Abstract
The first amidoboronic acids were identified that show significant fluorescent property changes upon binding with various carbohydrates.
Keywords: Amidoboronic acid, Fluorescent, Sugar, Sensor
Boronic acids are known to form tight complexes with compounds containing the diol moiety. Such properties form basis for the design and synthesis of boronic acid-based carbohydrate sensors and transporters.1–11 So far, essentially all carbohydrate sensing studies have been done with arylboronic acids, which often have water solubility and stability problems.3 α-Amidoboronic acids is an important class of boronic acids that have been used for the development of enzyme, especially hydrolytic enzyme, inhibitors.12–14 However, there has never been effort in using amidoboronic acids for the design and synthesis of carbohydrate sensors despite the fact that such boronic acids have very desirable properties, such as high stability, high water solubility, modular synthesis, and easily controlled stereochemistry, for sensor work.12, 13, 15 One reason for the lack activities in using amidoboronic acids in carbohydrate sensing could be the lack of amidoboronic acids that change spectroscopic properties upon sugar binding. Such fluorescent boronic acid “reporters” are essential building blocks for the construction of fluorescent sensors. In addition, there has never been effort in examining the binding between amidoboronic acids and diols in a systematic and quantitative fashion. Herein we report the very first amidoboronic acids that change fluorescent properties upon sugar binding. In addition, the binding affinities these amidoboronic acids with three representative sugars were examined and were found to be comparable to that of arylboronic acids. We hope that publication of such work will stimulate further and extensive work in using amidoboronic acid for sensor design.
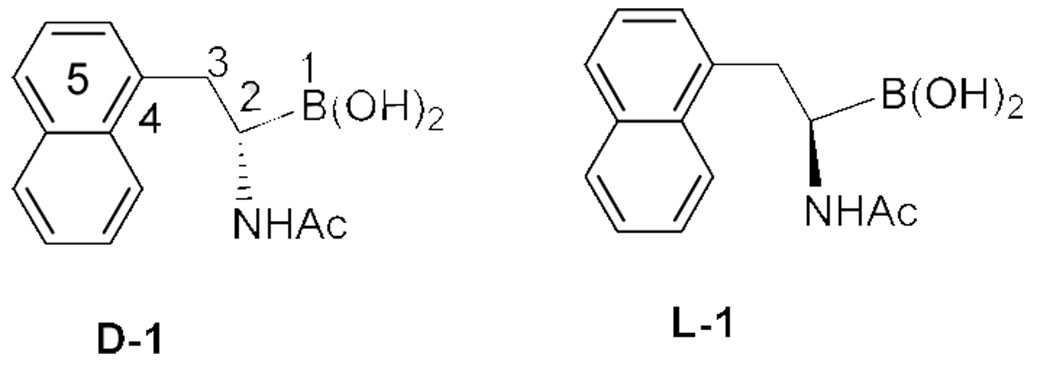
In designing amidoboronic acid-based fluorescent reporters, one does have to keep in mind of their differences from arylboronic acids. In the case of arylboronic acids, the boron is directly attached to the aryl group. Therefore, diol binding and the subsequent boron hybridization changes often directly affect the chromophoric properties of the aryl group. Such a direct effect has been explored for the design and synthesis of many fluorescent boronic acid reporter compounds.3, 16 However, in the case of amidoboronic acids the boron open shell is not in direct conjugation with the chromophore. Therefore, any effect that binding could have on the fluorescent properties of a tethered fluorophore would be through space. Consequently, it would be desirable to have a short “linker” between the boronic acid unit and the fluorophore. We envisioned that a pseudo-1,5 or 1,6 relationship (Figure 1) between the boron atom and the fluorophore would give the best chance for through space interactions. Therefore, we designed compounds 1 (Figure 1) which has a naphthalene fluorophore. We made this selection because of our past experience with this chromophore17–20 and the ready avalability of synthetic procedures.15
Figure 1.
