Abstract
Oxidative stress is a candidate mechanism for ethanol neuropathology in Fetal Alcohol Spectrum Disorders. Oxidative stress often involves production of reactive oxygen species (ROS), deterioration of the mitochondrial membrane potential (MMP), and cell death. Previous studies have produced conflicting results regarding the role of oxidative stress and the benefit of antioxidants in ethanol neuropathology in the developing brain. This study investigated the hypothesis that ethanol neurotoxicity involves production of ROS with negative downstream consequences for MMP and neuron survival. This was modeled in neonatal rats at postnatal day 4 (P4) and P14. It is well established that granule neurons in the rat cerebellar cortex are more vulnerable to ethanol neurotoxicity on P4 than at later ages. Thus, it was hypothesized that ethanol produces more oxidative stress and its negative consequences on P4 than on P14. A novel experimental approach was employed in which ethanol was administered to animals in vivo (gavage 6g/kg), granule neurons were isolated 2-24 hr post-treatment, and ROS production and relative MMP were immediately assessed in the viable cells. Cells were also placed in culture and survival was measured 24 hr later. The results revealed that ethanol did not induce granule cells to produce ROS, cause deterioration of neuronal MMP, or cause neuron death when compared to vehicle controls. Further, granule neurons from neither P4 nor P14 animals mounted an oxidative response to ethanol. These findings do not support the hypothesis that oxidative stress is obligate to granule neuron death following ethanol exposure in the neonatal rat brain. Other investigators have reached a similar conclusion using either brain homogenates or cell cultures. In this context, it is likely that oxidative stress is not the sole and perhaps not the principal mechanism of ethanol neurotoxicity for cerebellar granule neurons during this stage of brain development.
Keywords: alcohol, neurotoxicity, reactive oxygen species, cerebellum, brain development
Introduction
Ethanol exposure during brain development causes the neuropathology underlying Fetal Alcohol Syndrome and Alcohol-Related Neurodevelopmental Disorder (Stratton et al., 1996). The pathology of Fetal Alcohol Syndrome includes significant malformation and dysfunction in the brain including learning, behavioral, social, and psychiatric disorders that persist into adulthood (Sowell et al., 2002; Warren and Bast, 1988). Animal models (Bonthius and West, 1991; Driscoll et al., 1990; Green et al., 2002; Kane et al., 1997; Klintsova et al., 2002; Light et al., 2002b; Light et al., 1998) demonstrate that ethanol exposure during brain development causes significant neuronal loss (Hamre and West, 1993; Maier et al., 1999; Pierce et al., 1989; Pierce et al., 1997; Pierce et al., 1993; Pierce and West, 1987; Pierce et al., 1999). The neonatal rodent model of maternal drinking in the third trimester demonstrates striking Purkinje cell and granule neuron loss in the cerebellum (Goodlett et al., 1990; Light et al., 2002a; Pierce et al., 1999). A particular feature of this neurotoxicity is the differential temporal vulnerability of neuronal loss. When ethanol exposure occurs during the period postnatal day (P) 4-6, significant loss of neurons occurs reproducibly in the cerebellum (Goodlett and Eilers, 1997; Hamre and West, 1993; Miki et al., 1999; Pierce et al., 1999). In contrast, the periods prior to P4 and after P6 are less sensitive to ethanol neurotoxicity. The basis for this differential vulnerability has not been clearly established.
Several direct, as well as indirect, cellular mechanisms may contribute to ethanol-induced neuronal apoptosis during brain development. These include: altered neuronal metabolism (de la Monte et al., 2005; Li et al., 2002), glutamate excitotoxicity (Ikonomidou et al., 2000), disruption of apoptotic regulators (Heaton et al., 2006; Siler-Marsiglio et al., 2005a), limited neurotrophin support (Heaton et al., 2000b; Light et al., 2002b; Light et al., 2001), restricted synaptic development (Klintsova et al., 2002; West et al., 1994), and oxidative stress (Heaton et al., 2003; Heaton et al., 2002; Henderson et al., 1995; Ramachandran et al., 2003; Reyes et al., 1993; Siler-Marsiglio et al., 2005b; Smith et al., 2005). Oxidative stress can cause mitochondrial membrane depolarization, which is followed by cytochrome c release, caspase activation, and apoptosis (Bernardi et al., 2006; O’Rourke et al., 2005). In fact, ethanol treatment in cell culture as well as in the developing brain in vivo increases levels of ROS and free radicals, decreases endogenous antioxidant enzyme and metabolite concentrations, and produces lipid peroxidation (Heaton et al., 2003; Heaton et al., 2002; Henderson et al., 1995; Ramachandran et al., 2003; Siler-Marsiglio et al., 2005a; Siler-Marsiglio et al., 2004; Siler-Marsiglio et al., 2005b; Smith et al., 2005).
Although the involvement of ROS in ethanol-induced neurotoxicity has attracted much attention (Sun et al., 2001), a consensus of understanding has not been achieved. This important issue merits resolution because the existing observations provide potential for a pivotal relationship between ethanol-induced oxidative stress and ethanol-induced neurotoxicity.
The present study investigated the hypothesis that the developmental pattern of neuronal vulnerability to ethanol in the cerebellum is a function of the oxidative stress response in the tissue. It may be expected that ethanol exposure in neonatal animals would stimulate ROS production and that the level of ROS produced would be greater with exposure on P4 than on P14. This study contrasted, at P4 and P14, the oxidative response of granule neurons following ethanol exposure in vivo. To quantify changes in oxidative stress and determine the timing of cellular response after treatment, granule neurons were isolated 2, 12, and 24 hr after in vivo ethanol exposure and the viable neurons were immediately assayed for ROS generation. Mitochondrial membrane potential and cell survival were also measured because detrimental affects of ROS on these cellular mechanisms represent downstream consequences of oxidative stress.
