972). This phenomenon was consistent with the failure of back-propagating APs at dendritic branching points, a discovery enabled by rapid fluorescence Ca2+ imaging (Spruston and others 1995). More recently, it was proposed that AP failures at axonal branches in cultured hippocampal neurons could contribute to synaptic depression (Debanne and others 1997). However, AP-evoked fluorescent Ca2+ transients seem to propagate reliably through axonal collaterals of neocortical pyramidal neurons (Cox and others 2000; Koester and Sakmann 2000) and hippocampal interneurons (Rusakov and others 2004) in acute brain slices. In addition, no failures in AP propagation have been reported to occur at the neuromuscular junction of smooth muscle cells in the mouse (Brain and others 2002). Under what circumstances the conduction block of APs contributes significantly to synaptic function requires therefore further investigation.
Ca2+-dependent Ca2+ channel deactivation
It has long been established that a sustained Ca2+ current through various subtypes of VGCCs deactivates the channel (Brehm and Eckert 1978). That substituting Ca2+ for Ba2+ reduces dramatically this deactivation has been a hallmark of the Ca2+ ion specificity with respect to the underlying mechanism (reviewed in (Budde and others 2002)). Classically, these phenomena have been investigated using prolonged depolarization pulses in electrophysiological experiments carried out in whole-cell voltage-clamp mode. More recently, however, advances in single-terminal recording techniques, both electrophysiological and optical, have provided the opportunity to directly monitor AP-evoked Ca2+ transients in virtually intact individual presynaptic terminals. It has emerged that deactivation of rapid AP-evoked Ca2+ current in individual terminals of the calyx of Held does not contribute signficantly to use-dependent depression of synaptic transmission (von Gersdorff and Borst 2002) (however, see (Xu and Wu 2005)). Whole-terminal recordings in giant mossy fiber boutons in the hippocampus indicated no detectable decrease in Ca2+ current in response to a series of the command voltage pulses reproducing the AP waveform (Geiger and Jonas 2000). A similar conclusion was suggested by the analysis of Ca2+ dependent fluorescent transients evoked by repetitive APs in presynaptic axonal boutons of neocortical pyramidal cells (Cox and others 2000; Koester and Sakmann 2000), hippocampal interneurons (Rusakov and others 2004) or cerebellar basket cells (Rusakov and others 2005).
The most parsimonious explanation for the apparent lack of detectable VGCC deactivation in such cases is that Ca2+ ions entering the terminal in the course of a brief (~1 ms long) AP rapidly bind to endogenous Ca2+ buffers (see below). Indeed, the role of Ca2+ buffering as an attenuating mechanism for Ca2+-dependent deactivation of VGCCs has long been recognized (Brehm and Eckert 1978). This mechanism, however, is not expected to act during prolong periods of depolarization: local Ca2+ buffers are likely to be saturated near the channel mouth in the course of sustained Ca2+ influx. Nevertheless, sustained depolarization of the mossy fiber bouton membrane, in whole-terminal mode, failed to deactivate the recorded Ca2+ current (Bischofberger and others 2002). This suggests that rapid Ca2+ buffering alone cannot explain the absence of detectable Ca2+-dependent inactivation at endogenous presynaptic VGGCs at mossy fiber terminals.
Taken together, these observations point to a divergence in the Ca2+ -dependent inactivation properties of VGCCs expressed in different experimental systems and activated in different conditions. Clearly, the role of Ca2+-dependent inactivation of presynaptic VGCCs cannot be completely understood without investigating the channels in question in their natural habitat in situ, in a physiological context.
Rapid Ca2+ release from internal stores
The role of presynaptic ER Ca2+ stores in maintaining Ca2+ homeostasis had emerged when intracellular Ca2+ uptake was detected in conditions of suppressed mitochondrial activity (Blaustein and others 1978). Rapid release of Ca2+ from the ER occurs through two types of Ca2+ channels. The first type is the Ca2+-activated channels commonly known as the ryanodine receptors. The second type is represented by the inositol-3-phosphate (InsP3)-gated channels, or InsP3 receptors. Although the molecular composition and pharmacological profile of both Ca2+channel types have been studied in detail (reviewed in (Berridge 2002; Verkhratsky 2005)), their role in presynaptic signaling remains incompletely understood.
In the past decade, combining confocal or two-photon microscopy with single-cell electrophysiology has enabled monitoring of rapid Ca2+-dependent fluorescence at individual axonal varicosities in the course of synaptic activity. Studies that employed this approach have proposed that spontaneous Ca2+ discharges from presynaptic stores could trigger spontaneous synaptic release of glutamate in hippocampal area CA1 (Emptage and others 2001) and release of GABA from cerebellar interneurons (Llano and others 2000) producing miniature synaptic events in the postsynaptic cell. In mossy fiber - CA3 pyramidal cell synapses, synchronization of spontaneous release was associated with pharmacologically induced Ca2+ release from ER stores (Sharma and Vijayaraghavan 2003). In layer II neurons of the rat barrel cortex, pharmacological manipulation of ryanodine receptors has demonstrated that a significant proportion of spontaneous miniature EPSC events rely on Ca2+ release from the ryanodine receptor-controlled stores (Simkus and Stricker 2002).
