Abstract
The progressive debilitation of motor functions in Parkinson's disease (PD) results from degeneration of dopaminergic neurons within the substantia nigra pars compacta of the midbrain. Long-term inflammatory activation of microglia and astrocytes plays a central role in the progression of PD and is characterized by activation of the nuclear factor-κB (NF-κB) signaling cascade and subsequent overproduction of inflammatory cytokines and nitric oxide (NO). Suppression of this neuroinflammatory phenotype has received considerable attention as a potential target for chemotherapy, but there are no currently approved drugs that sufficiently address this problem. The data presented here demonstrate the efficacy of a novel anti-inflammatory diindolylmethane class compound, 1,1-bis(3′-indolyl)-1-(p-t-butylphenyl)methane (DIM-C-pPhtBu), in suppressing NF-κB-dependent expression of inducible nitric-oxide synthase (NOS2) and NO production in astrocytes exposed to the parkinsonian neurotoxicant 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) through a mechanism distinct from that described for the thiazolidinedione-class compound, rosiglitazone. Chromatin immunoprecipitations revealed that micromolar concentrations of DIM-C-pPhtBu prevented association of the p65 subunit of NF-κB with enhancer elements in the Nos2 promoter but had little effect on DNA binding of either peroxisome proliferator-activated receptor-γ (PPAR-γ) or the nuclear corepressor NCoR2. Treatment with DIM-C-pPhtBu concomitantly suppressed NO production and protein nitration in MPTP-activated astrocytes and completely protected cocultured primary striatal neurons from astrocyte-dependent apoptosis. These data demonstrate the efficacy of DIM-C-pPhtBu in preventing the activation of NF-κB-dependent inflammatory genes in primary astrocytes and suggest that this class of compounds may be effective neuroprotective anti-inflammatory agents in vivo.
Parkinson's disease (PD) is a severely debilitating movement disorder resulting from progressive degeneration of dopaminergic neurons within the substantia nigra pars compacta of the midbrain. Unfortunately, pharmacological treatment for PD has not progressed beyond the use of dopamine mimetics, such as l-DOPA, that only transiently alleviate motor symptoms. Furthermore, long-term use of l-DOPA is associated with its own array of resultant pathologies, such as dyskinesia (Lang and Lozano, 1998), cardiac arrhythmia and ischemic injury, and cerebral vascular dysfunction (Ben-Shlomo and Marmot, 1995). Ultimately, patients with PD will progress to the end stage of the disease, which is characterized by significant gait abnormalities and frequent falls, as well as a deficit in nonmotor functions resulting in dementia, psychosis, and other autonomic disturbances (Djaldetti et al., 2004).
Currently, a precise etiology explaining PD remains to be discovered, but recent research has revealed features of the disease that represent realistic targets for neuroprotective chemotherapeutic intervention that could mitigate the progressive loss of dopaminergic neurons. Among these observations are the presence of long-term inflammation and sustained expression of inducible nitric oxide (NOS2), accompanied by activation of the surrounding astrocytes and microglia (Hirsch et al., 2003; Teismann and Schulz, 2004; Pekny and Nilsson, 2005).
Astrocytes have diverse and critical functions in the central nervous system that include providing energetic (Pellerin and Magistretti, 2004), antioxidant (Pekny and Nilsson, 2005), and other trophic support essential for the survival and function of neurons. However, many neurological disease states, including PD, Alzheimer's disease, and ischemic injury, are typically accompanied by varying degrees of astrocyte activation or astrogliosis (Hirsch et al., 2003; Teismann and Schulz, 2004; Pekny and Nilsson, 2005). Although the exact cause of astrogliosis in PD is unknown, several reports have suggested that the activation of astrocytes is due to secretion of inflammatory cytokines, such as TNF-α and IFN-γ, by the surrounding microglial cells (Hunot et al., 1999; Liberatore et al., 1999). Although some degree of activation is probably beneficial, reactive astrogliosis results in neuronal injury (Hirsch and Hunot, 2000; Pekny and Nilsson, 2005).
Astrogliosis results in increased production of various neurotoxic inflammatory mediators, including nitric oxide (NO), that contribute to progressive loss of nigrostriatal neurons. Supporting a deleterious role for excessive NO production in PD are postmortem observations of increased NOS2 expression in patients diagnosed with PD (Ebadi and Sharma, 2003), as well as reports that deletion of the Nos2 gene in mice confers protection against MPTP-mediated neurotoxicity (Liberatore et al., 1999). Expression of NOS2 in diverse cell types is highly dependent on the NF-κB signaling pathway (Karin et al., 2002), and we demonstrated previously a requirement for NF-κB in the expression of NOS2 in activated astrocytes after stimulation with inflammatory cytokines and manganese (Tjalkens et al., 2008). Multiple signaling pathways activate NF-κB through the IκB kinase complex, leading to phosphorylation and degradation of the inhibitory IκB subunit and nuclear translocation of the transcriptionally active p65 subunit (Karin et al., 2002). Ensuing induction of Nos2 then typically requires binding of p65 to enhancer sequences on the Nos2 promoter and removal of constitutively bound nuclear corepressor proteins such as NCoR2 by the nuclear proteosome (Pascual et al., 2005).
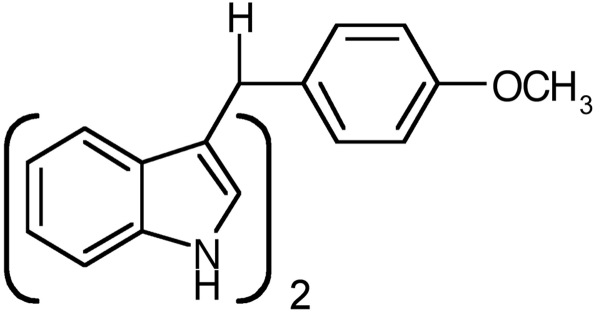
Suppressing neuroinflammation has emerged as a potential strategy for treating disorders such as PD. In particular, modulation of nuclear orphan receptors has been examined as a possible approach for suppressing inflammatory gene expression in astrocytes using traditional thiazolidinedione (TZD) ligands of PPAR-γ (Dehmer et al., 2004; Luna-Medina et al., 2005). The TZD ligand rosiglitazone [5-((4-(2-(methyl-2-pyridinylamino) ethoxy)phenyl)methyl)-2,4-thiazolidinedione] seems to antagonize NF-κB by stabilizing NCoR2 at the proximal p65 enhancer element in RAW macrophages (Pascual et al., 2005), and another drug in this series, pioglitazone, confers partial neuroprotection in the 1-methyl-MPTP model of Parkinson's disease, preserving dopaminergic cell bodies in the substantia nigra but not dopaminergic fibers in the striatum (Dehmer et al., 2004). The work presented here demonstrates that a novel methylene-substituted C-DIM-class compound, 1,1-bis(3′-indolyl)-1-(p-t-butylphenyl)-methane (DIM-C-pPhtBu; Fig. 1) (Chintharlapalli et al., 2004; Qin et al., 2004; Kassouf et al., 2006), suppresses expression of NOS2 with similar efficacy to rosiglitazone in astrocytes exposed to TNF-α, IFN-γ, and MPTP. DIM-C-pPhtBu also prevented apoptosis of primary striatal neurons cocultured with activated astrocytes. Furthermore, we demonstrate that the mechanism behind DIM-C-pPhtBu-mediated inflammatory suppression differs from the transrepressive mechanism described for rosiglitazone (Pascual et al., 2005), presenting a novel avenue for inflammatory gene suppression and neuroprotection using diindolylmethane-class therapeutics.
