Abstract
Objectives
To investigate the prevalence of non-alcoholic fatty liver disease (NAFLD) in children and its relationship to metabolic syndrome, insulin resistance, and waist circumference (WC).
Methods
This was a population-based cross-sectional, case–control study. Cases were selected among students of a primary and junior high school, respectively, and age- and sex-matched control subjects were selected randomly (ratio of cases to control subject was 37:113).
Results
Of the 846 students, aged between 6 and 15 years, enrolled in the study and screened by ultrasonography, 37 children were diagnosed as having NAFLD (score ≥ 1). There was a significant sex difference in the prevalence of NAFLD(P = 0.003). The trend test revealed a strong dose–response relationship (P < 0.001) between pediatric NAFLD and the number of the proposed components of pediatric metabolic syndrome in Japan (MetS-JC), such as a clustering of the components of MetS-JC. Additionally, the linear trend of the odds ratios (ORs) with increasing percentile of the homeostasis model assessment-insulin resistance (HOMA-IR) was statistically significant (P < 0.001). However, when WC was added to the logistic model, the ORs were no longer significant, whereas WC turned out to be an independent risk factor for NAFLD regardless of the HOMA-IR index.
Conclusion
The prevalence of NAFLD in children and adolescents is closely related to metabolic syndrome, insulin resistance, and WC.
Keywords: Children, Insulin resistance, Metabolic syndrome, Non-alcoholic fatty liver disease, Waist circumference
Introduction
The global epidemic of childhood obesity has become a serious public health concern [1], and recent studies suggest that the prevalence of non-alcoholic fatty liver disease (NAFLD) is increasing in children [2, 3], especially in obese children [4, 5]. This condition is considered to be the most common form of chronic liver disease in the pediatric population [6]. Although the natural history of NAFLD has yet to be defined, it ranges from simple steatosis, which is a slowly progressing disorder, to steatohepatitis, fibrosis, and even to cirrhosis [2, 3]. Rashid et al. [7] reported cirrhosis in children as young as 10 years. Although results from population-based epidemiologic studies are limited, the prevalence of NAFLD seems to be higher than expected [8], and an earlier epidemiological survey carried out by our group found the prevalence of this condition among school children (4–12 years of age), as detected by ultrasonography, to be 2.6%, increasing to 22.5% among obese children [9].
The aim of the study reported here was: (1) to determine whether each component of metabolic syndrome is positively correlated with the degree of NAFLD (dose–response relationship); (2) to determine whether pediatric NAFLD is closely related to metabolic syndrome, insulin resistance, and waist circumference (WC).
As the components of metabolic syndrome, we used the provisional diagnostic criteria of pediatric metabolic syndrome in Japan proposed by the study group of the Ministry of Health, Labor and Welfare of Japan (MetS-JC) [10, 11]. The homeostasis model assessment-insulin resistance (HOMA-IR) was used as an indicator of the strength of insulin resistance. Three obesity indices, WC, waist-to-height ratio (WC/H), and body mass index (BMI), were examined and compared.
Methods
Study design
This was a population-based cross-sectional, case–control study. Cases and controls were selected among students attending a primary and junior high school, respectively. The controls were selected randomly and were age- and sex-matched (case:control is 37:113). Anthropometric measurements and ultrasonographic examinations were carried out on the entire study cohort. Biochemical parameters and blood pressure were also measured for both the case and control groups.
Participants and demographics
The study cohort comprised 846 students, ages 6–15 years, attending a public elementary school and a junior high school, respectively, in Asakawa, a town located in Fukushima Prefecture, northern Japan. Almost 100% of the offspring of the residents of this prefecture attend the district public schools; therefore, the participants can be considered to be representative of the general pediatric population of rural northern Japan. At the beginning of the first trimester in 1994, we distributed informed consent documents to the guardians of the students at both schools. The participation rate was 846/1006(83.1%). Based on an ultrasonographic examination and anthropometric measurements, we identified 37 students with NAFLD and subsequently randomly selected 113 age- and sex-matched controls. The ratio of cases to controls (37:113) was not precisely 1:3 due to factors beyond our control. The protocol was approved by the joint institutional review boards of the Health Educational Committee of the Town of Asakawa and the Fukushima Medical University in June 1994.
