Abstract
Background:
Studies of octreotide in hepatocellular carcinoma have yielded conflicting results. Since past studies have excluded patients with highly advanced disease and given the fact that octreotide offers several potential physiologic benefits in patients with advanced cirrhosis, such as improving renal physiology and decreasing portal venous pressure, we designed a trial to examine the survival of patients with both advanced HCC and advanced cirrhosis as defined by a CLIP score of 3 or higher.
Patients and Methods:
The study was designed as a phase-II, multicenter trial, enrolling patients with advanced HCC in three tertiary care academic centers in the United States. The primary objective was to verify whether long-acting octreotide will extend median survival from 5 months to 8.75 months for patients with CLIP scores of 3 or higher, representing a 75% increase in median survival time. Secondary objectives included assessing safety and tolerability in this patient population.
Results:
Twenty-two patients were enrolled from 2003 to 2005. The mean age was 66, with the majority of patients being men. The median CLIP score was 4 with a median KPS of 80%. Ten of 22 patients died without evidence of progression of HCC. The median TTP was 5.7 months (95% confidence interval [CI], 2.8–10.7). The median PFS time was approximately 3 months (95% CI, 1.7–5.7). The median OS time was 4.5 months (95% CI, 2.3–8) and therefore did not meet the established primary end point. Six of 22 patients achieved an OS of greater than 10 months. One patient experienced a radiographic partial response.
Conclusions:
Long-acting octreotide was not associated with a survival benefit in patients with significant liver disease related to HCC. The identification of one patient with disease regression and a subgroup of patients with significantly greater survival underscores the need to gain a better understanding of the role of somatostatin receptors on HCC cells before further clinical testing of this drug in HCC patients.
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide; it is the fifth most common malignancy in men and the eighth most common in women.1 In the United States alone, over 21,000 new cases of HCC were projected for 2008, with an estimated 18,000 deaths expected.2 Until recently, no standard therapy was available for patients with advanced HCC. Systemic chemotherapy has generally been ineffective, and until the introduction of sorafenib, an inhibitor of vascular endothelial growth factor (VEGF)-receptor and raf-kinase, no therapy had shown a survival benefit.3 In two large randomized studies, sorafenib has been tested in patients with well-preserved liver function; however, therapies applicable to patients with poorer liver function and interventions targeting other pathways are needed.
Somatostatin receptors have been variably identified on HCC cells.4 In vitro and animal tumor model studies have shown inhibition of tumor growth by somatostatin in human tumor cell lines, including human HCC.5,6 Octreotide, a semisynthetic somatostatin analog used for the management of neuroendocrine tumors, may have antitumor effects against HCC cells.7,8 A small randomized study suggested a survival benefit over best supportive care for HCC patients treated with short-acting octreotide (median overall survival [OS], 4 vs. 11 months).9 A second randomized trial including 127 patients with cirrhosis and advanced HCC who overexpressed somatostatin receptors compared long-acting octreotide with placebo. Again, a significantly higher OS was noted in the group that received long-acting octreotide, though no reduction in alpha-fetoprotein (AFP) levels or disease burden occurred.10 These positive results, however, were not confirmed in three separate randomized, controlled, double-blind trials of octreotide vs. placebo.11–13
Doses of long-acting octreotide were similar across most trials; ie, 30 mg given intramuscularly every 4 weeks. Reasons for the discordant results are likely multifactorial, attributable to differences in sample size and study design as well as selection criteria. For example, Barbare and colleagues, with 272 enrolled patients, conducted the largest of the randomized studies and their results failed to demonstrate a survival benefit with octreotide,12 whereas those trials showing benefit have been considerably smaller. The Dimitroulopoulos study of octreotide in HCC enrolled 127 patients; however, only 61 patients were eligible to be randomized to treatment or placebo based on overexpression of somatostatin receptors.10 A criticism of the initial trial by Kouroumalis of short-acting octreotide has been the absence of a placebo comparator arm to octreotide.9
Given these disparate results in trials that excluded patients with highly advanced disease (CLIP [Cancer of the Liver Italian Program] score > 3 or Okuda stage III) and the fact that octreotide offers several potential physiologic benefits in patients with advanced cirrhosis, such as improving renal physiology and decreasing portal venous pressure,14–16 we designed a trial to examine the survival of patients with both advanced HCC and advanced cirrhosis as defined by a CLIP score of 3 or higher, a population with essentially no treatment options.