Fig. 1.
Chemical structure of 1,1-bis(3′-indolyl)-1-(p-t-butylphenyl)methane (DIM-C-pPhtBu).
Materials and Methods
Materials. Unless otherwise stated, all reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). DIM-C-pPhtBu was synthesized as described previously (Qin et al., 2004), and rosiglitazone was purchased from Cayman Chemical (Ann Arbor, MI). The NOS2 inhibitor 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine (AMT) was purchased from Calbiochem (San Diego, CA). Cell culture media, antibiotics, and fluorescent antibodies and dyes were purchased from Invitrogen (Carlsbad, CA). Monoclonal antibodies against NOS2 were purchased from BD Biosciences (San Jose, CA). Horseradish peroxidase-conjugated goat anti-mouse secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). For immunofluorescence studies, antibodies against glial fibrillary acidic protein (GFAP), 3-nitrotyrosine, ionized calcium binding adapter molecule 1 (Iba1), and Gad63/67 and p65 were purchased from Sigma Chemical Co. (St. Louis, MO), Millipore Bioscience Research Reagents (Temecula, CA), Wako Pure Chemical Industries, Ltd. (Tokyo, Japan), and Santa Cruz Biotechnology, respectively. Antibodies used for chromatin immunoprecipitation (ChIP) analysis of p65, PPAR-γ, and NCoR2 were purchased from Santa Cruz Biotechnology, Cell Signaling Technology (Danvers, MA), and Abcam (Cambridge, MA), respectively.
Primary Cell Isolation. Cortical astrocytes were isolated from 1-day-old C57BL/6 or transgenic mouse pups according to procedures described previously (Aschner and Kimelberg, 1991), and purity was confirmed through immunofluorescent staining using antibodies against GFAP and Iba1. In brief, pups were euthanized by decapitation under isoflurane anesthesia, and cortices (astrocytes) or striatum (neurons) were rapidly dissected out, meninges removed. Tissue was subject to digestion with Dispase (1.5 U/ml), and selection of astrocytes was performed by complete media change 24 h after plating to remove nonastroglial cell types by serum shock. This method routinely results in cultures that are approximately 99% pure astrocytes, with less than 1% contaminating microglial cells (Supplementary Fig. 1). Astrocyte cultures were maintained at 37°C and 5% CO2 in minimum essential media supplemented with 10% heat-inactivated fetal bovine serum and a penicillin (0.001 mg/ml), streptomycin (0.002 mg/ml), and neomycin (0.001) antibiotic cocktail. Cell media was changed 24 h before all treatments. Primary neuronal cultures were seeded onto poly(l-lysine)-coated 30-mm glass coverslips at 4 × 105 cells/well and maintained in neurobasal media supplemented with 2 mM l-glutamine, a B27 supplement, and a penicillin (0.001 mg/ml), streptomycin (0.002 mg/ml), and neomycin (0.001) antibiotic cocktail. Neuronal culture media was changed 24 h after isolation and every 2 days afterward, and culture purity was confirmed through cell morphology and immunostaining against GAD65/67 (Supplementary Fig. 2). Neurons were used within 2 weeks of plating. All animal procedures were approved by the Colorado State University Institutional Animal Care and Use Committee and were conducted in accordance with published National Institutes of Health guidelines.
Reverse Transcriptase and Real-Time RT-PCR. Astrocytes were treated with MPTP (10 μM) and the inflammatory cytokines TNF-α (10 pg/ml) and IFN-γ (1 μg/μl), with or without DIM-C-pPhtBu (1, 10, or 100 μM), rosiglitazone (10 μM), or a DMSO vehicle control for 3 h before RNA isolation. RNA was isolated using the RNEasy Mini kit (QIAGEN, Valencia, CA), and purity and concentration were determined using a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). After purification, 1 μg of RNA was used as a template for reverse transcriptase reactions using the iScript RT kit (Bio-Rad, Hercules CA). The resulting cDNA was immediately profiled for Nos2 gene expression (forward, 5′-TCACGCTTGGGTCTTGTT-3′; reverse, 5′-CAGGTCACTTTGGTAGGATTT-3′) using β-Actin as a housekeeping gene (forward, 5′-GCTGTGCTATGTTGCTCTAG-3′; reverse: 5′-CGCTCGTTGCCAATACTG-3′) according to the 2-ΔΔCT method (Livak and Schmittgen, 2001).
Western Blotting. Astrocytes were treated with MPTP (10 μM) and the inflammatory cytokines TNF-α (10 pg/ml) and IFN-γ (1 ng/ml), with or without DIM-C-pPhtBu (10 μM), rosiglitazone (10 μM), or a DMSO vehicle control for 8 h before protein harvesting. Cells were lysed using a triple detergent lysis buffer (50 mM Tris-HCl, pH 8.1, 150 mM NaCl, 0.1% SDS, 1.0% Nonidet P-40, and 0.5% sodium deoxycholate) supplemented with Complete protease inhibitor (Roche, Indianapolis IN). Protein was quantified using the BCA assay (Pierce, Rockford IL), and 20 μg of protein was separated by standard SDS-polyacrylamide gel electrophoresis using a 6% slab gel (Bio-Rad) followed by semidry transfer to polyvinylidene fluoride membrane (Pall Corporation, Pensacola, FL). All blocking and antibody incubations were performed in 5% nonfat dry milk in Tris-buffered saline containing 0.2% Tween 20. A positive control consisting of 0.5 μg of activated macrophage lysate (BD Biosciences) was included to confirm results. Protein was visualized on film using enhanced chemiluminescence (Pierce).
Immunofluorescence. Primary astrocytes were grown to confluence on 20-mm serum-coated glass coverslips and treated with saline or MPTP (10 μM), TNF-α (10 pg/ml), and IFN-γ (1 ng/ml) with or without DIM-C-pPhtBu (10 μM), rosiglitazone (10 μM), the NOS2 inhibitor AMT (25 nM) or NOS1 inhibitor 7-NI (10 μM), or a DMSO vehicle control 8 h before analysis. Immunofluorescence to confirm culture purity was conducted on cells grown to confluence on the 20-mm glass coverslips. Blocking and antibody hybridization was conducted in 1% BSA (w/v) in PBS, and all washes were conducted in PBS. Images were acquired using a Zeiss 20× air plan apochromatic objective (Carl Zeiss Inc., Thornwood, NY), and six to eight microscopic fields were examined per treatment group over no less than three independent experiments. Fluorescent secondary antibodies were used to detect GFAP (excitation at 488 nm, emission at 519 nm) and Iba1 or nitrosylated protein (excitation at 647 nm; emission at 668 nm), respectively, whereas mounting medium containing 4,6-diamidino-2-phenylindole (excitation at 360 nm; emission at 460 nm) was used to identify cell nuclei.