Less is known about the role of Ca2+ stores in the evoked, as opposed to spontaneous, synaptic signals. It has been reported that the amplitude of Ca2+ induced Ca2+ release (CICR) from internal Ca2+ stores depends, in a graded manner, on the amount of Ca2+ influx and probably on the cytosolic Ca2+ level (Solovyova and others 2002). These relationships could represent a mechanism that prompts use-dependent, progressive engagement of CICR in the course of neural activity, as opposed to “all-or-none” contribution of CICR to a single-AP Ca2+ response. Indeed, neither postsynaptic responses nor presynaptic Ca2+ -sensitive fluorescent transients (recorded in multiple presynaptic axons) in response to single or paired APs have been reported to depend on Ca2+ stores in six types of hippocampal and cerebellar synapses (Carter and others 2002). However, Ca2+ release from stores has been found to contribute to the presynaptic Ca2+ transient and, correspondingly, to the postsynaptic EPSCs at hippocampal mossy fiber - CA3 pyramidal cell synapses in response to a short train of APs at a moderate frequency (Lauri and others 2003). The authors have attributed the triggering of Ca2+ release to synaptic activation of presynaptic kainate receptors (KARs): this mechanism cannot be activated during a single AP but initiates activity-dependent contribution of Ca2+ stores to synaptic transmission (Lauri and others 2003). In addition, because the functioning of ER stores is important for controlling low resting Ca2+ (Lomax and others 2002), use-dependent inhibition of store function could increase presynaptic Ca2+ concentration. As in the case of residual Ca2+ influx (see above), the buffering capacity of the terminal, which is determined by the amount of Ca-free (high-affinity) buffer, would be decreased, to a roughly proportional degree. Again, this would imply that the free Ca2+ transient following AP-evoked Ca2+ entry will be enhanced compared to that under the low resting Ca2+. This mechanism could therefore provide a simple causal link between the condition of presynaptic Ca2+ stores and the neurotransmitter release probability. However, because changes in the background Ca2+ levels are difficult to monitor without holding the cell in whole-cell mode, establishing their functional role is likely to require experimental probing at the single-terminal level (Ruiz and others 2003; Awatramani and others 2005).
Rapid Ca2+-buffering
A large proportion of Ca2+ ions that enter the presynaptic terminal are rapidly bound by endogenous Ca2+ buffers. In most cases, the endogenous Ca2+ buffers are represented by Ca2+ binding proteins from the EF-hand protein family: these molecules feature two or more well-conserved helix-loop-helix motives equipped with high-affinity binding sites for Ca2+ ions. Among the Ca2+ binding proteins, сalretinin (CR), calbindin D-28k (CB) and parvalbumin (PV) are commonly expressed in nerve terminals (see (Schwaller and others 2002) for detailed review). Their distinct biophysical properties determine their relatively fast (CR and CB) or slow (PV) Ca2+ buffering action and their relatively high (PV and CR) or low (CB) cytosolic solubility / mobility. These distinctions appear to determine the functional specificity of Ca2+ buffering, by providing a differential regulatory control of presynaptic Ca2+ dynamics at different synaptic types. In the cerebellum, for instance, CR is in large part expressed by granule cells and their parallel fiber axons whereas PV and CB are present throughout the axon, soma, dendrites and spines of Purkinje cells; PV is also found in several types of inhibitory interneurons including stellate and basket cells (Bastianelli 2003). In fact, electrophysiological recordings (Blatow and others 2003) and rapid two-photon fluorescence imaging at individual presynaptic terminals (Koester and Johnston 2005) in connected cell pairs have demonstrated that the mode of presynaptic Ca2+ buffering could be specific to the type / modality of synaptic connection. This specificity could therefore constitute an important factor of synaptic identity.
The complex relationship arising between the Ca2+ entry kinetics, the spatio-temporal dynamics of free Ca2+ and the amount, affinity and distribution of endogenous Ca2+ buffers has long been recognized (Neher and Augustine 1992). This relationship has been studied extensively in the giant terminals of the calyx of Held (reviewed in (Meinrenken and others 2003)). Among the multiple regulatory factors operating in this system, one general mechanism that could provide use-dependent control of presynaptic Ca2+ dynamics lies in progressive buffer saturation during repetitive synaptic activation (Rozov and others 2001; Blatow and others 2003). Because repetitive Ca2+ entry increases the proportion of Ca-bound buffer, the remaining buffering capacity of the terminal decreases progressively. Similar to the case of the increased resting Ca2+ (due to Ca2+ leakage through partially activated VGCCs or from ER stores, see above), gradual AP-induced accumulation of cytosolic Ca2+ will result to a greater transient of free Ca2+ generated by the same amount of AP-driven total Ca2+ entry.
Conversely, the persistence of high Ca2+ concentration near the channel mouth during Ca2+ entry implies that local Ca2+ buffers will be rapidly saturated in the course of repetitive APs. The rapid saturation of local buffers could curtail the facilitatory effect of buffer saturation on the extent of Ca2+ domains during a train of APs. However, even modest facilitation of Ca2+ transients could result in a substantial increase in the release probability at the calyx of Held synapses where the Ca2+ -dependent triggers of synaptic release operate in a highly supra-linear manner (Schneggenburger and Neher 2000; Meinrenken and others 2003). In contrast, an increase in presynaptic Ca2+ entry in hippocampal mossy fibers seems to correspond to a roughly proportional enhancement of single-pulse EPSCs in the postsynaptic CA3 pyramidal cells (Geiger and Jonas 2000; Blatow and others 2003; Mori-Kawakami and others 2003). In the latter case, therefore, buffer saturation is likely to have a less strong impact on the frequency-dependent facilitation of transmission.