PATIENTS AND METHODS
Study Design
The study was designed as a phase-II, multicenter trial, enrolling patients with advanced HCC in three tertiary care academic centers in the United States. The primary objective was to verify that long-acting octreotide will extend median survival from 5 months to 8.75 months for patients with CLIP scores of 3 or higher, representing a 75% increase in median survival time. Secondary objectives included assessing safety and tolerability in this patient population. Inclusion criteria were advanced HCC confirmed either by tissue diagnosis or AFP > 1000 ng/mL with compatible mass on imaging study, not a candidate for transplant, resection, or ablation, CLIP score ≥ 3, alanine aminotransferase/aspartate aminotransferase (AST/ALT) < 5 × upper limit of normal (ULN), total bilirubin < 5 × ULN, platelet count > 50 × 109/L, creatinine < 2 × ULN, prothrombin time < 15 seconds, hemoglobin greater than or equal to 8.5 g/dL, and a life expectancy of at least 8 weeks.
Patients were excluded from the study if they had fibrolammelar HCC, active variceal bleeding during the prior 3 months, Karnofsky performance status (KPS) < 60%, grade 3/4 encephalopathy, pregnant/breast feeding, presence of clinically apparent central nervous system metastases or carcinomatous meningitis, patients who are receiving concomitant chemotherapy, radiotherapy or immunotherapy, or ongoing ethanol or intravenous drug abuse. Patients were allowed any number of prior therapies except previous treatment with octreotide. The CLIP scoring system was used to stratify patients because it accounts for liver function as well as HCC characteristics, including portal vein thrombosis and AFP level. The Childs-Pugh staging system was not used in the inclusion criteria. All patients provided written informed consent approved by the respective institutions’ Institutional Review Boards.
Follow Up
Baseline assessment included computed tomography (CT) scans with contrast or magnetic resonance imaging (MRI) of the abdomen, chest x-ray, electrocardiogram, complete history and physical exam with documentation of KPS. Laboratory assessments included complete blood cell (CBC) count, serum electrolyte levels, blood urea nitrogen (BUN) and creatinine, serum total bilirubin, AST, ALT, alkaline phosphatase, albumin, glucose, prothrombin and partial thromboplastin times, and serum calcium, magnesium, and phosphorus. Imaging was repeated at 12, 24, and 36 weeks and laboratory data were collected every 4 weeks.
Treatment Schedule
Tolerability of octreotide was assessed with subcutaneous octreotide 200 μg three times daily for 1 week (dose reduction to 150 μg was allowed if poorly tolerated). After an initial run-in trial of subcutaneous octreotide, long-acting octreotide was begun at 30 mg IM monthly (or 20 mg monthly for those requiring dose reduction of octreotide). Treatment was continued until day 21. Patients were treated with long-acting octreotide every 28 days until discontinued due to toxicity, symptomatic progression, or patient/physician discretion. Treatment beyond RECIST (Response Evaluation Criteria in Solid Tumors) progression was allowed.
Statistical Analysis
A sample size of 48 was initially planned based on increasing median OS to 8.75 months compared to an expected median survival of 5 months with no therapy (α = 0.05, β = 0.10) An interim analysis was originally planned in 24 patients. However, secondary to relatively slow enrollment and the release of data from the randomized trial of Barbare et al12 showing absence of benefit, the study was analyzed early (17 patients accrued) with the potential to close the trial if the likelihood of achieving the target survival was low.
Enrollment began in late 2003, and was significantly slower than originally planned (partly due to a large number of screen failures and withdrawals after consent due to disease-related complications). Overall survival was defined as time from study enrollment to death due to any cause. Progression-free survival (PFS) was defined as the time from enrollment to HCC disease progression in accordance with RECIST criteria or death, while time to progression (TTP) was defined as time from study entry to progression or death due to HCC, censoring other causes of death.
