Abstract
Physiological evidence indicates that vestibular signals modulate the activity of motoneurons innervating the masseter muscle. Recently, experiments using transynaptic retrograde transport of pseudorabies virus provided anatomical evidence that many neurons concentrated in the dorsomedial part of the parvicellular division of the medial vestibular nucleus (MVePC) and the caudal prepositus hypoglossi (PH) provide inputs to motoneurons innervating the lower third of the superficial layer of the masseter muscle. However, it was not clear whether this vestibulotrigeminal projection was monosynaptic or polysynaptic. The present study sought to determine whether neurons in the MVePC or PH project directly to motoneurons controlling the masseter muscle in rats. For this purpose, an anterograde tracer (biotinylated dextran amine, BDA) was injected into vestibular nuclei (mainly MVePC) or PH and a retrograde tracer (the β-subunit of cholera toxin, b-CT) was injected into the masseter muscle ipsilateral or contralateral to the BDA injection site. Following injections of BDA into the vestibular nuclei or PH, anterogradely-labeled axon terminals were observed bilaterally in the motor trigeminal nucleus (Mo5), particularly in the ventral, medial, and lateral portions of the nucleus; projections to dorsal Mo5 were sparse. In addition, retrogradely-labeled motoneurons were located in the ventral and lateral portions of the ipsilateral Mo5. Moreover, anterogradely-labeled terminals were observed to make contact with motoneurons in the Mo5 that were retrogradely labeled from b-CT injections into the masseter muscle. This study provides direct evidence that a monosynaptic pathway exists between the MVePC and PH and masseter motoneurons.
Keywords: vestibular nuclei, prepositus hypoglossi, motor trigeminal nucleus, jaw musculature, biotinylated dextran amine, cholera toxin
Introduction
A variety of studies have shown that the activity of mandibular muscles is modulated by the vestibular system (Hickenbottom et al. 1985; Tolu and Pugliatti 1993; Tolu et al. 1994; Tolu et al. 1996; Deriu et al. 1999; Deriu et al. 2003; Deriu et al. 2005). In particular, experiments performed on guinea pigs demonstrated that stimulation of receptors in the semicircular canal ampullae elicits bilateral excitatory responses of masseter and digastric motoneurons and that macular inputs exert bilateral asymmetrical influences on jaw muscle activity. Furthermore, the presence of vestibular inputs to trigeminal motoneurons has been demonstrated in healthy humans (Hickenbottom et al. 1985; Deriu et al. 2000). More recently, short-duration masseter muscle responses that were elicited by vestibular stimulation at latencies consistent with no more than 2-3 synaptic relays have been described in human subjects (Deriu et al. 2003; Deriu et al. 2005). Presumably these vestibulotrigeminal responses serve to maintain the jaw in a fixed position as head position changes with respect to gravity.
A recent study that employed pseudorabies virus to transneuronally trace the polysynaptic pathways providing inputs to jaw musculature demonstrated that neurons concentrated in the dorsomedial part of the parvicellular division of medial vestibular nucleus (MVePC) and the caudal prepositus hypoglossi (PH) project bilaterally to motoneurons innervating the lower third of the masseter muscle (Giaconi et al. 2006). A limitation of this experiment was that it was not possible to ascertain whether the projections of MVePC and PH to masseter motoneurons were direct or multisynaptic. The present study made use of conventional monosynaptic anterograde and retrograde tracing techniques to ascertain whether neurons in the vestibular nuclei and PH make monosynaptic connections with masseter motoneurons. We tested the hypothesis that a neural substrate exists to relay vestibular signals directly from MVePC and PH to motoneurons innervating this jaw-closing muscle.
Methods and materials
All procedures involving animals were reviewed and approved by the University of Pittsburgh's Institutional Animal Care and Use Committee, and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments were conducted on 20 adult male Sprague Dawley® rats weighing 220-280 g, which were obtained from Hilltop Laboratories (Scottdale, PA). However, the injection sites were confined to the target regions in only 11 of the animals, and data from just these cases will be discussed in this manuscript.