Materials and Methods
The potential for ethanol to induce oxidative stress when administered in vivo was evaluated in P4 or P14 rats, an age of either relatively high or low ethanol sensitivity, respectively, in the cerebellum. Animals of each age were divided into three treatment groups: handled control (H); vehicle treated control (V); or ethanol treated (E). The treatment paradigm was designed to optimize detection of an acute alteration in the level of oxidative stress as well as its post-treatment time course. To this end, treatment with ethanol or vehicle was performed once per animal. Analyses were performed 2, 12, or 24 hr after the treatment in order to specifically encompass the period of time (1) a few hours prior to neuronal apoptosis, (2) at initiation of significant increase in neuronal apoptosis, and (3) at mid-peak level of neuronal apoptosis, in the cerebellum (Light et al., 2002a). Loss of granule neurons is essentially complete by 24 hr. Because quantitative measurement of ROS production can be more accurately performed in vitro than in the intact animal, granule neurons were isolated from fresh cerebella. Oxidative stress was determined in the freshly dissociated granule neurons immediately upon isolation from the tissue and measured as change in the level of ROS production or mitochondrial depolarization.
In the present report, in vivo refers to treatment of live animals. In vitro refers to assay of intact viable cells that were freshly isolated from treated animals; it does not refer to assay of cultured cells.
Ethanol Administration
Sprague Dawley CD rats were purchased from Charles River Laboratories and housed in the AAALAC-approved campus facility in the Division of Laboratory Animal Medicine. Animal protocols were approved by the Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult rats were bred to provide postnatal day 4 and day 14 (designated P4 and P14 respectively) pups for treatment.
Ethanol treated animals were administered a 15% w/v solution of ethanol (6 g/kg) in Intralipid-20%® (Fresenius Kabi Clayton, L.P.) vehicle by intragastric gavage (Kane et al., 1997; Light et al., 2002b; Light et al., 1998; Pierce et al., 1993; Pierce et al., 1999). The blood ethanol concentration was 355-385 mg/dl (n=6; Diagnostic Chemicals Limited kit) 1 hr post-treatment. Vehicle control animals were administered the same volume and concentration of vehicle by gavage. Handled control animals were weighed. In all treatment groups, the dam and pups were separated 5-10 min for weighing and gavage. Animals were sacrificed 2, 12, or 24 hr after treatment. On a given day, 6 pups from 1-2 litters were processed with 3 animals in each of 2 treatment groups, e.g., 3 E and 3 V. Each treatment group at each postnatal age and post-treatment interval contained 11-12 animals from 4 or more litters.
Cerebellar Granule Neurons
Cerebella were dissociated and cerebellar granule neurons were isolated as described previously (Chang et al., 1996, 1997; Chang and Liu, 1997, 1998; Kane et al., 1996a, b). Animals were sacrificed 2 hr, 12 hr, or 24 hr after ethanol treatment by an overdose of CO2 and cervical transection. The cerebellum of each animal was minced, digested with trypsin (0.3 mg/ml) for 5 min, and mixed with an equal volume of dissociation buffer (80% DMEM medium, 10% F12 medium, 10 % fetal bovine serum, and 0.1 mg/ml DNase). The tissue was centrifuged (110xg, 5 min) and the pellet was dissociated with 1 ml dissociation buffer in a 12×75 mm tissue culture tube. Dissociated cells were collected in the supernatant after the tissue settled by gravity in the tube for 10 min. The dissociation process was repeated on the tissue pellet. The dissociated cells were pooled, centrifuged, and resuspended in 1.5 ml Hank’s Balanced Salt Solution (HBSS). The neurons isolated by this method are >95% granule neurons based on staining of astrocytes and microglia, morphological differentiation of small granule neurons and larger Purkinje neurons, and cell counts (data not shown).
Viability of the cell isolates was assessed by trypan blue dye exclusion and cell counts. This was performed in order to standardize the number of viable cells for each sample and each assay. Thus, the results within each assay are based on an equivalent number of viable cells from each sample. Freshly isolated cerebellar granule neurons from handled control P4 (n=11) and P14 (n=12) animals exhibited similar viability of 97.9 ± 0.3% and 98.6 ± 0.2% respectively. There was no difference in the viability of freshly isolated cells due to treatment. Each cell isolate in the study contained greater than 95% viable cells.
Using parallel aliquots of each cell isolate, the production of reactive oxygen species (ROS) and the mitochondrial membrane potential (MMP) were analyzed immediately, within 1 hr. In addition, aliquots of each cell isolate were placed in culture and neuron survival was measured 24 hr later. All three experimental assays (ROS, MMP, and 24-hr survival) were performed on aliquots of the same sample; that is, every sample was subjected to all three biological assays.
Production of Reactive Oxygen Species (ROS)
Quantitative analysis of ROS production by granule neurons was performed with oxidative conversion of the fluorescent marker H2DCF-DA (2′, 7′-dichlorodihydrofluorescein diacetate; Molecular Probes, Eugene, OR). Aliquots of the granule neuron suspensions described above were used in this assay. Viable cells (50,000 in 50 μl HBSS) were mixed with 50 μl of 20 μM H2DCF-DA in 96-well plates and incubated at 37°C for 1 hr. H2DCF-DA is converted to DCF-DA in the presence of ROS. Thus, relative DCF-DA fluorescence corresponds to the relative level of ROS production (Rosenkranz et al., 1992). Fluorescence of DCF-DA was quantified with a Spectra Max Gemini XS (Molecular Devices, Sunnyvale, CA) at an excitation of 485 nm, emission of 535 nm, and cutoff of 530 nm. The results were expressed as mean ± SE fluorescence units (FU) of DCF-DA at 535 nm.