The kinetics of Ca2+ buffering is intricately linked to the experimental methodology of real-time fluorescence imaging of intracellular Ca2+. That the fluorescent Ca2+ indicators rely on Ca2+ binding to generate their emission signal introduces an additional buffering capacity to the terminal. This might interfere with neurotransmitter release also affecting interpretation of the Ca2+ kinetics. It is therefore important to obtain an assessment of presynaptic Ca2+ kinetics in the absence of Ca2+ buffers, by constraining the system parameters using fluorescence indicators of different affinities and at different concentrations (Sabatini and Regehr 1997; Jackson and Redman 2003; Rusakov and others 2005). Finally, imaging experiments have to account for the minimum effective volume (point-spread function) within which fluorescence emission can be collected. This volume, normally in the region of 0.1-0.2 μm3, is determined by the diffraction-limited excitation probability function and additional optical parameters of the imaging system. The recorded signal therefore always represents volume-integrated fluorescence, which tends to mask the extent of local buffer saturation and underestimates “hot spots” of free Ca2+ on the sub-micron scale.
Ca2+ removal
A large Ca2+ sequestration capacity has classically been attributed to mitochondria which seem to provide a substantial proportion of Ca2+ uptake at presynaptic terminals (Blaustein and others 1978). The main route through which Ca2+ is sequestrated is believed to involve the mitochondrial Ca2+ uniporter located on the inner mitochondrial membrane (see (Parekh 2003) for review). However, the role of mitochondria in regulating neurotransmitter release during rapid synaptic events remains incompletely understood. In the calyx of Held terminals, Ca2+ sequestration by mitochondria can be detected on a scale of seconds. However, pharmacological disruption of mitochondrial Ca2+ uptake unveils a considerable Ca2+ buffering capacity of this process, which could in principle interfere with fast concentration transients of the ion (Billups and Forsythe 2002). In retinal amacrinal cells, inhibition of mitochondrial Ca2+ uptake removes the Ca2+ buffering effect caused by prolonged depolarization and facilitates the occurrence of exocytosis events (Medler and Gleason 2002). Conversely, leakage of Ca2+ ions from mitochondria increases the probability of neurotransmitter release (Billups and Forsythe 2002), which is consistent with the role of mitochondiral buffering in maintaining low resting Ca2+ (Medler and Gleason 2002). These mechanisms are likely to play a role in facilitation of synaptic transmission following tetanic stimulation (Tang and Zucker 1997). In summary, it appears that contribution of mitochondria to presynaptic regulation of Ca2+ comes to prominence during repetitive synaptic activity, on a relatively slow timescale.
On a more rapid timescale, excess Ca2+ is removed from the presynaptic cytosol mainly through the action of two families of Ca2+ pumps, the plasma membrane Ca2+-ATPase (PMCA) and the intracellular sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) families, and also by a relatively low affinity Na+/Ca2+ exchanger (reviewed in (Mata and Sepulveda 2005; Verkhratsky 2005)). In the mammalian brain, four genes correspond to PMCA1-4 isoforms and three other genes correspond to SERCA1-3 isoforms whereas the ATP hydrolysis is coupled with Ca2+ transport through a proton/Ca2+ counter-transport. Fluorescence imaging experiments indicate that the presynaptic Ca2+ extrusion rate, which is likely to rely on the cumulative action of pumps and exchangers, is relatively fast. Its estimated value could vary among and within different types of presynaptic terminals, from ~0.03 ms-1 in the proximal axonal varicosities of dentate granule cells (Jackson and Redman 2003) to ~3 ms-1 in nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex (Koester and Sakmann 2000) or in the axonal boutons of hippocampal interneurons (Rusakov and others 2004). Towards the upper limit of the range, Ca2+ removal may directly interfere, on a scale of milliseconds, with rapid Ca2+ transients generated by AP-driven influx.
In addition to mitochondria and ER stores, the ability to remove and store Ca2+ is now attributed, on a larger cellular scale, to multiple cellular organelles including the nucleus, Golgi apparatus and lysosomes (reviewed in (Annunziato and others 2004)). While operating as Ca2+ stores, many of these organelles interact with each other, adding complexity to Ca2+ signalling events inside the synaptic terminal.
Presynaptic metabotropic receptors
Presynaptic receptors activated by fast neurotransmitters, in particular glutamate and GABA, have attracted much attention because their actions are expected to contribute to fast signal processing in the brain. Retrograde messengers, such as adenosine or the endocannabinoids, could also suppress transmission, albeit on a slower timescale. Because the mechanisms of glutamate release, receptor activation and uptake have been studied in detail, much effort has been concentrated on presynaptic glutamate receptors (GluRs). Amongst metabotropic GluRs (mGluRs), eight subtypes have been cloned and classified into three groups (I, mGluRs 1 and 5; II, mGluRs 2 and 3; and III, mGluRs 4, 6, 7 and 8), according to their sequence homologies and pharmacological profiles (reviewed in (El Far and Betz 2002)).