To perform tracer injections, animals were anesthetized with a mixture of xylazine (6 mg/kg), ketamine (50 mg/kg) and acepromazine (0.5 mg/kg) injected intramuscularly. An incision was made under the chin to access the lower third of one masseter muscle, and 40 μl of a 0.5 mg/ml solution of the β-subunit of cholera toxin (b-CT, List Biological Laboratories, Campbell, CA) was injected at multiple sites in the superficial layer of the muscle using a 10 μl Hamilton syringe. To reduce the possibility of b-CT spread, the needle was left in place for 1 min after each injection, and the area around the injection sites was swabbed with saline. After the injections were completed, the skin was sutured closed. Subsequently, animals were placed in a stereotaxic apparatus and a scalp incision was made from the supraorbital process to the interparietal bone. A small hole was drilled in the skull, and a 7.5% solution of 10,000 molecular weight biotinylated dextran amine (BDA; Molecular Probes, Eugene, OR) dissolved in 0.01 M phosphate buffer (pH 7.2-7.4) was iontophoretically injected through a glass micropipette (10-50 μm tip diameter) lowered into the vestibular nuclei or PH on the basis of stereotaxic coordinates obtained from the atlas of Paxinos and Watson (Paxinos and Watson 1998). Iontophoresis was performed using a 5 μA positive current applied for 7 s and then discontinued for 7 s over a period of 15-20 min. After all injections, electrodes were left in place for 5 min before being removed. The hole in the skull was subsequently closed with bone wax and the skin overlying the injection sites was sutured closed. An analgesic, Ketoprofen (5 mg/kg), was injected intramuscularly at 12-h intervals for 72 h to prevent any indications of pain and distress.
After a survival time of 7 days, the animals were anesthetized using sodium pentobarbital (100 mg/kg, intraperitoneal) and perfused transcardially using 300 ml of saline followed by 300 ml of a 4% paraformaldehyde-lysine-periodate (PLP) fixative solution (McLean and Nakane 1974). Subsequently, the brain was postfixed in 4°C PLP for 1 day, and transferred to 30% sucrose in phosphate-buffered saline (PBS) at 4°C for two days. Transverse frozen sections (35 μm thick) of the pons and medulla were cut using a freezing microtome. Tissue was collected in six sequential bins, such that each well contained a rostrocaudal series of sections spaced 210 μm apart, and was stored at -20°C in cryoprotectant (Watson et al. 1986) until it was processed immunohistochemically to visualize BDA and b-CT.
The tissue was first processed to visualize BDA. After rinsing with PBS, the tissue was processed using the avidin-biotin modification of the peroxidase-antiperoxidase procedure (Hsu et al. 1981) and the sections were reacted for 1-2 min with a nickel-enhanced diaminobenzidine tetrahydrochloride (DAB) solution. Once the detection of BDA was completed, an avidin-biotin blocking step was performed (Avidin-Biotin Blocking Kit, Vector Laboratories, Burlingame, CA). Subsequently, after rinsing with PBS, the sections were incubated for 48 h at 4°C in a primary antiserum that contained 1:100 goat anti-b-CT (List Biological Laboratories). The tissue was then incubated for 1 h in 1:200 donkey anti-goat antibody (Jackson ImmunoResearch Laboratories, West Grove, PA), and subsequently placed for 1 h in Vectastain ABC peroxidase reagent (Vector Laboratories). The visualization of b-CT was completed by incubating the sections in a solution containing DAB chromagen, or by employing the Vector Nova Red Substrate Kit (Vector Laboratories). Upon completion of the immunohistochemical processing, the tissue sections in which b-CT was visualized using DAB chromagen were counterstained with methyl green. Finally, the tissue was mounted on gelatin-coated slides, dehydrated through a graded series of ethanol, cleared using xylene, and coverslipped using DPX (Sigma-Aldrich, St. Louis, MO).