Data collection
Data were collected on three school-day mornings (October 26–28, 1994). Weight (kg), WC (cm), height (m), and blood pressure (BP: mmHg) were measured following the guideline of the Japanese Ministry of Health, Welfare and Labor [12]. Ultrasonography is widely accepted as a surrogate method of liver biopsy in the survey of large populations [13] and is widely used as a tool for grading NAFLD [5, 14, 15]. Real-time ultrasonographic examination of the liver (3.5 MHz) was performed by three experienced ultrasonographers using an ALOKA-SSD650 unit.
The scoring of each of the three examiners was combined to make a composite score [9]. Each criterion was assigned a score that indicated the level of fatty liver infiltration, with a score of 2 indicating a distinctly positive (++) finding of fatty liver infiltration for that criterion, and a score of 1 indicating a positive (+) finding based on the criterion. A score of zero was assigned to the criterion when a negative (−) evaluation for fatty liver was found based on the criterion. The sum of the scores for the three criteria was then considered to indicate the degree of fatty liver infiltration. Thus, the score for the fatty liver indicator ranged from zero to 6, with a total score of 1 and 2 indicating a fatty liver, associated with a diagnosis of mild to moderate NAFLD, and a total score of ≥3 associated with a diagnosis of serious NAFLD.
The criteria and scoring assignments were as follows. (1) The echo levels for the liver parenchyma and kidney parenchyma were compared. An evaluation was made of the relative increase in the liver versus kidney surface echo level accompanied by a high contrast between liver parenchyma and kidney parenchyma (liver–kidney contrast or liver–kidney echo discrepancy). A large discrepancy between hepatic and renal echoes resulted in a (++) finding. A slight increase in liver echogenicity, that is, a slight exaggeration of liver and kidney echo discrepancy, was assigned a (+) evaluation. A negative (−) evaluation of fatty liver was made when the echo level was homogeneous, and the contrast between the liver parenchyma and the kidney parenchyma was unclear. (2)An assessment was made of echo penetration into the deep portion of the liver. Ultrasonic penetration into the deepest portion of the liver is reduced due to interference from enhanced liver surface echogenicity. This results in opacity of the lower part of the liver and the loss of visibility of the diaphragm. A (++) evaluation was given when both indications were definitely present. The probable presence of both indications resulted in a (+) score, while a (−) evaluation meant that the liver structure was clearly defined from the surface of the liver to the diaphragm. (3) The clarity of liver blood vessel structures visualized in the sonograph, especially the veins, was evaluated. An (++) evaluation was assigned when liver vessel structures were obscure and the identification of the vessel walls could not be made. A (+) score was given if a loss of echoes from the vessel wall structures was observed. A (−) score resulted when the vessel structures and vessel walls could be clearly defined.
Measurement of obesity indices
The body mass index was calculated as the body weight divided by the square of height (kg/m2). Waist circumference was measured at the umbilical level, with subjects standing and in the exhalation phase. The WC/H was calculated as waist/height. These measurements were taken after a 12-h fast, with the students dressed in light-weight clothing and without shoes. A recent report from Japanese researchers indicates that WC/H is a simple and practical index for assessing central fat distribution and metabolic risk [16]. To examine the biochemical parameters, we drew blood samples after a 12-h fast. The sample was sent to a commercial medical laboratory center for testing (SRL, Japan).
The provisional diagnostic criteria of MetS-JC have been proposed by the study group of the Ministry of Health, Labor and Welfare of Japan. The details of these criteria are described by Ohzeki [10], but essentially children meeting at least three of the following criteria qualify as having the metabolic syndrome: (1) WC ≥ 80 cm (prerequisite); (2) elevated BPs [systolic BP (SBP) ≥ 125 mmHg and/or diastolic blood pressure (DBP) ≥ 70 mmHg]; (3) low high-density lipoprotein-cholesterol (HDL-C < 40 mg/dl) and/or high fasting serum triglyceride levels (TG ≥ 120 mg/dl); (4) high fasting plasma glucose levels (FPG ≥ 100 mg/dl). In terms of an obesity index, WC/H is also an acceptable alternative to WC. As mentioned above, immuno-reactive insulin (IRI) and the HOMA-IR index were included as biochemical/metabolic parameters.
Insulin resistance was evaluated using the HOMA-IR index and computed using the formula HOMA-IR = FPG (mg/dl) × IRI(mU/ml)/405, where FPG is the fasting plasma glucose level and IRI is the immuno-reactive insulin level. This value has been shown to correlate with euglycemic-hyperinsulinemic cramp studies and can be applied to children and adolescents [17, 18].
Continuous variables were expressed as mean values ± the standard error (SE). A significance level of P = 0.05 was selected for all statistical tests. Data was analyzed by SPSS ver. 15 J for Windows (SPSS, Chicago, IL).