RESULTS
Patient Characteristics
Twenty-two patients were enrolled from 2003 to 2005. Patient characteristics are described in Table 1. The mean age was 66, with the majority of patients being men. The median CLIP score was 4 with a median KPS of 80% (Table 1).
Table 1.
Patient characteristics
| N = 22 | |
|---|---|
| Age (mean) | 66 (46–82) |
| Male | 20 (90%) |
| CLIP Score | 4.0 (mean) |
| 3 | 9 (40.9%) |
| 4 | 6 (27.3%) |
| 5 | 3 (13.6%) |
| 10 | 1 (6.2%) |
| Missing | 3 |
| Karnofsky performance status | 80 (median) |
| 100 | 2 (9.1%) |
| 90 | 5 (22.7%) |
| 80 | 9 (40.1%) |
| 70 | 3 (13.6%) |
| 60 | 1 (4.5%) |
| Missing | 2 |
| Prior treatment | 6 (27%) |
| TACE | 1 |
| Capecitabine | 2 |
| Doxorubicin | 1 |
| Not recorded | 2 |
Outcomes
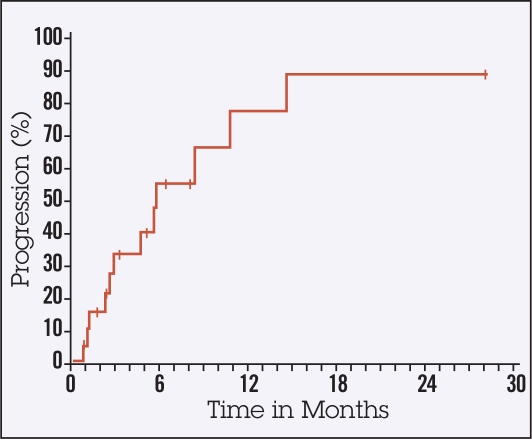
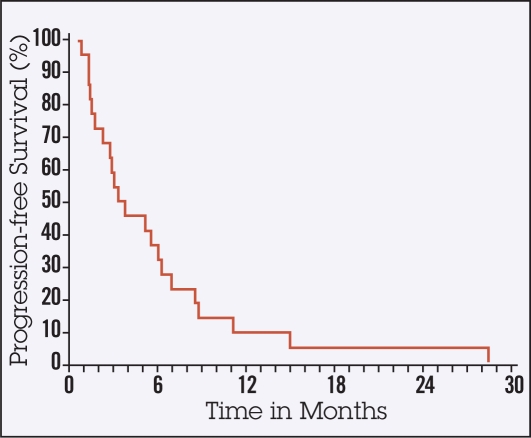
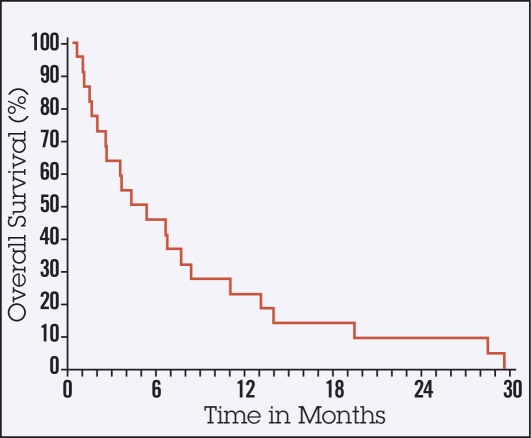
Ten of 22 patients died without evidence of progression of HCC. The median TTP was 5.7 months (95% confidence interval [CI], 2.8–10.7, Figure 1). The median PFS time was approximately 3 months (95% CI, 1.7–5.7, Figure 2). The median OS time was 4.5 months (95% CI, 2.3–8, Figure 3) and therefore did not meet the established primary end point. However, 6 out of 22 patients achieved an OS of greater than 10 months. All 22 patients enrolled have died (Figure 1). One patient experienced a radiographic partial response.
Figure 1.
Time to progression.
Figure 2.
Progression-free survival.
Figure 3.
