Abstract
Objective
This study examined whether exposure to pesticides, including DDT, was associated with longer time to pregnancy (TTP).
Methods
Pregnant women (N = 402) living in a migrant farmworker community were asked how many months they took to conceive. Women reported their and their partners' occupational and home pesticide exposure preceding conception. In a subset (N = 289), levels of DDT and DDE, were measured in maternal serum.
Results
No associations were seen with p,p'-DDT, o,p'-DDT, or p,p'-DDE. Maternal occupational pesticide exposure (fOR=0.8, 95% CI: 0.6, 1.0), home pesticide use (fOR=0.6, 95% CI: 0.4, 0.9), and residence within 200 feet of an agricultural field (fOR=0.7, 95% CI: 0.5, 1.0) were associated with reduced fecundability (i.e. longer TTP).
Conclusions
Longer TTP was seen among women, but not men, reporting exposure to agricultural and home pesticides.
The effects of pesticides on human fertility has raised considerable concern, beginning with the discovery thirty years ago of azoospermia and oliogospermia among workers exposed to the pesticide 1,2-dibromo-3-choloropropane (DBCP).1 Since then, several other pesticides have been associated with toxicologic effects on spermatogenesis in humans, including 2,4-D,2 carbaryl,3 and organophosphate pesticides,4, 5 but less is known about possible effects of pesticides on female reproductive capacity. With studies showing worldwide decline in sperm counts6, 7 and on-going discussion about whether infertility rates are changing over time,8 there is considerable concern about whether widespread exposure to pesticides, many of which may be endocrine disruptors, is impacting human fecundability.
The pesticide, dichlorodiphenyltrichloroethane (DDT), which was banned for use in the United States in 1973, and its breakdown product dichlorodiphenyldichloroethylene (DDE), have been shown to have endocrine disrupting properties.9 DDT and DDE exist in two isomeric forms; the o,p' and p,p' isomers of DDT have been shown to have estrogenic effects10, 11, while p,p'-DDE appears to have anti-androgenic properties.12 Two studies have examined DDT or DDE levels and menstrual cycle characteristics, both finding an association with shorter cycle length.13, 14 Two studies have also found DDT or DDE levels to be associated with lower levels of urinary progesterone 14, 15 and estrogen metabolites,15 and a rigorous study in China found DDT exposure to be associated with an increased odds of unrecognized fetal loss.16
Time to pregnancy, or the number of menstrual cycles required to achieve a recognized pregnancy, is the final endpoint of multiple biological processes in male and female partners and is a useful outcome for investigating the effects of environmental exposures on fecundability.17 Two studies have examined time to pregnancy in relation to maternal levels of serum DDE, but not DDT. Axmon et al.18 measured p,p'-DDE in cohorts of women in Sweden, Poland, Ukraine and Greenland and found current DDE levels to be associated with longer time to pregnancy only in the Greenland (Inuit) cohort. Median DDE levels in the Greenland cohort were 300 ng/g of lipid (range: 26 - 1700 ng/g), which was the lowest of the four locations. Law et al.19 examined p,p'-DDE levels in maternal serum during pregnancy in women participating in the Collaborative Perinatal Project in 12 U.S. cities from 1959 to 1965. Although DDE levels were considerably higher than in the Inuit cohort (median approximately 2500 ng/g of lipid (range: 0 - >7,685 ng/g)), the authors found no association with time to pregnancy.
Although DDT has been largely banned, a wide variety of other pesticides are currently in use. Several epidemiologic studies have examined the association of male and female exposure to currently-used pesticides with time to pregnancy. The term pesticide refers to hundreds of products with differing chemistry and toxicity, and most studies have used agricultural employment as a surrogate for pesticide exposure. Using paternal agricultural or greenhouse work, some studies have found longer time to pregnancy20, 21 or increased odds of delayed conception,22 but other studies have found no association.23-26 Similarly, maternal work in agriculture has been associated with longer time to pregnancy in some studies27, 28 but not others.23, 29, 30 Abell et al 27 retrospectively gathered time to pregnancy information from 492 female members of the Danish gardeners union. Although they found no difference in time-to-pregnancy comparing greenhouse workers with non-greenhouse workers, they observed reduced fecundability among those greenhouse workers reporting pesticide spraying, high pesticide exposure, and not wearing gloves. Idrovo et al 28 interviewed 2085 female floriculture workers in Colombian greenhouses about previous pregnancies and found that work in flowers during the year before pregnancy was associated with decreased fecundability. However, Curtis et al. found no significant association with reported pesticide use among Ontario farm families23 and two other studies found no association with maternal greenhouse work.29,30 Most previous studies of both men and women examined pregnancies and exposures occurring several years earlier, and many used external control groups, such as retail workers, for comparison.
To minimize recall time and improve accuracy of exposure assessment, we examined time to pregnancy in a cohort of pregnant women living in a farmworker community in California. California is home to approximately 700,000 migrant and seasonal farmworkers,31 most of whom are immigrants from Mexico.32 This group is at high risk of occupational and residential exposure to a variety of pesticides, including organophosphate and carbamate compounds.33 They are also likely to have high levels of DDT and DDE, because DDT was used in Mexico as recently as the year 2000.34 Thus, California farmworkers have the potential for high exposure to a variety of currently used, non-persistent pesticides as well as discontinued, persistent pesticides like DDT.