The Mantel–Haentzel chi-square was used to examine whether there was any difference in the prevalence of NAFLD according to sex (see Table 1).
Selected anthropometric and metabolic risk factors for NAFLD were analyzed by univariate logistic regression (results not shown in Table 1).
A weighted kappa index was computed to show the degree of agreement between the three ultrasonographic examiners who scored positive for NAFLD.
The Kruskal–Wallis test was used for the anthropometric and metabolic parameters to test for differences according to the three categories of NAFLD (absence, mild–moderate, serious). Additionally, Shaffe’s multiple comparison was used to show the pairwise difference of the same parameters between each of the three categories of NAFLD (see Table 2). Each subject was assigned a fatty infiltration score that represented the degree of NAFLD. The sum of the fatty infiltration scores resulted in the subject being categorized as non-NAFLD (category 1; score = 0); mild–moderate NAFLD (category 2; score = 1, 2); serious NAFLD (category 3; score = 3–6). For the above three categories of NAFLD, mean values ± SE of each component of pediatric metabolic syndrome, such as WC, WC/H, BMI, SBP, DBP, TG, HDL-C, and FPG, were measured and analyzed. The IRI level and HOMA-IR index were included to explore the relationship between NAFLD and insulin resistance.
Multiple logistic regression analysis was used to examine the linear trend of MetS-JC and insulin resistance (HOMA-IR) in terms of identifying NAFLD (see Tables 3, 4).
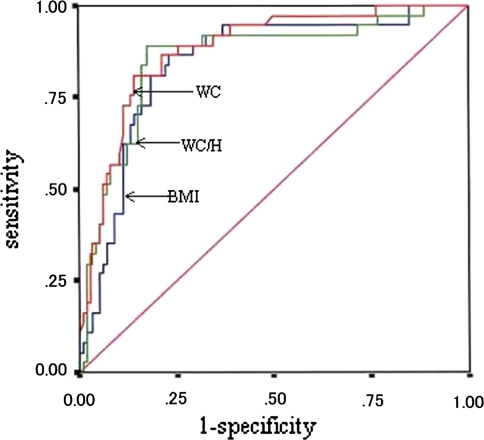
Receiver operating characteristic (ROC) curve analysis was used to compare the three different obesity indices to estimate the presence of pediatric NAFLD (see Fig. 1).
Table 1.
Number of subjects with non-alcoholic fatty liver disease according to sex
| Sexa | Subjects (n) aged 6–10 years with NAFLDb | Subjects (n) aged 11–15 years with NAFLDb | Cases (n) of NAFLD in total study cohortb |
|---|---|---|---|
| Male | 10/194 (5.2) | 19/244 (7.8) | 29/438 (6.6) |
| Female | 3/194 (1.5) | 5/214 (2.3) | 8/408 (2.0) |
| Total | 13/388 (3.4) | 24/458 (5.2) | 37/846 (4.4) |
NAFLD, Non-alcoholic fatty liver disease (NAFLD)
aSex differences in terms of presence of NAFLD was examined by the Mantel–Haentzel χ2 test (Yaetes correction, P = 0.003)
bValues are given as the number of each group with NAFLD relative to the study population under consideration. The percentage of the subjects with NAFLD are given in parenthesis
Table 2.