An enantiomeric pair of α-amidoboronic acids
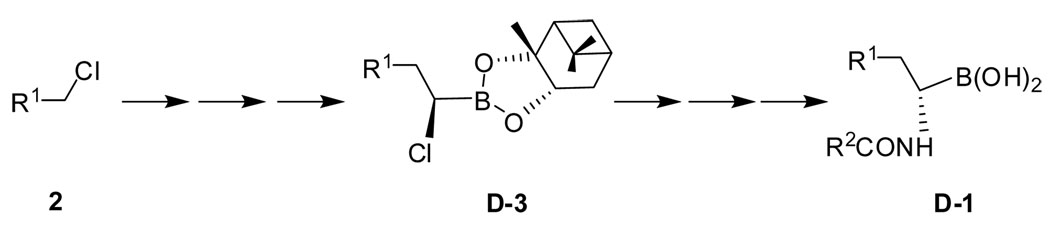
Amidoboronic acids 1 (Figure 1) were prepared by following literature procedures (Scheme 1, R1 = naphthyl).12, 15 The enantiomeric purity was established by determining the optical rotation of each isomer [L-(R)-1: [α]20 D = −140.3 (c 1.22, MeOH) and D-(S)-1: [α]20 D = +143.8 (c 1.35, MeOH)]15 and HPLC. It is important to note that established synthetic procedures for amidoboronic acids allow for the incorporation of various functional groups for future work in constructing sensors with various scaffolds. Specifically, synthesis can start with simple alkyl halides (Scheme 1). Various functional groups can be introduced at the R1 and R2-positions including fluorophores such as a naphthalene group. The R2 position especially has a high degree of flexibility to allow for the incorporation of functional groups such as a alkyne or azido group, which can later be used for conjugation through click chemistry.21–23 The stereochemistry of the chiral center can be carefully controlled through the use of pinanediol, which is commercially available in both enantiomeric forms.12, 15 The reaction de is generally very high. All these indicate the versatility and modular nature of the chemistry available for the future synthesis of amidoboronic acid-based sensors.
Scheme 1.
Synthesis route of D-1 (R1 = naphthyl).
Before any binding studies, we first examined the water solubility of the synthesized boronic acids using UV. The UV absorbance of both enantiomers showed good linearity in the concentration range of 1×10−4 to 1×10−6 M, indicating good solubility for the purpose of sensing. This is a sufficient concentration range for sensor work.
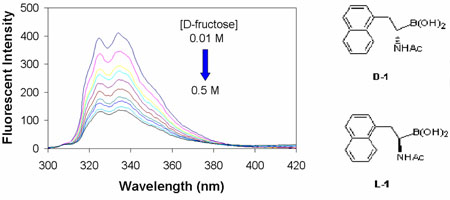
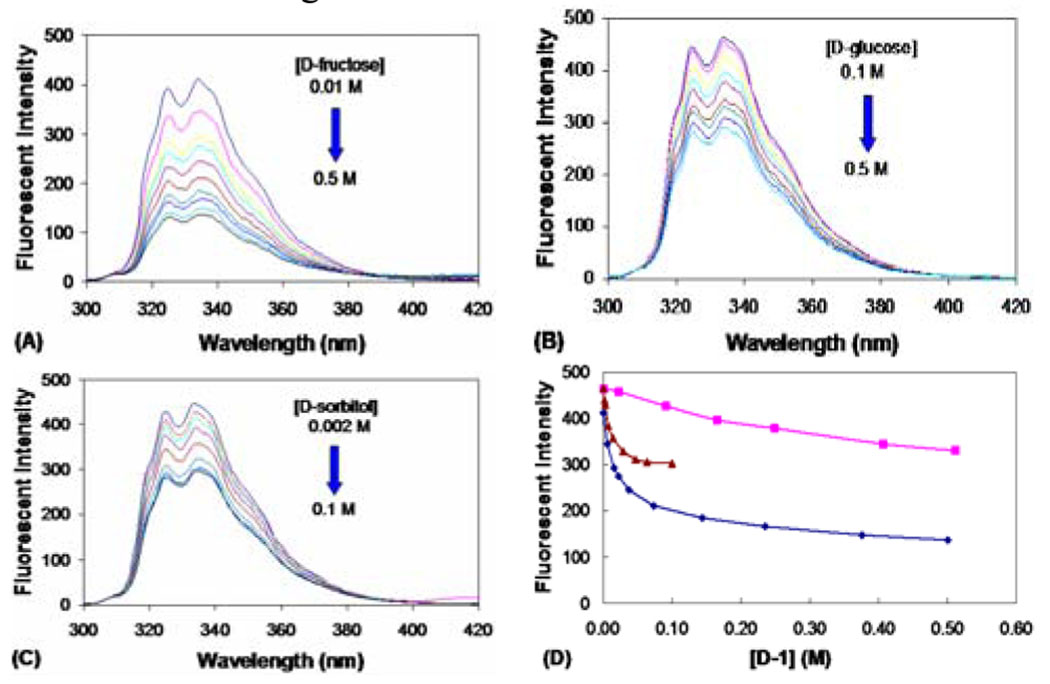
Next we were interested in studying whether diol binding would trigger fluorescent changes. Much to our delight, addition of sugars to β-naphthyl-α-amidoboronic acid induced significant fluorescent intensity changes in phosphate buffer (Figure 2). Specifically, for boronic acid D-1, the fluorescent intensity decreased by 80%, 34%, and 33% upon binding with D-fructose (0.5 M), D-glucose (0.5 M) and D-sorbitol (0.1 M) (Figure 2). It is interesting to note that no noticeable differences were observed between the D and L isomers