This study examines whether time to pregnancy is associated with maternal levels of the persistent pesticide DDT in serum during pregnancy and maternal or paternal exposure to non-persistent, current-use pesticides, estimated by work and home behaviors during the time before pregnancy.
MATERIALS AND METHODS
Study subjects
The women in this study were participants in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) project, a longitudinal cohort study of pesticides and other environmental exposures among pregnant women and children living in the Salinas Valley, California. The Salinas Valley is an agricultural region with large-scale production of salad and cole crops, an eleven-month growing season, and extensive use of agricultural pesticides. Pregnant women were recruited from five local prenatal clinics that serve a low-income, predominantly farmworker population. Women were eligible to participate if they were less than 20 weeks gestation at enrollment, spoke English or Spanish, were eligible for Medicaid, and were 18 years of age or older. Written informed consent was obtained, and study procedures were approved by the Committee for the Protection of Human Subjects at U.C. Berkeley.
Of 601 participants enrolled in this study, we excluded eight women missing time-to-pregnancy data, 177 women using contraception at the time of conception (contraceptive failures), and two women using fertility medication during the month of conception. Twelve women were missing information on agricultural work or pesticide use, resulting in a sample size of 402. DDT and DDE levels were available for a subset of 289 women who provided blood samples of adequate volume.
Time to pregnancy
An in-person interview was conducted at the time of enrollment (median: 13 weeks gestation, IQR: 10-18 weeks). The beginning of the pregnancy was determined by asking women the date of their last menstrual period (LMP). Women who did not know their LMP date were asked their due date and whether it had been estimated by ultrasound. If the due date was determined by ultrasound, LMP was estimated by back-calculation. The LMP date was then marked on a calendar which was referred to throughout the interview to clarify the time periods of interest and to aid with recall. Participants were asked, “How many months did it take to become pregnant? In other words, for how many months had you been having sexual intercourse without doing anything to prevent pregnancy?” Pregnancies occurring immediately were coded as one month.
Additional questions covered whether the couple had been trying to get pregnant, using infertility medication, or using contraceptives (either regularly or inconsistently) during the month of conception. Women were also asked about frequency of intercourse and prior contraceptive use.
Pesticide exposure
Exposure to DDT and DDE was measured in maternal blood collected during the second trimester of pregnancy (N = 265), or just before delivery (N = 24). Maternal serum was analyzed for p,p'-DDT, o,p'-DDT, and p,p'-DDE using gas chromatography and high resolution mass spectrometry. Levels below the limit of detection (LOD) were assigned the value LOD/2. Quality control samples were included in each run. Maternal serum was analyzed for total cholesterol and triglyceride levels using standard enzymatic methods (Roche Chemicals, Indianapolis, IN). DDT and DDE values were lipid-adjusted and reported as ng/g of lipid 35. Statistical analyses included DDT and DDE as continuous variables, both with lipid-adjustment and without (data not shown).
Women were asked about their potential exposure to current-use pesticides at work and at home during the time-to-pregnancy period (i.e. the time from when they stopped using contraception until LMP) using the calendar to aid recall. Women who had been using contraception were asked about exposures during the six months before conception. Women were asked whether they worked in agriculture (defined as planting, picking, thinning or other work in the fields; packing or handling of produce or flowers; or nursery or greenhouse work), whether they worked in gardening or landscaping, and whether they had applied pesticides. Women answering yes to any of these jobs at any time during the time-to-pregnancy period were categorized as having potential occupational pesticide exposure. Similar questions were asked about the child's father's work. To assess home pesticide exposure, each woman was asked whether her home was located within 200 ft of an agricultural field (interviewers pointed out a landmark located 200 ft away for reference) and whether anyone had applied pesticides in her home.
Covariates
Demographic information included maternal and paternal age, education, race, country of birth, and years of residence in the United States. Women were asked about their reproductive histories, including previous pregnancies, contraceptive use, menstrual cycle characteristics, and breastfeeding history. History of sexually transmitted infection, gynecological conditions, urogenital surgeries, or thyroid problems were combined into a single variable indicating a history of relevant medical conditions. Body mass index was calculated from mother's self-reported pre-pregnancy weight and measured height. Information was gathered about smoking, alcohol, and caffeine consumption from coffee, tea, and caffeinated soda in the three months before conception.
Statistical Analyses
Statistical analyses were performed using STATA 10 (Stata Corporation, College Station, TX). Cox proportional hazards models adapted for discrete time data were used to estimate fecundability odds ratios (fOR) for the association of pesticide exposure with time-to-pregnancy.36 The fOR compares the odds of achieving pregnancy during a cycle in the exposed versus unexposed women, conditional on survival to that cycle. An fOR less than one indicates longer time to pregnancy and decreased fecundability in the exposed group. Time to pregnancy was censored at 13 months for most analyses, although alternate censoring scenarios were explored in post hoc validation analyses.