Change in Mitochondrial Membrane Potential (MMP)
The change in MMP was quantified with the dye JC-1 (5, 5′, 6, 6′-tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazolocarbocyanine iodide, Molecular Probes, Eugene, OR) as described previously (Garg and Chang, 2004, 2006). To prepare JC-1, a 5 mM stock solution was prepared in dimethyl sulfoxide, subsequently diluted 1:4 with 5% BSA, further diluted 1:199 in HBSS, and filtered through a 0.2 μm membrane. Binding of the dye to mitochondria was detected by fluorescence spectroscopy as two emission peaks. A green peak at 545 nm represents JC-1 monomers. A red peak at 595 nm represents JC-1 aggregates, which form at the mitochondrial membrane in the presence of a negative membrane potential in healthy cells. The intensity ratio of these two peaks is a relative measure of mitochondrial potential. An increased 545/595 ratio represents deterioration of the MMP, depolarization, and reduced mitochondrial integrity. The results were expressed as mean ± SE of the ratio.
Aliquots of the granule neuron suspensions described above were used in this assay. Viable cells (250,000 in 250 μl HBSS) were collected by centrifugation (110xg, 5 min), resuspended in 250 μl of 5 μM JC-1, and incubated at 37°C for 15 min. Cells were centrifuged, resuspended and centrifuged again. Then the cell pellet was resuspended in 200 μl HBSS and transferred to a 96-well plate. Fluorescence was quantified with a Spectra Max Gemini XS with an excitation of 485 nm, emission of 545 nm and 595 nm, and cutoff of 530 nm. The relative intensity of the two peaks was expressed as the ratio of FU at 545nm/595nm.
Granule Neuron Survival
Survival of isolated cells following culture for 24 hr was assessed with the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay as previously described (Chang and Liu, 2001; Garg and Chang, 2003; Kane et al., 1996a, b; Liu et al., 1997). Freshly isolated cell aliquots were plated in poly-L-lysine (25 mg/l) coated 96-well plates (150,000 viable cells/well) in medium containing 80% DMEM medium, 10% F12 medium, 10% fetal bovine serum, 1.4 mM glutamine, and 25 mM KCl. After overnight culture, the cells were incubated in DMEM containing 100 μg/ml of MTT for 1 hr. The dye was solubilized with dimethyl sulfoxide. The optical density at 570 nm was quantified with a Spectra Max 190 (Molecular Devices, Sunnyvale, CA).
Data Analysis
This set of experiments was performed in 161 animals. Each experimental group contained 11-12 animals, providing sufficient power for analyses. The impact of age (P4 vs. P14), treatment (H vs. V vs. E), and post-treatment time interval (2 hr vs. 12 hr vs. 24 hr) on each of the three outcomes was analyzed using three-way analysis of variance (ANOVA) including all possible interaction terms. The design did not include repeated measures. Each outcome measure (relative ROS production, relative MMP, and 24-hr survival) was analyzed separately.
Three-factor interactions terms were not statistically significant (DF 4) for any of the three outcome measures. Because the design had an unequal n, and the ANOVA models for ROS production and MMP contained statistically significant two-factor interaction terms, these variables were compared as tests of simple effects of least squares means. The least squares means are a model adjustment for the imbalance in the design, and the simple effects compare the effect of one factor while controlling for the other two. Nominal p-values were determined, and Bonferroni adjusted significance determinations were made, maintaining hypothesis-wide alpha levels at 0.05. The adjusted significance levels are noted in footnotes accompanying the tables. Because the only significant effect in the ANOVA model for 24-hr survival was a main effect for age, this variable was reported according to the observed means ± SE and compared between age groups using a t-test. All statistical evaluations were performed with SAS 9.1 (SAS Institute, Cary, NC).
9.1 (SAS Institute, Cary, NC).
Results
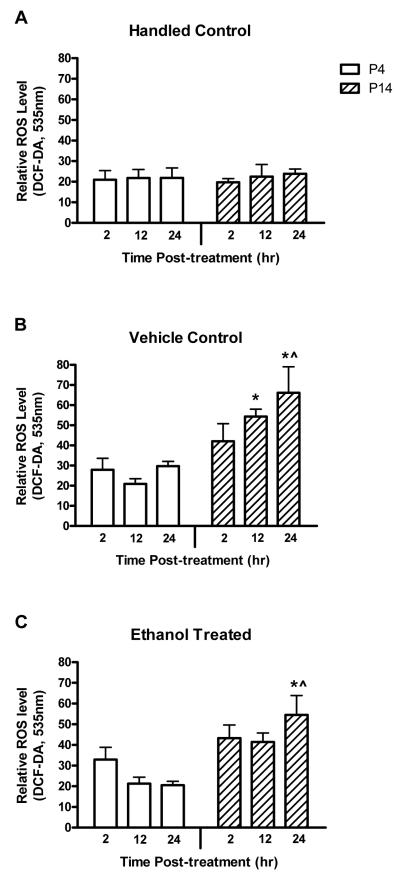
The experiments and analyses tested the hypotheses that cerebellar granule neurons from P4 and P14 animals mount distinct oxidative responses to ethanol exposure. To this end, neurons were freshly isolated from rats that were handled controls, vehicle controls, or were given ethanol in vivo. The cells were isolated 2, 12 or 24 hr post-treatment. ROS production, change in MMP, and 24-hr survival were measured to evaluate oxidative stress and its downstream consequences. Cellular levels of each of these biomarkers varied between animals that were treated at 4 versus 14 days of age (p<0.0001 ROS and MMP; p=0.0033 survival) — because of a singular difference found in P14 animals 12-24 hr after treatment as detailed below.
