Abstract
The type 1 chemokine monocyte chemoattractant protein (MCP-1) has been implicated in the generation of inflammatory and neuropathic pain, but the underlying mechanism remains poorly understood. Here we show that mechanical hyperalgesia induced by intradermal injection of MCP-1 in the rat is blocked by the intrathecal administration of IB4-saporin, a selective neurotoxin for IB4+/Ret+-nociceptors. MCP-1 induced hyperalgesia is also attenuated by intrathecal antisense oligodeoxynucleotides targeting mRNA for versican, a molecule that binds MCP-1 and that also renders the Ret-expressing nociceptors IB4-positive (+). Finally, peripheral administration of ADAMTS-4 or chondroitinase ABC, two enzymes that disrupt versican integrity by the degradation of the versican core-protein or its chondroitin sulfate glycosaminoglycan side chains, respectively, also attenuated MCP-1 hyperalgesia at the site of nociceptive testing. We suggest that versican’s glycosaminoglycan side chains present MCP-1 to a CCR2 expressing cell type in the skin that, in turn, selectively activates IB4+/Ret+-nociceptors, thereby contributing to enhanced mechanical sensitivity under inflammatory conditions.
Introduction
Monocyte chemoattractant protein 1 (MCP-1) is a member of the CCβ chemokine family with potent chemotactic activity for monocytes/macrophages, which expresses its cognate receptor, CC chemokine receptor 2 (CCR2) (Randolph and Furie, 1995, Rollins, 1996). It has been shown that MCP-1 is expressed by a subset of sensory neurons and that its expression is strongly up-regulated by peripheral nerve injury or inflammation (Tanaka et al., 2004, White et al., 2005b, Jeon et al., 2008). Sensory afferents that express MCP-1 have small to medium sized cell bodies and are characterized by the expression of the receptor tyrosine kinase Ret (rearranged during transfection, Ret+) and binding to the isolectin B4 (IB4+) (White et al., 2005a, Jeon et al., 2008), which we have previously been shown to bind the extracellular matrix molecule versican (Bogen et al., 2005).
Monocytes/macrophages, which can contribute to pain (White et al., 2005a), migrate to the site of inflammation, attracted by a chemotactic gradient. It has been proposed that sulfated glycosaminoglycans at the inflammatory site retain the secreted chemokines and create the localized high concentrations that are necessary to direct the migration of monocytes/macrophages (Bernfield et al., 1999, Kuschert et al., 1999). Versican is a chondroitin sulfate proteoglycan (Wight, 2002). Its association with the plasma membrane of Ret+/IB4+-sensory afferents is mediated by its binding to hyaluronan (Bogen et al., 2005). Versican has been shown to bind several different chemokines that are involved in the recruitment of mononuclear leukocytes such as RANTES (regulated upon activation, normal T-cell expressed) and MCP-1, interactions, that are dependent on versicans sulfated glycosaminoglycan side chains (Hirose et al., 2001). Since versican also interacts with several different adhesion molecules on the surface of mononuclear leukocytes such as L- and P-selectin or the P-selectin glycoprotein ligand-1 (PSGL-1) it has been suggested that versican functions by regulating interactions between chemokines and leukocytes under inflammatory conditions (Kawashima et al., 2000, Zheng et al., 2004, Wu et al., 2005). We therefore hypothesized that versican’s glycosaminoglycan side chains immobilize MCP-1 released by the peripheral terminals of Ret+/IB4+-sensory afferents under inflammatory conditions thereby creating the chemotactic gradient that directs the migration of CCR2-expressing monocytes/macrophages to the site of injury or infection.
Beside its function as a chemoattractant for CCR2-expressing monocytes/macrophages MCP-1 might also be able to sensitize sensory neurons in an autocrine or paracrine manner as sensory neurons also up-regulate the expression of CCR2 under inflammatory conditions (White et al., 2005b, Jung et al., 2008), a G-protein coupled receptor (GPCR) that has recently been shown to activate phosholipase C beta (PLCβ) and protein kinase C (PKC) (Qin et al., 2005), two second messengers that are known to mediate nociceptor sensitization (Aley et al., 2000, Joseph et al., 2007). To address this question we analyzed whether acute administration of MCP-1 is able to induce nociceptor sensitization and mechanical hyperalgesia. Because it has been shown that MCP-1 is selectively expressed by Ret+/IB4+-sensory afferents and known to bind to versican (Hirose et al., 2001, Jeon et al., 2008), the molecule that renders these neurons IB4-positive (Bogen et al., 2005), we additionally evaluated the role of the Ret+/IB4+-afferents and versican. We found that intradermal injection of MCP-1 produces mechanical hyperalgesia in the rat hindpaw that is mediated by the Ret-expressing, IB4-binding subpopulation of nociceptors and dependent on the presence of the extracellular matrix molecule versican at the sensory neurons peripheral terminals.