in their binding and spectral changes upon addition of a carbohydrate. The binding constants between D-sugars and L-1 and D-1 were very similar, if not identical (Table 1). For example, the apparent association constants (Ka) of L-1 with D-fructose, D-glucose, and D-sorbitol were 46, 1.5, and 102 M−1 respectively. On the other hand, the apparent association constants (Ka) of D-1 with D-fructose, D-glucose, and D-sorbitiol were 55, 1.6, and 100 M−1 respectively. These binding constants are comparable, but slightly lower than those observed with phenylboronic acid.24, 25 The fact that the D- and L-enantiomers of 1 showed similar binding affinities suggests that binding involved simple boronic acid-diol interactions and other groups on the amidoboronic acid did not engage in significant interactions. Such results also suggest that if we were to take advantage of the chiral nature of amidoboronic acid for enantiomeric discrimination, additional interaction points are needed. Currently, we are working on the synthesis of bisboronic acids using amidoboronic acids as the basic building blocks.
Figure 2.
Fluorescent spectral changes of boronic acid D-1 upon addition of different diols in phosphate buffer (0.1 M) at pH 7.4: λex = 280 nm, λem = 334 nm. (A) D-fructose; (B) D-glucose; (C) D-sorbitol; (D) plots of fluorescent intensity changes of D-1 as a function of sugar concentration: D-fructose (◆), D-glucose (■), D-sorbitol (▲). [D-1] = 5 × 10−6 M.
Table 1.
Apparent association constants (Ka) of boronic acids L-1 and D-1 with different diols
|
Ka[a] (M−1) |
D-fructose | D-glucose | D-sorbitol |
|---|---|---|---|
| L-1 | 46 ± 2 | 1.5 ± 0.4 | 102 ± 2 |
| D-1 | 55 ± 8 | 1.6 ± 0.1 | 100 ± 6 |
Binding studies were conducted in phosphate buffer (0.1 M) at pH 7.4 ([boronic acid] = 5 × 10−6 M)
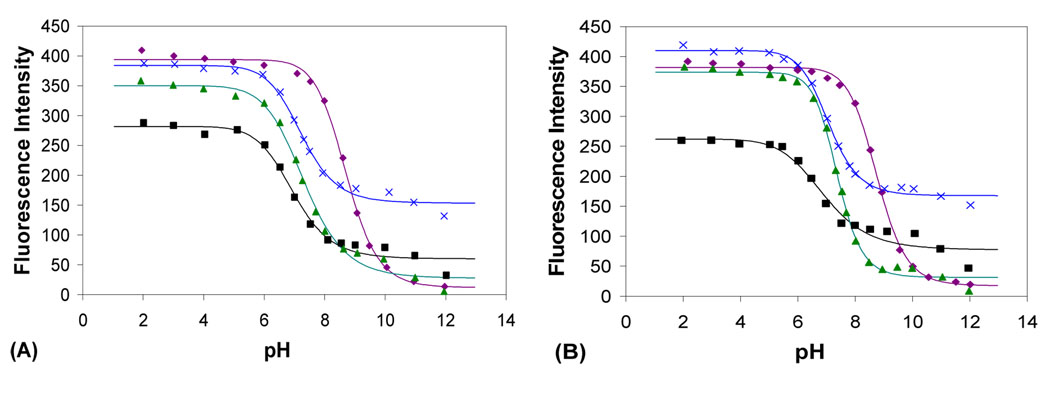
Aimed at understanding the basic mechanism through which fluorescent intensity changes occur, we also studied the pH profiles of the fluorescence intensity in the absence and presence of sugars. When tested in the absence of any sugar, the emission intensity of D-1 decreased by 30-fold at 334 nm upon changing the pH from 2 to 12 (Figure 3), with an apparent pKa of about 8.8, which was assigned to the boronic acid moiety (Table 2). The pH titration of the enantiomer L-1 gave similar results.
