Abstract
Background. In the diabetic kidney, stimulation of mitogen-activated protein kinases (MAPKs) leads to extracellular matrix protein synthesis. In the proximal tubule, angiotensin-(1–7) [Ang-(1–7)] blocks activation of MAPKs by angiotensin II. We studied the effect of Ang-(1–7) on signalling responses in LLC-PK1 cells in normal (5 mM) or high (25 mM) glucose.
Methods. The p38 MAPK was assayed by immunoblot, Src homology 2-containing protein-tyrosine phosphatase-1 (SHP-1) activity was measured after immunoprecipitation, cell protein synthesis was determined by [3H]-leucine incorporation and transforming growth factor-β1 (TGF-β1), fibronectin and collagen IV were assayed by immunoblots and/or ELISA.
Results. High glucose stimulated p38 MAPK. This response was inhibited by Ang-(1–7) in a concentration-dependent fashion, an effect reversed by the receptor Mas antagonist A-779. Ang-(1–7) increased SHP-1 activity, via the receptor Mas. An inhibitor of tyrosine phosphatase, phenylarsine oxide, reversed the inhibitory effect of Ang-(1–7) on high glucose-stimulated p38 MAPK. Ang-(1–7) inhibited high glucose-stimulated protein synthesis, and blocked the stimulatory effect of glucose on TGF-β1. Conversely, Ang-(1–7) had no effect on glucose-stimulated synthesis of fibronectin or collagen IV.
Conclusions. These data indicate that in proximal tubular cells, binding of Ang-(1–7) to the receptor Mas stimulates SHP-1, associated with the inhibition of glucose-stimulated p38 MAPK. Ang-(1–7) selectively inhibits glucose-stimulated protein synthesis and TGF-β1. In diabetic nephropathy, Ang-(1–7) may partly counteract the profibrotic effects of high glucose.
Keywords: angiotensin, diabetic nephropathy, proximal tubule, receptor Mas, renin–angiotensin system
Introduction
Diabetic nephropathy is a leading cause of end-stage renal disease world-wide. The pathogenesis of this condition is complex, but exposure of cells to high glucose activates a variety of signalling pathways, including mitogen-activated protein kinases (MAPKs), reactive oxygen species and production of cytokines such as transforming growth factor-β1 (TGF-β1) and extracellular matrix proteins, which promote progressive glomerulosclerosis and tubulointerstitial fibrosis [1].
The proximal tubule contains all components of the renin–angiotensin system (RAS), and intratubular levels of angiotensin II (Ang II) exceed circulating levels by 100- to 1000-fold [2,3]. Diabetes stimulates intrarenal production of Ang II, which causes progressive nephron injury, as suggested in animal models and human studies that have demonstrated the beneficial effects of angiotensin-converting enzyme (ACE) inhibitors and AT1 receptor blockers [4].
The discovery of angiotensin-converting enzyme 2 (ACE2) has added complexity to the role of the RAS in diabetic nephropathy. ACE2 is a monocarboxypeptidase that is not blocked by ACE inhibitors, and cleaves Ang II to the heptapeptide fragment angiotensin-(1–7) [Ang-(1–7)] [5,6]. ACE2 is highly expressed in the kidney, with abundant levels in the proximal tubule, where it generates Ang-(1–7) [7–9]. Ang-(1–7) is present in the kidney at concentrations comparable to Ang II [10] and is associated with vasodilatation and modulation of sodium and water transport [11–14]. Some of these effects occur via binding to the Mas protein, identified as the receptor for Ang-(1–7) [15]. Information is limited on the signalling pathways associated with Ang-(1–7) in the kidney. However, in proximal tubular cells Ang-(1–7) inhibits Ang II-stimulated MAPK activation, via binding to the receptor Mas [16]. Ang-(1–7) may thereby counterbalance the effects of Ang II, and could serve a protective role in states of RAS activation, such as diabetic nephropathy.
In LLC-PK1 proximal tubular cells, high glucose activates MAPKs, which may be involved in the downstream stimulation of responses associated with nephron injury [17]. We therefore determined the effect of Ang-(1–7) on high glucose-mediated signalling responses in LLC-PK1 cells. Our data reveal that Ang-(1–7) blocks high glucose-stimulated p38 MAPK, an effect associated with activation of the Src-homology 2-containing protein-tyrosine phosphatase-1 (SHP-1). Moreover, we show that Ang-(1–7) inhibits high glucose-stimulated cell hypertrophy and TGF-β1 production.
Materials and methods
Cell culture
The LLC-PK1 cell line (American Type Culture Collection (ATCC), Manassas, VA, USA) was used for all studies. The cells were grown at 37°C in a humidified environment of 5% CO2 in Dulbecco's modified Eagle's medium nutrient mixture-Ham's F-12 (DMEM/F-12), supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml). The cells were rendered quiescent 24 h prior to experimentation by removal of serum, or, in cases where the experiments were performed after 48–72 h, by reduction of FBS to 0.5%, to prevent cell detachment.