NF-κB Reporter Assays in cis-NF-κBEGFP Transgenic Astrocytes and Expression of Mutant IκBα. To measure the activation of NF-κB in live cells, astrocytes were isolated from a unique transgenic mouse expressing a reporter construct consisting of three human immunodeficiency virus NF-κB consensus elements inserted 5′ to a minimal c-fos promoter that drives expression of enhanced green fluorescent protein (EGFP) (Magness et al., 2004) (provided by Dr. Christian Jobin, University of North Carolina at Chapel Hill, Chapel Hill, NC). NF-κB activity was determined by live-cell imaging using a Zeiss 20× air plan apochromatic objective using SlideBook v 4.2 (Intelligent Imaging Innovations, Inc., Denver, CO). Image saturation was prevented by 1) minimizing exposure time, 2) using the digital gain on the charge-coupled device to enhance sensitivity, and 3) carefully monitoring the exposure histogram to ensure that pixel intensities did not approach saturation. This approach was consistent between all replicates. At least four microscopic fields were examined per treatment group in each of at least three independent experiments and are reported as the percentage of activated cells 8 h after treatments. A phosphorylation-deficient mutant of IκBα,IκBα-(S32,36A)-hemagglutinin, was overexpressed in primary astrocytes using an adenoviral vector delivered for 24 h at 2 × 106 viral particles per milliliter of culture medium, with a multiplicity of infection of 1 × 103 virions per cell shown previously by us to result in expression of the mutant protein by more than 99% of the astrocytes (Barhoumi et al., 2004). Parallel control experiments used the same adenoviral construct lacking the insert. After incubation with the mutant IκBα construct for 24 h, astrocytes were washed with PBS to remove viral particles and cultured in fresh medium for 24 h before use.
ChIP. ChIP procedures were adapted from a report published previously (Weinmann and Farnham, 2002) and optimized for primary astrocytes according to recent studies from our laboratory. Astrocytes were grown to confluence in 10-cm plates (approximately 8.8 × 106 cells) and were treated for 3 h with MPTP (10 μM), TNF-α (10 pg/ml), and IFN-γ (1 ng/ml) with or without DIM-C-pPhtBu (10 μM), rosiglitazone (10 μM), or a DMSO vehicle control for 3 h before analysis. DNA was sheared into approximate 500-base pair fragments by three 10-s pulses using a Tekmar Sonic Disrupter (Tekmar Co., Cincinnati, OH) set at 30% output, followed by collection of 10% input controls and the addition of 2 μg of precipitating antibody. Immune complexes were allowed to form overnight at 4°C with gentle agitation, followed by the addition of protein G magnetic beads (Active Motif, Carlsbad, CA) for an additional 90 min. Immunopurified DNA was isolated via phenol/chloroform extraction and subjected to PCR using primers designed around the proximal murine Nos2 NF-κB binding region (Xie et al., 1992) (forward, 5′-ATG GCC TTG CAT GAG GAT ACA CCA-3′; reverse, 5′-GAG TCT CAG TCT TCA ACT CCC TGT-3′). Amplicons were separated by 2% agarose gel electrophoresis and stained with ethidium bromide.
Astrocyte Neuron Coculture. Cells were isolated as described above, and astrocytes were grown to confluence on permeable cell culture inserts (BD Biosciences) before treatment. Astrocytes were then treated with MPTP (10 μM), TNF-α (10 pg/ml), and IFN-γ (1 ng/ml) in the presence or absence of DIM-C-pPhtBu (10 μM), Rosiglitazone (10 μM), AMT (25 nM), or DMSO vehicle control for 8 h. Media were then removed, the astrocytes were washed three times with sterile PBS to prevent carryover of treatment medium to the neurons, and the inserts were placed directly in each well over cultured neurons. Astrocyte-containing inserts were removed after 6 h of coculture, and neuronal caspase activity and phosphatidylser-ine translocation were measured by widefield fluorescence microscopy using the general cell-permeable fluorescent caspase substrate rhodamine 110, bis-(l-aspartic acid amide) and an Annexin V Alexa Fluor 680 conjugate, respectively. Images were acquired using a Zeiss 20× air plan apochromatic objective, and at least 10 microscopic fields were examined per treatment group over three independent experiments.
Statistical Comparisons. Experiments were performed at least three times, with replicates consisting of independent cultures using a minimum of four plates or coverslips per replicate study. Comparison of two means was performed by Student's t test, whereas comparison of three or more means was performed using one-way analysis of variance followed by the Tukey-Kramer multiple comparison post hoc test using Prism software (version 4.0c; GraphPad Software, Inc., San Diego, CA). For all experiments, p < 0.05 was considered significant, although the level of significance was often much greater. Statistically different groups are identified in the figures by the assignment of a unique letter (e.g., a, b, c, d).
Results
Isolation of Primary Astrocytes and Neurons. The purity of primary astrocyte cultures isolated according to the procedures described previously (Aschner and Kimelberg, 1991) were assayed for microglial contamination through dual immunofluorescent staining against GFAP (fluorescein isothiocyanate) or Iba1 (Cy5) (Supplementary Fig. 1A), demonstrating approximately 99% culture purity (Supplementary Fig. 1B), with less than 1% Iba1-positive microglial cells.
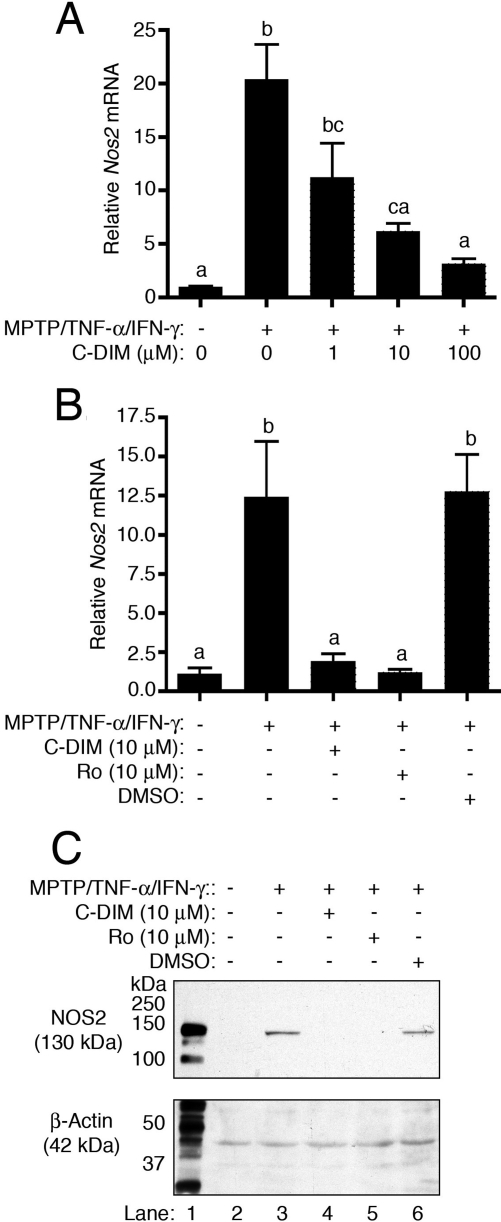
Modulation of NOS2 Expression by DIM-C-pPhtBu. Semiquantitative real-time RT-PCR analysis indicated that exposure of astrocytes to MPTP and TNF-α/IFN-γ resulted in a large increase in expression of Nos2 mRNA that was dose-dependently suppressed by concentrations of DIM-C-pPhtBu ranging from 1 to 100 μM (Fig. 2A). Because cotreatment with 10 μM DIM-C-pPhtBu represented the lowest dose that suppressed Nos2 induction to levels statistically indistinguishable from saline-treated control astrocytes, this concentration was used to compare efficacy with an equivalent concentration of the traditional thiazolidinedione PPAR-γ agonist, rosiglitazone. Real-time PCR demonstrated equivalent suppression of Nos2 to levels observed in saline-treated control astrocytes by both DIM-C-pPhtBu and rosiglitazone, whereas the DMSO vehicle control had no suppressive effect on induction (Fig. 2B). Immunoblotting demonstrated similar suppression of NOS2 protein by both DIM-C-pPhtBu and rosiglitazone in astrocytes exposed to MPTP and TNF-α/IFN-γ (Fig. 2C).