Material and methods
Animals
All experiments were performed on male Sprague Dawley rats (220-240 g; Charles River Laboratories, Hollister, CA). The animals were housed in a controlled environment in the animal care facility of the University of California, San Francisco, under a 12 h light/dark cycle. Food and water were available ad libitum. Experiments were approved by the Institutional Animal Care and Use Committee at UCSF and adhered to the guidelines of the American Association of Laboratory Animal Care, National Institutes of Health and the Committee for Research and Ethical Issues of the International Association for the Study of Pain. Effort was made to minimize the number of animals used and their suffering.
Drugs and enzymes
NGF and chondroitinase ABC were purchased from Sigma-Aldrich (Saint-Louis, MO, USA), MCP-1 from Fitzgerald Industries (Fitzgerald Industries International, Concord, MA, USA) ADAMTS-4 from Calbiochem (EMD Biosciences Inc., La Jolla, CA, USA) and IB4-saporin from Advanced Targeting Systems (San Diego, CA, USA).
MCP-1 and NGF were solubilized in sterile isotonic saline (Baxter Healthcare Corp., Deerfield, IL, USA). Chondroitinase ABC was solubilized in sterile filtered 0.1 M NaOAc containing 0.1 M Tris-HCl, pH 8 and ADAMTS-4 was used as delivered by the manufacturer.
IB4-saporin was diluted in sterile filtered PBS (concf= 160 ng/μl) prior to being used and intrathecally administered by using a 30-gauge needle inserted on the midline between the fifth and sixth lumbar vertebrae. We and others have shown that this protocol reduces the number of IB4-binding sensory neurons in the affected dorsal root ganglia and their central processes terminating in the dorsal horn of the spinal cord (Nishiguchi et al., 2004, Joseph et al., 2008).
Mechanical nociceptive threshold testing
The nociceptive flexion reflex was quantified using the Ugo Basile analgesymeter (Stoelting, Chicago, IL, USA), which applies a linearly increasing mechanical force to the dorsum of the rats hind paw. The nociceptive threshold was defined as the force in grams at which the rat withdrew its paw, and baseline paw-pressure threshold was defined as the mean of three readings before the test agents were injected. Each paw was treated as an independent measure and each experiment performed on a separate group of rats.
Antisense and mismatch preparation
Antisense and mismatch oligodeoxynucleotides (ODNs) for Trk A were prepared as we have described previously (Malik-Hall et al., 2005). The antisense ODN sequence 5’-CAT CAA CGA AGT CAC CAG ACC G-3’ was directed against a unique sequence of the rat Trk A receptor. The corresponding GenBank accession number and ODN position within the cDNA sequence are M85214 and 121-142, repectively. The mismatch ODN sequence 5’-CAA CAT CGA AGT GAC GAG ACC G-3’ corresponds to the Trk A antisense with four bases mismatched (denoted in bold).
Antisense and mismatch oligodeoxynucleotides (ODNs) for versican were also prepared as we have described previously (Bogen et al., 2008). The antisense ODN sequence 5’-CAC ACA TAG GAA GTC TCA GTA GG-3’ was directed against a unique rat sequence of exon 9 which is present in all known versican splice variants. The corresponding GenBank accession number and ODN position within the cDNA sequence are AF072892 and 1373-1395, respectively. The mismatch ODN sequence 5’-AAA ACA TTG GTA GTA TCA GTC AG-3’ corresponds to the versican antisense sequence with seven bases mismatched (denoted in bold).