Figure 3.
pH profile of the fluorescence intensity of boronic acid in the absence and presence of sugars in 0.1 M aqueous phosphate buffer with 1% MeOH, λex = 280 nm, λem = 334 nm. Boronic acid alone (◆), in the presence of D-fructose (0.5 M) (■), in the presence of D-glucose (0.5 M) (▲), in the presence of D-sorbitol (0.1 M) (×). (A) D-1 (5 × 10−6 M); (B) L-1 (5 × 10−6 M).
Table 2.
Apparent pKa values of the boronic acids in the absence and presence of sugars
| pKa | Boronic acid | D-fructose (0.5 M) |
D-glucose (0.5 M) |
D-sorbitol (0.1 M) |
|---|---|---|---|---|
| D-1 | 8.75 ± 0.05 | 6.92 ± 0.06 | 7.38 ± 0.07 | 7.17 ± 0.08 |
| L-1 | 8.76 ± 0.00 | 6.83 ± 0.11 | 7.33 ± 0.06 | 7.08 ± 0.01 |
Boronic acid concentration: 5 × 10−6 M in 0.1 M buffer solution at pH 7.4 with 1% MeOH (All experiments were duplicated).
When the pH was changed from 2 to 12, the fluorescence intensity of D-1 at 334 nm decreased by 8, 68 and 2 fold in the presence of D-fructose (0.5 M), D-glucose (0.5 M), and D-sorbitol (0.1 M), respectively (Figure 3). The apparent pKa values observed were 6.9, 7.4, and 7.2 for the esters of fructose, glucose, and sorbitol, respectively. The apparent pKa values trend with both enantiomers followed the order of boronic acid alone > D-glucose ester > D-sorbitol ester > D- fructose ester (Table 2). The lower pKa values for the sorbitol and fructose esters than the glucose esters and boronic acids alone are consistent with previous findings.24–26 However, it is interesting to note that the pKa value of sorbitol esters were higher than that of the fructose esters. This is in contrast to earlier reports that boronic esters of arylboronic acids with D-sorbitol tend to have the lowest pKa values among the three sugars tested.3, 25, 27
In order to achieve some initial understanding of the origin of the fluorescent intensity changes, we studied the pH profiles of the UV absorbance in the absence and presence of sugars. For both enantiomers and their sugar complexes, the UV intensities did not change at 280 nm and 334 nm when the solution pH increased from 2 to 10. The absorbance intensity showed a slight increase at 280 nm when the solution pH increased from 10 to 12. Such results suggest that the observed fluorescent intensity changes by varying pH could not be attributed to the absorption changes at the excitation wavelength.
The fluorescent quantum yields for these boronic acids and their sugar esters were determined with L-(−)-tryptophan as a reference compound.28 The quantum yields of boronic acids were determined by equation (1), where Q represents quantum yield; A means UV absorbance; OD represents optical density (fluorescence); and subscript R denotes reference compound.29, 30
| (1) |
The results showed that the quantum yields trend followed the order of boronic acids alone > D-sorbitol esters > D-glucose esters > D-fructose esters (Table 3). However, these quantum yields are not directly correlated with the apparent pKa of each compound. This is understandable since many other factors such as flexibility, solvation, and excited state electron density distribution are expected to affect the quantum yields of these compounds as well.
Table 3.
Fluorescence quantum yields of the boronic acids alone and in the presence of various sugars
| FQY (%) | Boronic acid |
D-fructose (0.5 M) |
D-glucose (0.5 M) |
D-sorbitol (0.1 M) |
|---|---|---|---|---|
| D-1 | 15.3 ± 0.74 | 0.50 ± 0.03 | 5.95 ± 0.51 | 8.51 ± 0.37 |
| L-1 | 14.5 ± 0.01 | 0.56 ± 0.03 | 5.88 ± 0.73 | 8.06 ± 0.16 |
Boronic acid concentration: 5 × 10−6 M in 0.1 M buffer solution at pH 7.4 with 1% MeOH (All experiments were duplicated)
We also determined molar extinction coefficients of 1 and its esters in order to allow for correlation of the fluorescent intensity variations with quantum yields. The results showed that the molar extinction coefficients of the amidoboronic acids and their sugar esters followed the order of D-fructose esters> D-sorbitol esters > D-glucose esters> boronic acids alone (Table 4). The molar extinction coefficients of D-fructose esters were much larger than that of others, while D-fructose gave the lowest fluorescent quantum yield. Such results suggest that the large fluorescent intensity decrease of the amidoboronic acids upon binding with D-fructose was largely due to the low quantum yield of the complex, mitigated by the fairly high extinction coefficient.