Sections were examined using an Olympus BH-2 microscope to visualize the distribution of b-CT-labeled motoneurons and BDA-labeled fibers and terminals in the brainstem. Camera lucida drawings were made of every section of the brainstem that was found to contain BDA-labeled axons, and the number of axonal branches in each brain region was counted. Digital images of sections were obtained using a Nikon Eclipse E600N microscope equipped with a Spot RT monochrome digital camera (model 2.1.1, Diagnostic Instruments, Sterling Heights, MI). The images were captured on a Pentium-based computer running MetaMorph software (Ver. 6.1r4, Universal Corporation, Downingtown, PA). Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA) was used to adjust brightness and contrast of digital images, although color balance was not altered.
Results
In 10 of the 11 animals from which data were obtained, the BDA injection sites were confined to the vestibular nuclei or PH and resulted in extensive axonal labeling in the brainstem. The only rat lacking labeled projections was a control animal (case 29) where BDA was iontophoresed into the MLF to determine whether axons coursing through this fiber bundle could have taken up the tracer. Camera lucida drawings of several of the BDA injection sites are provided in Fig. 1A, whereas Fig. 1B shows photomicrographs of a subset of the injection sites. Furthermore, b-CT-labeled motoneurons were observed in the lateral and ventral portions of the ipsilateral Mo5 in every rat; these portions of the nucleus have been demonstrated to contain masseter motoneurons (Mizuno et al. 1975; Lynch 1985; Giaconi et al. 2006).
Figure 1.
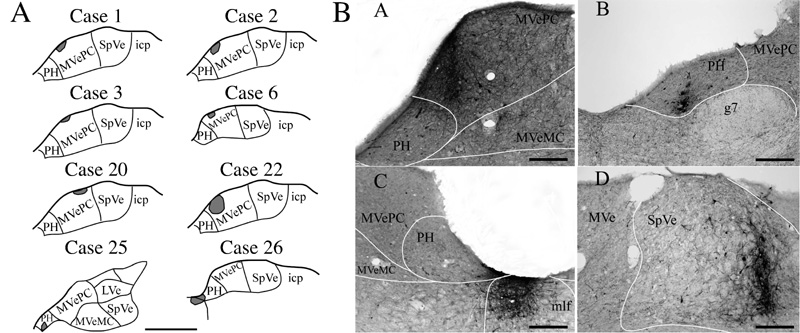
A: Schematic representations of BDA injection sites in the vestibular nuclei (cases 1, 2, 3, 6, 20, and 22) and the prepositus hypoglossi (PH) (cases 25 and 26). Anatomical templates were adapted from the atlas of Paxinos and Watson (1998). B: Photomicrographs of BDA injection sites made in the medial part of the parvicellular division of medial vestibular nucleus (MVePC) of case 22 (A), the ventrolateral part of the rostral PH of case 25 (B), between the ventromedial part of the caudal PH and the dorsal part of the medial longitudinal fasciculus (m/f) of case 26 (C), and in the lateral part of the spinal vestibular nucleus (SpVe) of case 27 (D). Abbreviations: g7, genu of the facial nerve; LVe, lateral vestibular nucleus; MVe, medial vestibular nucleus; MVeMC, magnocellular division of medial vestibular nucleus; icp, inferior cerebellar peduncle. Scale bars=1 mm in A and 200 μm in B.
For cases 1, 2, 3, 6 and 22, the BDA injection sites were confined to circumscribed regions of MVePC ipsilateral to the side containing motoneurons labeled by the injections of b-CT into the masseter muscle; the locations of these injection sites are shown in Fig. 1A. A photomicrograph of the injection site for rat 22 is provided in panel A of Fig. 1B. The injection sites for animals 1, 2 and 22 extended more medially within MVePC than did those in rats 3 and 6, which were limited to the most dorsal part of the nucleus. All of these injections labeled axonal projections to the lateral, ventral and medial portions of the Mo5, with only sparse anterograde labeling present in the dorsal part of the nucleus. The distribution of the labeled projections within Mo5 is indicated in Table 1; for this analysis, the Mo5 divided into 8 equivalent sectors, and the number of axons in each sector was counted. Bilateral labeling within Mo5 was observed, with no clear differences in the density of labeled axons on the ipsilateral and contralateral sides (see Table 1). Furthermore, in the three animals where the injections included medial MVePC (cases 1, 2, and 22), presumptive axonal terminals were observed in the ventral and lateral areas of Mo5 in apposition to motoneurons that were retrogradely labeled from b-CT injections into the masseter muscle; examples of such terminals from animal 22 are shown in panels A and B of Fig. 2.