Several covariates were identified a priori as potential confounders in the association of pesticide exposure and time to pregnancy, based on the literature. Covariates were retained in Cox models if they were independently associated with both time to pregnancy and pesticide exposure (either DDT/DDE levels or mother's occupational exposure status) or if their exclusion from the model changed the coefficient on the main exposures by ≥10 percent. Covariates retained in the final models were hormonal contraceptive use in the year before pregnancy; breastfeeding within two months before pregnancy; history of relevant medical condition; years of residence in the U.S. at the time of pregnancy; caffeine consumption during the three months before pregnancy; and whether the couple was actively trying to conceive. Maternal age at the beginning of the time-to-pregnancy period was not associated with the outcome, but was also included in all models. Because menstrual cycle irregularity might be on the causal pathway, final models were tested with and without this variable. Categories for covariates are as shown in Table 1, unless otherwise specified. Variables that were tested but were ultimately not retained in the model included paternal age (at pregnancy and at the beginning of the time-to-pregnancy period), maternal and paternal education, parity, marital status, maternal body mass index, maternal smoking, and length of menstrual cycle. Frequency of intercourse was missing for 42 women, but was not associated with time to pregnancy and did not change the coefficient on the main effects. To test for a “reproductively unhealthy” worker bias, we added maternal non-agricultural work to final models.
Table 1.
Selected characteristics of study population and their association with time to pregnancy, CHAMACOS Study, 1999-2000 (N = 402)
| Time-to-pregnancy |
||
|---|---|---|
| Unadjusted fOR (95% CI) | ||
| Time to Pregnancy (months), Median (IQR) | 2 (1, 6) | |
| Maternal age1 (years), Median (IQR) | 25 (21, 28) | 0.99 (0.97, 1.02) |
| Intercourse (times per month), Median (IQR) | 13 (9, 17) | 1.0 (0.99, 1.01) |
| Maternal ethnicity, N (%) | ||
| White, non-Latina | 6 (1.5) | 0.5 (0.2, 1.7) |
| Latina | 390 (97.0) | 1.0 |
| Other | 6 (1.5) | 0.7 (0.2, 3.0) |
| Length of residence in the U.S., N (%) | ||
| ≤ 5 years | 232 (57.7) | 1.0 |
| 6 to 10 years | 69 (17.2) | 0.6 (0.4, 0.8) ** |
| 11+ years | 60 (14.9) | 0.7 (0.5, 1.0) |
| Entire Life | 41 (10.2) | 0.8 (0.5, 1.2) |
| Maternal education, N (%) | ||
| ≤ 6th grade | 175 (43.5) | 1.0 |
| 7-12 grade | 145 (36.1) | 1.2 (0.9, 1.6) |
| Completed High School | 82 (20.4) | 1.0 (0.7, 1.4) |
| Prior pregnancy, N (%) | ||
| No | 169 (42.0) | 1.0 |
| Yes | 233 (58.0) | 0.8 (0.6, 1.0) |
| Trying to get pregnant, N (%) | ||
| No | 194 (48.3) | 1.0 |
| Yes | 208 (51.7) | 1.3 (1.0, 1.6) |
| Menstrual cycles, N (%) | ||
| Regular | 320 (79.6) | 1.0 |
| Irregular | 82 (20.4) | 0.5 (0.4, 0.7)** |
| Hormonal contraceptives in past year, N (%) | ||
| No | 279 (69.4) | 1.0 |
| Yes | 123 (30.6) | 1.8 (1.4, 2.2)** |
| Breastfeeding in past 2 months, N (%) | ||
| No | 384 (95.5) | 1.0 |
| Yes | 18 (4.5) | 0.3 (0.2, 0.6)** |
| History of gynecologic conditions, N (%) | ||
| No | 331 (82.3) | 1.0 |
| Yes | 71 (17.7) | 0.7 (0.5, 1.0)* |
| Caffeine consumption, N (%) | ||
| No | 59 (14.7) | 1.0 |
| Yes | 343 (85.3) | 0.8 (0.5, 1.1) |
| Maternal occupational pesticide exposure2, N (%) | ||
| No | 211 (52.5) | 1.0 |
| Yes | 191 (47.5) | 0.7 (0.5, 0.9)** |
| Paternal occupational pesticide exposure2, N (%) | ||
| No | 155 (38.6) | 1.0 |
| Yes | 247 (61.4) | 1.1 (0.9, 1.5) |
| Lived <200 feet from agricultural field, N (%) | ||
| No | 336 (83.6) | 1.0 |
| Yes | 66 (16.4) | 0.6 (0.4, 0.9) ** |
| Pesticides applied in home, N (%) | ||
| No | 357 (88.8) | 1.0 |
| Yes | 45 (11.2) | 0.6 (0.4, 0.9) * |
Maternal age at beginning of the time-to-pregnancy period
Includes work in agriculture, landscaping/gardening, or jobs involving application of pesticides.
p-value <0.05
p-value <0.01
After final models were identified, additional sensitivity analyses were conducted to check for possible biases, as recommended by Joffe 37. Models were repeated after expanding the study population to include women with contraceptive failures and exploring different ways to assign time to pregnancy among consistent and irregular contraceptive users (i.e. time to pregnancy = 0 or 1 for regular contraceptive users, time to pregnancy = 0, 1, or months of use/2 for irregular users). Models were also run: 1) changing the censoring of time to pregnancy to 7, 10, or 15 months, rather than 13 months; 2) limiting the study population to primiparous women; and 3) limiting the study population to couples who were actively trying to conceive. Results of these models were compared to the main models to check consistency of results.