The calyx of Held terminals express group III mGluRs (as well as GABAB , adenosine and noradrenaline receptors) which can inhibit synaptic release (reviewed in (Trussell 2002)). Presynaptic KARs, arguably acting in a metabotropic receptor capacity, contribute to modulation of mossy fiber- CA3 pyramidal cell and interneuron-interneuron GABAergic transmission in the hippocampus (reviewed in (Kullmann 2001; Lerma 2003)). Intriguingly, activation of group III mGluRs depresses GABA release at interneuron-interneuron synapses to a greater degree than at synapses made on principal cells. Group III (but not group I or II) mGluRs suppress synaptic release from retinal bipolar cells (Awatramani and Slaughter 2001). Several studies have indicated that presynaptic group II and III mGluRs regulate excitatory transmission by reducing AP-evoked presynaptic Ca2+ transients at area CA1 synapses in the hippocampus (Faas and others 2002). This is in line with the immunelectron microscopy evidence suggesting that group III mGluRs, in particular mGluR7 isoform, have a preferentially presynaptic localization (Bradley and others 1996). The presynaptic mGluR7 isoforms have been found inside the synaptic cleft of glutamatergic hippocampal synapses (Shigemoto and others 1997; Dalezios and others 2002) and in GABAergic interneuron terminals in the rat somatosensory cortex (Dalezios and others 2002).
In terms of the molecular machinery involved, mGluRs, as well as metabotropic GABAB receptors, represent a family of G-protein coupled receptors (reviewed in (El Far and Betz 2002)). These receptors could act on both cytoplasmic and plasmalemma-bound effectors by differential activation of either the diffusible Gα or the membrane-bound Gβγ subunits of G-proteins, correspondingly, or both. This dual action could potentially allow mGluRs to affect both the release cascade directly (mainly through Gα signaling) and by inactivating of Ca2+ channels (mainly through Gβγ-signaling). Indeed, it has been shown that the cytoplasmic C-terminal tail regions of mluR7 release the Gβγ subunit and thus modulate Ca2+ influx dependent synaptic release (O’Connor and others 1999).
Although inhibition of presynaptic Ca2+ entry by mGluRs occurs at many types of synapses (see above), predicting the identity of the VGCCs involved is not straightforward. In the calyx of Held terminals, agonists of mGluRs suppressed a high voltage-activated P/Q-type calcium conductance, thus inhibiting glutamate release (Takahashi and others 1996). In the nucleus accumbens, the effect of mGluR2/3 activation occluded with that of the P/Q-type channel blockade (Robbe and others 2002). Activation of K+ channels via an mGluR4 dependent mechanism was required to inhibit presynaptic Ca2+ influx in the cerebellar parallel fibers (Daniel and Crepel 2001). In cerebro-cortical terminals, activation of mGluR7 sub-type inhibited Ca2+ influx by affecting N-type channels (Millan and others 2002). In hippocampal interneurons, the group III mGluRs mediated depression of synaptic transmission by reducing Ca2+ influx mainly through the N-type VGCCs (Rusakov and others 2004).
In some synaptic types, presynaptic mGluRs can control the release machinery downstream of Ca2+ entry. In the calyx of Held, mGluRs depress transmission independently of presynaptic Ca2+ or K+ currents (Barnes-Davies and Forsythe 1995) whereas recovery from depression at these synapses is mediated by G-proteins (Takahashi and others 2000). Miniature EPSCs in hippocampal CA3 pyramidal cells are suppressed by mGluR agonists, but unaffected by the blockade of VGCCs (Scanziani and others 1995). At stratum radiatum CA1 synapses, the mGluR-dependent long-term depression of synaptic transmission seems to involve changes in the machinery of synaptic vesicle recycling (Zakharenko and others 2002).
In summary, the plethora of regulatory pathways provided by metabotropic receptors allows for complex adaptation of presynaptic control to the versatile patterns of synaptic activity. However, principles that govern the expression of these presynaptic mechanisms require further investigation. One plausible paradigm is the target-cell dependence: indeed, the presynaptic mechanisms expressed at individual synapses appear to adapt a particular mode of transmission depending on the postsynaptic target (Somogyi and others 2003; Koester and Johnston 2005). In line with this concept, data in Figure 2 illustrate that, in axonal varicosities of hippocampal interneurons, regulation of Ca2+ entry by group III mGluRs is not uniformly expressed at all presynaptic boutons supplied by the same axon. Whether the source of such variation strictly follows the postsynaptic target cell type remains to be established.
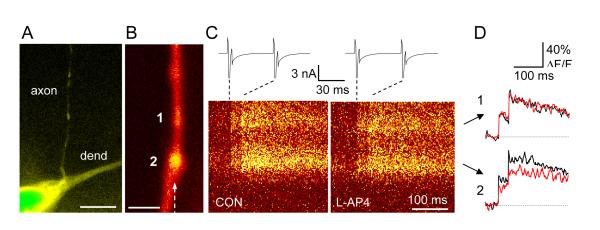
Figure 2.
Presynaptic modulation by metabotropic glutamate receptors varies among axonal varicosities supplied by the same axon. (Modified from (Rusakov and others 2004)).