Table 1.
Regions of the trigeminal motor nucleus (Mo5) containing BDA-labeled axons. Labeling was quantified in all sections processed for a particular animal. For this analysis, the Mo5 was divided into the following 8 equivalent sectors: V, ventral; VL, ventrolateral; VM, ventromedial; D, dorsal; DL, dorsolateral; DM, dorsomedial; M, medial; L, lateral. The number of labeled axons was counted both on the side ipsilateral (ipsi) and contralateral (contra) to the BDA injections. Abbreviations: Mo5, motor trigeminal nucleus; MVePC, parvocellular division of medial vestibular nucleus; PH, prepositus hypoglossi; SpVe, spinal vestibular nucleus.
| Case | Region of Mo5 Containing BDA-Labeled Axons | |||||||
|---|---|---|---|---|---|---|---|---|
| V ipsi/contra | VL ipsi/contra | VM ipsi/contra | D ipsi/contra | DL ipsi/contra | DM ipsi/contra | M ipsi/contra | L ipsi/contra | |
| BDA injected into MVePC | ||||||||
| 1 | 5/3 | 7/2 | -/- | 1/- | -/1 | -/- | 4/1 | 6/4 |
| 2 | 4/3 | 6/2 | -/- | 1/3 | 2/3 | 2/2 | 3/1 | 10/7 |
| 3 | 2/2 | 2/2 | 1/- | 3/1 | -/- | -/- | 2/- | 2/3 |
| 6 | 2/2 | 1/1 | 1/- | 1/- | -/- | -/- | 1/- | 3/2 |
| 20 | -/- | -/- | -/- | -/- | -/- | -/- | -/- | -/- |
| 22 | 13/11 | 6/4 | 1/- | 2/1 | 2/- | 1/- | 3/1 | 8/5 |
| BDA injected into SpVe | ||||||||
| 27 | 9/6 | 8/6 | 1/2 | 5/5 | 3/2 | 2/- | 5/5 | 10/10 |
| BDA injected into PH | ||||||||
| 21 | 15/17 | 7/7 | 11/10 | 11/9 | 8/5 | 8/6 | 25/21 | 33/29 |
| 25 | -/- | -/- | -/- | -/- | -/- | -/- | -/- | -/- |
| 26 | 16/17 | 11/7 | 12/8 | 12/9 | 10/5 | 12/7 | 30/25 | 36/33 |
Figure 2.