The level of ROS production by the neurons, as assessed by H2DCF-DA conversion, was significantly different between the P4 and P14 animals. ROS production was also significantly different between the three treatment groups. This was revealed as a main effect of age (p<0.0001, F-factor 19.92, DF 1) and treatment (p=0.0016, F-factor 6.75, DF 2) in the ANOVA. The statistical analysis also revealed a significant interaction between age and treatment (p=0.0216). The post-treatment time interval did not affect production of ROS and this was reflected in the lack of a main effect or interaction of this factor.
Because the effect of treatment on ROS production differed by the age of the animal, as evidenced by the statistically significant age by treatment interaction, change within each age group was investigated. The constitutive level of ROS production was equivalent in H animals at P4 and P14 (Fig. 1). There was elevated ROS production in V and E animals at P14 compared to P4. The difference was significant at 12 and 24 hr post-treatment in the V group and at 24 hr in the E group (Table 1). These analyses found that treatment with either vehicle or ethanol produced a distinct ROS response at P14.
Figure 1.
Ethanol treatment did not increase ROS production in either P4 or P14 animals relative to vehicle controls. Neurons were isolated from handled control (A), vehicle control (B), or ethanol treated (C) animals 2-24 hr post-treatment. The level of ROS was determined by H2DCF-DA conversion to DCF-DA. Increased DCF-DA fluorescence at 535nm reflects an increased ROS level. The experimental means ± SE are illustrated. The least squares means and the results of the statistical comparisons are detailed in Table 1. No effect specific to ethanol treatment was observed. Higher ROS production was found 24 hr after treatment in P14 animals given either vehicle or ethanol when compared to P4 animals or handled controls. Significant compared to *P4, ^H.
Table 1.
Comparison of ROS Levels between Ages by Treatment and Time
| Treatment Group |
Post-Treatment Interval (hr) |
Age | Nominal p-value |
|
|---|---|---|---|---|
| P4 | P14 | |||
| H | 2 | 21.95 (±10.43) |
19.80 (±10.43) |
0.884 |
| 12 | 21.75 (±10.43) |
22.48 (±10.43) |
0.961 | |
| 24 | 21.88 (±10.43) |
23.83 (±10.43) |
0.895 | |
| V | 2 | 27.92 (±6.95) |
42.10 (±6.02) |
0.126 |
| 12 | 20.89 (±6.02) |
54.33 (±6.02) |
0.0001* | |
| 24 | 29.69 (±6.29) |
66.04 (±6.02) |
<0.0001* | |
| E | 2 | 32.89 (±6.95) |
43.33 (±6.02) |
0.258 |
| 12 | 21.31 (±6.02) |
41.43 (±6.02) |
0.020 | |
| 24 | 20.50 (±6.02) |
54.54 (±6.02) |
0.0001* | |
Results are presented as least-squares mean (± standard error).
Significant, compared to adjusted p-value = 0.0056.
Similarly, change within individual treatment groups was evaluated, thus exploring interaction between age and treatment. The results were clear; changes in ROS production based on treatment were due primarily to effects within the P14 group and not the P4 group (Fig. 1). Potential differences between treatment groups for specific combinations of age and post-treatment interval were explored. This revealed that neurons from the P4 animals produced the same amount of ROS regardless of the treatment group and the post-treatment interval. In P14 animals, 12-24 hr after treatment, the three treatment groups produced different amounts of ROS (p=0.0027), with the highest values in the V and E groups (p=0.0006 and p=0.0118 respectively). There was no difference in ROS production between V and E animals at any post-treatment interval. Thus, the effect of treatment and/or age on ROS production by granule neurons was specific to P14 animals and was based on treatment with either vehicle or ethanol.
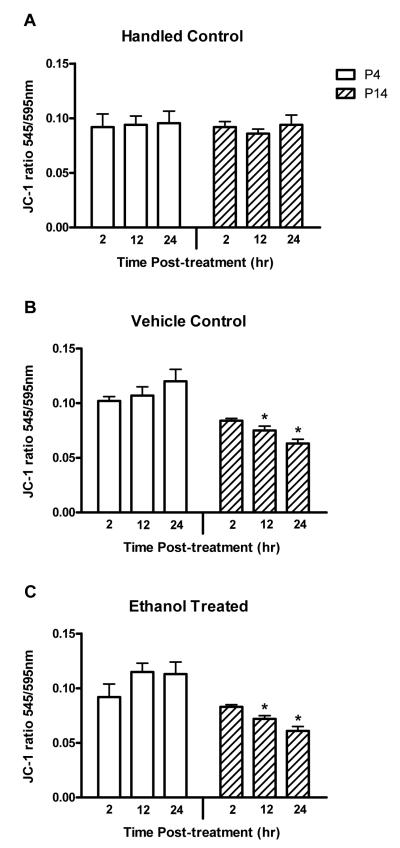
Change in the mitochondrial membrane potential was assayed in aliquots of the same cell isolates used to determine ROS levels. MMP, as assessed with the JC-1 545/595 nm emission intensity ratio, was significantly different between the 4- and 14-day old animals. This was revealed as a main effect in the ANOVA (p<0.0001, F-factor 39.38, DF 1). There was no difference in MMP between the three treatment groups. Likewise, MMP did not vary with the post-treatment time interval. There was no difference in MMP between treatment groups for particular combinations of age and post-treatment interval. Interactions between age and treatment (p=0.0060) and between age and post-treatment interval (p=0.0151) were found. Thus, age and the relationship of age to treatment or to post-treatment interval determined the MMP in isolated granule neurons.