RESULTS
Women in the study were mostly Latina, immigrants to the United States, and had less than high school education (Table 1). Forty-eight percent had potential pesticide exposure at work and 61 percent reported partners with potential occupational exposure, with almost all occupational exposure (>90%) resulting from work in agriculture. The mean age at the beginning of the time-to-pregnancy period was 25 years (range: 15 - 44 years). Most of the women had been pregnant before, had regular menstrual cycles, had not used hormonal contraceptives in the year before the time-to-pregnancy period, and had no history of gynecologic conditions. Only half of women reported that they had been trying to get pregnant; the others were trying not to get pregnant (12 percent) or were not concerned whether they got pregnant (36 percent). Median time to pregnancy was 2 months (range: 1 - 180 months).
In crude analyses (Table 1), longer residence in the U.S., irregular menstrual cycles, recent breastfeeding, and history of gynecologic conditions were associated with longer time-to-pregnancy. Recent hormonal contraceptive use was associated with shorter time-to-pregnancy. Longer time to pregnancy was also seen among women with potential occupational pesticide exposure, who had pesticides applied in their homes, or who lived within 200 feet of an agricultural field.
Almost all women (96 - 100%) with available blood samples had detectable levels of DDT and DDE. DDT and DDE levels in this population were higher than in a national reference population of women of childbearing age participating in the National Health and Nutrition Examination Survey (NHANES) during the same years. In the study population, the geometric mean of p,p'-DDE was 1,500 ng/g of lipid (range: 49 - 159,303 ng/g), of p,p'-DDT was 24 ng/g (range: 2 - 33,174 ng/g) and of o,p'-DDT was 2 ng/g (range: 0.1 - 1,878 ng/g). In contrast, median levels in NHANES were 210.5 ng/g of lipid (range 5.4 - 17,900 ng/g) for p,p'-DDE and 6.8 (range 3.3 - 1,070 ng/g) for p,p'-DDT. Most women in NHANES had o,p'-DDT below the limit of detection. The subset of women with DDT and DDE measurements did not differ from the full study population, except that they were slightly more likely to be recent immigrants to the U.S. and to have breastfed in the past 2 months.
Adjusted fecundability odds ratios and 95% confidence intervals (CI) for log-transformed, continuous, lipid-adjusted p,p'-DDT, o,p'-DDT, and p,p'-DDE are shown in Table 2, with a separate model constructed for each analyte. Separate models were also conducted examining p,p'-DDT, o,p'-DDT, and p,p'-DDE continuously without lipid adjustment, as quartiles of exposure, and as the ratio of DDT/DDE (not shown). No associations were seen with time to pregnancy and p,p'-DDT, o,p'-DDT, or p,p'-DDE in any analyses.
Table 2.
Association between maternal DDT and DDE levels (log10 transformed) and time to pregnancy, CHAMACOS Study, 1999-2000 (N = 289)
| fOR* | 95% CI | |
|---|---|---|
| p,p'-DDT | 0.96 | (0.79, 1.18) |
| p,p'-DDE | 0.91 | (0.68, 1.22) |
| o,p'-DDT | 0.98 | (0.79, 1.21) |
Models adjusted for maternal age, irregular menstrual cycle, hormonal contraceptive use in previous year, breastfeeding in previous two months, history of gynecologic conditions, caffeine consumption, years of residence in the U.S., maternal and paternal occupational exposure, home pesticide use, proximity of home to agricultural fields, and whether the couple was trying to become pregnant.
Adjusted fORs for behavioral markers of pesticide exposure are shown in Table 3; the four exposure variables were included in a single model to examine independent effects of maternal occupational, paternal occupational, and home exposures. Maternal work in agriculture was associated with reduced fecundability (fOR=0.7, 95% CI: 0.6, 1.0), but paternal work in agriculture was not (fOR=1.2, 95% CI: 0.9, 1.5). Reduced fecundability was also observed if pesticides were applied in the home (fOR=0.6, 95% CI: 0.4, 0.9) and if the home was located within 200 feet of an agricultural field (fOR=0.7, 95% CI: 0.5, 1.0).
Table 3.
Association of maternal and paternal markers of pesticide exposure with time to pregnancy, CHAMACOS Study, 1999-2000 (N = 402)
| fOR1 | 95% CI | |
|---|---|---|
| Mother worked in agriculture | 0.76 | (0.59, 0.99)* |
| Father worked in agriculture | 1.13 | (0.85, 1.49) |
| Pesticides used in home | 0.64 | (0.43, 0.94)* |
| Lived ≤200 feet from agricultural field | 0.69 | (0.48, 0.99)* |
Models adjusted for maternal age, irregular menstrual cycle, hormonal contraceptive use in previous year, breastfeeding in previous two months, history of gynecologic conditions, caffeine consumption, years of residence in the U.S., and whether the couple was trying to become pregnant.