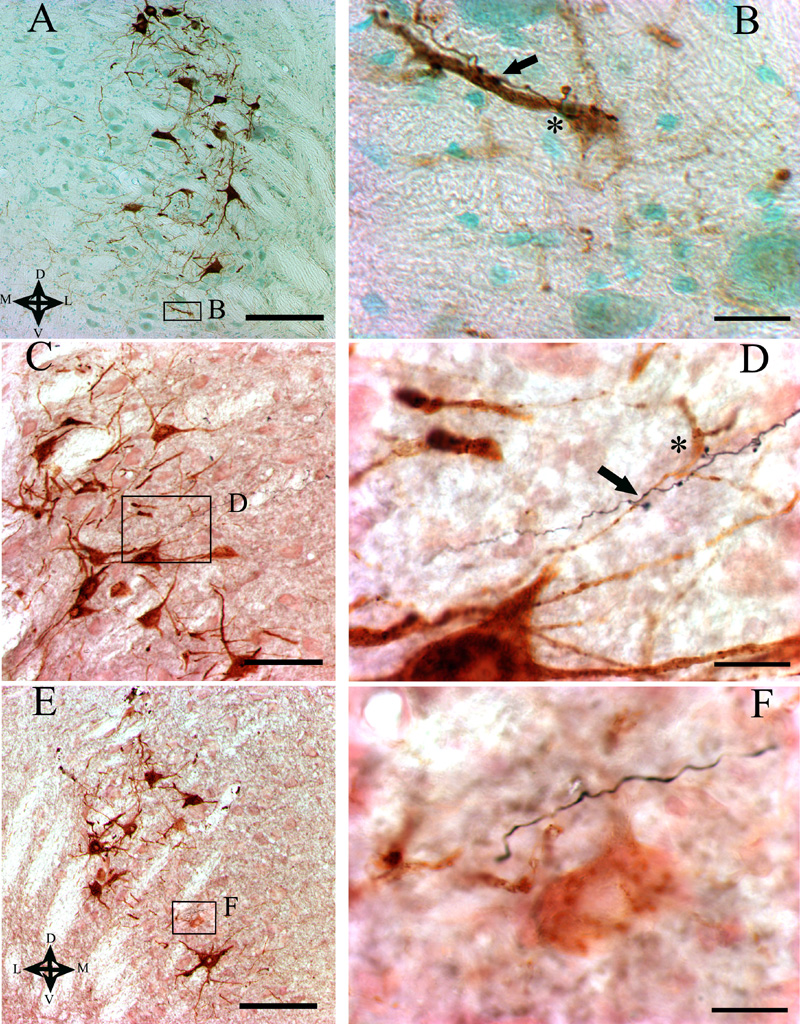
Photomicrographs of the trigeminal motor nucleus showing b-CT-labeled masseter motoneurons and axons that were anterogradely labeled by the injection of BDA into the vestibular nucleus complex. The area designated by a box in panels A, C, and E is represented at higher power in panels B, D, and F, respectively. The dorsal (D), ventral (V), medial (M), and lateral (L) aspects of the micrographs are designated by arrows in panel A. The BDA injections were made in the following locations: ipsilateral MVePC of case 22 (A, B), caudal PH of case 26 (C, D), and contralateral SpVe of case 27 (E, F). Asterisks indicate locations where swellings that were presumed to be boutons along the BDA-labeled fibers made contacts with the cell bodies of motoneurons. Arrows designate contacts between presumptive axon terminals and the dendrites of motoneurons. In D, the motoneuron cell body was in a different plane of focus than the BDA-labeled axon, and is evident in the background of the image. Note in F that whereas BDA-labeled axons coursed in the proximity of masseter motoneurons, no apparent contacts between the axons and motoneurons were present. Scale bars represent 200 μm in A, C, and E and 20 μm in B, D, and F.
Two injections were made into the contralateral vestibular nuclei. As indicated in Fig. 1A, the injection site in case 20 was small and located in dorsolateral MVePC, more laterally than the injections on the ipsilateral side (cases 1, 2, 3, 6 and 22). No anterogradely-labeled fibers were found in Mo5 in this animal, although axons that were positive for BDA were observed in the vestibular nuclei and reticular formation (see Table 2). In case 27, the injection was made into the lateral part of SpVe, with no spread to any other nuclei (see panel D of Fig. 1B). In this rat, anterogradely-labeled fibers were scattered in the ventral, ventrolateral and lateral subdivisions of Mo5 on both sides (Table 1). However, none of the BDA-labeled fibers were observed to be in contact with either the dendrites or the cell bodies of b-CT-labeled motoneurons (Fig. 2E, F).
Table 2.