Fig. 2.
A, semiquantitative real-time RT-PCR demonstrating dose-responsive suppression of Nos2 mRNA by concentrations of DIM-C-pPhtBu ranging from 1 to 10 μM in astrocytes challenged with MPTP, TNF-α, and IFN-γ. B, semiquantitative real-time PCR demonstrating equivalent suppression of Nos2 mRNA by either DIM-C-pPhtBu or rosiglitazone in astrocytes challenged with MPTP, TNF-α, and IFN-γ. C, immunoblotting demonstrates the suppression of NOS2 protein expression by either DIM-C-pPhtBu or rosiglitazone in astrocytes exposed to MPTP and TNF-α/IFN-γ (lanes 4 and 5). Activated macrophage lysate was used as a positive control for identification of NOS2 (lane 1). All quantitative PCR and Western blotting experiments were performed three times (n = 3).
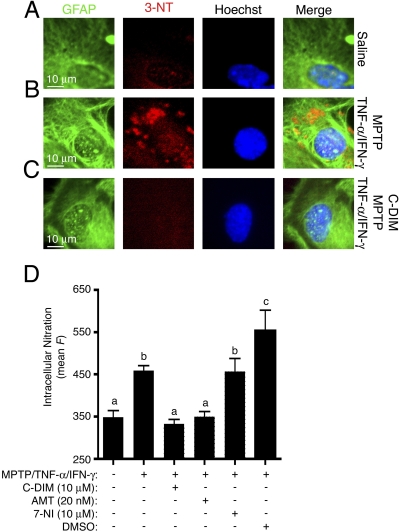
Suppression of NOS2-Mediated Protein Nitration. The effect of NOS2 induction by MPTP and inflammatory cytokines on global protein nitration after NO/peroxynitrite (ONOO-) formation in astrocytes was examined by immunofluorescence (Fig. 3). Immunofluorescence using antibodies against GFAP (fluorescein isothiocyanate; green) and 3-nitrotyrosine adducts (Cy5; red) was used to measure the extent of protein nitration within astrocytes treated with MPTP and cytokines, with or without inclusion of DIM-C-pPhtBu (10 μM), rosiglitazone (10 μM), the high-affinity NOS2 inhibitor AMT (25 nM), the NOS1 inhibitor 7-NI (10 μM), or a DMSO vehicle control. Minimal levels of protein nitration were detected in saline-treated control astrocytes (Fig. 3A), whereas astrocytes challenged with MPTP and cytokines demonstrated a significant elevation in internal protein nitration (Fig. 3B). Cotreatment of astrocytes with DIM-C-pPhtBu prevented MPTP-induced increases in nitration, resulting in similar levels of 3-nitrotyrosine adducts as saline-treated control cells (Fig. 3C). Mean intracellular fluorescence for 3-nitrotyrosine was quantified and compared among the treatment groups (Fig. 3D). The MPTP-induced increase in 3-nitrotyrosine formation was prevented in astrocytes cotreated with either DIM-C-pPhtBu (10 μM) or the NOS2 inhibitor AMT (25 nM), but not the NOS1 inhibitor 7-NI (10 μM), to levels observed in saline-treated astrocytes, demonstrating suppression of internal protein nitration by DIM-C-pPhtBu. The DMSO vehicle control did not suppress MPTP-induced increases in the 3-nitrotyrosine fluorescence signal but rather increased the signal somewhat above that induced by treatment with MPTP and TNF-α/IFN-γ alone.
Fig. 3.
Immunofluorescence detection of GFAP (green) and 3-nitrotyrosine (3-NT; red) reveal an increase in nitration in astrocytes treated with MPTP and TNF-α/IFN-γ (B) and suppression of nitration to control levels after cotreatment with DIM-C-pPhtBu (C). D, quantitation of 3-NT fluorescence (red channel) demonstrates a significant elevation in nitration in activated astrocytes and suppression of this effect by DIM-C-pPhtBu or the NOS2 inhibitor AMT, but not the NOS1 inhibitor 7-NI, implicating NOS2 in the elevated nitration. Imaging studies presented here were performed three times (n = 3), and between four and six images were captured per experiment for internal repetition.
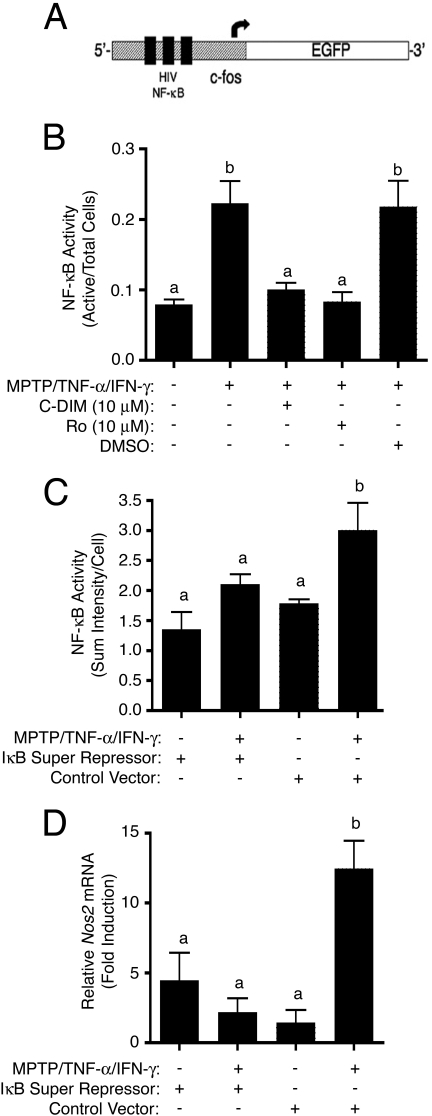
Inhibition of the NF-κB Signaling Pathway. A wide array of inflammatory genes, including Nos2, are driven by the NF-κB signaling cascade, and we have demonstrated in previous studies that another compound in the C-DIM series effectively prevented activation of NF-κB and expression of NOS2 in activated astrocytes (Tjalkens et al., 2008). To explore the efficacy of DIM-C-pPhtBu in modulating this pathway, we used astrocytes isolated from a transgenic mouse that expresses an EGFP construct driven by multiple cis-acting NF-κB domains (Fig. 4A) (Magness et al., 2004). Real-time imaging of GFP fluorescence was performed in control transgenic astrocytes exposed to saline and in cells exposed to MPTP and TNF-α/IFN-γ in combination with DIM-C-pPhtBu (10 μM), rosiglitazone (10 μM), or DMSO (Fig. 4B). Exposure to MPTP + TNF-α/IFN-γ resulted in an approximate 2.5-fold increase in total cellular GFP fluorescence compared with control cells that was completely suppressed by either DIM-C-pPhtBu or rosiglitazone but not by DMSO. As a control for the specificity of the cis-NF-κBEGFP construct for NF-κB-dependent signaling, transgenic astrocytes were transfected with mutant IκBα (S32/36A) to prevent activation of NF-κB. Cells expressing mutant IκBα failed to respond to MPTP and TNF-α/IFN-γ with an increase in GFP fluorescence, whereas GFP fluorescence increased markedly in transgenic astrocytes transfected with a control adenoviral vector (Fig. 4C). Likewise, challenge with MPTP and cytokines in astrocytes isolated from wild-type C57/Bl6 mice expressing mutant IκBα did not result in an increase in Nos2 mRNA, whereas a similar exposure in astrocytes expressing an empty control vector resulted in a significant increase in Nos2 mRNA (Fig. 4D).