Overall survival
Toxicity
Long-acting octreotide appeared to be well tolerated with manageable toxicities. Common grade-3 toxicities included hypoglycemia, elevation in transaminases or bilirubin, confusion, and abdominal pain/cramping. There were seven episodes of grade 4 toxicities, including small-bowel obstruction, liver dysfunction, hepatorenal syndrome, cord compression with lower extremity paralysis, and hepatic encephalopathy. Of note, hypoglycemia was the most common grade 3/4 toxicity, accounting for 17% of all grade 3/4 toxicities (Table 2).
Table 2.
All grade 3/4 toxicities
| Toxicity | Frequency (%) |
|---|---|
| Abdominal pain or cramping | 2 (6.67) |
| Alkaline phosphatase elevation | 1 (3.33) |
| Bilirubin elevation | 2 (6.67) |
| Thrombocytopenia | 1 (3.33) |
| Fatigue | 1 (3.33) |
| Dizziness/lightheadedness | 1 (3.33) |
| Bloating | 1 (3.33) |
| Hyperglycemia | 1 (3.33) |
| Hypoglycemia | 5 (16.67) |
| Hyponatremia | 1 (3.33) |
| Lymphatics | 1 (3.33) |
| Ascites | 1 (3.33) |
| Transaminitis or Confusion | 4 (13.33) |
| Hepatic Encephalopathy | 2 (6.67) |
| Seizure(s) | 1 (3.33) |
| Small bowel obstruction | 1 (3.33) |
| Liver dysfunction/hepatorenal syndrome | 2 (6.67) |
| Cord compression | 1 (3.33) |
| Speech impairment (eg, dysphasia or aphasia) | 1 (3.33) |
DISCUSSION
We report here the results of a phase-II trial designed to evaluate the efficacy of long-acting octreotide in previously treated patients with HCC who were no longer candidates for other therapies. Interim analysis after accrual of 22 patients revealed that there was no overall survival benefit to long-acting octreotide according to predefined criteria. Median OS was 4.5 months and historically, patients with CLIP scores greater than 3 and 4 have a median OS of 4.5 and 2.5 months, respectively.14 It is interesting to note that the TTP was longer than OS, characterizing the fact that this was a significantly ill population who died of causes other than directly attributable to HCC.
In their randomized controlled trial of 272 patients with advanced HCC treated with long-acting octreotide or placebo, Barbare et al reported a median OS of 6.5 months in the octreotide arm and 7.3 months in the placebo arm.12 However, in contrast to our study enrolling patients with a mean CLIP score of 4, they excluded those with CLIP scores > 3. We did note that one patient had a partial radiographic response to long-acting octreotide and 6 of 22 patients achieved an OS of greater than 10 months.
The subgroup of patients who appeared to have the longest OS were of Asian descent with a history of hepatitis B viral infection. Two trials that had reported positive results evaluated somatostatin receptor status with either scintigraphy or immunohistochemistry as part of the inclusion criteria.9,10 However, other trials have shown no correlation of outcomes with somatostatin receptor status.17 It is possible that a small subset of patients with HCC may benefit from treatment with long-acting octreotide. Unfortunately, such predictive factors are not currently known; thus, it is not possible at present to identify patients likely to respond to such treatment.
Long-acting octreotide demonstrated an acceptable toxicity profile; however, it was associated with grade 3 and 4 hypoglycemia requiring discontinuation of the drug. Barbare et al, while excluding patients with hyperglycemia or hypoglycemia, reported symptomatic grade 3 or 4 hyperglycemia in 7% of patients in the octreotide arm compared to 5% in the placebo arm.12 While hyperglycemia is seen more commonly with octreotide, hypoglycemia has also been reported in 2%–4% of patients. The mechanism behind hypoglycemia in this setting is not well described. It is surmised that patients with more advanced liver disease could be at higher risk of drug-associated hypoglycemia or that hypoglycemia occurs because of the underlying liver disease in these patients.
Treatment options in advanced HCC remain limited, though the results of the trial by the SHARP (Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol) investigators demonstrated a 10.7month median OS with sorafenib compared to 7.9 months with placebo.3 This has led to sorafenib becoming the standard of care in a non-trial setting for advanced HCC. Strictly speaking, experience with sorafenib has been limited to mostly Childs-Pugh A patients with some evidence in class B. However, the safety and benefit of this targeted therapy in patients with significant hepatic dysfunction remains unproven.