Number of BDA-labeled axons observed in brain regions other than Mo5 containing an appreciable number of such projections. This analysis considered labeled axons both on the side ipsilateral (ipsi) and contralateral (contra) to the BDA injections. The number of fibers in a particular region was quantified only in the section containing the highest density of labeling. Symbols designate the relative number of fibers counted in each area of each animal: +, 1-7 fibers; ++, 8-30 fibers; +++, 31-40 fibers; ++++, > 40 fibers; —, no fibers. Abbreviations: IRt, intermediate reticular nucleus; LVe, lateral vestibular nucleus; mlf, medial longitudinal fasciculus; MVeMC, magnocellular division of medial vestibular nucleus; MVePC, parvocellular division of medial vestibular nucleus; PH, prepositus hypoglossi; Sp5, spinal trigeminal nucleus; SpVe, spinal vestibular nucleus; SuVe, superior vestibular nucleus.
| Case | Side Observed | Region Containing BDA-Labeled Axons | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MVePC | MVeMC | PH | IRt | SuVe | SpVe | LVe | Sp5 | mlf | ||
| BDA injected into MVePC | ||||||||||
| 1 | ipsi | ++++ | +++ | +++ | ++ | ++ | ++ | ++ | + | + |
| contra | ++ | ++ | ++ | ++ | + | + | + | + | + | |
| 2 | ipsi | +++ | +++ | +++ | ++ | +++ | +++ | +++ | + | + |
| contra | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | + | |
| 3 | ipsi | +++ | ++ | ++ | ++ | ++ | + | ++ | - | + |
| contra | ++ | ++ | + | ++ | + | + | ++ | - | + | |
| 6 | ipsi | ++ | + | + | + | ++ | + | + | - | + |
| contra | + | + | + | + | + | + | + | - | + | |
| 20 | ipsi | +++ | ++ | +++ | + | + | ++ | + | - | + |
| contra | ++++ | ++ | +++ | ++ | + | +++ | ++ | + | ++ | |
| 22 | ipsi | ++++ | +++ | +++ | +++ | +++ | ++ | +++ | ++ | ++ |
| contra | +++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | |
| BDA injected into SpVe | ||||||||||
| 27 | ipsi | +++ | +++ | ++ | ++ | +++ | +++ | +++ | + | + |
| contra | ++++ | ++++ | ++ | +++ | ++++ | ++++ | ++++ | ++ | ++ | |
| BDA injected into PH | ||||||||||
| 21 | ipsi | +++ | +++ | ++++ | ++ | ++ | ++ | + | ++ | +++ |
| contra | +++ | ++ | +++ | ++ | + | + | + | + | ++ | |
| 25 | ipsi | ++ | ++ | +++ | + | + | + | + | - | ++ |
| contra | ++ | ++ | ++++ | + | + | + | + | - | ++ | |
| 26 | ipsi | +++ | ++++ | ++++ | ++ | + | + | + | ++ | ++++ |
| contra | ++ | ++ | ++++ | ++ | - | - | - | + | ++++ | |
BDA injections were made into PH in three animals. The injection in case 25 included the ventrolateral part of rostral PH, contralateral to the b-CT-labeled masseter motoneurons (Fig. 1A and panel B of Fig. 1B), whereas the injection in animal 21 involved the ventral part of PH, midway along the anterior-posterior extent of the vestibular nuclei, ipsilateral to the b-CT-labeled motoneurons. In rat 26, BDA was injected between the ventromedial part of the caudal PH and the dorsal part of the MLF, ipsilateral to the b-CT injections into the masseter muscle (Fig. 1A and panel C of Fig. 1B). In animal 25, where BDA was injected more rostrally than in the other animals, no anterograde labeling was observed in Mo5. In contrast, in cases 21 and 26, BDA-labeled fibers were observed bilaterally in the Mo5, mainly in the ventral, medial, and lateral parts of the nucleus (Table 1). Furthermore, in both of these rats, presumptive BDA-labeled terminals were observed to be in contact with the dendrites and/or the cell bodies of b-CT-labeled motoneurons (see Fig. 3C and D for examples from case 26). However, the number of anterogradely-labeled axonal projections to Mo5 was larger in case 26 (the most caudal injection) than in case 21 (which was positioned more rostrally). As noted above, injections of BDA into the ipsilateral MLF of a control animal (case 29) did not result in the labeling of projections to Mo5 or other regions of the brainstem, suggesting that the axons observed in rat 26 were labeled through the uptake of BDA by cell bodies and dendrites in PH.