Anthropometric and metabolic parameters by graded NAFLD
| Parametersa | Non-NAFLD, category 1 (n = 113) | Mild–moderate NAFLD, category 2 (n = 25) | Serious NAFLD, category 3 (n = 12) | P for trendb | P valuec |
|---|---|---|---|---|---|
| WC (cm) | 63.5 ± 0.8 | 76.5 ± 1.8 | 85.1 ± 2.1 | <0.001 | <0.001 (1 vs. 2) |
| 0.026 (2 vs. 3) | |||||
| <0.001 (1 vs. 3) | |||||
| WC/H (index) | 0.4 ± 0.005 | 0.5 ± 0.01 | 0.5 ± 0.01 | <0.001 | <0.001 (1 vs. 2) |
| <0.001 (1 vs. 3) | |||||
| BMI (index) | 18.9 ± 0.3 | 23.2 ± 0.7 | 26.5 ± 1.1 | <0.001 | <0.001 (1 vs. 2) |
| <0.001 (1 vs. 3) | |||||
| 0.035 (2 vs. 3) | |||||
| SBP (mmHg) | 105.6 ± 1.2 | 111.0 ± 2.3 | 116.4 ± 3.1 | 0.008 | 0.028 (1 vs. 3) |
| DBP (mmHg) | 51.3 ± 0.9 | 56.0 ± 2.0 | 59.7 ± 2.6 | 0.004 | 0.024 (1 vs. 3) |
| TG (mg/dl) | 56.2 ± 2.6 | 80.7 ± 14.6 | 90.5 ± 10.1 | 0.001 | 0.022 (1 vs. 2) |
| 0.019 (1 vs. 3) | |||||
| HDLC (mg/dl) | 36.1 ± 0.6 | 30.5 ± 1.0 | 29.1 ± 2.3 | <0.001 | 0.002 (1 vs. 2) |
| 0.006 (1 vs. 3) | |||||
| FPG (mg/dl) | 91.7 ± 0.7 | 91.7 ± 1.2 | 94.7 ± 1.6 | 0.232 | NS |
| IRI (μU/ml) | 8.3 ± 0.3 | 10.8 ± 0.8 | 13.8 ± 1.4 | <0.001 | 0.007 (1 vs. 2) |
| <0.001 (1 vs. 3) | |||||
| HOMA-IR (index) | 1.9 ± 0.1 | 2.4 ± 0.2 | 3.2 ± 0.3 | <0.001 | 0.018 (1 vs. 2) |
| <0.001 (1 vs. 3) |
aFor definition of the terms, see the Abbreviation section
bThe kruskal–Wallis test was performed to test for the difference among the three categories
cPairwise difference between the three categories by Shaffe’s Multiple Comparison
Table 3.
Odds ratio for NAFLD according to the number of MetS-JC components
| Number of MetS-JC components | ||||
|---|---|---|---|---|
| 0 | 1 | 2 | ≥3 | |
| Number of subjects without NAFLD (%) | 30 (96.8) | 65 (81.2) | 15 (60) | 3 (21.4) |
| Number of subjects with NAFLD (%) | 1 (3.2) | 15 (18.8) | 10 (40) | 11 (78.6) |
| Odds ratio (95% CI) | 1.0 | 7.0 (0.09–55.82) | 19.8 (2.30–169.61) | 122.3 (11.22–1333.69) |
MetS-JC Pediatric metabolic syndrome in Japan, CI confidence interval
Values are sex- and age-adjusted; trend test (P < 0.001)
Table 4.
Odds ratio for NAFLD by HOMA-IR
| HOMA-IR (percentile) | ||||
|---|---|---|---|---|
| ≤25 | 25–50 | 51–75 | ≥75 | |
| Number of subjects without NAFLD (%) | 35/36 (97.2) | 30/38 (78.9) | 25/37 (67.6) | 23/39 (59.0) |
| Number of subjects with NAFLD (%) | 1/36 (2.8) | 8/38 (21.1) | 12/37 (32.4) | 16/39 (41.0) |
| Odds ratio (95% CI)a | 1.0 | 10.2 (1.18–88.33) | 19.3 (2.31–161.19) | 31.6 (3.69–271.17) |
| Odds ratio (95% CI)b | 1.0 | 2.4 (0.23–24.9) | 3.9 (0.40–38.12) | 3.6 (0.33–39.16) |
HOMA-IR, Homeostasis model assessment-insulin resistance
aAge- and sex-adjusted, trend test (P < 0.001)
bAge-, sex-, and WC-adjusted
Fig. 1.
Receiver operating characteristic curves of waist circumference (WC), waist circumference/height (WC/H), and body mass index (BMI)
Results
As shown in Table 1, of the 846 students, aged 6–15 years, enrolled in the study and screened by ultrasonography, 37 children were diagnosed as having NAFLD (score ≥ 1). The prevalence of mild to serious NAFLD (score ≥ 1) was 4.4% among the entire study cohort, and 6.6% in males and 2.0% in females when categorized according to sex. The prevalence of serious NAFLD (score ≥ 3) was 1.4% among the entire study cohort, and 2.3% in males and 0.5% in females when categorized according to sex. As shown in Table 1, among the study cohort, there was a sex difference in the prevalence of NAFLD (Mantel–Haentzel chi-square = 8.58, P = 0.003).
This study was designed on the basis of a large-scale ultrasonographic survey. Therefore, it was essential that the independent ultrasonographers agreed with each other in terms of their evaluation of NAFLD. Agreement between the independent observers in terms of their numerical scoring for the presence of fatty liver was highly consistent (weighted kappa index = 0.866, P < 0.0005; percentage kappa = 87.3%).