In summary, long-acting octreotide was not associated with a survival benefit in patients with significant liver disease related to HCC. The identification of one patient with disease regression and a subgroup of patients with significantly greater survival underscores the need to gain a better understanding of the role of somatostatin receptors on HCC cells before further clinical testing of this drug in HCC patients.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1.Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005, National Cancer InstituteBethesda, MD, http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER web site, 2008
- 3.Llovet J, Ricci S, Mazzaferro V, et al. SHARP Investigator: Sorafenib improves survival in advanced Hepatocellular Carcinoma (HCC): Results of a Phase III randomized placebo-controlled trial (SHARP trial). 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 2007;25:18S. (abstr LBA1) [Google Scholar]
- 4.Reubi JC, Zimmermann A, Jonas S, et al. Regulatory peptide receptors in human hepatocellular carcinomas. Gut. 1999;45:766–774. doi: 10.1136/gut.45.5.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papalampros E, Felekouras ES, Filis K, et al. Liver pathology and cell proliferation after octreotide administration following partial hepatectomy in rats: an experimental study. Dig Dis Sci. 2002;47:1953–1958. doi: 10.1023/a:1019687804859. [DOI] [PubMed] [Google Scholar]
- 6.Schindel DT, Grosfeld JL.Hepatic resection enhances growth of residual intrahepatic and subcutaneous hepatoma, which is inhibited by octreotide J Pediatr Surg 32995–997.discussion 997–998, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Liu HL, Huo L, Wang L. Octreotide inhibits proliferation and induces apoptosis of hepatocellular carcinoma cells. Acta Pharmacol Sin. 2004;25:1380–1386. [PubMed] [Google Scholar]
- 8.Oberg K. Management of neuroendocrine tumours. Ann Oncol. 2004;15(suppl 4):293–298. doi: 10.1093/annonc/mdh942. [DOI] [PubMed] [Google Scholar]
- 9.Kouroumalis E, Skordilis P, Thermos K, et al. Treatment of hepatocellular carcinoma with octreotide: a randomised controlled study. Gut. 1998;42:442–447. doi: 10.1136/gut.42.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimitroulopoulos D, Xinopoulos D, Tsamakidis K, et al. Long acting octreotide in the treatment of advanced hepatocellular cancer and overexpression of somatostatin receptors: randomized placebo-controlled trial. World J Gastroenterol. 2007;13:3164–3170. doi: 10.3748/wjg.v13.i23.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker G, Allgaier H-P, Olschewski M, et al. Long-acting octreotide versus placebo for treatment of advanced HCC: a randomized controlled double-blind study. Hepatology. 2007;45:9–15. doi: 10.1002/hep.21468. [DOI] [PubMed] [Google Scholar]
- 12.Barbare JC, Bouché O, Bonnetain F, et al. Treatment of advanced hepatocellular carcinoma with long-acting octreotide: preliminary results of a randomized placebo-controlled trial (FFCD-ANGH 2001-01 CHOC). 2005 ASCO Annual Meeting Proceedings. J Clin Oncol. 2005;23:16. (abstr 4036) [Google Scholar]
- 13.Yuen MF, Poon RT, Lai CL, et al. A randomized placebo-controlled study of long-acting octreotide for the treatment of advanced hepatocellular carcinoma. Hepatology. 2002;36:687–691. doi: 10.1053/jhep.2002.35071. [DOI] [PubMed] [Google Scholar]
- 14.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 15.Reynaert H, Geerts A. Pharmacological rationale for the use of somatostatin and analogues in portal hypertension. Aliment Pharmacol Ther. 2003;18:375–386. doi: 10.1046/j.1365-2036.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- 16.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Cebon J, Findlay M, Hargreaves C, et al. Somatostatin receptor expression, tumour response, and quality of life in patients with advanced hepatocellular carcinoma treated with long-acting octreotide. Br J Cancer. 2006;95:853–861. doi: 10.1038/sj.bjc.6603325. [DOI] [PMC free article] [PubMed] [Google Scholar]