Immunoblotting
Subconfluent LLC-PK1 cells were exposed to normal glucose (5 mM d-glucose) or high glucose (25 mM d-glucose) in media various times, with or without Ang-(1–7) (Bachem Bioscience Inc., King of Prussia, PA, USA), or bradykinin (Sigma-Aldrich, St Louis, MO, USA) for 30 min, followed by immunoblot assays for phosphorylated p38 MAPK. In all experiments, l-glucose (20 mM) was added to the normal glucose media to control for osmolality. In some experiments, the cells were preincubated for 15–30 min with either the AT1 receptor antagonist losartan (10−6–10−5 M, Merck Research Laboratories, Rahway, NJ, USA), the AT2 receptor antagonist PD123319 (10−6–10−5 M, Sigma-Aldrich), the receptor Mas antagonist D-Ala7-Ang-(1–7) (A-779) (10−6–10−5 M, Bachem Bioscience Inc.), the bradykinin B2 receptor antagonist HOE-140 (10−6 M) (Sigma-Aldrich) or the protein tyrosine phosphatase inhibitor phenylarsine oxide (PAO) (10−7 M, Sigma-Aldrich) prior to incubation with Ang-(1–7). Immunoblotting was performed as described [16]. Membranes were incubated for 16 h at 4°C with a 1:1000 dilution of anti-phosphospecific antibodies to p38 MAPK (Cell Signaling Technology, New England Biolabs Ltd, Pickering, Ontario, Canada). The p38 antibody recognizes the α, β, γ and δ isoforms of p38. In some experiments, the primary antibody was a rabbit polyclonal antibody to the receptor Mas (Abcam, Cambridge, MA, USA). For fibronectin immunoblots, the membranes were incubated for 16 h at 4°C with a 1:2000 dilution of a rabbit anti-human fibronectin antibody (Sigma-Aldrich).
To control for protein loading on phosphorylated p38 (pp38) MAPK immunoblots, membranes were stripped and re-probed with antibodies to total p38 MAPK (Cell Signaling Technology). Fibronectin immunoblots from cell lysates were reprobed with antibody against β-actin (Sigma-Aldrich), or by Ponceau staining for cell supernatants. Immunoblot signals were quantified by densitometry and corrected for total protein levels, using an image-analysis software programme (Kodak Densitometer 1S440CF) [16].
Immunoprecipitation and protein tyrosine phosphatase assay
The activity of the protein tyrosine phosphatase SHP-1 was measured by an immunoprecipitation assay, as described [18]. After 24 h in a serum-free medium, LLC-PK1 cells were exposed to normal glucose (5 mM d-glucose + 20 mM l-glucose) or high glucose (25 mM d-glucose) and stimulated with Ang-(1–7) (10−7 M) for 30 min. In some experiments, the cells were preincubated for 15–30 min with the receptor Mas antagonist A-779, or PAO (10−7 M) prior to incubation with Ang-(1–7). After washing with PBS, the cells were lysed with immunoprecipitation buffer (15 mM KCl, 10 mM HEPES (pH 7.6), 2 mM MgCl2, 0.1% NP40, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, 1 mM PMSF) for 10 min on ice. After centrifugation at 10 000 × g for 5 min at room temperature, cell supernatants were incubated with an antibody to SHP-1 (Transduction Laboratories, Lexington, KY, USA) at 4°C overnight followed by addition of 30 μl Protein A-agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and incubation for an additional 2 h at 4°C. The beads were washed and mixed with 100 μl of protein tyrosine phosphatase buffer (25 mM imidazole (pH 7.2), 45 mM NaCl, 1 mM EDTA) containing 30 mM p-nitrophenyl phosphate (pNPP), followed by incubation at 37°C for 30 min. The rate of formation of p-nitrophenol from pNPP was determined by spectrophotometry at 410 nm. For each assay of protein tyrosine phosphatase activity, the absorbance of pNPP hydrolyzed from beads containing control IgG (normal IgG) (Santa Cruz Biotechnology) alone was subtracted from the absorbance of lysate immunoprecipitates. The absorbance for the experimental groups was then normalized to the absorbance obtained with control IgG immunoprecipitates. After measurement of protein tyrosine phosphatase activity, the beads were recovered and subjected to SDS–PAGE for immunoblotting to detect the presence of SHP-1.
Collagen IV ELISA
After incubation of LLC-PK1 cells for 48–72 h in normal or high glucose, collagen IV was measured in the cell culture supernatant, using a commercial ELISA kit (Chemicon Int., Temecula, CA, USA).
TGF-β1 ELISA
The cells were incubated with agonists, and aliquots of the culture media were collected. TGF-β1 was activated by treating the media with 1 N HCl for 10 min at room temperature, followed by neutralization with 1.2 N NaOH/0.5 M HEPES. The levels of TGF-β1 were determined by a quantitative sandwich enzyme immunoassay using a commercial TGF-β1 ELISA kit (R&D Systems, TWC Biosearch International, Hong Kong).
[3H]-Leucine incorporation
After reaching 50–60% confluence, LLC-PK1 cells were exposed to media containing 5 mM d-glucose or 25 mM d-glucose, supplemented with 0.5% FBS, with or without Ang-(1–7) (10−7 M) or A-779 (10−5 M) for 48 h. To measure cell protein synthesis, 1 μCi of l-[4,5-3H]-leucine (73.0 Ci/mmol) (Amersham, Mississauga, Ontario, Canada) was added to each well. After 16 h, the cells were washed four times in ice-cold PBS and lysed in 0.5 N NaOH with 0.1% SDS. The cell-associated radioactivity was measured in a liquid scintillation counter. Leucine incorporation is reported as cpm/cell, with cell counts determined with a haematocytometer.
Statistical analysis
Results are presented as means ± SE. For immunoblots, control lanes were normalized to a value of unity as a reference, and the means ± SE are presented for the other lanes. Significance, considered as P < 0.05, was determined by one-way ANOVA with Bonferroni correction, in all cases involving multiple comparisons.
Results
Effect of Ang-(1–7) on p38 MAPK phosphorylation
Initial experiments were performed to determine if LLC-PK1 cells express the Mas protein, identified as the receptor for Ang-(1–7) [15]. Immunoblot assay in lysates of these cells revealed a single protein band of ∼37 kDa, confirming the expression of the receptor Mas (data not shown).
