Abstract
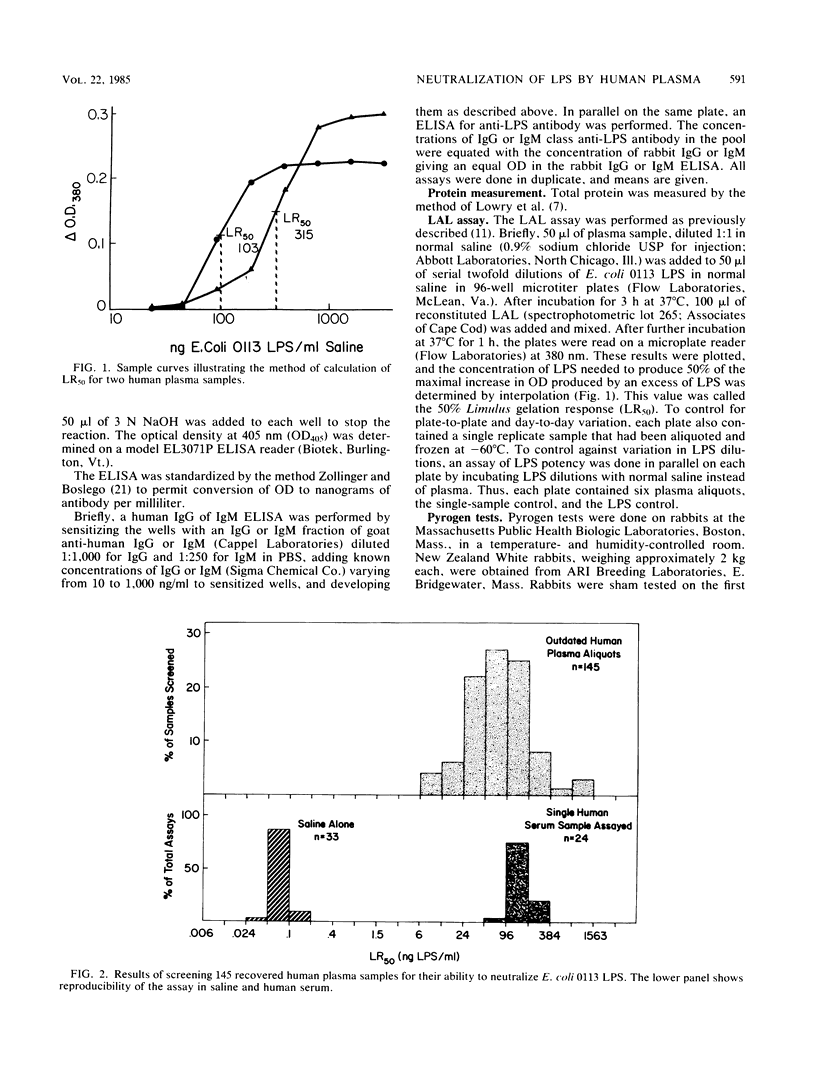
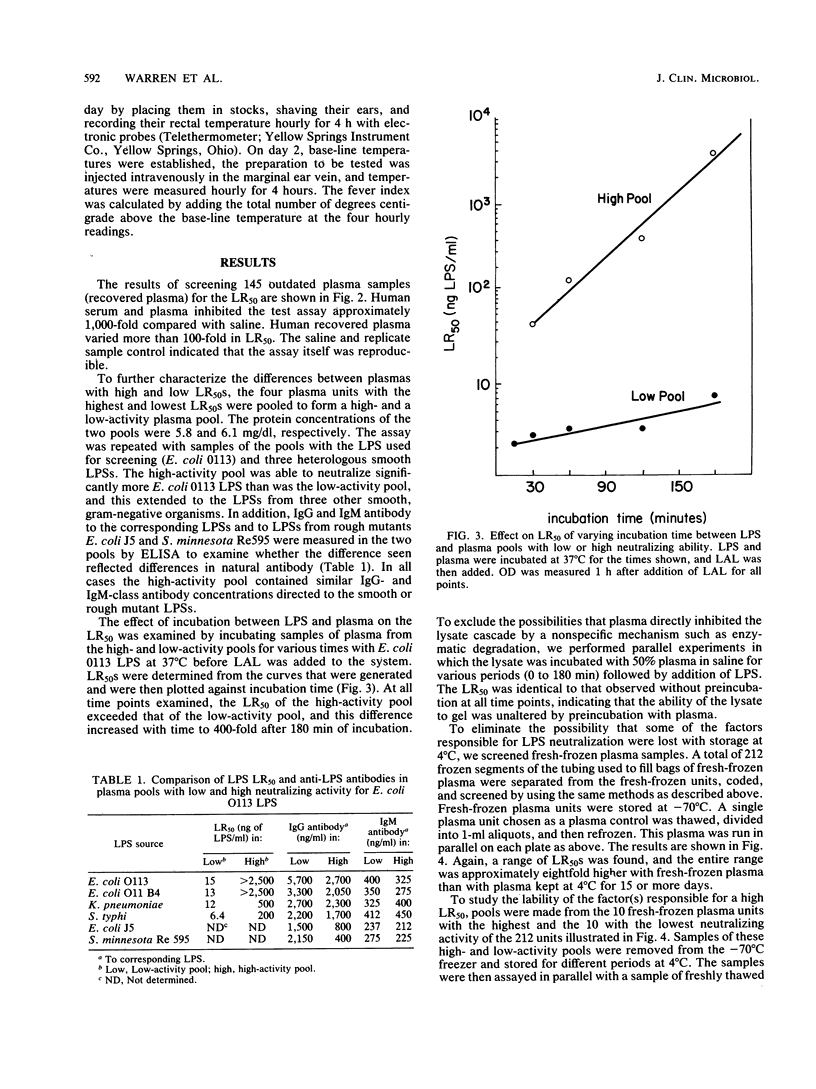
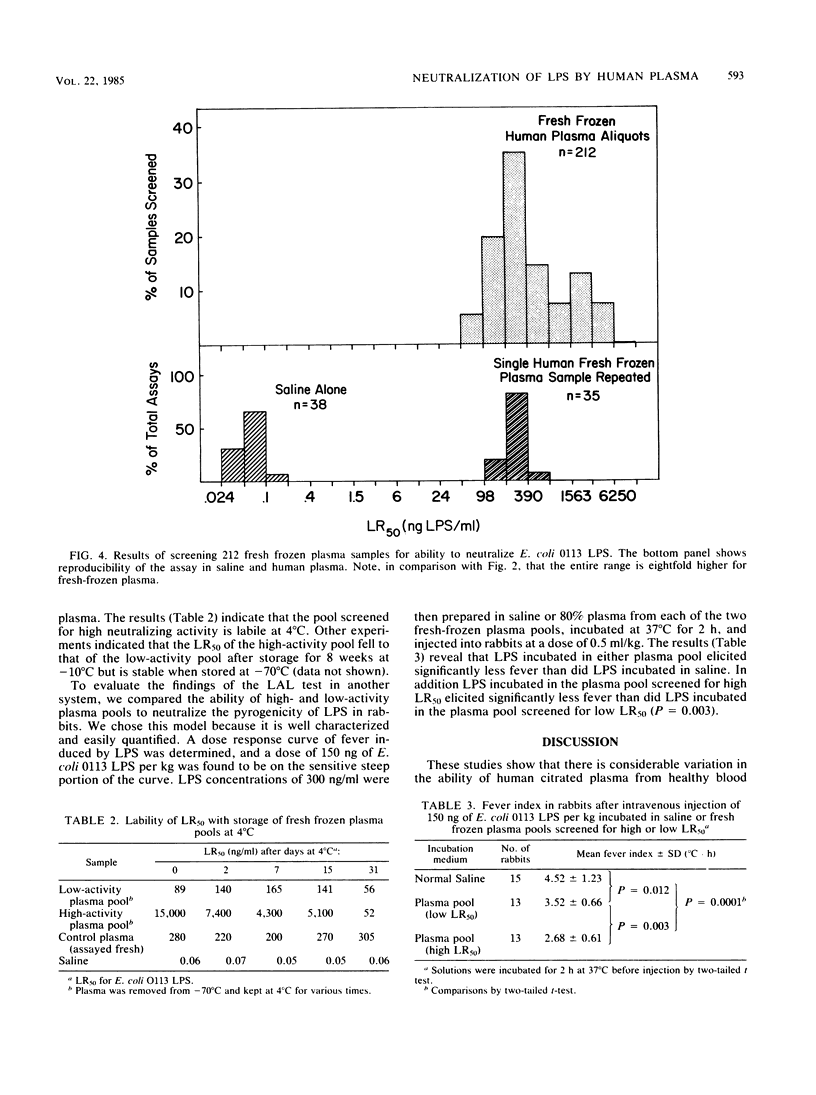
To quantify the neutralization of bacterial lipopolysaccharide (LPS) by human plasma, dilutions of Escherichia coli O113 LPS were incubated with plasma, followed by the addition of Limulus amebocyte lysate (LAL). The reaction between the LPS and LAL was monitored spectrophotometrically, and the concentration of LPS resulting in 50% lysate response (LR50) was determined. Analysis of 145 outdated plasma samples yielded a range of LR50 between 6 and 1,500 ng/ml. Pools of plasma with high and low LR50 were prepared. The pool with high LR50 neutralized 166-fold more E. coli 0113 LPS, 190-fold more E. coli 0111B4 LPS, 42-fold more Klebsiella pneumoniae LPS, and 29-fold more Salmonella typhimurium LPS than did the pool with low LR50. Each pool had similar immunoglobulin G (IgG) and IgM antibody levels to homologous LPS, measured by an enzyme-linked immunosorbent assay. Analysis of 212 fresh-frozen plasma units revealed a range of LR50 between 48 and 6,000 ng/ml. Incubation of LPS in a pool of fresh-frozen plasma with high LR50 elicited significantly less fever in the rabbit pyrogen test than did LPS incubated in plasma with low LR50 (fever index, 2.68 +/- 0.61 degrees C X h and 3.52 +/- 0.66 degrees C X h, respectively; P = 0.003). We conclude that there is a 100-fold range in the endotoxin-neutralizing capacity of human plasma and that this variation is not due to LPS-specific IgG or IgM antibodies. Further investigations are needed to determine whether differing susceptibility of patients to the effects of LPS is due to differences in the endotoxin-neutralizing capacity of their plasma and whether plasma screened for high endotoxin-neutralizing capacity may be therapeutically useful in endotoxemia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis C. E., Ziegler E. J., Arnold K. F. Neutralization of meningococcal endotoxin by antibody to core glycolipid. J Exp Med. 1978 Apr 1;147(4):1007–1017. doi: 10.1084/jem.147.