Table 2 indicates the relative density of BDA-labeled axons in brain regions other than Mo5 containing an appreciable number of such projections. The areas containing labeled projections varied in accordance with the injection site location, but included all regions of the vestibular nucleus complex and PH, the spinal trigeminal nucleus (Sp5), and the intermediate reticular nucleus (IRt) including the region immediately adjacent to Mo5. The labeling was bilateral, with a similar distribution of efferents on each side.
Discussion
Physiological experiments have shown that the vestibular system contributes to regulating the activity of the masseter muscle (Hickenbottom et al. 1985; Tolu and Pugliatti 1993; Tolu et al. 1994; Tolu et al. 1996; Deriu et al. 1999; Deriu et al. 2000; Deriu et al. 2003; Deriu et al. 2005). In addition, a prior anatomical study using the transneuronal transport of pseudorabies virus revealed that a neural pathway links the caudal vestibular nuclei and PH with masseter motoneurons (Giaconi et al. 2006). The present study extended these findings by showing that at least some of the neural connections between the vestibular nucleus complex and Mo5 are monosynaptic. Most injections of the anterograde tracer BDA into the vestibular nucleus complex resulted in the bilateral labeling of efferents to Mo5; exceptions were a small injection confined to the lateral portion of MVePC (case 20) and an injection into the rostral PH (case 25). Furthermore, BDA-labeled axonal swellings presumed to be terminals were observed in close proximity to masseter motoneurons in the 5 rats (cases 1, 2, 21, 22 and 26) in which the BDA injections included the medial MVePC or caudal PH, but were not detected when the injections were placed elsewhere. Although it is possible that some axonal appositions with masseter motoneurons were present in the other animals, but were not noted because of limited sampling, it seems likely that most of the direct projections from the vestibular nuclei to these motoneurons arise from the medial MVePC and caudal PH.
The present experiments focused on direct connections from the vestibular nucleus complex to Mo5, but our results do not exclude the possibility of multisynaptic vestibular influences on jaw musculature. For example, BDA-labeled projections were noted in the pontomedullary reticular formation, including the zone of IRt adjacent to Mo5, which has been reported to contain interneurons that modulate trigeminal motoneuron activity (Donga and Lund 1991; Westberg et al. 1998). Vestibular inputs might also reach masseter motoneurons through relays in Sp5, since this nucleus contained anterogradely-labeled axons following the injection of BDA into the MVePC or PH (see Table 2) and is known to provide efferents to Mo5 (Westberg and Olsson 1991; Inoue et al. 2002). These data are consistent with findings that neurons in the pontomedullary reticular formation and Sp5 are infected at short survival times following the injection of pseudorabies virus into the masseter muscle (Giaconi et al. 2006).
Electrophysiological studies have concluded that vestibular influences on masseter motoneurons are mediated through two separate pathways: 1) an excitatory polysynaptic pathway that is most strongly directed to the contralateral side and produces tonic changes in excitability (Tolu and Pugliatti 1993; Tolu et al. 1994; Tolu et al. 1996; Deriu et al. 1999; Deriu et al. 2000) and 2) a bilateral inhibitory oligosynaptic pathway that elicits phasic responses (Deriu et al. 2003; Deriu et al. 2005). The present results provide a possible neural substrate to mediate both responses. However, further studies will be needed to determine if the direct projections from the vestibular nucleus complex to Mo5 are inhibitory, as suggested by electrophysiological studies (Deriu et al. 2003; Deriu et al. 2005), or whether excitatory connections not predicted by former experiments also exist. The multiplicity of connections relaying signals from the vestibular nuclei to masseter motoneurons underscores the likely physiological importance of labyrinthine influences on jaw musculature.
Acknowledgements
The authors thank Lucy Cotter, Jen-Shew Yen, Tom d'Arville, Bob Sabol, and Robin Holtje for providing valuable technical assistance in completing this study and Dr. Adam Halberstadt for supplying valuable advice. Funding was provided by Grant R01-DC003732 from the National Institutes of Health (USA), as well as Ministero dell Università della Ricerca Scientifica e Tecnologica and Regione Autonoma della Sardegna (Italy).