Table 4.
Molar extinction coefficients of the boronic acids alone and in the presence of various sugars
| ε × 10−3 (L·cm−1·mol−1) |
Boronic acid |
D-fructose (0.5 M) |
D-glucose (0.5 M) |
D-sorbitol (0.1 M) |
|---|---|---|---|---|
| D-1 | 13.6 ± 0.3 | 56.8 ± 0.5 | 15.9 ± 0.4 | 14.0 ± 0.3 |
| L-1 | 13.2 ± 0.3 | 56.9 ± 1.6 | 15.7 ± 0.6 | 14.6 ± 0.3 |
Boronic acid concentration: 5 × 10−6 M in 0.1 M buffer solution at pH 7.4 with 1% MeOH (All experiments were duplicated)
Overall, the observed fluorescent pH-dependent changes can be largely attributed to the quantum yield variations in different complexes and at different ionization states of the boronic acids. Such a mechanism is also generally consistent with those for many arylboronic acid-based fluorescent reporters reported previously.27, 31–34 Therefore, it seems that positioning the boronic acid in a pseudo 1,5-relationship with a fluorophore does seem to allow for the modulation of the fluorescent properties of the fluorophore by sugar complexation and ionization state changes as designed. However, the exact electronic mechanism through which the fluorescent intensity changes is not clear. In a previous Czarnik paper34 and others,33 excited state photoelectron transfer was cited as a likely mechanism. It is reasonable to assume that in this case, the same mechanism is one distinct possibility.
In conclusion, we have described the very first water soluble amidoboronic acids that change fluorescent properties upon binding. Binding of these amidoboronic acids to three representative sugars, fructose, glucose, and sorbitol, is comparable to that of phenylboronic acids. The observed fluorescent pH profiles, quantum yield studies and molar extinction coefficient determination were conducted as well. We believe that the following features will make similar fluorescent amidoboronic acids very useful in carbohydrate sensor design. First, amidoboronic acids are known to be water soluble, stable, and biocompatible as demonstrated by an FDA-approved drug, bortezomib.13, 14 Second, there are well-established synthesis of amidoboronic acids that allow for careful control of the chiral center with high ee. Third, the synthesis is modular, which should allow for the introduction of functional groups for the easy synthesis of bisboronic acid compounds with various structural scaffolds and functional groups. We hope that publication of this work would trigger more extensive work in using amidoboronic acids for the development of sensors for carbohydrates, fluoride, cyanide, and other species known to interact with boronic acids. Work is underway in our lab to use amidoboronic acids for the synthesis of bisboronic acids using click chemistry. Work is also underway to develop amidoboronic acids that fluoresce at a longer wavelength.