Fig. 4.
A, activation of NF-κB in response to the MPTP and cytokine insult was measured by live-cell fluorescence imaging using primary astrocytes isolated from transgenic mice, which express an EGFP reporter construct driven by multiple cis-acting NF-κB domains. B, NF-κB is activated in astrocytes exposed to MPTP and TNF-α/IFN-γ, but cotreatment with either DIM-C-pPhtBu or rosiglitazone suppresses this activation, whereas DMSO vehicle control had no effect. C, expression of mutant IκBα (S32/36A; an NF-κB “super repressor”) by adenoviral transfection suppressed induction of Nos2 mRNA, but an empty control vector had no suppressive effect. D, expression of EGFP in transgenic astrocytes exposed to MPTP and inflammatory cytokines in the presence of mutant IκBα or control construct. Imaging studies using transgenic cells were performed three times (n = 3), and four images per chamber were captured for internal replicate.
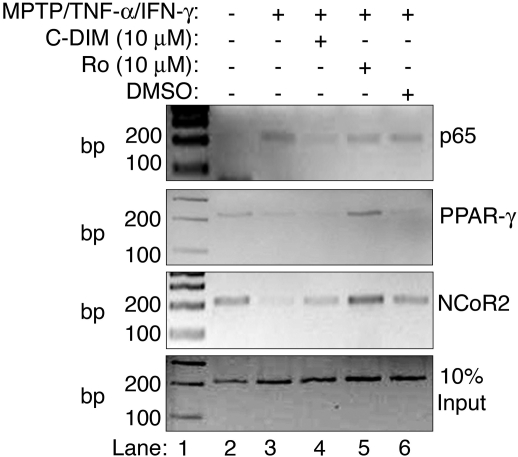
ChIP Analysis of the Nos2 Promoter. To determine the mechanism by which DIM-C-pPhtBu modulates NF-κB activity, the ChIP assay was used to identify specific DNA-protein interactions at the proximal NF-κB enhancer element (-86 to -76) of the Nos2 promoter. Based on previous studies demonstrating a requirement for degradation of the nuclear corepressor 2 protein (NCoR2) at the p65 binding site during NF-κB-dependent transactivation (Pascual et al., 2005), we examined binding of this factor, as well as that of p65 and PPAR-γ, to the proximal NF-κB response element during challenge with MPTP and cytokines in the absence and presence of DIM-C-pPhtBu or rosiglitazone (Fig. 5). These data demonstrate that treatment with MPTP and cytokines induced binding of p65 to the Nos2 promoter (Fig. 5, lane 3) that was blocked by cotreatment with DIM-C-pPhtBu (10 μM) but not rosiglitazone (10 μM) (Fig. 5, lanes 4 and 5). However, DIM-C-pPhtBu did not recruit PPAR-γ to this promoter region or stabilize NCoR2 (Fig. 5, lanes 2, 3, and 4). In contrast, rosiglitazone recruited PPAR-γ to the proximal NF-κB response element and prevented degradation of NCoR2 (Fig. 5, top two panels, lane 5). These data demonstrate a mechanism of inflammatory suppression by DIM-C-pPhtBu distinct from that of rosiglitazone.
Fig. 5.
ChIP reveals binding of p65 to the proximal NF-κB enhancer element in the Nos2 promoter along with removal of NCoR2 after challenge of astrocytes with MPTP and TNF-α/IFN-γ (lane 3). Cotreatment of the astrocytes with DIM-C-pPhtBu resulted in the prevention of p65 docking (lane 4); however, no effect on PPAR-γ recruitment or NCoR2 stabilization was observed. In contrast, cotreatment of astrocytes with rosiglitazone (lane 5) did not affect p65 docking; however, this treatment did result in recruitment of PPAR-γ and ensuing stabilization of NCoR2. These data demonstrate different mechanisms of Nos2 gene suppression by DIM-C-pPhtBu and rosiglitazone. ChIP experiments were repeated three times (n = 3) using individual cultures, and the presented data are representative of the results.
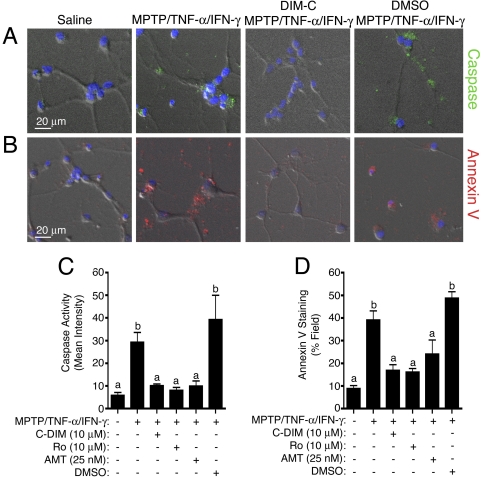
Astrocyte and Neuron CoCulture. The efficacy of DIM-C-pPhtBu in preventing astrocyte-dependent neuronal apoptosis is examined in Fig. 6. Astrocytes were grown to confluence in cell culture inserts that are permeable to small molecules but prevent direct cell-cell contact. After treatment of astrocytes with MPTP and TNF-α/IFN-γ in the presence or absence of DIM-C-pPhtBu (10 μM), rosiglitazone (10 μM), the NOS2 inhibitor AMT (25 nM), or DMSO, astrocytes were washed with PBS and coincubated directly above primary striatal neurons in neurobasal medium. Indices of neuronal apoptosis were then measured using live-cell fluorescence imaging of caspase activity (Fig. 6A) and Annexin V binding (Fig. 6B). Quantification of caspase activity and Annexin V-binding indicated that astrocytes treated with MPTP and TNF-α/IFN-γ caused apoptosis in cocultured striatal neurons that was prevented by prior cotreatment of the astrocytes with DIM-C-pPhtBu, rosiglitazone, or AMT (Fig. 6, C and D). The DMSO vehicle control had no suppressive effect.