p-value < 0.05
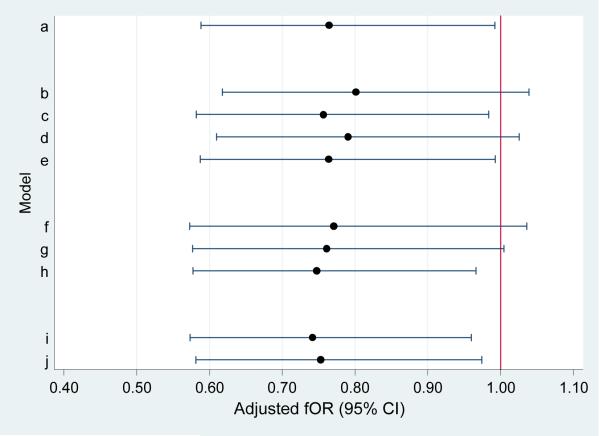
We conducted additional sensitivity analyses to determine whether the findings persisted under different conditions. Figure 1 shows the fOR and 95% CI for maternal occupational pesticide exposure: in the main model (a), when women who were using contraception were included in the sample (b-e), when the censoring cut-point was changed (f-h), and when the sample was restricted to primiparous women (N=170) (i), or to women actively trying to get pregnant (N=210) (j). For the most part, maternal occupational exposure continued to be associated with decreased fecundability, with a statistically significant fOR of 0.75 or less. Similarly, sensitivity analyses showed that findings of reduced fecundability associated with home pesticide application and residence near a field were robust under different conditions. Paternal occupational exposure was not associated with time to pregnancy in any analyses. Adding frequency of intercourse to final models did not change observed associations and was not itself associated with time to pregnancy (not shown). In addition, no associations were observed in models using DDT and DDE values unadjusted for lipids or adjusting for lipids separately in the model.
FIGURE 1.
Adjusted fOR (points) and 95% confidence intervals (lines) for association of maternal work in agriculture with time to pregnancy, according to various sensitivity analyses. (time-to-pregnancy is censored at 13 months unless otherwise specified.)
DISCUSSION
In an immigrant, farmworker community, we found increased time to pregnancy among women with potential occupational exposure to pesticides, who had pesticides used in their homes, and who lived within 200 feet of an agricultural field. Paternal occupational pesticide exposure was not associated with time-to-pregnancy. Maternal levels of p,p'-DDT, o,p'-DDT, and p,p'-DDE were also unassociated with time-to-pregnancy.
Our DDE findings are consistent with those of Law et al.19 who found no association between maternal DDE and time to pregnancy in 390 women pregnant between 1959 and 1965. Axmon et al 18 found no association between maternal DDE and time to pregnancy among women in three European cohorts, but found reduced fecundability with higher DDE exposure in a population of 497 Inuit women from Greenland. However, the DDE findings in the Inuit population might be attributable to PCBs, which were highly correlated to DDE and also associated with reduced fecundability in that population. Although DDE levels in our study population were lower than those from the early 1960s, they were higher than any of the cohorts studied by Axmon et al. Although other studies have found elevated DDT or DDE levels to be associated with shorter menstrual cycles,13, 14 decreased progesterone and estrogen levels,14, 15 and increased risk of early fetal loss,16 this did not translate to overall decreased fecundability in our population.
Three previous studies have found markers of paternal exposure to currently-used pesticides to be associated with increased time-to-pregnancy.20, 21, 38 In two,21, 38 reduced fecundability was not seen with paternal work in greenhouses in general, but was under specific circumstances: Bretveld et al. reported decreased fecundability only among male greenhouse workers with primiparous partners and, Sallmen et al. found decreased fecundability only among male greenhouse workers who reported exposure to pyrethroid pesticides. However, several studies, including the current study, have found no association with paternal work in agriculture.23-26 A limitation of the current study is that male pesticide exposure information was ascertained by interviewing the female partner. However, it is likely that she would know the answer to broad questions, such as whether her partner was working in agricultural fields.
Our finding that potential maternal occupational exposure, largely through work in agriculture, was associated with decreased fecundability is consistent with findings in two previous time-to-pregnancy studies conducted among female greenhouse workers in Denmark27 and Colombia.28 Although women are generally less likely to be involved in direct spraying than men, female greenhouse workers and agricultural field workers often enter the area following pesticide application and may have considerable “re-entry” exposure through handling plants and breathing contaminated air. Thus, like our study, these two studies represent women with high potential for a wide variety of pesticide exposures.
The term pesticide encompasses many different compounds with differing modes of action. Women participating in this study were unable to identify specific pesticides that were used in their jobs or their homes. However, the most widely used agricultural pesticides in this region include almost 4 million pounds per year of soil fumigants (including methyl bromide and chloropicrin), 500,000 pounds per year of organophosphates (including diazinon, malathion, and chlorpyrifos), and 300,000 pounds per year of dithiocarbamate fungicides (primarily maneb).39 Comparison with previous studies, generally conducted with greenhouse workers, is difficult as few studies report specific pesticides. However, Ividro et al28 report considerable use of dithiocarbamates in the Colombian floriculture industry where maternal greenhouses work was also associated with longer time-to-pregnancy. In contrast, the home pesticides used in this cohort were primarily pyrethroids, which were found to be present in 31% of the homes in this cohort.40 Despite the different formulations, we found longer time to pregnancy associated with reported exposure to both home use and agricultural use of pesticides.