Because the treatment differences in MMP varied based on the age of the animal, change within each age group was investigated. Control H animals in the P4 group and the P14 group exhibited equivalent MMP (Fig. 2). A difference was observed in the V group and in the E group when P4 and P14 animals were compared. P14 animals showed a decrease in the 545/595 ratio, indicating increased mitochondrial polarity and integrity, compared to P4 animals 12-24 hr after treatment with vehicle or ethanol (Table 2).
Figure 2.
Ethanol treatment did not cause depolarization of the mitochondrial membrane in either P4 or P14 animals relative to vehicle controls. Neurons were isolated from handled control (A), vehicle control (B), or ethanol treated (C) animals 2-24 hr post-treatment. Change in the MMP was quantified with the fluorescent dye JC-1. A JC-1 emission shift from 595 nm to 545 nm (increase in the 545/595 ratio) reflects depolarization and deterioration of mitochondrial integrity. The experimental means ± SE is illustrated. The least squares means and the results of the statistical comparisons are detailed in Table 2. No effect specific to ethanol treatment was observed. The MMP was more negative, more stable, in P14 animals compared to P4 animals 12 and 24 hr after they were given either vehicle or ethanol. Significant compared to *P4.
Table 2.
Comparison of Relative MMP between Ages by Treatment and Time
| Treatment Group |
Post-Treatment Interval (hr) |
Age | Nominal p-value |
|
|---|---|---|---|---|
| P4 | P14 | |||
| H | 2 | 0.092 (±0.011) |
0.092 (±0.011) |
0.987 |
| 12 | 0.094 (±0.011) |
0.086 (±0.011) |
0.585 | |
| 24 | 0.096 (±0.011) |
0.094 (±0.011) |
0.923 | |
| V | 2 | 0.102 (±0.007) |
0.084 (±0.006) |
0.072 |
| 12 | 0.107 (±0.006) |
0.075 (±0.006) |
0.0005* | |
| 24 | 0.120 (±0.007) |
0.063 (±0.006) |
<0.0001* | |
| E | 2 | 0.092 (±0.007) |
0.083 (±0.006) |
0.358 |
| 12 | 0.115 (±0.006) |
0.072 (±0.006) |
<0.0001* | |
| 24 | 0.113 (±0.006) |
0.061 (±0.006) |
<0.0001* | |
Results are presented as least-squares mean (± standard error).
Significant, compared to adjusted p-value = 0.0056.
To assess the potential parallel between oxidative stress and neuron survival, aliquots of the same cell isolates used above were placed in culture and their survival was measured 24 hr later. Analysis established that the ability of the isolated cells to survive in culture depended solely on the age of the animal. Better survival was observed (p=0.0033) in 4-day-old animals (0.269 ± 0.085; n=84) compared to 14-day-old animals (0.225 ± 0.097; n=84). Neither the treatment group nor the post-treatment time interval had an influence on neuron survival, either as main effects or in interaction effects.
Discussion
Based on multiple reports, it has been hypothesized that ethanol induces production of ROS and, further, that antioxidants protect neurons from ethanol-induced death by blocking production of ROS (Heaton et al., 2000a; Heaton et al., 2003; Heaton et al., 2002; Henderson et al., 1995; Ramachandran et al., 2003; Siler-Marsiglio et al., 2004; Siler-Marsiglio et al., 2005b). However, other studies have failed to support the hypothesis (Edwards et al., 2002; Grisel and Chen, 2005; Pierce et al., 2006; Smith et al., 2005; Tran et al., 2005). The present study employed a unique combination of features to address the hypothesis. In this study, exposure to ethanol occurred in living animals at either 4 or 14 days of age. Treatment was followed by isolation of fresh, viable neurons over a time course of 2-24 hr and immediate assay of oxidative biomarkers. Previous investigations have analyzed brain homogenates from treated animals or have analyzed cultured cells. Although homogenates provide analyses of mixtures of neurons and glia, and cultures provide analyses of rapid changes in a single cell type, it is important to extend our understanding to the level of individual cell types within the context of the living animal. The design of this study provided not only the complexity of ethanol effects that occur in the whole animal but also provided analysis of neuron-specific consequences.
Based on the hypothesis that ethanol neurotoxicity is mediated by oxidative stress, and given differential granule neuron vulnerability to ethanol in vivo at P4 versus P14, it was predicted that ethanol would generate an increased oxidative response at P4 relative to P14. However, this was not observed. In fact, ethanol did not produce an oxidative response in granule neurons at either age. Ethanol treatment did not increase neuronal ROS production when compared to vehicle control in either P4 or P14 animals. Ethanol did not cause mitochondrial depolarization in neurons from either P4 or P14 animals. Furthermore, the ability of the neurons to survive was not impaired by ethanol at either age.
These results suggest that oxidative stress is not obligate to granule neuron death associated with neonatal ethanol exposure. This was notable based on previous work of other investigators and current hypotheses (Heaton et al., 2000a; Heaton et al., 2003; Heaton et al., 2002; Henderson et al., 1995; Ramachandran et al., 2003; Siler-Marsiglio et al., 2004; Siler-Marsiglio et al., 2005b). However, it is consistent with reports from several investigators suggesting that oxidative stress is not involved in ethanol neurotoxicity (Edwards et al., 2002; Grisel and Chen, 2005; Pierce et al., 2006; Smith et al., 2005; Tran et al., 2005). That being said, the present findings do not exclude the possibility that ethanol produces oxidative stress that contributes to neuronal death. Although the post-treatment sampling intervals were selected to precede and encompass the period of granule neuron apoptosis that occurs in this model (Light et al., 2002a), a different sampling strategy may provide different results. Perhaps the process of tissue isolation, although rapid, altered oxidative events that were occurring in the animal. Glial cells are a significant source of ROS in situ; their removal during the preparation of a purified population of granule neurons alters the neuronal environment relative to the intact brain. Alternatively, perhaps granule neurons are more resistant to pro-oxidative insult than other neuronal populations. It would be interesting to employ the model used in the present study to examine ROS production, MMP, and survival in other neuronal populations following ethanol exposure in vivo.