A, A low-magnification fluorescence image of an area CA1 hippocampal interneuron (fragment) held in whole-cell mode. Acute slice, two-photon excitation (λx = 810 nm; Alexa Fluo 594 emission channel). Dendrite and axons are clearly distinguishable, as indicated. Scale bar, 10 μm.
B, A high-magnification fluorescence image of an axon fragment from cell depicted in A. Two varicosities are shown (denoted 1 and 2; Alexa emission channel; increased brightness in the middle of varicosity 1 implies that its size in the Z direction exceeds the axon diameter 2-3-fold). Dotted arrow, line-scan positioning. Scale bar, 2 μm.
C, Line-scan images (Ca2+-sensitive Fluo-4 emission channel) of the axon fragment shown in B in response to a pair of evoked action potentials (escape action currents, shown in upper traces). Left panel, control recording; right panel, 20 min following application of group III mGluR agonist L-AP4 (50 μM; average of 10 line-scans).
D, Digitized traces of line-scan images in C, as indicated. Black line, control recording; red line, L-AP4 application. No effect of L-AP4 is seen in varicosity 1 whereas it is substantial in varicosity 2.
Presynaptic ionotropic receptors
The importance of direct ionotropic actions of presynaptic GluRs is only beginning to emerge (reviewed in (Engelman and MacDermott 2004)), with the role of KARs drawing much attention. In the hippocampus, interneuron - principal cell paired recordings show a dose-dependent, biphasic relationship between activation of presynaptic KARs and synaptic release, a switch from enhanced to inhibited transmission; at the same time, presynaptic depolarization by KARs could induce spontaneous firing of interneurons, probably by reducing the threshold for ectopic APs (reviewed in (Kullmann 2001; Lerma 2003)). In addition to direct ionotropic influences, KARs appear to induce G-protein dependent metabotropic actions, as briefly discussed above, but how exactly these two modes of operation interact remains poorly understood. For instance, wide-field fluorescent imaging in hippocampal area CA3 suggests that activation of KARs reduces Ca2+ transients in multiple axons of mossy fibers (Kamiya and Ozawa 2000); at the same time, electrophysiological recordings point to a KAR-dependent triggering of Ca2+ release from internal stores at mossy fiber synapses (Lauri and others 2003).
Other types of “classical” ionotropic receptors have also been implicated in presynaptic modulation of transmission at central synapses. In the cerebellum, activation of presynaptic NMDA receptors potentiates GABA release, on a scale of minutes, at inhibitory inputs to Purkinje cells (Duguid and Smart 2004) and also contributes to long-term depression at parallel fiber- Purkinje cell synapses (Casado and others 2002). An important role of presynaptic NMDA receptors in induction of synaptic depression has also been reported in neocortical pyramidal cells (Sjostrom and others 2003). Presynaptic AMPA receptors in cerebellar basket cell axons can be activated by glutamate discharges from the climbing fibers resulting in short-term suppression of GABA release (Satake and others 2000; Rusakov and others 2005). This mechanism could involve a novel signaling cascade acting independently of Ca2+ entry regulation (Schenk and Matteoli 2004). Modulation of neurotransmitter release by presynaptic ionotorpic GABAA receptors, a classical mechanism of presynaptic inhibition in the spinal cord, has been found to occur in the calyx of Held (Turecek and Trussell 2001) and in developing cerebellar interneurons (Pouzat and Marty 1999). In individual axonal varicosities of hippocampal mossy fibers, activation of GABAA receptors reduces presynaptic AP-evoked Ca2+ entry and elevates the background Ca2+ level (Ruiz and others 2003). Depending on the intracellular concentration of Cl−, this activation could either depolarize or hyperpolarize presynaptic terminals allowing, in principle, bi-directional tuning of the release probability.
A distinct signaling concept arises when presynaptic glutamate receptors are expressed at GABAergic terminals or GABA receptors are expressed at glutamatergic terminals. This arrangement implies that receptor activation requires extrasynaptic escape of glutamate or GABA, respectively, from the corresponding synapses in the surrounding neuropil. Therefore, the extent to which these receptors are activated is likely to depend on the ongoing activity in the neighboring synaptic circuitry and on the parameters of the immediate synaptic environment (Min and others 1998). How this “network-dependent” presynaptic control contributes to the information flow in the brain remains to be investigated.
Concluding remarks
At central synapses, presynaptic terminals are equipped with a multitude of mechanisms capable of regulating Ca2+ dependent neurotransmitter release (Figure 1). By complementing each other, these mechanisms engage in complex interplay covering a wide dynamic range and a broad temporal domain of Ca2+ signaling inside the terminal (Figure 3A). The roles and respective contribution of the players could change depending on the pattern of the ongoing neural activity (Figure 3B), thus enabling use-dependent adaptation of synaptic strength. The fundamental issue for future studies would be to determine the principles by which the activity of synaptic circuits shapes the non-uniform expression of these regulatory mechanisms among synaptic populations.