Prior to being used, lyophilized ODNs were reconstituted in nuclease-free 0.9 % NaCl to a concentration of 12 μg/μl and stored at -20°C until use. A dose of 40 μg of Trk A or versican antisense or mismatch ODN was intrathecally administered in a volume of 20 μl once daily for three days. Using this protocol we have previously demonstrated down-regulation of the expression of several different proteins in dorsal root ganglion neurons including Trk A and versican (Alessandri-Haber et al., 2003, Dina et al., 2004, Dina et al., 2005, Malik-Hall et al., 2005, Joseph et al., 2007, Bogen et al., 2008, Dina et al., 2008). Prior to each injection rats were anaesthetized with 2.5% isofluorane containing oxygen. ODNs were injected by using a 30-gauge needle inserted intrathecally on the midline between the fifth and sixth lumbar vertebrae.
Statistical analysis
For the dose-response curve a repeated measures ANOVA was used. Because a significant main effect of dose was demonstrated post hoc pair wise contrasts were employed to determine where the differences occurred. The remaining data was analyzed using one-way or two-way ANOVAs as appropriate. All two-way ANOVAs demonstrating a significant interaction were further analyzed with one-way ANOVAs to determine the basis of the interaction. Scheffe post-hoc analysis was employed to determine the basis of significance in one-way ANOVAs involving more than two groups.
Results
MCP-1 induces mechanical hyperalgesia
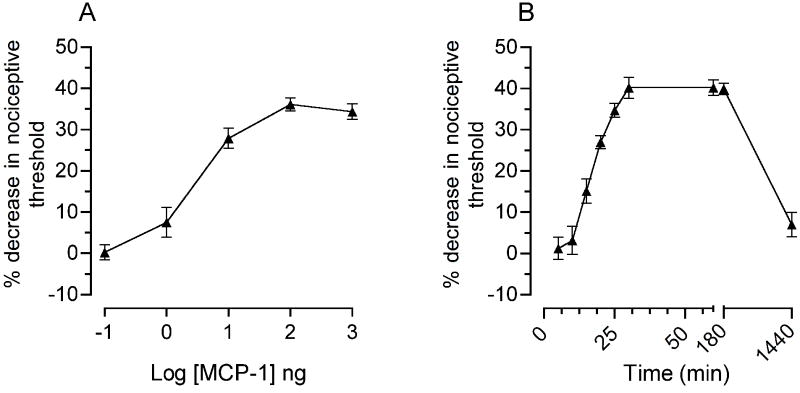
To determine the acute effect of MCP-1 on mechanical nociceptive threshold, we injected recombinant rat MCP-1 intradermally on the dorsum of the rat hind paw. As shown in figure 1A, MCP-1 produced dose-dependent mechanical hyperalgesia (100 pg - 1000 ng, cumulative dosing protocol with each sequentially higher dose administered at 30 min intervals). To study the time course of MCP-1 hyperalgesia we injected a single 100 ng dose. As shown in figure 1B this dose produced a decrease in mechanical nociceptive threshold that was already significant 15 min post-injection, reached its maximum (40 % decrease) by 30 min and lasted for 24 hours.
Figure 1. MCP-1 induces mechanical hyperalgesia.
(A) Dose-response curve for the effect of intradermal MCP-1 on mechanical nociceptive threshold following administration into the dorsum of the rat hind paw. Incremental doses from 100 pg to 1μg were administered at 30 min intervals. A one-way repeated measures ANOVA (5 different doses) showed a significant effect of dose [F(4,28) = 77.912, P < 0.001]. Within-subjects pair wise contrasts showed that only the two lowest doses were not significantly different from each other (P < 0.077); the highest three doses were each significantly different from the lowest dose. A maximal decrease in the nociceptive threshold was obtained at 100 ng MCP-1.
(B) The time-response curve for hyperalgesia induced by 100 ng MCP-1 was investigated from 5 min to 24 hrs (n = 12). Mechanical hyperalgesia was first detectable at 15 min, reached a maximum 30 min post-injection, and lasted for 24 hrs.