Although an ethanol-specific effect was not identified, neurons from P14 animals given either vehicle or ethanol produced more ROS 24 hr after treatment than handled controls. However, cellular integrity as assessed by MMP was stabilized 12 and 24 hr after treatment, and cell survival was not altered. No such effect was found in P4 animals. The P14-specific, 24 hr-specific increase in ROS may reflect differential cell vulnerability during tissue processing or reflect unique characteristics within the parenchyma. This model will be useful to further investigate the apparent dissociation of oxidative stress and mitochondrial depolarization in these neurons.
In conclusion, the basic question of cause and effect between oxidative stress and ethanol neurotoxicity has not yet been unraveled. The mechanisms underlying ethanol neuropathology in fetal development are likely multi-faceted and complex. Our current understanding suggests that oxidative stress is one of multiple mechanisms that can contribute to ethanol neurotoxicity. Probing causative mechanisms and understanding their role in fetal alcohol exposure continues to provide new insights, and non-oxidative mechanisms are likely to be uncovered.
Acknowledgements
This study was supported by grants from the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism (CJMK) and Research to Prevent Blindness (JYC).
Abbreviations
- DF
degree of freedom
- E
ethanol treated
- FU
fluorescence units
- H
handled control
- HBSS
Hank’s balanced salt solution
- H2DCF-DA
2′, 7′-dichlorodihydrofluorescein diacetate
- JC-1
5, 5′, 6, 6′-tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazolocarbocyanine iodide
- MMP
mitochondrial membrane potential
- MTT
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- OD
optical density
- P
postnatal day
- ROS
reactive oxygen species
- V
vehicle control
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Permanent neuronal deficits in rats exposed to alcohol during the brain growth spurt. Teratology. 1991;44:147–163. doi: 10.1002/tera.1420440203. [DOI] [PubMed] [Google Scholar]
- Chang JY, Korolev VV, Wang JZ. Cyclic AMP and pituitary adenylate cyclase-activating polypeptide (PACAP) prevent programmed cell death of cultured rat cerebellar granule cells. Neurosci Lett. 1996;206:181–184. doi: 10.1016/s0304-3940(96)12468-x. [DOI] [PubMed] [Google Scholar]
- Chang JY, Korolev VV, Wang JZ. Neurotoxicity of tunicamycin on cultured cerebellar granule cells. Neurotoxicology. 1997;18:129–135. [PubMed] [Google Scholar]
- Chang JY, Liu LZ. 25-Hydroxycholesterol causes death but does not prevent nerve growth factor-induced neurite outgrowth in PC12 cells. Neurochem Int. 1997;31:517–523. doi: 10.1016/s0197-0186(97)00020-x. [DOI] [PubMed] [Google Scholar]
- Chang JY, Liu LZ. Neurotoxicity of cholesterol oxides on cultured cerebellar granule cells. Neurochem Int. 1998;32:317–323. doi: 10.1016/s0197-0186(97)00103-4. [DOI] [PubMed] [Google Scholar]
- Chang JY, Liu LZ. Peroxisome proliferator-activated receptor agonists prevent 25-OH-cholesterol induced c-jun activation and cell death. BMC Pharmacol. 2001;1:10. doi: 10.1186/1471-2210-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Xu XJ, Wands JR. Ethanol inhibits insulin expression and actions in the developing brain. Cell Mol Life Sci. 2005;62:1131–1145. doi: 10.1007/s00018-005-4571-z. [DOI] [PubMed] [Google Scholar]
- Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: comparability of effects in humans and animal models. Neurotoxicol Teratol. 1990;12:231–237. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- Edwards RB, Manzana EJ, Chen WJ. Melatonin (an antioxidant) does not ameliorate alcohol-induced Purkinje cell loss in the developing cerebellum. Alcohol Clin Exp Res. 2002;26:1003–1009. doi: 10.1097/01.ALC.0000021148.70836.75. [DOI] [PubMed] [Google Scholar]
- Garg TK, Chang JY. Oxidative stress causes ERK phosphorylation and cell death in cultured retinal pigment epithelium: Prevention of cell death by AG126 and 15-deoxy-delta 12, 14-PGJ2. BMC Ophthalmol. 2003;3:5. doi: 10.1186/1471-2415-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg TK, Chang JY. 15-deoxy-delta 12, 14-Prostaglandin J2 prevents reactive oxygen species generation and mitochondrial membrane depolarization induced by oxidative stress. BMC Pharmacol. 2004;4:6. doi: 10.1186/1471-2210-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg TK, Chang JY. Methylmercury causes oxidative stress and cytotoxicity in microglia: attenuation by 15-deoxy-delta 12, 14-prostaglandin J2. J Neuroimmunol. 2006;171:17–28. doi: 10.1016/j.jneuroim.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Eilers AT. Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: a stereological study of temporal windows of vulnerability. Alcohol Clin Exp Res. 1997;21:738–744. [PubMed] [Google Scholar]
- Goodlett CR, Marcussen BL, West JR. A single day of alcohol exposure during the brain growth spurt induces brain weight restriction and cerebellar Purkinje cell loss. Alcohol. 1990;7:107–114. doi: 10.1016/0741-8329(90)90070-s. [DOI] [PubMed] [Google Scholar]