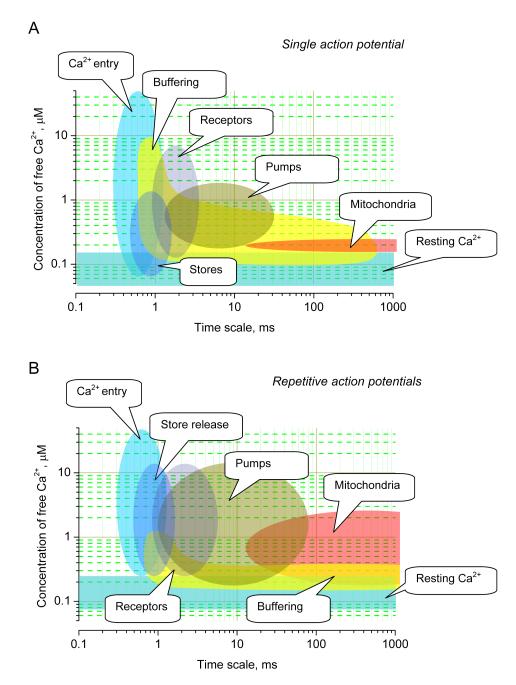
Figure 3.
A diagram illustrating the dynamic range - time domain relationship for major cellular mechanisms that shape the dynamics of presynaptic Ca2+.
The abscissa indicates relative contribution in terms of the affected concentration range of free intra-terminal Ca2+ in response to a single AP (A) and repetitive APs (B).
Acknowledgements
This work is supported by Wellcome Trust, Medical Research Council (UK) and European Commission (PROMEMORIA LSHM-CT-2005-512012). The author thanks Dimitri Kullmann for valuable suggestions.
REFERENCES
- Annunziato L, Pignataro G, Di Renzo GF. Pharmacology of brain Na+/Ca2+ exchanger: from molecular biology to therapeutic perspectives. Pharmacol Rev. 2004;56:633–654. doi: 10.1124/pr.56.4.5. [DOI] [PubMed] [Google Scholar]
- Awatramani GB, Slaughter MM. Intensity-dependent, rapid activation of presynaptic metabotropic glutamate receptors at a central synapse. J Neurosci. 2001;21:741–749. doi: 10.1523/JNEUROSCI.21-02-00741.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Barnes-Davies M, Forsythe ID. Presynaptic and postsynaptic glutamate receptors at a giant excitatory synapse in rat auditory brain stem slices. J Physiol. 1995;488:387–406. doi: 10.1113/jphysiol.1995.sp020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastianelli E. Distribution of calcium-binding proteins in the cerebellum. Cerebellum. 2003;2:242–262. doi: 10.1080/14734220310022289. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Billups B, Forsythe ID. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J Neurosci. 2002;22:5840–5847. doi: 10.1523/JNEUROSCI.22-14-05840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger J, Geiger JR, Jonas P. Timing and efficacy of Ca2+ channel activation in hippocampal mossy fiber boutons. J Neurosci. 2002;22:10593–10602. doi: 10.1523/JNEUROSCI.22-24-10593.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron. 2003;38:79–88. doi: 10.1016/s0896-6273(03)00196-x. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Ratzlaff RW, Kendrick NC, Schweitzer ES. Calcium buffering in presynaptic nerve terminals. I. Evidence for involvement of a nonmitochondrial ATP-dependent sequestration mechanism. J Gen Physiol. 1978;72:15–41. doi: 10.1085/jgp.72.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst JGG, Helmchen F, Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J Physiol. 1995;489:825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, Levey AI, Hersch SM, Conn PJ. Immunocytochemical localization of group III metabotropic glutamate receptors in the hippocampus with subtype-specific antibodies. J Neurosci. 1996;16:2044–2056. doi: 10.1523/JNEUROSCI.16-06-02044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain KL, Jackson VM, Trout SJ, Cunnane TC. Intermittent ATP release from nerve terminals elicits focal smooth muscle Ca2+ transients in mouse vas deferens. J Physiol. 2002;541:849–862. doi: 10.1113/jphysiol.2002.019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P, Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978;202:1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Budde T, Meuth S, Pape HC. Calcium-dependent inactivation of neuronal calcium channels. Nature Rev Neurosci. 2002;3:873–883. doi: 10.1038/nrn959. [DOI] [PubMed] [Google Scholar]
- Carter AG, Vogt KE, Foster KA, Regehr WG. Assessing the role of calcium-induced calcium release in short-term presynaptic plasticity at excitatory central synapses. J Neurosci. 2002;22:21–28. doi: 10.1523/JNEUROSCI.22-01-00021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado M, Isope P, Ascher P. Involvement of presynaptic N-methyl-D-aspartate receptors in cerebellar long-term depression. Neuron. 2002;33:123–130. doi: 10.1016/s0896-6273(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Cox CL, Denk W, Tank DW, Svoboda K. Action potentials reliably invade axonal arbors of rat neocortical neurons. Proc Natl Acad Sci USA. 2000;97:9724–9728. doi: 10.1073/pnas.170278697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalezios Y, Lujan R, Shigemoto R, Roberts JDB, Somogyi P. Enrichment of mGluR7a in the presynaptic active zones of GABAergic and non-GABAergic terminals on interneurons in the rat somatosensory cortex. Cereb Cortex. 2002;12:961–974. doi: 10.1093/cercor/12.9.961. [DOI] [PubMed] [Google Scholar]
- Daniel H, Crepel F. Control of Ca2+ influx by cannabinoid and metabotropic glutamate receptors in rat cerebellar cortex requires K+ channels. J Physiol. 2001;537:793–800. doi: 10.1111/j.1469-7793.2001.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Action-potential propagation gated by an axonal I(A)-like K+ conductance in hippocampus. Nature. 1997;389:286–289. doi: 10.1038/38502. [DOI] [PubMed] [Google Scholar]
- Dodge FA, Rahamimoff R. Co-operative action of calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid IC, Smart TG. Retrograde activation of presynaptic NMDA receptors enhances GABA release at cerebellar interneuron-Purkinje cell synapses. Nat Neurosci. 2004;7:525–533. doi: 10.1038/nn1227. [DOI] [PubMed] [Google Scholar]