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Greisman S. E., Young E. J., DuBuy B. Mechanisms of endotoxin tolerance. 8. Specificity of serum transfer. J Immunol. 1973 Nov;111(5):1349–1360. [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A., Goralnick S., Osborn M. J. Isolation from human serum of an inactivator of bacterial lipopolysaccharide. Am J Pathol. 1977 Sep;88(3):559–574. [PMC free article] [PubMed] [Google Scholar]
- LANDY M., SKARNES R. C., ROSEN F. S., TRAPANI R. J., SHEAR M. J. Inactivation of biologically active endotoxic polysaccharides by fresh human serum. Proc Soc Exp Biol Med. 1957 Dec;96(3):744–747. doi: 10.3181/00379727-96-23596. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin J., Poore T. E., Young N. S., Margolis S., Zauber N. P., Townes A. S., Bell W. R. Gram-negative sepsis: detection of endotoxemia with the limulus test. With studies of associated changes in blood coagulation, serum lipids, and complement. Ann Intern Med. 1972 Jan;76(1):1–7. doi: 10.7326/0003-4819-76-1-1. [DOI] [PubMed] [Google Scholar]
- McCabe W. R. Immunization with R mutants of S. Minnesota. I. Protection against challenge with heterologous gram-negative bacilli. J Immunol. 1972 Mar;108(3):601–610. [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Dietschy J. M. Binding of Salmonella typhimurium lipopolysaccharides to rat high-density lipoproteins. Infect Immun. 1981 Dec;34(3):835–843. doi: 10.1128/iai.34.3.835-843.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky T. J., Roslansky P. F., Siber G. R., Warren H. S. Turbidimetric method for quantifying serum inhibition of Limulus amoebocyte lysate. J Clin Microbiol. 1985 Feb;21(2):211–216. doi: 10.1128/jcm.21.2.211-216.