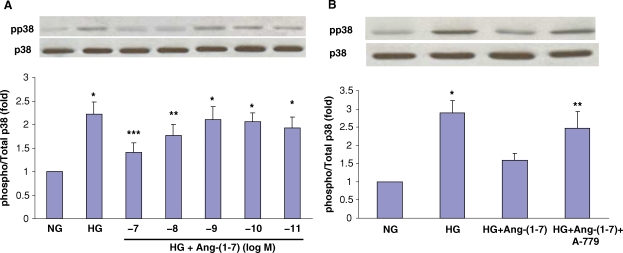
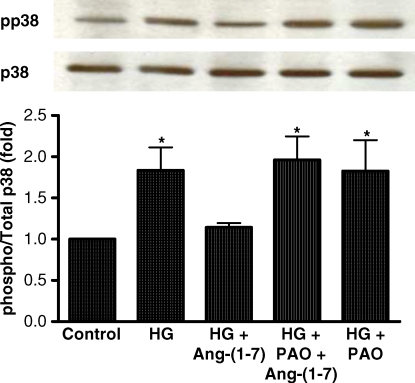
High concentrations of extracellular glucose activate p38 MAPK in LLC-PK1 cells [17]. Similarly, we found that exposure of LLC-PK1 cells to media containing high glucose (25 mM) increased p38 MAPK phosphorylation compared to the cells in normal glucose (5 mM). In preliminary experiments, this response peaked after 30 min incubation in high glucose and persisted for 3–6 h. Preincubation of cells with Ang-(1–7) inhibited the stimulatory effect of high glucose on p38 phosphorylation, in a concentration-dependent fashion, with a maximal effect at 10−7 M (Figure 1A). Furthermore, incubation of cells with A-779 (10−5 M) reversed the inhibitory effect of Ang-(1–7) on high glucose-stimulated p38 MAPK phosphorylation, suggesting that the effect of Ang-(1–7) is mediated via the receptor Mas (Figure 1B).
Fig. 1.
Effect of Ang-(1–7) on high glucose-stimulated p38 MAPK phosphorylation. (A) Graph depicts the concentration-dependent inhibitory effect of 30 min incubation with Ang-(1–7) (10−11–10−7 M) on high glucose (HG, 25 mM)-stimulated p38 MAPK phosphorylation in LLC-PK1 cells. NG, normal glucose (5 mM). *P < 0.005 versus NG, **P < 0.05 versus NG, ***P < 0.02 versus HG and P = NS versus NG; n = 10. (B) Graph depicts the effect of normal glucose (NG, 5 mM) or high glucose (HG, 25 mM) for 30 min with or without Ang-(1–7) (10−7 M) or D-Ala7-Ang-(1–7) (A-779, 10−5 M), on p38 MAPK phosphorylation. Control cells were incubated in media supplemented with l-glucose (20 mM) to control for osmolality. *P < 0.001 versus NG and HG + Ang-(1–7), **P < 0.001 versus NG, and P < 0.05 versus HG + Ang-(1–7); n = 6–13. Results are presented as the ratio of phosphorylated p38/total p38, in arbitrary units. Representative blots are depicted above the graphs, showing phosphorylated p38 (pp38) and total p38 (p38).
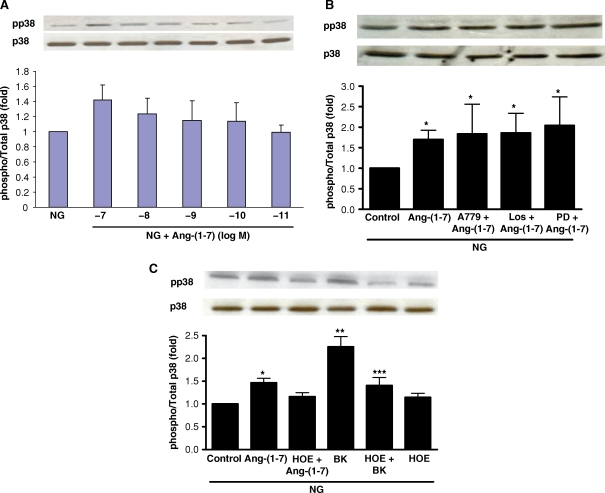
In normal glucose (5 mM), incubation of cells with Ang-(1–7) caused small concentration-dependent activation of p38 MAPK, with a peak effect at 10−7 M (Figure 2A). Pretreatment with A-779 (10−5 M), the AT1 receptor antagonist losartan (10−6 M), or the AT2 receptor antagonist PD123319 (10−6 M) did not reverse this activation (Figure 2B). In contrast, the bradykinin B2 receptor antagonist HOE-140 (10−6 M) partly inhibited the Ang-(1–7)-stimulated p38 MAPK phosphorylation in normal glucose. Bradykinin also stimulated p38 MAPK phosphorylation in normal glucose, and this was reversed by HOE-140 (Figure 2C).
Fig. 2.
Effect of Ang-(1–7) on p38 MAPK phosphorylation in normal glucose. (A) Graph depicts the concentration-dependent stimulatory effect of incubation of Ang-(1–7) (10−11–10−7 M) on phosphorylation of p38 MAPK in normal glucose (NG, 5 mM). By ANOVA, the effect of Ang-(1–7) (10−7 M) was not significant compared to NG alone in this series of experiments; n = 7–8. Results are presented as the ratio of phosphorylated p38/total p38, in arbitrary units. Representative blot is depicted above the graph, showing phosphorylated p38 (pp38) and total p38 (p38). (B) Graph depicts the effect of Ang-(1–7) (10−7 M) in normal glucose (NG, 5 mM) with or without A-779 (10−5 M), Losartan (Los, 10−6 M) or PD123319 (PD, 10−6 M). *P < 0.05 versus control; n = 5–6. To control for the possible effects of osmolality in these experiments, incubations in NG were supplemented with l-glucose (20 mM). Representative blot is depicted above the graph, showing phosphorylated p38 (pp38) and total p38 (p38). (C) Graph depicts the effect of Ang-(1–7) (10−7 M) and bradykinin (BK) (10−7 M) in normal glucose (NG, 5 mM) with or without the B2 receptor antagonist HOE-140 (HOE, 10−6 M). *P < 0.05 versus control, **P < 0.001 versus control ***P < 0.01 versus BK; n = 7–16. Representative blot is depicted above the graph, showing phosphorylated p38 (pp38) and total p38 (p38).