MCP-1 induced hyperalgesia is mediated by IB4+/Ret+-nociceptors
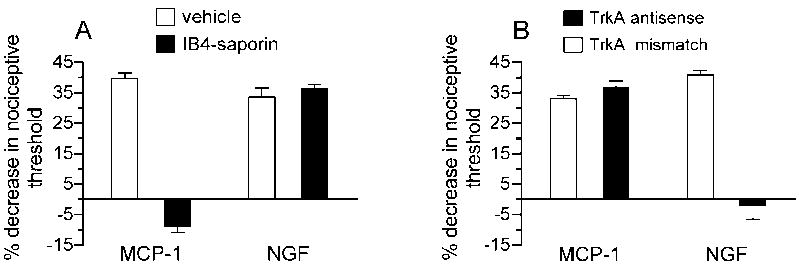
C-fiber nociceptors have been divided into two classes based on their neurotrophic factor dependence, the different termination areas of their peripheral and central projections and the expression of different subsets of immunocytochemical markers (Snider and McMahon, 1998, Zylka et al., 2005). Based on these differences it has been suggested that they might represent different functional classes that sense and transmit different aspects of pain related information to the spinal cord (Snider and McMahon, 1998, Stucky and Lewin, 1999). In order to analyze if MCP-1 induced hyperalgesia is mediated by the IB4+/Ret+-subset of C-fibers, we determined the effect of IB4-saporin, a selective neurotoxin for IB4+/Ret+-nociceptors (Vulchanova et al., 2001, Gerke and Plenderleith, 2004, Nishiguchi et al., 2004, Joseph et al., 2008), on MCP-1 hyperalgesia. Animals received a single intrathecal injection of 3.2 μg IB4-saporin, to destroy IB4 (+) -nociceptors, 10 days prior to intradermal treatment with MCP-1. As shown in figure 2A IB4-saporin significantly attenuated hyperalgesia induced by MCP-1 suggesting that MCP-1 hyperalgesia is, indeed, mediated by the IB4+/Ret+-subset of nociceptors. In contrast, the mechanical hyperalgesia induced by 1 μg NGF was not attenuated by the pretreatment with IB4-saporin. NGF hyperalgesia was, however, blocked by the intrathecal injection of antisense oligodeoxynucleotides to Trk A, the high affinity receptor for NGF, while MCP-1 hyperalgesia was not affected (Fig. 2B).
Figure 2. MCP-1 hyperalgesia is dependent on IB4 (+)-nociceptors.
(A) MCP-1 but not NGF hyperalgesia was inhibited by intrathecal administration of IB4-saporin. A two-way ANOVA with two between subjects factors (intrathecal vehicle or IB4-saporin; and intradermal MCP-1 or NGF) showed a significant intrathecal × intradermal treatment interaction [F(1,20) = 144.882, P < 0.001], a significant main effect of intrathecal treatment [F(1,20) = 115.09, P < 0.001] and a significant effect of intradermal treatment [F(1,20) = 84.638, P < 0.001]. Since the interaction was significant the groups receiving MCP-1 were analyzed separately from those receiving NGF with one-way ANOVAs. The effect of MCP-1 was significantly attenuated in the group that received IB4-saporin compared to the vehicle treated group [F(1,10) = 304.357, P < 0.001], indicating the dependence of MCP-1 induced hyperalgesia on IB4-binding sensory neurons. There was no significant difference between the two groups receiving NGF.
(B) NGF but not MCP-1 hyperalgesia could be blocked by intrathecal administration of antisense oligodeoxynucleotides to Trk A mRNA. A two-way ANOVA with two between subjects factors (intrathecal antisense or mismatch ODNs; and intradermal MCP-1 or NGF) showed a significant intrathecal × intradermal treatment interaction [F(1,20) = 81.728, P < 0.001], a significant effect of intrathecal treatment [F(1,20) = 57.015, P < 0.001] and a significant effect of intradermal treatment [F(1,20) = 36.039, P < 0.001]. Because the interaction was significant, the groups receiving NGF were analyzed separately from those receiving MCP-1 with one-way ANOVAs. The effect of NGF was significantly attenuated in the group that received antisense ODNs for trk A mRNA compared to the group treated with mismatch ODNs [F(1,10) = 84.747, P < 0.001], indicating the dependence of NGF induced hyperalgesia on trk A-expressing sensory neurons. There was no significant differences between the two groups receiving MCP-1.