Our primary focus was an exploration of the relationship between NAFLD and anthropometric and metabolic parameters in the pediatric population. We first performed a univariate analysis using a logistic model to identify the estimated anthropometric and metabolic risk factors for NAFLD. The results of this analysis were: WC [odds ratio (OR) 1.2, 95% confidence interval (CI) 1.11–1.24], IRI (OR 1.3, 95% CI 1.12–1.40), HOMA-IR (OR 2.3, 95% CI 1.51–3.50), respectively.
We then investigated the risk factors for NAFLD increase in terms of the degree of fatty infiltration. As shown in Table 2, we conducted a Kruskal–Wallis test and a Schaffe’s multiple comparison to examine the difference between the three categories of NAFLD. The results of the Kruskal–Wallis test showed a statistically significant difference for WC, WC/H, BMI, SBP, DBP, TG, HDL-C, IRI, HOMA-IR, but not for FPG, according to the three categories of the degree of NAFLD—that is, there were significant linear trends with increasing degree of NAFLD. In addition, the results of the Schaffe’s multiple comparison showed that WC increases linearly with increasing degree of NAFLD, with each pairwise comparison [non-NAFLD (category 1) vs. mild–moderate NAFLD (category 2), 2 vs. 3 (serious NAFLD), 1 vs. 3] showing significant differences. In terms of the trend of BPs according to fatty infiltration level, both the SBP and DBP were significantly higher in the serious NAFLD group compared to the control (normal) group (1 vs. 3). These results suggest that BPs are related to only serious NAFLD. With respect to the changes in lipid profiles, increasing TG levels were associated with an accumulation of fat in the liver, and a linear reduction of HDL-C was also observed (the 1 vs. 2, 1 vs. 3 comparisons both showed statistically significant differences in TG and HDL). However, the FPG level was not statistically significant between the three groups. Overall, these results strongly suggest that there were significant linear trends for the anthropometric and metabolic parameters, with the exception of FPG, with increasing degree of NAFLD.
We performed a multiple logistic regression analysis to determine whether there was a relationship between the clustering of MetS-JC components and the risk of NAFLD. The results were an OR = 7.0 (95% CI 0.09–55.82), OR = 19.8 (95% CI 2.30-169.61), and OR = 122.3 (95% CI 1.22–1,333.69) for one, two, and three or more components, respectively (Table 3). The ORs for NAFLD increased with the clustering of the number of components (the linear trend was significant, P < 0.001). A multiple logistic regression analysis was also performed to explore the relationship between insulin resistance and pediatric NAFLD. Table 4 shows that the ORs in terms of increasing grade of HOMA-IR index (compared to the ≤25 percentile). The fourth quartile compared with the first quartile was OR = 31.6 (95% CI 3.69–271.17). The trend test was statistically significant (P < 0.001); however, when WC was added to the logistic model, it was no longer statistically significant, while WC was an independent risk factor for NAFLD (WC: OR 1.16, 95% CI1.09–1.23; results not shown in Table 4). In general, model fitting in a logistic regression was sensitive to multicolinearlities among the independent variables. We found a correlation between WC and the HOMA-IR index of r = 0.467 (P < 0.001), which indicates that both variables can be placed together in the logistic models.
As shown in Fig. 1, we applied an ROC curve analysis of NAFLD according to the three obesity indices. The area under the curves in terms of identifying pediatric NAFLD according to WC, WC/H, and BMI were: WC (0.880, 95% CI 0.818–0.941), WC/H (0.856, 95% CI 0.781–0.932), BMI (0.842, 95% CI 0.768–0.916), respectively. The best discriminating points (cut-offs) would have been: WC = 74 (sensitivity 81.8%, specificity 85.8%), WC/H = 0.5 (sensitivity 89.0%, specificity 81.0%), and BMI (sensitivity 83.8%, specificity 77.0%), respectively.
Discussion
Prevalence of pediatric NAFLD
The prevalence of NAFLD in the pediatric population is increasing with lifestyle changes that are leading to obesity. However, only a few population-based epidemiological studies have been carried out on pediatric NAFLD and its relationship to metabolic syndrome, insulin resistance, and WC. Among the entire pediatric population enrolled in this study, the prevalence of mild–serious NAFLD (score ≥ 1) was 4.4%, with a prevalence of 6.6% in males and 2.0% in females. This result confirms the existence of a gender difference in the prevalence of NAFLD, which has also been shown in previous studies [2, 9].