- Green JT, Tran T, Steinmetz JE, Goodlett CR. Neonatal ethanol produces cerebellar deep nuclear cell loss and correlated disruption of eyeblink conditioning in adult rats. Brain Res. 2002;956:302–311. doi: 10.1016/s0006-8993(02)03561-8. [DOI] [PubMed] [Google Scholar]
- Grisel JJ, Chen WJ. Antioxidant pretreatment does not ameliorate alcohol-induced Purkinje cell loss in the developing rat cerebellum. Alcohol Clin Exp Res. 2005;29:1223–1229. doi: 10.1097/01.alc.0000171932.13148.cf. [DOI] [PubMed] [Google Scholar]
- Hamre KM, West JR. The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles. Alcohol Clin Exp Res. 1993;17:610–622. doi: 10.1111/j.1530-0277.1993.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Mitchell JJ, Paiva M. Amelioration of ethanol-induced neurotoxicity in the neonatal rat central nervous system by antioxidant therapy. Alcohol Clin Exp Res. 2000a;24:512–518. [PubMed] [Google Scholar]
- Heaton MB, Mitchell JJ, Paiva M. Overexpression of NGF ameliorates ethanol neurotoxicity in the developing cerebellum. J Neurobiol. 2000b;45:95–104. [PubMed] [Google Scholar]
- Heaton MB, Moore DB, Paiva M, Madorsky I, Mayer J, Shaw G. The role of neurotrophic factors, apoptosis-related proteins, and endogenous antioxidants in the differential temporal vulnerability of neonatal cerebellum to ethanol. Alcohol Clin Exp Res. 2003;27:657–669. doi: 10.1097/01.ALC.0000060527.55252.71. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Madorsky I, Siler-Marsiglio K, Shaw G. Effect of bax deletion on ethanol sensitivity in the neonatal rat cerebellum. J Neurobiol. 2006;66:95–101. doi: 10.1002/neu.20208. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Mayer J, Miller R. Ethanol-mediated generation of reactive oxygen species in developing rat cerebellum. Neurosci Lett. 2002;334:83–86. doi: 10.1016/s0304-3940(02)01123-0. [DOI] [PubMed] [Google Scholar]
- Henderson GI, Devi BG, Perez A, Schenker S. In utero ethanol exposure elicits oxidative stress in the rat fetus. Alcohol Clin Exp Res. 1995;19:714–720. doi: 10.1111/j.1530-0277.1995.tb01572.x. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Kane CJM, Brown GJ, Phelan KD. Transforming growth factor-beta2 increases NMDA receptor-mediated excitotoxicity in rat cerebral cortical neurons independently of glia. Neurosci Lett. 1996a;204:93–96. doi: 10.1016/0304-3940(96)12332-6. [DOI] [PubMed] [Google Scholar]
- Kane CJM, Brown GJ, Phelan KD. Transforming growth factor-beta 2 both stimulates and inhibits neurogenesis of rat cerebellar granule cells in culture. Brain Res Dev Brain Res. 1996b;96:46–51. doi: 10.1016/0165-3806(96)00092-2. [DOI] [PubMed] [Google Scholar]
- Kane CJM, Pierce DR, Nyamweya NN, Yang H, Kasmi Y, Mosby R, Serbus DC, Light KE. Nutritional factors modify the inhibition of CNS development by combined exposure to methadone and ethanol in neonatal rats. Pharmacol Biochem Behav. 1997;56:399–407. doi: 10.1016/s0091-3057(96)00239-0. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Scamra C, Hoffman M, Napper RM, Goodlett CR, Greenough WT. Therapeutic effects of complex motor training on motor performance deficits induced by neonatal binge-like alcohol exposure in rats: II. A quantitative stereological study of synaptic plasticity in female rat cerebellum. Brain Res. 2002;937:83–93. doi: 10.1016/s0006-8993(02)02492-7. [DOI] [PubMed] [Google Scholar]
- Li Z, Miller MW, Luo J. Effects of prenatal exposure to ethanol on the cyclin-dependent kinase system in the developing rat cerebellum. Brain Res Dev Brain Res. 2002;139:237–245. doi: 10.1016/s0165-3806(02)00573-4. [DOI] [PubMed] [Google Scholar]
- Light KE, Belcher SM, Pierce DR. Time course and manner of Purkinje neuron death following a single ethanol exposure on postnatal day 4 in the developing rat. Neuroscience. 2002a;114:327–337. doi: 10.1016/s0306-4522(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Light KE, Brown DP, Newton BW, Belcher SM, Kane CJM. Ethanol-induced alterations of neurotrophin receptor expression on Purkinje cells in the neonatal rat cerebellum. Brain Res. 2002b;924:71–81. doi: 10.1016/s0006-8993(01)03224-3. [DOI] [PubMed] [Google Scholar]
- Light KE, Ge Y, Belcher SM. Early postnatal ethanol exposure selectively decreases BDNF and truncated TrkB-T2 receptor mRNA expression in the rat cerebellum. Brain Res Mol Brain Res. 2001;93:46–55. doi: 10.1016/s0169-328x(01)00182-6. [DOI] [PubMed] [Google Scholar]
- Light KE, Kane CJM, Pierce DR, Jenkins D, Ge Y, Brown G, Yang H, Nyamweya N. Intragastric intubation: important aspects of the model for administration of ethanol to rat pups during the postnatal period. Alcohol Clin Exp Res. 1998;22:1600–1606. doi: 10.1111/j.1530-0277.1998.tb03954.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peterson DA, Kimura H, Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem. 1997;69:581–593. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- Maier SE, Miller JA, Blackwell JM, West JR. Fetal alcohol exposure and temporal vulnerability: regional differences in cell loss as a function of the timing of binge-like alcohol exposure during brain development. Alcohol Clin Exp Res. 1999;23:726–734. doi: 10.1111/j.1530-0277.1999.tb04176.x. [DOI] [PubMed] [Google Scholar]