Fig. 6.
Caspase activity (A) and annexin V binding (B) in primary striatal neurons cocultured with astrocytes activated by exposure to MPTP and TNF-α/IFN-γ. Cotreatment of astrocytes with DIM-C-pPhtBu before incubation with neurons suppressed neuronal apoptosis. Quantitation of fluorescence intensity demonstrates elevated neuronal caspase (C) and annexin V (D) binding after coculture with astrocytes challenged with MPTP and TNF-α/IFN-γ and suppression of this effect by cotreating astrocytes with DIM-C-pPhtBu, rosiglitazone, or the NOS2 inhibitor AMT. Coculture experiments were performed three times (n = 3), and between four and six images were captured per replicate for internal repetition.
Discussion
More than one million people currently have a diagnosis of PD, with an additional 50,000 expected diagnoses annually, making this disease the second most prevalent neurological disorder behind Alzheimer's disease (Teismann and Schulz, 2004). Although the reason for selective neuronal loss in PD remains poorly explained, chronic inflammation and activation of glial cells has been consistently observed in PD models as well as after postmortem evaluation and may provide a realistic target for slowing the progression of neuronal injury. Nuclear orphan receptor ligands, especially those belonging to the thiazolidinedione class that bind PPAR-γ, have been explored as candidates for halting this inflammation (Dehmer et al., 2004; Luna-Medina et al., 2005). Research in this laboratory has identified recently a member of the PPAR-γ-active C-DIM class of compounds that suppressed manganese-induced NOS expression (Tjalkens et al., 2008), and one of these C-DIM compounds, DIM-C-pPhtBu, which has been documented previously to suppress endothelial cell activation in a model of atherosclerosis (Calabro et al., 2005), was used in this study.
Inflammation and astrogliosis in models of PD are characterized by induction of NOS2, which has received considerable attention as a potential etiological factor in PD because of its association with nigral degeneration in humans (Knott et al., 2000), as well as the induction of this enzyme in chemical (e.g., MPTP) models of the disease (Liberatore et al., 1999). Pathology from excess NO is postulated to occur through multiple mechanisms, including reaction with superoxide to form the peroxynitrite anion, which can modify tyrosine residues through the formation of covalent 3-nitrotyrosine adducts (Dawson and Dawson, 1996). Indeed, studies characterizing the effects of increased protein nitration have documented deleterious consequences, including conformational changes (Cassina et al., 2000) and prevention of phosphorylation resulting in abnormal loss or gain of function, such as that reported for glutamine synthetase (Berlett et al., 1996) and protein kinase C (Hink et al., 2003). Excessive production of NO and subsequent formation of peroxynitrite could therefore interfere with critical homeostatic intracellular processes in astrocytes and damage adjacent neurons. Supporting this hypothesis are observations that astrocytes can secrete NO into extracellular matrix at concentrations reaching 1 μM (Brown, 1995). Furthermore, in addition to elevating nitrosative stress in neighboring neurons, astrocyte-derived NO has also been hypothesized to exacerbate neuronal excitotoxicity through the potentiation of glutamate release (Duncan and Heales, 2005) and directly inhibits mitochondrial respiration in neurons (Bolanos et al., 1995),
Using NOS2 as a marker for astrocyte activation, the efficacy of a novel anti-inflammatory diindolylmethane compound, DIM-C-pPhtBu, was compared with that of an existing thiazolidinedione-class compound, rosiglitazone, that has been shown previously to suppress induction of Nos2 (Pascual et al., 2005). Initial studies in primary cultured astrocytes activated by an MPTP (10 μM), TNF-α (10 pg/ml), and IFN-γ (1 ng/ml) combination demonstrated a clear dose-responsive suppression of Nos2 by DIM-C-pPhtBu at concentrations ranging form 1 to 100 μM, where 10 μM was the lowest tested dose that suppressed induction to levels statistically similar to the saline-treated controls (Fig. 2A) and was comparable with the Nos2 suppression observed upon including rosiglitazone (10 μM), shown in Fig. 2B. Finally, suppression of NOS2 protein levels was also demonstrated by both 10 μM DIM-C-pPhtBu or rosiglitazone (Fig. 2C) in primary astrocytes activated with MPTP and inflammatory cytokines.
Intracellular nitration stemming from NOS2 expression is hypothesized to play a role in protein dysregulation (Dawson and Dawson, 1996). Because astrocytes perform tasks vital to the survival and function of neurons, increased protein nitration within this cell could negatively affect neuronal survival. Therefore, nitration in primary cultured astrocytes was assayed by immunofluorescence using antibodies against 3-nitrotyrosine, revealing an increase in global protein nitration after treatment of the cells with the MPTP and TNF-α/IFN-γ (Fig. 3, A and B) and suppression of this effect by DIM-C-pPhtBu (Fig. 3C). These results paralleled the elevated NOS2 protein levels observed by immunoblotting (Fig. 2C). Quantification of 3-nitrotyrosine fluorescence demonstrated equivalent suppression of nitration by cotreatment with either DIM-C-pPhtBu or the NOS2 inhibitor AMT, whereas the NOS1 inhibitor 7-NI had no suppressive effect (Fig. 3D), suggesting that the increase in nitration is primarily due to NO derived from NOS2. It is interesting that DMSO somewhat enhanced MPTP-mediated protein nitration (Fig. 3D) but had no effect by itself (data not shown), suggesting that DMSO exacerbated MPTP and cytokine toxicity, possibly through facilitation of MPTP movement across the cell membrane (Fig. 3D).
Activation of the NF-κB signaling cascade is linked to the induction of a wide array of stress-response genes (Pahl, 1999) and is recognized to be a key event in the expression of Nos2 (Xie et al., 1992). Activation of NF-κB in astrocytes treated with MPTP and inflammatory cytokines was measured in astrocytes isolated from a transgenic mouse, which expresses EGFP in response to multiple cis-acting NF-κB domains (Fig. 4A) (Magness et al., 2004). Live-cell fluorescence imaging revealed increased NF-κB-dependent GFP expression in astrocytes challenged with MPTP and TNF-α/IFN-γ cytokines that was suppressed by both DIM-C-pPhtBu (10 μM) and rosiglitazone (10 μM) (Fig. 4B). Involvement of NF-κB in the induction of Nos2 in astrocytes was confirmed using a phosphorylation-resistant mutant of IκBα (an NF-κB “super repressor”) that prevents the release of the active p65/p50 NF-κB dimer and ensuing activation of NF-κB-responsive genes. Expression of mutant IκBα prevented both MPTP-induced increases in GFP fluorescence in transgenic astrocytes and induction of Nos2 mRNA in wild-type astrocytes, directly implicating this pathway in inducible expression of NOS2 in MPTP-activated astrocytes (Fig. 4, C and D).