Metabolic syndrome and pediatric NAFLD
The primary aim of our study was to examine the relationship between NAFLD and metabolic syndrome. Metabolic syndrome is a complex condition and consists of interrelated metabolic abnormalities that increase the risk for cardiovascular disease, diabetes mellitus, and polycystic ovary syndrome in women [19]. Based the results of our study, the components of metabolic syndrome, with the exception of FPG, showed a linear trend in accordance to the degree of fat accumulation of the liver. These observations support the hypothesis that ultrasonographic grading of NAFLD is a useful diagnostic tool for estimating the grade of metabolic syndrome.
If pediatric metabolic syndrome is an early symptom of NAFLD [20], or vice versa, school-based screening for this condition would be a useful approach for detecting students at risk of developing cardiovascular disease and diabetes later in life. Population-based prospective studies are needed to clarify the causal relationship between NAFLD and metabolic syndrome.
Although FPG is one of the components of MetS-JC, it was not statistically significant between the three groups of NAFLD. Park et al. [21] also found that fasting blood glucose was not significantly different between pediatric NAFLD and a normal control group. Further, Genuth et al. [22] reported that only 1% of the overweight children demonstrated impaired FPG even when the new World Health Organization definition was applied. Further studies are needed to determine whether FPG should be included as a diagnostic criteria of pediatric metabolic syndrome or not.
The results of our study show that the risk of NAFLD increases according to the clustering of the components of MetS-JC (trend test, P < 0.001).
Insulin resistance and pediatric NAFLD
Although pediatric metabolic syndrome has been proven to be a risk factor for NAFLD [21], it is still unclear whether insulin resistance is an independent risk factor for pediatric NAFLD. Kawasaki et al. [23] suggested that hyperinsulinemia in obese children is the most important predictor of NAFLD; however, it has not been fully explored whether hyperinsulinemia is the primary disorder of NAFLD in children. Bugiaesi et al. [24] conducted an experimental study, and their results suggest that adipose tissue is an important site for insulin resistance development and resultant NAFLD, although this remains to be confirmed by further studies.
Based on the Kruskal–Wallis test and Shaffe’s multiple comparison, the subjects of our study showed significant linear increases of IRI and HOMA-IR levels with increasing degree of NAFLD. The HOMA-IR index has been widely accepted as an indicator of the insulin-resistant state. We showed that the ORs for NAFLD increased with increasing percentile of HOMA-IR index following an adjustment for age and sex. However, when WC was added to the logistic model, this trend of ORs was no longer significant.
Our results confirm the inference that there is a strong association between insulin resistance and NAFLD. However, the question of whether hyperinsulinemia or insulin resistance is the primary disorder of NAFLD in children remains unanswered.
Waist circumference and NAFLD
Waist circumference is recognized as a simple and variable parameter for estimating visceral fat accumulation and metabolic syndrome [25–27]. In the ROC curves analysis of NAFLD in relation to the obesity indices, such as WC, WC/H, and BMI, the area under the curve (AUC) showed only a slight difference, but WC appears to be the most sensitive obesity index for detecting pediatric NAFLD.
Interrelationships between NAFLD, metabolic syndrome, insulin resistance, and WC
Manco et al. [28] conducted a cohort study and concluded that metabolic syndrome, insulin resistance, and NAFLD in children were interrelated. In their cohort study, Hamaguchi et al. [29] showed that metabolic syndrome is a predictor for NAFLD. In our study, we showed high ORs and a linear trend between clustering MetS-JC’s components and the prevalence of NAFLD. In addition, we found that HOMA-IR appears to be a sensitive risk factor for NAFLD. This result indicates a dose–response relationship between NAFLD and insulin resistance. These findings support the importance of an ultrasonographic examination of NAFLD in children as a simple and useful tool to detect the insulin-resistant condition as well as metabolic syndrome because NAFLD may be a hepatic symptom of insulin resistance and/or metabolic syndrome. Thus, our cross-sectional, case–control study revealed a relationship between fat accumulation in the liver and the magnitude of insulin resistance and metabolic syndrome in the pediatric population.
A number of studies have shown that obesity per se is not always related to the development of NAFLD. Nobili et al. [30] reported that children with NAFLD are almost always insulin resistant regardless of BMI. Conversely, in our population-based study, WC was an independent risk factor for NAFLD regardless of HOMA-IR index. In addition, WC linearly increased with the degree of NAFLD.