- El Far O, Betz H. G-protein-coupled receptors for neurotransmitter amino acids: C-terminal tails, crowded signalosomes. Biochemical Journal. 2002;365:329–336. doi: 10.1042/BJ20020481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- Engelman HS, MacDermott AB. Presynaptic ionotropic receptors and control of transmitter release. Nat Rev Neurosci. 2004;5:135–145. doi: 10.1038/nrn1297. [DOI] [PubMed] [Google Scholar]
- Faas GC, Adwanikar H, Gereau RW, Saggau P. Modulation of presynaptic calcium transients by metabotropic glutamate receptor activation: A differential role in acute depression of synaptic transmission and long-term depression. J Neurosci. 2002;22:6885–6890. doi: 10.1523/JNEUROSCI.22-16-06885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS in vitro. J Physiol. 1994;479:381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JRP, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Jackson MB, Redman SJ. Calcium dynamics, buffering, and buffer saturation in the boutons of dentate granule-cell axons in the hilus. J Neurosci. 2003;23:1612–1621. doi: 10.1523/JNEUROSCI.23-05-01612.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Konnerth A, Augustine GJ. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci USA. 1991;88:380–384. doi: 10.1073/pnas.88.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S. Kainate receptor-mediated presynaptic inhibition at the mouse hippocampal mossy fibre synapse. J Physiol. 2000;523(Pt 3):653–665. doi: 10.1111/j.1469-7793.2000.t01-1-00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester HJ, Sakmann B. Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex. J Physiol. 2000;529:625–646. doi: 10.1111/j.1469-7793.2000.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester HJ, Johnston D. Target cell-dependent normalization of transmitter release at neocortical synapses. Science. 2005;308:863–866. doi: 10.1126/science.1100815. [DOI] [PubMed] [Google Scholar]
- Kullmann DM. Presynaptic kainate receptors in the hippocampus: Slowly emerging from obscurity. Neuron. 2001;32:561–564. doi: 10.1016/s0896-6273(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Nistico R, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. A role for Ca2+ stores in kainate receptor-dependent synaptic facilitation and LTP at mossy fiber synapses in the hippocampus. Neuron. 2003;39:327–341. doi: 10.1016/s0896-6273(03)00369-6. [DOI] [PubMed] [Google Scholar]
- Lerma J. Roles and rules of kainate receptors in synaptic transmission. Nature Rev Neurosci. 2003;4:481–495. doi: 10.1038/nrn1118. [DOI] [PubMed] [Google Scholar]
- Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- Lomax RB, Camello C, Van Coppenolle F, Petersen OH, Tepikin AV. Basal and physiological Ca(2+) leak from the endoplasmic reticulum of pancreatic acinar cells. Second messenger-activated channels and translocons. J Biol Chem. 2002;277:26479–26485. doi: 10.1074/jbc.M201845200. [DOI] [PubMed] [Google Scholar]
- Mata AM, Sepulveda MR. Calcium pumps in the central nervous system. Brain Res Brain Res Rev. 2005;49:398–405. doi: 10.1016/j.brainresrev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Medler K, Gleason EL. Mitochondrial Ca(2+) buffering regulates synaptic transmission between retinal amacrine cells. J Neurophysiol. 2002;87:1426–1439. doi: 10.1152/jn.00627.2001. [DOI] [PubMed] [Google Scholar]
- Meinrenken CJ, Borst JGG, Sakmann B. Local routes revisited: the space and time dependence of the Ca2+ signal for phasic transmitter release at the rat calyx of Held. J Physiol. 2003;547:665. doi: 10.1113/jphysiol.2002.032714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan C, Lujan R, Shigemoto R, Sanchez-Prieto J. The inhibition of glutamate release by metabotropic glutamate receptor 7 affects both [Ca2+](c) and cAMP -Evidence for a strong reduction of Ca2+ entry in single nerve terminals. J Biol Chem. 2002;277:14092–14101. doi: 10.1074/jbc.M109044200. [DOI] [PubMed] [Google Scholar]
- Min MY, Rusakov DA, Kullmann DM. Activation of AMPA, kainate, and metabotropic receptors at hippocampal mossy fiber synapses: Role of glutamate diffusion. Neuron. 1998;21:561–570. doi: 10.1016/s0896-6273(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Mori-Kawakami F, Kobayashi K, Takahashi T. Developmental decrease in synaptic facilitation at the mouse hippocampal mossy fibre synapse. J Physiol. 2003;553:37–48. doi: 10.1113/jphysiol.2003.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Augustine GJ. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor V, El Far O, Bofill-Cardona E, Nanoff C, Freissmuth M, Karschin A, Airas JM, Betz H, Boehm S. Calmodulin dependence of presynaptic metabotropic glutamate receptor signaling. Science. 1999;286:1180–1184. doi: 10.1126/science.286.5442.1180. [DOI] [PubMed] [Google Scholar]
- Parekh AB. Store-operated Ca2+ entry: dynamic interplay between endoplasmic reticulum, mitochondria and plasma membrane. J Physiol. 2003;547:333. doi: 10.1113/jphysiol.2002.034140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas I. Differential block at high frequency of branches of a single axon innervating two muscles. J Neurophysiol. 1972;35:903–914. doi: 10.1152/jn.1972.35.6.903. [DOI] [PubMed] [Google Scholar]