- Miki T, Harris S, Wilce P, Takeuchi Y, Bedi KS. The effect of the timing of ethanol exposure during early postnatal life on total number of Purkinje cells in rat cerebellum. J Anat. 1999;194(Pt 3):423–431. doi: 10.1046/j.1469-7580.1999.19430423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke B, Cortassa S, Aon MA. Mitochondrial ion channels: gatekeepers of life and death. Physiology (Bethesda) 2005;20:303–315. doi: 10.1152/physiol.00020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce DR, Cook CC, Hinson JA, Light KE. Are oxidative mechanisms primary in ethanol induced Purkinje neuron death of the neonatal rat? Neurosci Lett. 2006;400:130–134. doi: 10.1016/j.neulet.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Pierce DR, Goodlett CR, West JR. Differential neuronal loss following early postnatal alcohol exposure. Teratology. 1989;40:113–126. doi: 10.1002/tera.1420400205. [DOI] [PubMed] [Google Scholar]
- Pierce DR, Kane CJM, Serbus DC, Light KE. Microencephaly and selective decreases in cerebellar Purkinje cell numbers following combined exposure to ethanol and methadone during rat brain development. Dev Neurosci. 1997;19:438–445. doi: 10.1159/000111241. [DOI] [PubMed] [Google Scholar]
- Pierce DR, Serbus DC, Light KE. Intragastric intubation of alcohol during postnatal development of rats results in selective cell loss in the cerebellum. Alcohol Clin Exp Res. 1993;17:1275–1280. doi: 10.1111/j.1530-0277.1993.tb05241.x. [DOI] [PubMed] [Google Scholar]
- Pierce DR, West JR. Differential deficits in regional brain growth induced by postnatal alcohol. Neurotoxicol Teratol. 1987;9:129–141. doi: 10.1016/0892-0362(87)90089-4. [DOI] [PubMed] [Google Scholar]
- Pierce DR, Williams DK, Light KE. Purkinje cell vulnerability to developmental ethanol exposure in the rat cerebellum. Alcohol Clin Exp Res. 1999;23:1650–1659. [PubMed] [Google Scholar]
- Ramachandran V, Watts LT, Maffi SK, Chen J, Schenker S, Henderson G. Ethanol-induced oxidative stress precedes mitochondrially mediated apoptotic death of cultured fetal cortical neurons. J Neurosci Res. 2003;74:577–588. doi: 10.1002/jnr.10767. [DOI] [PubMed] [Google Scholar]
- Reyes E, Ott S, Robinson B. Effects of in utero administration of alcohol on glutathione levels in brain and liver. Alcohol Clin Exp Res. 1993;17:877–881. doi: 10.1111/j.1530-0277.1993.tb00857.x. [DOI] [PubMed] [Google Scholar]
- Rosenkranz AR, Schmaldienst S, Stuhlmeier KM, Chen W, Knapp W, Zlabinger GJ. A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescin-diacetate. J Immunol Methods. 1992;156:39–45. doi: 10.1016/0022-1759(92)90008-h. [DOI] [PubMed] [Google Scholar]
- Siler-Marsiglio KI, Paiva M, Madorsky I, Pan Q, Shaw G, Heaton MB. Functional mechanisms of apoptosis-related proteins in neonatal rat cerebellum are differentially influenced by ethanol at postnatal days 4 and 7. J Neurosci Res. 2005a;81:632–643. doi: 10.1002/jnr.20591. [DOI] [PubMed] [Google Scholar]
- Siler-Marsiglio KI, Paiva M, Madorsky I, Serrano Y, Neeley A, Heaton MB. Protective mechanisms of pycnogenol in ethanol-insulted cerebellar granule cells. J Neurobiol. 2004;61:267–276. doi: 10.1002/neu.20057. [DOI] [PubMed] [Google Scholar]
- Siler-Marsiglio KI, Pan Q, Paiva M, Madorsky I, Khurana NC, Heaton MB. Mitochondrially targeted vitamin E and vitamin E mitigate ethanol-mediated effects on cerebellar granule cell antioxidant defense systems. Brain Res. 2005b;1052:202–211. doi: 10.1016/j.brainres.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Smith AM, Zeve DR, Grisel JJ, Chen WJ. Neonatal alcohol exposure increases malondialdehyde (MDA) and glutathione (GSH) levels in the developing cerebellum. Brain Res Dev Brain Res. 2005;160:231–238. doi: 10.1016/j.devbrainres.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 2002;12:856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention and Treatment. Institute of Medicine, National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Sun AY, Ingelman-Sundberg M, Neve E, Matsumoto H, Nishitani Y, Minowa Y, Fukui Y, Bailey SM, Patel VB, Cunningham CC, et al. Ethanol and oxidative stress. Alcohol Clin Exp Res. 2001;25:237S–243S. doi: 10.1097/00000374-200105051-00038. [DOI] [PubMed] [Google Scholar]
- Tran TD, Jackson HD, Horn KH, Goodlett CR. Vitamin E does not protect against neonatal ethanol-induced cerebellar damage or deficits in eyeblink classical conditioning in rats. Alcohol Clin Exp Res. 2005;29:117–129. doi: 10.1097/01.alc.0000150004.53870.e1. [DOI] [PubMed] [Google Scholar]
- Warren KR, Bast RJ. Alcohol-related birth defects: an update. Public Health Rep. 1988;103:638–642. [PMC free article] [PubMed] [Google Scholar]
- West JR, Chen WJ, Pantazis NJ. Fetal alcohol syndrome: the vulnerability of the developing brain and possible mechanisms of damage. Metab Brain Dis. 1994;9:291–322. doi: 10.1007/BF02098878. [DOI] [PubMed] [Google Scholar]