We conclude that WC should be recognized as a simple and variable parameter to estimate liver fat accumulation. Hamaguchi et al. recently reported the results of a cohort study in which those participants with NAFLD and metabolic syndrome at baseline developed cardiovascular disease faster than their control counterparts. These researchers concluded that NAFLD—but not metabolic syndrome—retained a statistically significant correlation with cardiovascular disease [31].
Although further studies are needed to elucidate the interrelationship between NAFLD, metabolic syndrome, insulin resistance, and WC, it is clearly important to detect NAFLD in the early stage of life to prevent cardiovascular disease at a later stage. This should be clarified through well-designed prospective epidemiological studies and biochemical investigations.
Limitations of the present study
There are several potential limitations to our study. Firstly, because of its cross-sectional nature, the causal relationship between the pediatric NAFLD and MetS-JC, insulin resistance, and WC was not fully explored. Secondly, the prevalence of pediatric NAFLD was low at the time of the study. This may reflect the large confidence intervals of ORs in the multiple logistic regression analysis.
Conclusions
In conclusion, NAFLD in children and adolescents has a close relationship with metabolic syndrome, insulin resistance, and WC. Ultrasonographic examination can be considered to be a valid and reliable procedure in any population-based screening for NAFLD. Among the anthropometric indices or biochemical markers, the most sensitive, simplest, and cost-effective index for the pediatric NAFLD is a WC measurement, although the MetS-JC and HOMA-IR index are also highly sensitive risk factors for NAFLD. However, in school-based surveys, cost-effectiveness considerations may recommend against the latter. The natural history of pediatric NAFLD has not yet been fully explored; however, children with pediatric NAFLD can develop cirrhosis. Although details of the disease pathogenesis in pediatric NAFLD remain unclear, it is necessary to explore the interrelationship between NAFLD, central obesity, insulin resistance, and resultant metabolic syndrome. To reduce the risk for cardiovascular disease and/or diabetes mellitus in adulthood, healthcare providers will have to find ways of preventing pediatric NAFLD. Such measures are urgently needed as part of child healthcare strategies.
Acknowledgments
This study was supported, in part, by a Grant-in-Aid for Scientific Research of the Ministry of Education, Science and Culture of Japan: (C).066-70388, in 1994. We appreciate the assistance of M. Melby, Noyuri Tominaga, and Edward Brawn with the English correction, and that of Abe Ichiro, Kusano Yoshimasa, Miyagawa Shouhei, and the staffs of the Department of Hygiene, Fukushima Medical University, who assisted in the anthropometric and ultrasonographic measurements of the participants.
Abbreviations
- AUC
Area under the curve in ROC curve analyses
- DBP
Diastolic blood pressure
- FPG
Fasting plasma glucose
- HDL-C
High-density lipoprotein-cholesterol
- HOMA-IR
Homeostasis model assessment-insulin resistance
- IRI
Immuno-reactive insulin
- MetS-JC
Pediatric metabolic syndrome in Japan
- NAFLD
Non-alcoholic fatty liver disease
- OR
Odds ratio
- SBP
Systolic blood pressure
- TG
Triglyceride
- WC
Waist circumference
- WC/H
Waist circumference to height ratio
- ≥
Represents a cut off point
References
- 1.Kosti RI, Panagiotakos DB. The epidemic of obesity in children and adolescents in the world. Cent Eur J Public Health. 2006;14:151–9. [DOI] [PubMed]
- 2.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors. Gastroenterology. 2007;133:1814–20. [DOI] [PMC free article] [PubMed]
- 3.Mager DR, Roberts EA. Nonalcoholic fatty liver disease in children. Clin Liver Dis. 2006;10:109–31. [DOI] [PubMed]
- 4.Chan DF, Li AM, Chu WC, Chan MH, Wong EM, Liu EK, et al. Hepatic steatosis in obese Chinese children. Int J Obes Relat Metab Disord. 2004;28:1257–63. [DOI] [PubMed]
- 5.Sagi R, Reif S, Neuman G, Webb M, Phillip M, Shalitin S. Nonalcoholic fatty liver disease in overweight children and adolescents. Acta Paediatr. 2007;96:1209–13. [DOI] [PubMed]
- 6.Sherlock S, Dooley J. Diseases of the liver and biliary system, 11th edn. Oxford: Blackwell Science; 2002.