Suppression of NF-κB activation was observed in astrocytes challenged with MPTP and TNF-α/IFN-γ and cotreated with either DIM-C-pPhtBu or rosiglitazone. We initially postulated that this C-DIM analog acted through a transrepressive mechanism similar to that reported for rosiglitazone, in which the induction of macrophage Nos2 was prevented through recruitment of PPAR-γ to the promoter region that subsequently stabilized NCoR2 (Pascual et al., 2005). We observed a similar mechanism for rosiglitazone-mediated Nos2 suppression using the ChIP assay (Fig. 5, lane 5) but found a strikingly different mode of action for DIM-C-pPhtBu in primary astrocytes. The C-DIM analog failed to recruit PPAR-γ or stabilize NCoR2 and, in contrast to rosiglitazone, prevented the binding of p65 to the Nos2 promoter (Fig. 5, lane 4). This finding demonstrates a mechanism of transrepression of NF-κB distinct from that reported for TZD compounds such that interdiction of NF-κB signaling either may occur upstream of transcriptional repressor stabilization or may involve nuclear factors distinct from those currently identified as targets of PPAR-γ. These results are consistent with the ongoing studies with PPAR-γ-active C-DIMs, in which many of their activities are PPAR-γ-independent and involve the activation of kinases, decreased mitochondrial membrane potential, and induction of endoplasmic reticulum stress (Abdelrahim et al., 2006; Chintharlapalli et al., 2006, 2007; Hong et al., 2008; Lei et al., 2008). By blocking the binding of p65, DIM-C-pPhtBu may thereby suppress a larger array of NF-κB-responsive genes than TZD compounds because the transrepressive properties of this class of C-DIM compounds may not be limited to the same transcriptional repressors.
The neuroprotective efficacy of DIM-C-pPhtBu was examined in a model of astrocyte-mediated neurodegeneration (Fig. 6), in which astrocytes activated by exposure to MPTP and inflammatory cytokines were coincubated with GADD65/67-positive primary striatal neurons. Astrocytes stimulated in this manner caused increases in caspase activity and Annexin V reactivity in cocultured neurons, along with a consistently observed general decrease in the number of live neurons. Cotreatment with DIM-C-pPhtBu decreased neuronal caspase activity comparably with rosiglitazone or the NOS2 inhibitor AMT (Fig. 6C), with a similar trend observed after Annexin V staining (Fig. 6D). These data demonstrate that DIM-C-pPhtBu effectively counters the neuroinflammatory effects of MPTP and TNF-α/IFN-γ in astrocytes and thereby prevents the degeneration of cocultured striatal neurons. NO seems to be an important neurotoxic mediator produced by activated astrocytes after challenge with MPTP because inhibiting NOS2 activity or decreasing its expression preserved neuronal viability and prevented the activation of apoptotic signaling pathways.
The C-DIM compound explored here is a novel derivative of the diindolylmethane class of chemical found in cruciferous vegetables. This particular compound was demonstrated to be a potent agonist of the transcription factor PPAR-γ (Qin et al., 2004). The described compound was subsequently tested as a candidate for halting neuronal degeneration based on the existing body of literature linking PPAR-γ agonists to the suppression of inflammation (Dehmer et al., 2004; Luna-Medina et al., 2005; Pascual et al., 2005), with the latter providing a solid mechanism for the observed suppression of Nos2 by ligands of this orphan receptor. It is interesting that the mechanism of Nos2 suppression differed from that reported for the thiazolidinedione rosiglitazone (Pascual et al., 2005), in that recruitment of PPAR-γ was not observed, but a mechanism involving prevention of p65 docking was demonstrated. This has led to exciting speculation regarding a potential mechanism for this interdiction, which is currently under investigation. The alternate mechanism also suggests the possibility of gene regulation patterns different from that of rosiglitazone. However, very little data exist regarding the extent of gene regulation by DIM-C-pPhtBu, other than the initial characterization (Qin et al., 2004), in which a PPAR-γ/luciferase reporter construct was used in MCF-7 breast cancer cells. This same article described several other C-DIM derivatives with significantly different effects on this reporter construct, and it is therefore very difficult to predict gene regulation by this particular C-DIM. However, this does represent a critical knowledge gap, and experiments are planned for globally profiling gene expression in astrocytes treated with DIM-C-pPhtBu. The data described here suggest that DIM-C-pPhtBu inhibits NOS2 expression in astrocytes by a mechanism involving interdiction of NF-κB signaling at the level of p65 binding rather than through stabilization of NCoR2, although the involvement of other transcriptional corepressors cannot be ruled out yet. Together, these studies suggest that modulation of astrocyte inflammatory phenotype through novel therapeutics such as DIM-C-pPhtBu could be a realistic strategy for suppressing the deleterious effects of activated glia in PD.
Supplementary Material
This study was supported by ES012941 (to R.B.T.), NS055632 (to D.L.C.), CA108718 (to S.S.), and by an individual research grant from the American Parkinson Disease Association (to R.B.T.).
ABBREVIATIONS: PD, Parkinson's disease; DIM-C-pPhtBu 1,1-bis(3′-indolyl)-1-(p-t-butylphenyl)methane; C-DIM, methylene-substituted diindolylmethane; PPAR, peroxisome proliferator-activated receptor, MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NCoR2, nuclear corepressor complex-2; GFP, green fluorescent protein; NF-κB, nuclear factor-κB; NOS2, inducible nitric-oxide synthase; PCR, polymerase chain reaction; TNF, tumor necrosis factor; IFN, interferon; TZD, thiazolidinedione; AMT, 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine; GFAP, glial fibrillary acidic protein; Iba1, ionized calcium binding adapter molecule 1; ChIP, chromatin immunoprecipitation; DMSO, dimethyl sulfoxide; RT, reverse transcription; PBS, phosphate-buffered saline; EGFP, enhanced green fluorescent protein; 7-NI, 7-nitroindazole.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
References
- Abdelrahim M, Newman K, Vanderlaag K, Samudio I, and Safe S (2006) 3,3′-Diindolylmethane (DIM) and its derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis 27 717-728. [DOI] [PubMed] [Google Scholar]
- Aschner M and Kimelberg HK (1991) The use of astrocytes in culture as model systems for evaluating neurotoxic-induced-injury. Neurotoxicology 12 505-517. [PubMed] [Google Scholar]
- Barhoumi R, Faske J, Liu X, and Tjalkens RB (2004) Manganese potentiates lipopolysaccharide-induced expression of NOS2 in C6 glioma cells through mitochondrial-dependent activation of nuclear factor kappaB [published erratum appears in Brain Res Mol Brain Res 126:103-105, 2004]. Brain Res Mol Brain Res 122 167-179. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y and Marmot MG (1995) Survival and cause of death in a cohort of patients with parkinsonism: possible clues to aetiology? J Neurol Neurosurg Psychiatry 58 293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlett BS, Friguet B, Yim MB, Chock PB, and Stadtman ER (1996) Peroxynitrite-mediated nitration of tyrosine residues in Escherichia coli glutamine synthetase mimics adenylylation: relevance to signal transduction. Proc Natl Acad Sci U S A 93 1776-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolaños JP, Heales SJ, Land JM, and Clark JB (1995) Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. J Neurochem 64 1965-1972. [DOI] [PubMed] [Google Scholar]