Effect of Ang-(1–7) on SHP-1 activity
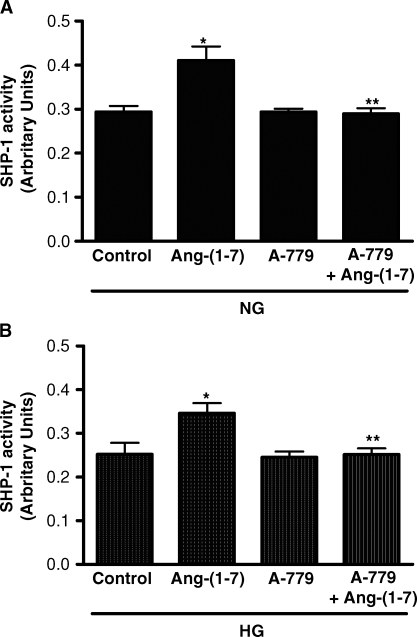
Further experiments focused on a possible mechanism for the inhibitory effect of Ang-(1–7) on high glucose-stimulated p38 MAPK. In this regard, the effects of MAPK signalling pathways may be regulated by the opposing actions of tyrosine kinases and tyrosine phosphatases. Recruitment of the protein tyrosine phosphatase SHP-1 to its targets may suppress downstream signalling events [19, 20]. In cells incubated under either normal (5 mM) or high glucose (25 mM) conditions for 30 min, co-incubation with Ang-(1–7) (10−7 M) significantly increased SHP-1 activity (Figure 3). No change in protein expression of SHP-1 was detected by immunoblot at this time point (not shown). Activation of SHP-1 by Ang-(1–7) was significantly inhibited when the cells were pretreated with A-779 (10−5 M) (Figure 3).
Fig. 3.
Effect of Ang-(1–7) on SHP-1 activity. (A) Graph depicts the effect of Ang-(1–7) (10−7 M) on SHP-1 activity in normal glucose (NG, 5 mM), with or without Ang-(1–7) (10−7 M) or A-779 (10−5 M). *P < 0.05 versus control, **P < 0.05 versus Ang-(1–7); n = 3. (B) SHP- 1 activity in high glucose (HG, 25 mM) is depicted with or without Ang-(1–7) (10−7 M) or A-779 (10−5 M). *P < 0.01 versus control; **P < 0.01 versus Ang-(1–7); n = 5. Results are presented in arbitrary units.
Inhibition of tyrosine phosphatase blocks the inhibitory effect of Ang-(1–7) on high glucose-stimulated p38 MAPK
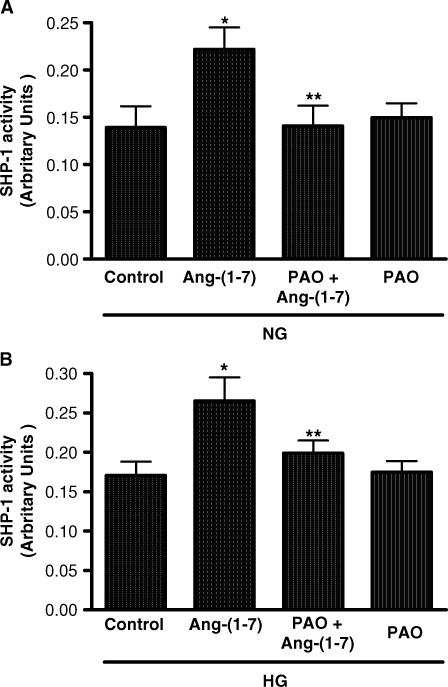
To determine the role of Ang-(1–7)-stimulated SHP-1 activation in mediating the inhibition of p38 phosphorylation in high glucose, the cells were preincubated for 15 min with the inhibitor of phosphotyrosine phosphatase PAO [21]. PAO completely inhibited activation of SHP-1 activity under both normal and high glucose conditions (Figure 4), and it also reversed the inhibitory effect of Ang-(1–7) on high glucose-stimulated p38 phosphorylation (Figure 5).
Fig. 4.
Effect of phenylarsine oxide (PAO) on SHP-1 activity in normal glucose or high glucose. (A) The effect of Ang-(1–7) (10−7 M) on SHP-1 activity in normal glucose is depicted (NG, 5 mM) with or without PAO (10−7 M). *P < 0.001 versus control, **P < 0.001 versus Ang-(1–7); n = 3. (B) The effect of Ang-(1–7) (10−7 M) on SHP-1 activity in high glucose is depicted (HG, 25 mM) with or without PAO (10−7 M). *P < 0.01 versus control, **P < 0.05 versus Ang-(1–7); n = 5. Results are presented in arbitrary units.
Fig. 5.
PAO blocks the inhibitory effect of Ang-(1–7) on HG-stimulated p38 MAPK. Graph depicts the effect of Ang-(1–7) (10−7 M) on high glucose (HG, 25 mM)-stimulated p38 MAPK with or without phenylarsine oxide (PAO, 10−7 M). *P < 0.05 versus control; n = 7. Results are presented as the ratio of phosphorylated p38/total p38, in arbitrary units. Representative blot is depicted above the graph, showing phosphorylated p38 (pp38) and total p38 (p38).