- Pouzat C, Marty A. Somatic recording of GABAergic autoreceptor current in cerebellar stellate and basket cells. J Neurosci. 1999;19:1675–1690. doi: 10.1523/JNEUROSCI.19-05-01675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Chaumont S, Bockaert J, Manzoni OJ. Role of P/Q-Ca2+ channels in metabotropic glutamate receptor 2/3-dependent presynaptic long-term depression at nucleus accumbens synapses. J Neurosci. 2002;22:4346–4356. doi: 10.1523/JNEUROSCI.22-11-04346.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Burnashev N, Sakmann B, Neher E. Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J Physiol. 2001;531:807–826. doi: 10.1111/j.1469-7793.2001.0807h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Fabian-Fine R, Scott R, Walker MC, Rusakov DA, Kullmann DM. GABAA receptors at hippocampal mossy fibers. Neuron. 2003;39:961–973. doi: 10.1016/s0896-6273(03)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov DA, Wuerz A, Kullmann DM. Heterogeneity and specificity of presynaptic Ca2+ current modulation by mGluRs at individual hippocampal synapses. Cereb Cortex. 2004;14:748–758. doi: 10.1093/cercor/bhh035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov DA, Saitow F, Lehre KP, Konishi S. Modulation of presynaptic Ca2+ entry by AMPA receptors at individual GABAergic synapses in the cerebellum. J Neurosci. 2005;25:4930–4940. doi: 10.1523/JNEUROSCI.0338-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse. J Neurosci. 1997;17:3425–3435. doi: 10.1523/JNEUROSCI.17-10-03425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake S, Saitow F, Yamada J, Konishi S. Synaptic activation of AMPA receptors inhibits GABA release from cerebellar interneurons. Nature Neurosci. 2000;3:551–558. doi: 10.1038/75718. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Gahwiler BH, Thompson SM. Inhibition of excitatory synaptic transmission by muscarinic and metabotropic glutamate-receptor activation in the hippocampus -are Ca2+ channels involved? Neuropharmacol. 1995;34:1549–1557. doi: 10.1016/0028-3908(95)00119-q. [DOI] [PubMed] [Google Scholar]
- Schenk U, Matteoli M. Presynaptic AMPA receptors: more than just ion channels? Biol Cell. 2004;96:257–260. doi: 10.1016/j.biolcel.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Schwaller B, Meyer M, Schiffmann S. ’New’ functions for ’old’ proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum. 2002;1:241–258. doi: 10.1080/147342202320883551. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron. 2003;38:929–939. doi: 10.1016/s0896-6273(03)00322-2. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkus CR, Stricker C. The contribution of intracellular calcium stores to mEPSCs recorded in layer II neurones of rat barrel cortex. J Physiol. 2002;545:521–535. doi: 10.1113/jphysiol.2002.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Solovyova N, Veselovsky N, Toescu EC, Verkhratsky A. Ca2+ dynamics in the lumen of the endoplasmic reticulum in sensory neurons: direct visualization of Ca2+-induced Ca2+ release triggered by physiological Ca2+ entry. Embo J. 2002;21:622–630. doi: 10.1093/emboj/21.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Dalezios Y, Lujan R, Roberts JDB, Watanabe M, Shigemoto R. High level of mGluR7 in the presynaptic active zones of select populations of GABAergic terminals innervating interneurons in the rat hippocampus. Eur J Neurosci. 2003;17:2503–2520. doi: 10.1046/j.1460-9568.2003.02697.x. [DOI] [PubMed] [Google Scholar]
- Spruston N, Schiller Y, Stuart G, Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science. 1995;268:297–300. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Hori T, Kajikawa Y, Tsujimoto T. The role of GTP-binding protein activity in fast central synaptic transmission. Science. 2000;289:460–463. doi: 10.1126/science.289.5478.460. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, Onodera K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science. 1996;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- Tang YG, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- Trussell LO. Modulation of transmitter release at giant synapses of the auditory system. Curr Opin Neurobiol. 2002;12:400–404. doi: 10.1016/s0959-4388(02)00335-5. [DOI] [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Borst JG. Short-term plasticity at the calyx of held. Nat Rev Neurosci. 2002;3:53–64. doi: 10.1038/nrn705. [DOI] [PubMed] [Google Scholar]
- Wu LG, Borst JGG, Sakmann B. R-type Ca2+ currents evoke transmitter release at a rat central synapse. Proc Natl Acad Sci USA. 1998;95:4720–4725. doi: 10.1073/pnas.95.8.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 2005;46:633–645. doi: 10.1016/j.neuron.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Zakharenko SS, Zablow L, Siegelbaum SA. Altered presynaptic vesicle release and cycling during mGluR-dependent LTD. Neuron. 2002;35:1099–1110. doi: 10.1016/s0896-6273(02)00898-x. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Ann Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]