- 7.Rashid M, Roberts EA. Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. 2000;30:48–53. [DOI] [PubMed]
- 8.Imhof A, Kratzer W, Boehm B, Meitinger K, Trischler G, Steinbach G, et al. Prevalence of non-alcoholic fatty liver and characteristics in overweight adolescents in the general population. Eur J Epidemiol. 2007;22:889–97. [DOI] [PubMed]
- 9.Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, et al. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. 1995;40:2002–9. [DOI] [PubMed]
- 10.Ohzeki T. Metabolic syndrome in children, vol. 423. International Medical News. 2007:6. Available at: http://www.imsj.or.jp/e/imn_e/e423.pdf.
- 11.Abe Y, Kikuchi T, Nagasaki K, Hiura M, Tanaka Y, Ogawa Y, et al. Lower birth weight associated with current overweight status is related with the metabolic syndrome in obese Japanese children. Hypertens Res. 2007;30:627–34. [DOI] [PubMed]
- 12.Ohkuni M, Murata M. Shoniseijinbyo handbook. Japan: Chugai Igaku-sha; 1991.
- 13.Saverymuttu SH, Joseph AEA, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J. 1986;292:13–5. [DOI] [PMC free article] [PubMed]
- 14.Fenkci S, Rota S, Sabir N, Akdag B. Ultrasonographic and biochemical evaluation of visceral obesity in obese women with non-alcoholic fatty liver disease. Eur J Med Res. 2007;12:68–73. [PubMed]
- 15.Colicchio P, Tarantino G, del Genio F, Sorrentino P, Saldalamacchia G, Finelli C, et al. Non-alcoholic fatty liver disease in young adult severely obese non-diabetic patients in South Italy. Ann Nutr Metab. 2005;49:289–95. [DOI] [PubMed]
- 16.Hsieh SD, Yoshinaga H, Muto T. Waist-to-height ratio, a simple and practical index for assessing central fat distribution and metabolic risk in Japanese men and women. Int J Obes. 2003;27:610–6. [DOI] [PubMed]
- 17.Atabek ME, Pirgon O. Assessment of insulin sensitivity from measurements in fasting state and during an oral glucose tolerance test in obese children. J Pediatr Endocrinol Metab. 2007;20:187–95. [DOI] [PubMed]
- 18.Tresaco B, Bueno G, Pineda I, Moreno LA, Garagorri JM, Bueno M. Homeostatic model assessment (HOMA) index cut-off values to identify the metabolic syndrome in children. J Physiol Biochem. 2005;61:381–8. [DOI] [PubMed]
- 19.Cerda C, Pérez-Ayuso RM, Riquelme A, Soza A, Villaseca P, Sir-Petermann T, et al. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. J Hepatol. 2007;47:412–7. [DOI] [PubMed]
- 20.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23. [DOI] [PubMed]
- 21.Park HS, Han JH, Choi KM, Kim SM. Relation between elevated serum alanine aminotransferase and metabolic syndrome in Korean adolescents. Am J Clin Nutr. 2005;82:1046–51. [DOI] [PubMed]
- 22.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7. [DOI] [PubMed]
- 23.Kawasaki T, Hashimoto N, Kikuchi T, Takahashi H, Uchiyama M. The relationship between fatty liver and hyperinsulinemia in obese Japanese children. J Pediatr Gastroenterol Nutr. 1997;24:317–21. [DOI] [PubMed]
- 24.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–42. [DOI] [PubMed]
- 25.Morimoto A, Nishimura R, Kanda A, Sano H, Matsudaira T, Miyashita Y, et al. Waist circumference estimation from BMI in Japanese children. Diabetes Res Clin Pract. 2007;75:96–8. [DOI] [PubMed]
- 26.Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference, blood pressure, and lipid components of the metabolic syndrome. J Pediatr. 2006;149:809–16. [DOI] [PubMed]
- 27.Taylor RW, Jones IE, Williams SM, Goulding A. Evaluation of waist circumference, waist-to-hip ratio, and the conicity index as screening tools for high trunk fat mass, as measured by dual-energy X-ray absorptiometry, in children aged 3–19 years. Am J Clin Nutr. 2000;72:490–5. [DOI] [PubMed]
- 28.Manco M, Marcellini M, Devito R, Comparcola D, Sartorelli MR, Nobili V, et al. Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int J Obes Lond. 2008;32:381–7. [DOI] [PubMed]
- 29.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–8. [DOI] [PubMed]
- 30.Nobili V, Marcellini M, Devito R, Ciampalini P, Piemonte F, Comparcola D, et al. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology. 2006;44:458–65. [DOI] [PubMed]
- 31.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–84. [DOI] [PMC free article] [PubMed]