Effect of Ang-(1–7) on cell protein synthesis
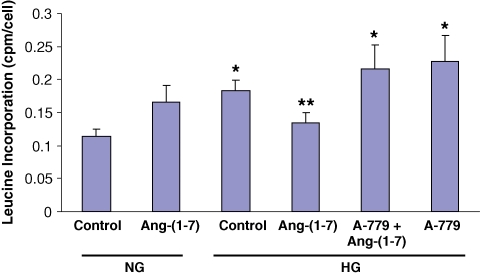
To determine the functional impact of the inhibitory effect of Ang-(1–7) on high glucose-stimulated p38 MAPK, we examined the effects on cell protein synthesis, measured by [3H]-leucine incorporation. Incubation of cells for 48 h in high glucose caused a significant stimulation of leucine incorporation, compared to normal glucose, and this was blocked by 48 h incubation with Ang-(1–7) (10−7 M) (Figure 6). The inhibitory effect of Ang-(1–7) was completely prevented by co-incubation with A-779 (10−5 M).
Fig. 6.
Ang-(1–7) inhibits high glucose-stimulated cell protein synthesis. Graph depicts the effect of Ang-(1–7) (10−7 M) in normal glucose (NG, 5 mM) and high glucose (HG, 25 mM) for 48 h, with or without A-779 (10−5 M) on cell protein synthesis, measured by [3H]-leucine incorporation. *P < 0.001 versus NG control, **P < 0.001 versus A-779 + Ang-(1–7), P < 0.010 versus HG control, and P < 0.030 versus HG + A-779; n = 5–11.
Effect of Ang-(1–7) on TGF-β1, fibronectin and collagen IV
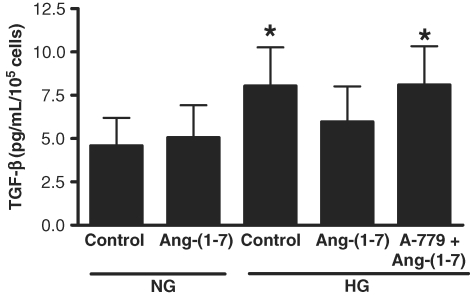
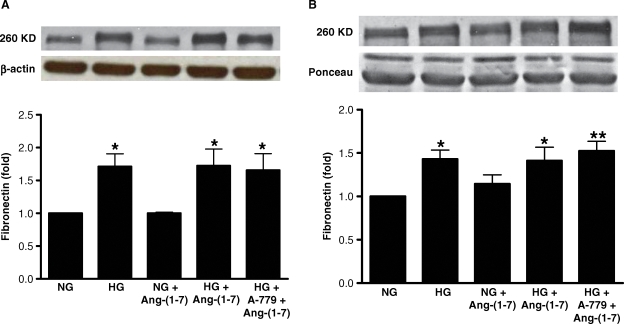
Diabetic nephropathy is associated with high glucose-induced synthesis of intrarenal TGF-β1, and extracellular matrix proteins [1]. Incubation of LLC-PK1 cells in high glucose for 72 h caused a small but significant stimulation of TGF-β1 production (Figure 7). This effect was partly inhibited by Ang-(1–7) (10−7 M), in a receptor Mas-dependent manner. In contrast, Ang-(1–7) had no significant effect on high glucose-stimulated production of fibronectin, either cell-associated or secreted into the media (Figure 8), and did not inhibit high glucose-stimulated synthesis of collagen IV (Figure 9). In normal glucose, Ang-(1–7) had no significant effect on TGF-β1, fibronectin or collagen IV (Figures 7–9).
Fig. 7.
Effect of Ang-(1–7) on high glucose-stimulated TGF-β1. Graph depicts the effect of Ang-(1–7) (10−7 M) on TGF-β1 in normal glucose (NG, 5 mM) and high glucose (HG, 25 mM) for 72 h, with or without A-779 (10−5 M). TGF-β1 synthesis was measured by ELISA. *P < 0.05 versus NG control; n = 5.
Fig. 8.
Effect of Ang-(1–7) on fibronectin production. Graphs depict effects of Ang-(1–7) (10−7 M) on high glucose-stimulated production of fibronectin, either cell-associated (A) or secreted into the media (B), in the presence or absence of A-779 (10−5 M). Sample immunoblots for fibronectin are shown above, with β-actin blot below in (A) and Ponceau staining of proteins below in (B). *P < 0.05 versus NG, **P < 0.01 versus NG; n = 4.
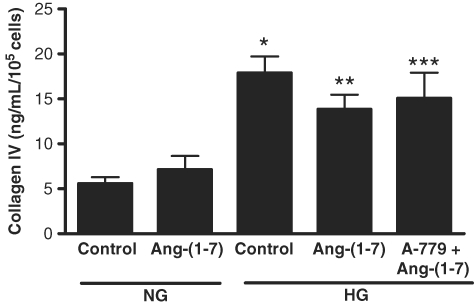
Fig. 9.
Effect of Ang-(1–7) on collagen IV production. Graph depicts the effect of Ang-(1–7) (10−7 M) on collagen IV production in normal glucose (NG, 5 mM) and high glucose (HG, 25 mM) for 72 h, with or without A-779 (10−5 M). Collagen IV synthesis was measured by ELISA. *P < 0.001 versus NG control, **P < 0.05 versus NG control, ***P < 0.01 versus NG control; n = 7.
Discussion
There are several major findings in the current study that are relevant to diabetic tubulointerstitial injury. First, in proximal tubular cells, high glucose-stimulated activation of p38 MAPK is blocked by Ang-(1–7) via binding to the receptor Mas. Second, Ang-(1–7) activates the protein tyrosine phosphatase SHP-1, which may mediate the inhibition of p38 MAPK phosphorylation. Third, Ang-(1–7) inhibits high glucose-stimulated cell protein synthesis, and blocks TGF-β1 production, but has no effect on production of the matrix proteins fibronectin or collagen IV. Finally, in normal glucose, Ang-(1–7) causes a small but significant stimulation of p38 MAPK, which is mediated not by the receptor Mas, but at least partly by a bradykinin B2 receptor-dependent pathway.