This study used biological measures of exposure to the persistent pesticide, DDT, but not to current-use pesticides. Because of the long half-life of DDT and DDE, measures of these compounds in blood collected during pregnancy were considered good indicators of levels during the time-to-pregnancy period. In contrast, most pesticides currently used in the study area are non-persistent compounds that are used intermittently and stay in the body for only a few days.41 Although we measured urinary metabolites of organophosphate and other pesticides during pregnancy, these levels were highly variable over the course of pregnancy and were not included in this analysis because they cannot accurately reflect exposure in the time-to-pregnancy period. Additionally, over 200 pesticides are registered for use in this agricultural region,42 many of which cannot be measured in biological matrices.43 Thus, self-reported information on agricultural work, pesticide application, and proximity to agricultural fields was considered the best available marker of total pesticide exposure for the relevant time period. Previous studies in this population have found that work in the fields is associated with urinary organophosphate pesticide metabolites on a short-term basis44 and that maternal work in agriculture and living near agricultural fields were associated with higher levels of urinary pesticide metabolites in infants and toddlers (in preparation), suggesting that these behavioral markers are valid proxies of pesticide exposure.
Although work in agriculture or with pesticides may be associated with higher pesticide exposure, they may also be proxies for other factors associated with fecundability. Women working in agriculture were more likely to be recent immigrants from Mexico with low educational attainment. However, recent immigrants had increased fecundability, on average, so this does not explain the reduced fecundability associated with agricultural work. Women working in agriculture also had lower frequency of intercourse than other women, but frequency of intercourse was not associated with time-to-pregnancy, and including frequency of intercourse in the models did not change the associations of pesticide-related behaviors and fecundability. However, it is possible that other unmeasured factors associated with work in agriculture or other exposure to pesticides are actually responsible for the observed decrease in fecundability.
This study is a cross-sectional study of time-to-pregnancy, since women were recruited early in pregnancy and asked about exposures in the recent period before conception.17 An advantage of this study design over retrospective studies is that recall time was short and was similar for all women. All pregnancies occurred in the years 1999 and 2000, eliminating the problem of time trends in fertility, contraceptive use, or abortion that can plague retrospective studies asking about pregnancies occurring years, or even decades, earlier. A disadvantage of this study design is that only pregnant women were eligible for participation, resulting in an under-representation of infertile and sub-fertile couples. However, excluding these couples would likely underestimate the true effect of an exposure, biasing the findings towards the null.
This study differed from prospective studies in that it was not limited to couples actively trying to conceive. Only 50% of the study population was actively trying to become pregnant, suggesting that prospective studies in this population could result in considerable selection bias. Sensitivity analyses showed that the findings persisted when the sample was expanded to include couples experiencing contraceptive failures. The findings also persisted when the sample was limited to couples actively trying and to primiparous women with untested fertility.
A concern in this study was that the finding of decreased fecundability among women with potential occupational pesticide exposure might actually reflect a “reproductively unhealthy worker effect”. More fertile women might be more likely to have young children and thus be less likely to be working. However, we found no evidence that work in general, as opposed to agricultural or pesticide-related work, was associated with fecundability in this study. Furthermore, increased time to pregnancy was associated with pesticide use in the home and proximity to agricultural fields, in addition to maternal occupational exposure.
This study included women from a culturally homogeneous population with sufficient variability in employment, pesticide use, proximity to fields, and DDT/DDE exposure that we did not need to use external controls. We found no association between maternal DDT or DDE exposure and time-to-pregnancy, but did find associations between maternal pesticide-related exposures at work and home and time-to-pregnancy. This study could be improved by prospectively measuring biomarkers of current use pesticides during the time-to-pregnancy period. However, such a study would be limited by the need for repeated measurements because of strong daily variability in exposure, a lack of available biomarkers for many pesticides, and the need for a highly motivated population, possibly introducing selection bias.
Models.
Main population (N=402)
Contraceptive use & Inclusion of contraceptive failures:
-
b
Main + all pregnancies resulting from contraceptive failure (time-to-pregnancy=0) (N=570)
-
c
Main + contraceptive failure with irregular contraceptive use (time-to-pregnancy=duration of irregular use/2) (N=447)
-
d
Main + contraceptive failure with regular contraception use (time-to-pregnancy=0) (N=528)
-
e
Main + all contraceptive failure (time-to-pregnancy=0 or duration/2) (N=570)
Censoring
-
f
Main, censored at 7 months (N=402)
-
g
Main, censored at 10 months (N=402)
-
h
Main, censored at 15 months (N=402)
Limiting the dataset
-
i
Limited to primiparas (N=170)
-
j
Limited to those actively trying (N=210)
Acknowledgments
Funding: This work was supported by grants from the U.S. EPA (RD 83171001), NIEHS (PO1 ES009605), and NIOSH (RO1 OH007400).