References
- Deriu F, Podda MV, Chessa G, Tolu E. Trigeminal integration of vestibular and forelimb nerve inputs. Arch Ital Biol. 1999;137:63–73. [PubMed] [Google Scholar]
- Deriu F, Podda MV, Milia M, Chessa G, Sau G, Pastorino M, Aiello I, Tolu E. Masseter muscle activity during vestibular stimulation in man. Arch Ital Biol. 2000;138:205–215. [PubMed] [Google Scholar]
- Deriu F, Tolu E, Rothwell JC. A short latency vestibulomasseteric reflex evoked by electrical stimulation over the mastoid in healthy humans. J Physiol. 2003;553:267–279. doi: 10.1113/jphysiol.2003.047274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriu F, Tolu E, Rothwell JC. A sound-evoked vestibulomasseteric reflex in healthy humans. J Neurophysiol. 2005;93:2739–2751. doi: 10.1152/jn.01005.2004. [DOI] [PubMed] [Google Scholar]
- Donga R, Lund JP. Discharge patterns of trigeminal commissural last-order interneurons during fictive mastication in the rabbit. J Neurophysiol. 1991;66:1564–1578. doi: 10.1152/jn.1991.66.5.1564. [DOI] [PubMed] [Google Scholar]
- Giaconi E, Deriu F, Tolu E, Cuccurazzu B, Yates BJ, Billig I. Transneuronal tracing of vestibulo-trigeminal pathways innervating the masseter muscle in the rat. Exp Brain Res. 2006;171:330–339. doi: 10.1007/s00221-005-0275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickenbottom RS, Bishop B, Moriarty TM. Effects of whole-body rotation on masseteric motoneuron excitability. Exp Neurol. 1985;89:442–453. doi: 10.1016/0014-4886(85)90103-7. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Inoue M, Nozawa-Inoue K, Donga R, Yamada Y. Convergence of selected inputs from sensory afferents to trigeminal premotor neurons with possible projections to masseter motoneurons in the rabbit. Brain Res. 2002;957:183–191. doi: 10.1016/s0006-8993(02)03662-4. [DOI] [PubMed] [Google Scholar]
- Lynch R. A qualitative investigation of the topographical representation of masticatory muscles within the motor trigeminal nucleus of the rat: a horseradish peroxidase study. Brain Res. 1985;327:354–358. doi: 10.1016/0006-8993(85)91535-5. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Konishi A, Sato M. Localization of masticatory motoneurons in the cat and rat by means of retrograde axonal transport of horseradish peroxidase. J Comp Neurol. 1975;164:105–115. doi: 10.1002/cne.901640109. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Tolu E, Caria MA, Chessa G, Melis F, Simula ME, Podda MV, Solinas A, Deriu F. Trigeminal motoneuron responses to vestibular stimulation in the guinea pig. Arch Ital Biol. 1996;134:141–151. [PubMed] [Google Scholar]
- Tolu E, Caria MA, Simula ME, Lacana P. Muscle spindle and periodontal trigeminal afferents modulate the hypoglossal motoneuronal activity. Arch Ital Biol. 1994;132:93–104. [PubMed] [Google Scholar]
- Tolu E, Pugliatti M. The vestibular system modulates masseter muscle activity. J Vestib Res. 1993;3:163–171. [PubMed] [Google Scholar]
- Watson RE, Jr., Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Westberg K, Clavelou P, Sandstrom G, Lund JP. Evidence that trigeminal brainstem interneurons form subpopulations to produce different forms of mastication in the rabbit. J Neurosci. 1998;18:6466–6479. doi: 10.1523/JNEUROSCI.18-16-06466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westberg KG, Olsson KA. Integration in trigeminal premotor interneurones in the cat. 1. Functional characteristics of neurones in the subnucleus-gamma of the oral nucleus of the spinal trigeminal tract. Exp Brain Res. 1991;84:102–114. doi: 10.1007/BF00231765. [DOI] [PubMed] [Google Scholar]