Treatment of streptozotocin (STZ)-diabetic rats with Ang-(1–7) has recently been shown to diminish proteinuria, and prevent abnormal vascular responsiveness [11], suggesting that activation of Ang-(1–7) signalling could represent a therapeutic strategy in diabetic nephropathy. Strategies to inhibit high glucose-stimulated MAPK activation and subsequent downstream signalling in the proximal tubule could be important in reducing tubulointerstitial injury. We observed that Ang-(1–7) inhibited high glucose-stimulated p38 MAPK phosphorylation, and this was reversed by administration of A-779, suggesting an effect mediated by binding of Ang-(1–7) to the receptor Mas. Mas is a G protein-coupled cell surface receptor (GPCR) of 325 amino acids, belonging to the Class A orphan GPCRs, which contains approximately 50 receptors related to Mas [22]. However, the intracellular signalling pathways associated with binding of Ang-(1–7) to Mas have been incompletely characterized. In NIH 3T3 cells, Mas stimulates the GTP-binding protein Rac1, associated with activation of p38 and c-jun N-terminal kinase (JNK) [23]. In a rat heart in vivo and in human endothelial cells, Ang-(1–7) stimulates Akt phosphorylation through Mas, which could contribute positively to the cardiac actions of insulin and to nitric oxide release, respectively [24,25]. On the other hand, in cardiac myocytes, Ang-(1–7) inhibits MAPK activation through the receptor Mas [26]. Studies in human endothelial cells indicate that Ang-(1–7) negatively regulates Ang II signalling by enhancing the association of c-Src with the Src homology 2-containing inositol phosphatase 2 (SHP-2), and by blocking the reduced phosphorylation of SHP-2 induced by Ang II [27].
Signalling associated with Ang-(1–7) in the proximal tubule is poorly understood. Ang-(1–7) activates phospholipase A2 in proximal tubular cells, associated with the inhibition of sodium flux [28]. In an adult pig proximal tubule, Ang-(1–7) blocks the stimulatory effect of Ang II on Na+-ATPase activity, an effect prevented by a receptor Mas antagonist [29]. Ang-(1–7) may also bind to Ang II AT1 receptors in the proximal tubule, associated with stimulation of phospholipase C-beta, protein kinase C and activation of Na+-ATPase [30].
The current studies, along with our previous demonstration that Ang-(1–7) completely inhibits Ang II-stimulated phosphorylation of the MAPKs p38, ERK and JNK [16], suggested that Ang-(1–7) signalling might involve activation of a tyrosine phosphatase in the proximal tubule. We investigated SHP-1 as a candidate tyrosine phosphatase, since SHP-1 is activated by proximal tubular AT2 receptors cloned from a variety of species [31]. SHP-1 is a protein tyrosine phosphatase first described in the cytoplasm of hematopoietic cells, and may function as a negative regulator of intracellular signal transduction [32]. In vascular smooth muscle cells, the Ang II AT2 receptor-mediated inhibition of JNK activation and c-Jun expression, and inhibition of ERK phosphorylation, involves activation of SHP-1 [19,20]. The current studies indicate for the first time that significant increases in SHP-1 activity are induced by Ang-(1–7) in proximal tubular cells, and this is inhibited by the receptor Mas antagonist A-779. Furthermore, SHP-1 activation by Ang-(1–7) was blocked by the tyrosine phosphatase antagonist PAO, and PAO reversed the inhibitory effect of Ang-(1–7) on p38 MAPK phosphorylation. Although our data are consistent with the hypothesis that Ang-(1–7)-stimulated SHP-1 mediates the inhibition of p38 MAPK phosphorylation in high glucose, PAO is a non-specific inhibitor of tyrosine phosphatases, and therefore, other tyrosine phosphatases or even dual-specificity phosphatases (DUSPs) could be involved in the regulation of MAPK signalling by Ang-(1–7) in proximal tubular cells. Thus, in LLC-PK1 cells, atrial natriuretic peptide stimulates expression of the DUSP MKP-1, a phosphatase involved in dephosphorylation of ERK [33], while in human embryonic 293T cells, coordinated activation of multiple DUSPs causes dephosphorylation of JNK [34].
High glucose stimulates proximal tubular cell hypertrophy, TGF-β production and synthesis of extracellular matrix proteins such as fibronectin and collagen IV, which contribute to tubulointerstitial fibrosis in diabetic nephropathy [17,35–37]. Ang-(1–7) inhibited cell protein synthesis induced by high glucose, as well as glucose-stimulated TGF-β1 production, through binding to the receptor Mas. However, we observed no significant inhibitory effect of Ang-(1–7) on the levels of fibronectin or collagen IV induced by high glucose. These data suggest that other proteins associated with cell hypertrophy are affected by Ang-(1–7) in the proximal tubule, and that TGF-β1 synthesis is not necessary for fibronectin or collagen IV production in these cells. Moreover, the role of SHP-1 activation in mediating the inhibitory effect of Ang-(1–7) on glucose-induced cell hypertrophy and TGFβ-1 production remains unclear. Use of PAO for 48–72 h was associated with cell toxicity in our studies, and other strategies (e.g. siRNA) will be necessary to address this question.
In a normal glucose medium, Ang-(1–7) caused a small but significant stimulation of p38 MAPK phosphorylation, which was not inhibited by the receptor Mas antagonist, nor by AT1 or AT2 receptor blockers. Similarly, we observed a non-significant stimulation of [3H]-leucine incorporation by Ang-(1–7) in normal glucose (Figure 6). In previous studies in rat proximal tubular cells, we observed that Ang-(1–7) alone caused a 19% increase in p38 MAPK phosphorylation, although this did not achieve statistical significance [16]. In the present studies, the bradykinin B2 receptor antagonist HOE-140 inhibited the small stimulatory effect of Ang-(1–7) on p38 MAPK, suggesting that Ang-(1–7) can interact with bradykinin signalling in these cells. The mechanism underlying this interaction remains obscure. In other tissues, complex interactions between Ang-(1–7) and bradykinin B2 receptor responses have been described. Endogenous Ang-(1–7) potentiates the bradykinin-induced vasodepressor effect induced by ACE inhibition [38], and this appears to involve a direct interaction between Ang-(1–7) and a B2 receptor pathway, without requirement for the receptor Mas [39]. Taken together, our data also suggest that under normal glucose conditions, Ang-(1–7) may activate a B2 receptor-dependent signalling pathway in LLC-PK1 cells, which is linked to p38 MAPK activation and downstream increases in protein synthesis. However, since we observed only a partial inhibitory effect of HOE-140 on the response to Ang-(1–7) in normal glucose, other pathways may be involved, also independent of the receptor Mas.