Footnotes
Other sources of support: none
REFERENCES
- 1.Whorton D, Krauss RM, Marshall S, Milby TH. Infertility in male pesticide workers. Lancet. 1977;2:1259–1261. doi: 10.1016/s0140-6736(77)92665-4. [DOI] [PubMed] [Google Scholar]
- 2.Lerda D, Rizzi R. Study of reproductive function in persons occupationally exposed to 2,4-dichlorophenoxyacetic acid (2,4-D) Mutat Res. 1991;262:47–50. doi: 10.1016/0165-7992(91)90105-d. [DOI] [PubMed] [Google Scholar]
- 3.Wyrobek AJ, Watchmaker G, Gordon L, Wong K, Moore D, 2nd, Whorton D. Sperm shape abnormalities in carbaryl-exposed employees. Environ Health Perspect. 1981;40:255–265. doi: 10.1289/ehp.8140255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padungtod C, Lasley BL, Christiani DC, Ryan LM, Xu X. Reproductive hormone profile among pesticide factory workers. J Occup Environ Med. 1998;40:1038–1047. doi: 10.1097/00043764-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Swan SH, Kruse RL, Liu F, Barr DB, Drobnis EZ, Redmon JB, et al. Semen quality in relation to biomarkers of pesticide exposure. Environ Health Perspect. 2003;111:1478–1484. doi: 10.1289/ehp.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934-1996. Environ Health Perspect. 2000;108:961–966. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sallmen M, Weinberg CR, Baird DD, Lindbohm ML, Wilcox AJ. Has human fertility declined over time?: why we may never know. Epidemiology. 2005;16:494–499. doi: 10.1097/01.ede.0000165391.65690.e1. [DOI] [PubMed] [Google Scholar]
- 9.U.S. DHHS . Toxicological profile for DDT, DDE, and DDD. U.S. Dept. of Health and Human Services Public Health Service, Agency for Toxic Substances and Disease Registry; Atlanta, GA: 2002. [PubMed] [Google Scholar]
- 10.Klotz DM, Beckman BS, Hill SM, McLachlan JA, Walters MR, Arnold SF. Identification of environmental chemicals with estrogenic activity using a combination of in vitro assays. Environ Health Perspect. 1996;104:1084–1089. doi: 10.1289/ehp.961041084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kojima H, Katsura E, Takeuchi S, Niiyama K, Kobayashi K. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ Health Perspect. 2004;112:524–531. doi: 10.1289/ehp.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p'-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- 13.Ouyang F, Perry MJ, Venners SA, Chen C, Wang B, Yang F, et al. Serum DDT, age at menarche, and abnormal menstrual cycle length. Occup Environ Med. 2005;62:878–884. doi: 10.1136/oem.2005.020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Windham GC, Lee D, Mitchell P, Anderson M, Petreas M, Lasley B. Exposure to organochlorine compounds and effects on ovarian function. Epidemiology. 2005;16:182–190. doi: 10.1097/01.ede.0000152527.24339.17. [DOI] [PubMed] [Google Scholar]
- 15.Perry MJ, Ouyang F, Korrick SA, Venners SA, Chen C, Xu X, et al. A prospective study of serum DDT and progesterone and estrogen levels across the menstrual cycle in nulliparous women of reproductive age. Am J Epidemiol. 2006;164:1056–1064. doi: 10.1093/aje/kwj329. [DOI] [PubMed] [Google Scholar]
- 16.Venners SA, Korrick S, Xu X, Chen C, Guang W, Huang A, et al. Preconception serum DDT and pregnancy loss: a prospective study using a biomarker of pregnancy. Am J Epidemiol. 2005;162:709–716. doi: 10.1093/aje/kwi275. [DOI] [PubMed] [Google Scholar]
- 17.Baird DD, Wilcox AJ, Weinberg CR. Use of time to pregnancy to study environmental exposures. Am J Epidemiol. 1986;124:470–480. doi: 10.1093/oxfordjournals.aje.a114417. [DOI] [PubMed] [Google Scholar]
- 18.Axmon A, Thulstrup AM, Rignell-Hydbom A, Pedersen HS, Zvyezday V, Ludwicki JK, et al. Time to pregnancy as a function of male and female serum concentrations of 2,2'4,4'5,5'-hexachlorobiphenyl (CB-153) and 1,1-dichloro-2,2-bis (p-chlorophenyl)-ethylene (p,p'-DDE) Hum Reprod. 2006;21:657–665. doi: 10.1093/humrep/dei397. [DOI] [PubMed] [Google Scholar]
- 19.Law DC, Klebanoff MA, Brock JW, Dunson DB, Longnecker MP. Maternal serum levels of polychlorinated biphenyls and 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and time to pregnancy. Am J Epidemiol. 2005;162:523–532. doi: 10.1093/aje/kwi240. [DOI] [PubMed] [Google Scholar]
- 20.de Cock J, Westveer K, Heederik D, te Velde E, van Kooij R. Time to pregnancy and occupational exposure to pesticides in fruit growers in The Netherlands. Occup Environ Med. 1994;51:693–699. doi: 10.1136/oem.51.10.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bretveld R, Kik S, Hooiveld M, van Rooij I, Zielhuis G, Roeleveld N. Time-to-pregnancy among male greenhouse workers. Occup Environ Med. 2007 doi: 10.1136/oem.2007.036269. [DOI] [PubMed] [Google Scholar]
- 22.Petrelli G, Figa-Talamanca I. Reduction in fertility in male greenhouse workers exposed to pesticides. Eur J Epidemiol. 2001;17:675–677. doi: 10.1023/a:1015511625099. [DOI] [PubMed] [Google Scholar]