MCP-1 hyperalgesia is versican dependent
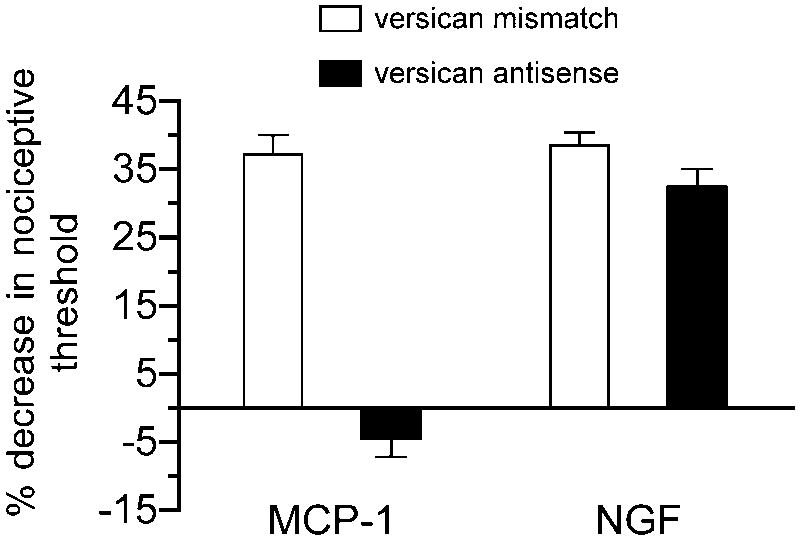
To evaluate the role of versican in MCP-1 hyperalgesia we treated rats intrathecally with antisense or mismatch oligodeoxynucleotides to versican. As shown in figure 3, MCP-1 hyperalgesia was completely blocked in animals that were pretreated with antisense ODNs, but was not affected in animals that had received mismatch ODNs. NGF-induced hyperalgesia was, however, not affected by the versican antisense.
Figure 3. MCP-1 hyperalgesia is dependent on versican.
MCP-1 but not NGF hyperalgesia could be blocked by intrathecal administration of antisense oligodeoxynucleotides to versican mRNA. A two-way ANOVA with two between subjects factors (intrathecal antisense or mismatch ODNs; and intradermal MCP-1 or NGF) showed a significant intrathecal × intradermal treatment interaction [F(1,20) = 49.083, P < 0.001], a significant effect of intrathecal treatment [F(1,20) = 88.387, P < 0.001] and a significant effect of intradermal treatment [F(1,20) = 56.913, P < 0.001]. Because the interaction was significant, the groups receiving MCP-1 were analyzed separately from those receiving NGF with one-way ANOVAs. The effect of MCP-1 was significantly attenuated in the group that received antisense ODNs for versican compared to the mismatch treated group [F(1,10) = 84.747, P < 0.001], indicating the dependence of MCP-1 induced hyperalgesia on versican expressing IB4-(+) sensory neurons. There was no significant difference between the two groups receiving NGF.
Peripheral role of versican
Enzymatic digestion of the versican core
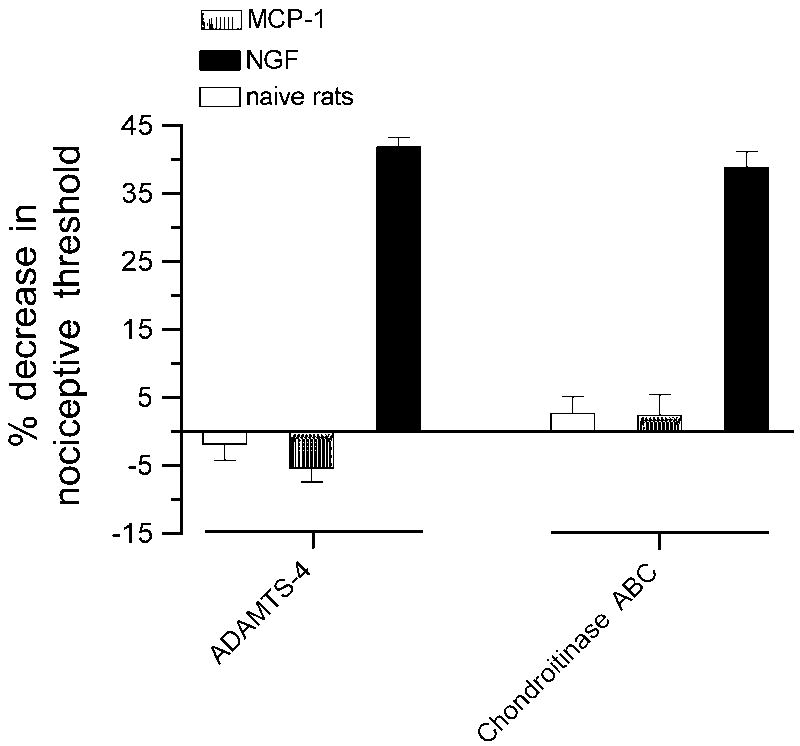
Knockdown of the expression of versican could attenuate MCP-1 hyperalgesia due to a downregulation of the versican level in the periphery, at the central terminals in the spinal dorsal horn or both. To evaluate a peripheral contribution of versican to the inhibition of hyperalgesia induced by injection of MCP-1 we analyzed the effect of intradermal ADAMTS-4, a matrix metalloprotease which has been shown previously to degrade versican by cleaving its glycosaminoglycan attachment domains at position E55-Q56 (GAGα) or E93-A94 (GAGβ) thereby releasing most of the molecule including all chondroitin sulfate side chains into the extracellular space (Sandy et al., 2001, Westling et al., 2004). As shown in figure 4 pre-administration of ADAMTS-4 (1.5 mU) totally blocked MCP-1 hyperalgesia but had no effect on NGF hyperalgesia. ADAMTS-4 alone had no effect on the mechanical nociceptive threshold.