- Brown GC (1995) Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett 369 136-139. [DOI] [PubMed] [Google Scholar]
- Calabrò P, Samudio I, Safe SH, Willerson JT, and Yeh ET (2005) Inhibition of tumor-necrosis-factor-alpha induced endothelial cell activation by a new class of PPAR-gamma agonists. An in vitro study showing receptor-independent effects. J Vasc Res 42 509-516. [DOI] [PubMed] [Google Scholar]
- Cassina AM, Hodara R, Souza JM, Thomson L, Castro L, Ischiropoulos H, Freeman BA, and Radi R (2000) Cytochrome c nitration by peroxynitrite. J Biol Chem 275 21409-21415. [DOI] [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, and Safe S (2006) 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through PPAR-gamma-dependent and PPARgamma-independent pathways. Mol Cancer Ther 5 1362-1370. [DOI] [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, and Safe S (2007) 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit growth, induce apoptosis, and decrease the androgen receptor in LNCaP prostate cancer cells through peroxisome proliferator-activated receptor γ-independent pathways. Mol Pharmacol 71 558-569. [DOI] [PubMed] [Google Scholar]
- Chintharlapalli S, Smith R 3rd, Samudio I, Zhang W, and Safe S (2004) 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes induce peroxisome proliferator-activated receptor gamma-mediated growth inhibition, transactivation, and differentiation markers in colon cancer cells. Cancer Res 64 5994-6001. [DOI] [PubMed] [Google Scholar]
- Dawson VL and Dawson TM (1996) Nitric oxide neurotoxicity. J Chem Neuroanat 10 179-190. [DOI] [PubMed] [Google Scholar]
- Dehmer T, Heneka MT, Sastre M, Dichgans J, and Schulz JB (2004) Protection by pioglitazone in the MPTP model of Parkinson's disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem 88 494-501. [DOI] [PubMed] [Google Scholar]
- Djaldetti R, Hellmann M, and Melamed E (2004) Bent knees and tiptoeing: late manifestations of end-stage Parkinson's disease. Mov Disord 19 1325-1328. [DOI] [PubMed] [Google Scholar]
- Duncan AJ and Heales SJ (2005) Nitric oxide and neurological disorders. Mol Aspects Med 26 67-96. [DOI] [PubMed] [Google Scholar]
- Ebadi M and Sharma SK (2003) Peroxynitrite and mitochondrial dysfunction in the pathogenesis of Parkinson's disease. Antioxid Redox Signal 5 319-335. [DOI] [PubMed] [Google Scholar]
- Hink U, Oelze M, Kolb P, Bachschmid M, Zou MH, Daiber A, Mollnau H, August M, Baldus S, Tsilimingas N, et al. (2003) Role for peroxynitrite in the inhibition of prostacyclin synthase in nitrate tolerance. J Am Coll Cardiol 42 1826-1834. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, and Michel PP (2003) The role of glial reaction and inflammation in Parkinson's disease. Ann N Y Acad Sci 991 214-228. [DOI] [PubMed] [Google Scholar]
- Hirsch EC and Hunot S (2000) Nitric oxide, glial cells and neuronal degeneration in parkinsonism. Trends Pharmacol Sci 21 163-165. [DOI] [PubMed] [Google Scholar]
- Hong J, Samudio I, Chintharlapalli S, and Safe S (2008) 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes decrease mitochondrial membrane potential and induce apoptosis in endometrial and other cancer cell lines. Mol Carcinog 47 492-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Dugas N, Faucheux B, Hartmann A, Tardieu M, Debré P, Agid Y, Dugas B, and Hirsch EC (1999) FcepsilonRII/CD23 is expressed in Parkinson's disease and induces, in vitro, production of nitric oxide and tumor necrosis factor-alpha in glial cells. J Neurosci 19 3440-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, and Li ZW (2002) NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2 301-310. [DOI] [PubMed] [Google Scholar]
- Kassouf W, Chintharlapalli S, Abdelrahim M, Nelkin G, Safe S, and Kamat AM (2006) Inhibition of bladder tumor growth by 1,1-bis(3′-indolyl)-1-(p-substituted-phenyl)methanes: a new class of peroxisome proliferator-activated receptor gamma agonists. Cancer Res 66 412-418. [DOI] [PubMed] [Google Scholar]
- Knott C, Stern G, and Wilkin GP (2000) Inflammatory regulators in Parkinson's disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci 16 724-739. [DOI] [PubMed] [Google Scholar]
- Lang AE and Lozano AM (1998) Parkinson's disease. Second of two parts. N Engl J Med 339 1130-1143. [DOI] [PubMed] [Google Scholar]
- Lei P, Abdelrahim M, Cho SD, Liu S, Chintharlapalli S, and Safe S (2008) 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through activation of c-jun N-terminal kinase. Carcinogenesis 29 1139-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, and Przedborski S (1999) Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 5 1403-1409. [DOI] [PubMed] [Google Scholar]
- Livak KJ and Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta CT) method. Methods 25 402-408. [DOI] [PubMed] [Google Scholar]
- Luna-Medina R, Cortes-Canteli M, Alonso M, Santos A, Martínez A, and Perez-Castillo A (2005) Regulation of inflammatory response in neural cells in vitro by thiadiazolidinones derivatives through peroxisome proliferator-activated receptor gamma activation. J Biol Chem 280 21453-21462. [DOI] [PubMed] [Google Scholar]
- Magness ST, Jijon H, Van Houten Fisher N, Sharpless NE, Brenner DA, and Jobin C (2004) In vivo pattern of lipopolysaccharide and anti-CD3-induced NF-kappa B activation using a novel gene-targeted enhanced GFP reporter gene mouse. J Immunol 173 1561-1570. [DOI] [PubMed] [Google Scholar]
- Pahl HL (1999) Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18 6853-6866. [DOI] [PubMed] [Google Scholar]
- Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, and Glass CK (2005) A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 437 759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M and Nilsson M (2005) Astrocyte activation and reactive gliosis. Glia 50 427-434. [DOI] [PubMed] [Google Scholar]
- Pellerin L and Magistretti PJ (2004) Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist 10 53-62. [DOI] [PubMed] [Google Scholar]
- Qin C, Morrow D, Stewart J, Spencer K, Porter W, Smith R 3rd, Phillips T, Abdelrahim M, Samudio I, and Safe S (2004) A new class of peroxisome proliferator-activated receptor gamma (PPARgamma) agonists that inhibit growth of breast cancer cells: 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes. Mol Cancer Ther 3 247-260. [PubMed] [Google Scholar]
- Teismann P and Schulz JB (2004) Cellular pathology of Parkinson's disease: astrocytes, microglia and inflammation. Cell Tissue Res 318 149-161. [DOI] [PubMed] [Google Scholar]
- Tjalkens RB, Liu X, Mohl B, Wright T, Moreno JA, Carbone DL, and Safe S (2008) The peroxisome proliferator-activated receptor-gamma agonist 1,1-bis(3′-indolyl)-1-(p-trifluoromethylphenyl)methane suppresses manganese-induced production of nitric oxide in astrocytes and inhibits apoptosis in cocultured PC12 cells. J Neurosci Res 86 618-629. [DOI] [PubMed] [Google Scholar]
- Weinmann AS and Farnham PJ (2002) Identification of unknown target genes of human transcription factors using chromatin immunoprecipitation. Methods 26 37-47. [DOI] [PubMed] [Google Scholar]
- Xie QW, Cho HJ, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Ding A, Troso T, and Nathan C (1992) Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 256 225-228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.