In summary, Ang-(1–7) activates SHP-1 in proximal tubular cells, and inhibits glucose-stimulated p38 MAPK, cell protein synthesis and TGF-β1 production, with no effect on the matrix proteins fibronectin or collagen IV. The results suggest that in the diabetic kidney, Ang-(1–7) may partly protect against the profibrotic effects of high glucose.
Acknowledgments
Parts of this work were presented in abstract form at the American Society of Nephrology Annual Meeting, San Francisco, CA, November 2007 (J Am Soc Nephrol 2007; 18: 138A). These studies were supported by grants from the Canadian Institutes of Health Research (CIHR) and the Kidney Foundation of Canada to KDB.
Conflict of interest statement. None declared.
References
- 1.Wolf G. New insights into the pathophysiology of diabetic nephropathy: from haemodynamic to molecular pathology. Eur J Clin Invest. 2004;34:785–796. doi: 10.1111/j.1365-2362.2004.01429.x. [DOI] [PubMed] [Google Scholar]
- 2.Braam B, Mitchell KD, Fox J, et al. Proximal tubular secretion of angiotensin II in rats. Am J Physiol Renal Physiol. 1993;264:F891–F898. doi: 10.1152/ajprenal.1993.264.5.F891. [DOI] [PubMed] [Google Scholar]
- 3.Seikaly MG, Arant BS, Jr, Seney FD., Jr Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest. 1990;86:1352–1357. doi: 10.1172/JCI114846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf G, Ritz E. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: pathophysiology and indications. Kidney Int. 2005;67:799–812. doi: 10.1111/j.1523-1755.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 5.Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 6.Tipnis SR, Hooper NM, Hyde R, et al. A human homolog of angiotensin-converting enzyme. cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 7.Brosnihan KB, Neves LAA, Joyner J, et al. Enhanced renal immunocytochemical expression of Ang-(1–7) and ACE2 during pregnancy. Hypertension. 2003;42:749–753. doi: 10.1161/01.HYP.0000085220.53285.11. [DOI] [PubMed] [Google Scholar]
- 8.Li N, Zimpelmann J, Cheng K, et al. The role of angiotensin converting enzyme 2 in the generation of angiotensin 1–7 by rat proximal tubules. Am J Physiol Renal Physiol. 2005;288:F353–F362. doi: 10.1152/ajprenal.00144.2004. [DOI] [PubMed] [Google Scholar]
- 9.Shaltout HA, Westwood BM, Averill DB, et al. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol. 2007;292:F82–F91. doi: 10.1152/ajprenal.00139.2006. [DOI] [PubMed] [Google Scholar]
- 10.Pendergrass KD, Averill DB, Ferrario CM, et al. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2 Lewis rat. Am J Physiol Renal Physiol. 2006;290:F1497–F1506. doi: 10.1152/ajprenal.00317.2005. [DOI] [PubMed] [Google Scholar]
- 11.Benter IF, Yousif MH, Cojocel C, et al. Angiotensin-(1–7) prevents diabetes-induced cardiovascular dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H6666–H6672. doi: 10.1152/ajpheart.00372.2006. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira A, Pinheiro SVB, Castro CH, et al. Renal function in transgenic rats expressing an angiotensin-(1–7)-producing fusion protein. Regul Pept. 2006;137:128–133. doi: 10.1016/j.regpep.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1–7): in vivo and in vitro studies. Am J Physiol Renal Physiol. 1996;270:F141–F147. doi: 10.1152/ajprenal.1996.270.1.F141. [DOI] [PubMed] [Google Scholar]
- 14.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1–7) on isolated rabbit afferent arterioles. Hypertension. 2002;39:799–802. doi: 10.1161/hy0302.104673. [DOI] [PubMed] [Google Scholar]
- 15.Santos RA, Simoes Silva AC, Maric C, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor mas. Proc Natl Acad Sci USA. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su Z, Zimpelmann J, Burns KD. Angiotensin-(1–7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int. 2006;69:2212–2218. doi: 10.1038/sj.ki.5001509. [DOI] [PubMed] [Google Scholar]
- 17.Fujita H, Omori S, Ishikura K, et al. ERK and p38 mediate high glucose-induced hypertrophy and TGF-β expression in renal tubular cells. Am J Physiol Renal Physiol. 2004;286:F120–F126. doi: 10.1152/ajprenal.00351.2002. [DOI] [PubMed] [Google Scholar]
- 18.Amiri F, Venema VJ, Wang X, et al. Hyperglycemia enhances angiotensin II-induced Janus-activated kinase/STAT signaling in vascular smooth muscle cells. J Biol Chem. 1999;274:32382–32386. doi: 10.1074/jbc.274.45.32382. [DOI] [PubMed] [Google Scholar]
- 19.Cui T, Nakagami H, Iwai M, et al. Pivotal role of tyrosine phosphatase SHP-1 in AT2 receptor-mediated apoptosis in rat fetal vascular smooth muscle cell. Cardiovasc Res. 2001;49:863–871. doi: 10.1016/s0008-6363(00)00299-6. [DOI] [PubMed] [Google Scholar]