- 23.Curtis KM, Savitz DA, Weinberg CR, Arbuckle TE. The effect of pesticide exposure on time to pregnancy. Epidemiology. 1999;10:112–117. [PubMed] [Google Scholar]
- 24.Larsen SB, Joffe M, Bonde JP. Time to pregnancy and exposure to pesticides in Danish farmers. ASCLEPIOS Study Group. Occup Environ Med. 1998;55:278–283. doi: 10.1136/oem.55.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thonneau P, Abell A, Larsen SB, Bonde JP, Joffe M, Clavert A, et al. Effects of pesticide exposure on time to pregnancy: results of a multicenter study in France and Denmark. ASCLEPIOS Study Group. Am J Epidemiol. 1999;150:157–163. doi: 10.1093/oxfordjournals.aje.a009975. [DOI] [PubMed] [Google Scholar]
- 26.Greenlee AR, Arbuckle TE, Chyou PH. Risk factors for female infertility in an agricultural region. Epidemiology. 2003;14:429–436. doi: 10.1097/01.EDE.0000071407.15670.aa. [DOI] [PubMed] [Google Scholar]
- 27.Abell A, Juul S, Bonde JP. Time to pregnancy among female greenhouse workers. Scand J Work Environ Health. 2000;26:131–136. doi: 10.5271/sjweh.522. [DOI] [PubMed] [Google Scholar]
- 28.Idrovo AJ, Sanin LH, Cole D, Chavarro J, Caceres H, Narvaez J, et al. Time to first pregnancy among women working in agricultural production. Int Arch Occup Environ Health. 2005;78:493–500. doi: 10.1007/s00420-005-0615-9. [DOI] [PubMed] [Google Scholar]
- 29.Lauria L, Settimi L, Spinelli A, Figa-Talamanca I. Exposure to pesticides and time to pregnancy among female greenhouse workers. Reprod Toxicol. 2006;22:425–430. doi: 10.1016/j.reprotox.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Bretveld R, Zielhuis GA, Roeleveld N. Time to pregnancy among female greenhouse workers. Scand J Work Environ Health. 2006;32:359–367. doi: 10.5271/sjweh.1031. [DOI] [PubMed] [Google Scholar]
- 31.Larson A. Migrant and Seasonal Farmworker Enumeration profiles study. 2000. [Google Scholar]
- 32.Aquirre International . The California Farm Labor Force Overview and Trends from the National Agricultural Workers Survey. Jul, 2005. [Google Scholar]
- 33.California Department of Pesticide Regulation Pesticide Use Report (PUR) 2005 [Online] Available at http://www.cdpr.ca.gov/docs/pur/purmain.htm.
- 34.Bradman AS, Schwartz JM, Fenster L, Barr DB, Holland NT, Eskenazi B. Factors predicting organochlorine pesticide levels in pregnant Latina women living in a United States agricultural area. J Expo Sci Environ Epidemiol. 2007;17:388–399. doi: 10.1038/sj.jes.7500525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips DL, Pirkle JL, Burse VW, Bernert JT, Jr., Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 36.Baird DD. Using time-to-pregnancy data to study occupational exposures: methodology. Reprod Toxicol. 1988;2:205–207. doi: 10.1016/0890-6238(88)90023-8. [DOI] [PubMed] [Google Scholar]
- 37.Joffe M, Key J, Best N, Keiding N, Scheike T, Jensen TK. Studying time to pregnancy by use of a retrospective design. Am J Epidemiol. 2005;162:115–124. doi: 10.1093/aje/kwi172. [DOI] [PubMed] [Google Scholar]
- 38.Sallmen M, Liesivuori J, Taskinen H, Lindbohm ML, Anttila A, Aalto L, et al. Time to pregnancy among the wives of Finnish greenhouse workers. Scand J Work Environ Health. 2003;29:85–93. doi: 10.5271/sjweh.709. [DOI] [PubMed] [Google Scholar]
- 39.DPR . Pesticide Use Report, Annual 2001, Indexed by Chemical and by Crop. Department of Pesticide Regulation, California Environmental Protection Agency; Sacramento, CA: 2001. [Google Scholar]
- 40.Bradman A, Chevrier J, Tager I, Lipsett M, Sedgwick J, Macher J, et al. Association of housing disrepair indicators with cockroach and rodent infestations in a cohort of pregnant Latina women and their children. Environ Health Perspect. 2005;113:1795–1801. doi: 10.1289/ehp.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradman A, Eskenazi B, Barr D, Bravo R, Castorina R, Chevrier J, et al. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Perspect. 2005;113:1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Department of Pesticide Regulation CEPA Pesticide Use Reporting 2001 Summary Data. 2002 Available at: http://www.cdpr.ca.gov/docs/pur/pur01rep/01_pur.htm.
- 43.Bradman A, Whyatt RM. Characterizing exposures to nonpersistent pesticides during pregnancy and early childhood in the National Children's Study: a review of monitoring and measurement methodologies. Environ Health Perspect. 2005;113:1092–1099. doi: 10.1289/ehp.7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradman A, Salvatore AL, Boeniger M, Castorina R, Snyder J, Barr DB, et al. Community-based intervention to reduce pesticide exposure to farmworkers and potential take-home exposure to their families. J Expo Sci Environ Epidemiol. 2008 doi: 10.1038/jes.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]