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudbach J. A., Anacker R. L., Haskins W. T., Johnson A. G., Milner K. C., Ribi E. Physical aspects of reversible inactivation of endotoxin. Ann N Y Acad Sci. 1966 Jun 30;133(2):629–643. doi: 10.1111/j.1749-6632.1966.tb52394.x. [DOI] [PubMed] [Google Scholar]
- Schultz D. R., Becker E. L. The alteration of endotoxin by postheparin plasma and its purified fractions. I. Comparison of the ability of guinea pig postheparin and normal plasma to detoxify endotoxin. J Immunol. 1967 Mar;98(3):473–481. [PubMed] [Google Scholar]
- Stumacher R. J., Kovnat M. J., McCabe W. R. Limitations of the usefulness of the Limulus assay for endotoxin. N Engl J Med. 1973 Jun 14;288(24):1261–1264. doi: 10.1056/NEJM197306142882402. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Isolation and characterization of a bacterial lipopolysaccharide-high density lipoprotein complex formed in rabbit plasma. J Clin Invest. 1981 Mar;67(3):827–837. doi: 10.1172/JCI110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIOKA M., JOHNSON A. G. Characteristics of endotoxin altering fractions derived from normal human serum. J Immunol. 1962 Sep;89:326–335. [PubMed] [Google Scholar]
- Young L. S. Opsonizing antibodies, host factors, and the limulus assay for endotoxin. Infect Immun. 1975 Jul;12(1):88–92. doi: 10.1128/iai.12.1.88-92.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Stevens P., Ingram J. Functional role of antibody against "core" glycolipid of Enterobacteriaceae. J Clin Invest. 1975 Oct;56(4):850–861. doi: 10.1172/JCI108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Boslego J. W. A general approach to standardization of the solid-phase radioimmunoassay for quantitation of class-specific antibodies. J Immunol Methods. 1981;46(2):129–140. doi: 10.1016/0022-1759(81)90130-7. [DOI] [PubMed] [Google Scholar]