Acknowledgements
Financial support from the Georgia Cancer Coalition, Georgia Research Alliance, and the National Institutes of Health (CA123329, CA113917 GM086925, and GM084933) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.James TD, Shinkai S. Top. Curr. Chem. 2002;218:159. [Google Scholar]
- 2.Lorand JP, Edwards JO. J. Org. Chem. 1959;24:769. [Google Scholar]
- 3.Yan J, Fang H, Wang B. Med. Res. Rev. 2005;25:490. doi: 10.1002/med.20038. [DOI] [PubMed] [Google Scholar]
- 4.Hall DG. Boronic Acids. Wiley-VCH; 2004. [Google Scholar]
- 5.Cao H, Diaz DI, DiCesare D, Lakowicz JR, Heagy MD. Org. Lett. 2002;4:1503. doi: 10.1021/ol025723x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu L, Shabbir SH, Gray M, Lynch VM, Sorey S, Anslyn EV. J. Am. Chem. Soc. 2006;128:1222. doi: 10.1021/ja055817c. [DOI] [PubMed] [Google Scholar]
- 7.Duggan PJ, Offermann DA. Aust. J. Chem. 2007;60:829. [Google Scholar]
- 8.Smith BD, Gardiner SJ, Munro TA, Paugam MF, Riggs JA. J. Incl. Phenom. Mol. Recogn. Chem. 1998;32:121. [Google Scholar]
- 9.Jiang S, Rusin O, Escobedo JO, Kim KK, Yang Y, Fakode S, Warner IM, Strongin RM. J. Am. Chem. Soc. 2006;128:12221. doi: 10.1021/ja063651p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartley JH, James TD, Ward CJ. J. Chem. Soc., Perkin Trans. I. 2000:3155. [Google Scholar]
- 11.Wang W, Gao X, Wang B. Curr. Org. Chem. 2002;6:1285. [Google Scholar]
- 12.Matteson DS. Med. Res. Rev. 2008;28:233. doi: 10.1002/med.20105. [DOI] [PubMed] [Google Scholar]
- 13.Yang W, Gao X, Wang B. Med. Res. Rev. 2003;23:346. doi: 10.1002/med.10043. [DOI] [PubMed] [Google Scholar]
- 14.Bross PF, Kane R, Farrell AT, Abraham S, Benson K, Brower ME, Bradley S, Gobburu JV, Goheer A, Lee SL, Leighton J, Liang CY, Lostritto RT, McGuinn WD, Morse DE, Rahman A, Rosario LA, Verbois SL, Williams G, Wang YC, Pazdur R. Clinical. Cancer Res. 2004;10:3954. doi: 10.1158/1078-0432.CCR-03-0781. [DOI] [PubMed] [Google Scholar]
- 15.Martichonok V, Jones JB. J. Am. Chem. Soc. 1996;118:950. [Google Scholar]
- 16.Cao HS, Heagy MD. J. Fluor. 2004;14:569. doi: 10.1023/b:jofl.0000039344.34642.4c. [DOI] [PubMed] [Google Scholar]
- 17.Gao X, Zhang Y, Wang B. Org. Lett. 2003;5:4615. doi: 10.1021/ol035783i. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Zhang Y, Wang B. Tetrahedron. 2005;61:9111. [Google Scholar]
- 19.Gao X, Zhang Y, Wang B. New J. Chem. 2005;29:579. [Google Scholar]
- 20.Zhang Y, Gao X, Hardcastle K, Wang B. Chem.-Eur. J. 2006;12:1377. doi: 10.1002/chem.200500982. [DOI] [PubMed] [Google Scholar]
- 21.Huisgen R. In: 1,3-Dipolar Cycloaddition Chemistry. Padwa A, editor. New York: John Wiley; 1984. p. 1. [Google Scholar]
- 22.Kolb HC, Sharpless KB. Drug Discov. Today. 2003;8:1128. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 23.Tornøe CW, Christensen C, Meldal M. J. Org. Chem. 2002;67:3057. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 24.Springsteen G, Wang B. Tetrahedron. 2002;58:5291. [Google Scholar]
- 25.Yan J, Springsteen G, Deeter S, Wang B. Tetrahedron. 2004;60:11205. [Google Scholar]
- 26.Wang J, Jin S, Akay S, Wang B. Eur. J. Org. Chem. 2007:2091. [Google Scholar]
- 27.Jin S, Wang J, Li M, Wang B. Chem. Eur. J. 2008;14:2795. doi: 10.1002/chem.200701785. [DOI] [PubMed] [Google Scholar]
- 28.Kirby EP, Steiner RF. J. Phys. Chem. 1970;74:4480. doi: 10.1021/j100846a015. [DOI] [PubMed] [Google Scholar]
- 29.Kronman MJ, Holmes LG, Robbins FM. J. Biol. Chem. 1971;246:1909–1921. [PubMed] [Google Scholar]
- 30.Liang JN, Chakrabarti B. Biochemistry. 1982;21:1847–1852. doi: 10.1021/bi00537a022. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Jin S, Wang B. Tetrahedron Lett. 2005;46:7003. [Google Scholar]
- 32.Wang J, Lin N, Jin S, Wang B. Chem. Biol. Drug Design. 2006;67:137. doi: 10.1111/j.1747-0285.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 33.Yang W, He H, Drueckhammer DG. Angew. Chem. Int. Ed. 2001;40:1714. [PubMed] [Google Scholar]
- 34.Yoon J, Czarnik AW. J. Am. Chem. Soc. 1992;114:5874. [Google Scholar]