- 20.Matsubara H, Shibasaki Y, Okigaki M, et al. Effect of angiotensin II type 2 receptor on tyrosine kinase Pyk2 and c-jun NH2-terminal kinase via SHP-1 tyrosine phosphatase activity: evidence from vascular-targeted transgenic mice of AT2 receptor. Biochem Biophys Res Commun. 2001;282:1085–1091. doi: 10.1006/bbrc.2001.4695. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Morales P, Minami Y, Luong E, et al. Tyrosine phosphorylation in T cells is regulated by phosphatase activity: studies with phenylarsine oxide. Biochemistry. 1990;87:9255–9259. doi: 10.1073/pnas.87.23.9255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alenina N, Xu P, Rentzsch B, et al. Genetically altered models for Mas and angiotensin-(1–7) Exp Physiol. 2008;93:528–537. doi: 10.1113/expphysiol.2007.040345. [DOI] [PubMed] [Google Scholar]
- 23.Zohn IE, Symons M, Chrzanowska-Wodnicka M, et al. Mas oncogene signaling and transformation require the small GTP-binding protein Rac. Mol Cell Biol. 1998;18:1225–1235. doi: 10.1128/mcb.18.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giani JF, Gironacci MM, Munoz MC, et al. Angiotensin-(1–7) stimulates the phosphorylation of JAK2, IRS-1 and Akt in rat heart in vivo: role of the AT1 and mas receptors. Am J Physiol Heart Circ Physiol. 2007;293:H1154–H1163. doi: 10.1152/ajpheart.01395.2006. [DOI] [PubMed] [Google Scholar]
- 25.Sampaio WO, Souza dos Santos RA, Faria-Silva R, et al. Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49:185–192. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- 26.Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol. 2005;289:H1560–H1566. doi: 10.1152/ajpheart.00941.2004. [DOI] [PubMed] [Google Scholar]
- 27.Sampaio WO, Henrique de Castro C, Santos RA, et al. Angiotensin-(1–7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension. 2007;50:1093–1098. doi: 10.1161/HYPERTENSIONAHA.106.084848. [DOI] [PubMed] [Google Scholar]
- 28.Andreatta-van Leyen S, Romero MF, Khosla MC, et al. Modulation of phospholipase A2 activity and sodium transport by angiotensin-(1–7) Kidney Int. 1993;44:932–936. doi: 10.1038/ki.1993.334. [DOI] [PubMed] [Google Scholar]
- 29.Lara LS, Bica RBS, Sena SLF, et al. Angiotensin-(1–7) reverts the stimulatory effect of angiotensin II on the proximal tubule Na+-ATPase activity via a A779-sensitive receptor. Regul Pept. 2002;103:17–22. doi: 10.1016/s0167-0115(01)00322-6. [DOI] [PubMed] [Google Scholar]
- 30.Lara LS, Correa JS, Lavelle AB, et al. The AT1R/Gq protein/PI-PLC(beta)/PKC pathway is involved in activation of proximal tubule Na+-ATPase activity by Ang-(1–7) Exp Physiol. 2008;93:639–647. doi: 10.1113/expphysiol.2007.040584. [DOI] [PubMed] [Google Scholar]
- 31.Feng Y-H, Zhou L, Sun Y, et al. Functional diversity of AT2 receptor orthologues in closely related species. Kidney Int. 2005;67:1731–1738. doi: 10.1111/j.1523-1755.2005.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hannken T, Schroeder R, Stahl RAK, et al. Atrial natriuretic peptide attenuates ANG II-induced hypertrophy of renal tubular cells. Am J Physiol Renal Physiology. 2001;281:F81–F90. doi: 10.1152/ajprenal.2001.281.1.F81. [DOI] [PubMed] [Google Scholar]
- 33.Teng C-H, Huang W-N, Meng T-C. Several dual specificity phosphatases coordinate to control the magnitude and duration of JNK activation in signaling response to oxidative stress. J Biol Chem. 2007;282:28395–28407. doi: 10.1074/jbc.M705142200. [DOI] [PubMed] [Google Scholar]
- 34.Shen SH, Bastien L, Posner BI, et al. A protein-tyrosine phosphatase with sequence similarity to the SH2 domain of the protein-tyrosine kinases. Nature. 1991;352:736–739. doi: 10.1038/352736a0. [DOI] [PubMed] [Google Scholar]
- 35.Morrissey K, Steadman R, Williams JD, et al. Renal proximal tubular cell fibronectin accumulation in response to glucose is polyol pathway dependent. Kidney Int. 1999;55:160–167. doi: 10.1046/j.1523-1755.1999.00248.x. [DOI] [PubMed] [Google Scholar]
- 36.Phillips AO, Steadman R, Morrissey K, et al. Exposure of human renal proximal tubular cells to glucose leads to accumulation of type IV collagen and fibronectin by decreased degradation. Kidney Int. 1997;52:973–984. doi: 10.1038/ki.1997.419. [DOI] [PubMed] [Google Scholar]
- 37.Ziyadeh FN, Snipes ER, Watanabe M, et al. High glucose induces cell hypertrophy and stimulates collagen gene transcription in proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol. 1990;28:F704–F714. doi: 10.1152/ajprenal.1990.259.4.F704. [DOI] [PubMed] [Google Scholar]
- 38.Maia LG, Ramos MC, Fernandes L, et al. Angiotensin-(1–7) antagonist A-779 attenuates the potentiation of bradykinin by captopril in rats. J Cardiovasc Pharmacol. 2004;43:685–691. doi: 10.1097/00005344-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Greco AJ, Master RG, Fokin A, Jr, et al. Angiotensin-(1–7) potentiates responses to bradykinin but does not change responses to angiotensin I. Can J Physiol Pharmacol. 2006;84:1163–1175. doi: 10.1139/y06-053. [DOI] [PubMed] [Google Scholar]