Figure 4. MCP-1 hyperalgesia is dependent on peripheral versican.
MCP-1 but not NGF hyperalgesia could be blocked by intradermal administration of ADAMTS-4 or chondroitinase ABC, two enzymes that are known to affect versican integrity either by the degradation of the versican core-protein or its chondroitin sulfate glycosaminoglycan side chains. The effect of ADAMTS-4 and chondroitinase ABC on MCP-1- and NGF-induced hyperalgesia was examined using separate one-way ANOVAs with one between subjects factors with 3 different levels (vehicle, MCP-1, NGF). The groups receiving ADAMTS-4 or chondroitinase ABC demonstrated significant differences [F(2,21) = 99.478, P < 0.001 for ADAMTS-4 and F(2,21) = 50.968, P < 0.001 for chondroitinase ABC]. Scheffe’s post hoc analysis showed that for both enzymes the groups receiving MCP-1 or vehicle were not significantly different from each other but both were significantly different from the group receiving NGF (P < 0.001 in both cases).
Enzymatic digestion of versicans glycosaminoglycan side chains
Finally, to determine if MCP-1 hyperalgesia could be dependent on versican’s chondroitin sulfate side chains we intradermally administered chondroitinase ABC which has previously been shown to digest chondroitin sulfate chains (Braunewell et al., 1995, Bradbury et al., 2002), 1 h prior to injecting MCP-1. As illustrated in figure 4 pre-administration of chondroitinase ABC (100 mU) also totally blocked MCP-1 hyperalgesia, but again, had no effect on NGF hyperalgesia, supporting the idea that MCP-1 is bound by versican glycosaminoglycan chains. Chondroitinase ABC, itself, does not affect the mechanical nociceptive threshold.
Discussion
Several recent reports suggest a role of MCP-1/CCR2 signaling in models of inflammatory and neuropathic pain syndromes. For example, the expression of both molecules by a subset of small-diameter sensory neurons is highly up-regulated in rodent models associated with neuropathic and inflammatory pain (Tanaka et al., 2004, White et al., 2005b, Jeon et al., 2008). Moreover, cultured rat dorsal root ganglion (DRG) neurons can be excited and sensitized by MCP-1 and the sensitization attenuated by inhibitors targeting PLCβ or PKC (Qin et al., 2005, Jung et al., 2008). However, since these studies were performed in vitro, it is not known whether MCP-1 acts on nociceptors to induce hyperalgesia. To determine whether MCP-1 can induce mechanical hyperalgesia we studied the acute effect of intradermally administered MCP-1. We found a rapid-onset mechanical hyperalgesia with a significant decrease in the paw-withdrawal threshold already detectable after 15 min, which lasted for 24 h. These results are consistent with a previous report showing that mice lacking CCR2 have impaired neuropathic pain (Abbadie et al., 2003).
The phenotypic differences between NGF- and GDNF- dependent nociceptors and the different termination areas of their peripheral and central projections suggesting that they represent different functional classes, which sense and transmit different aspects of pain-related information to the spinal cord (Snider and McMahon, 1998, Stucky and Lewin, 1999). To analyze if MCP-1 hyperalgesia is mediated by IB4+/Ret+- subset of primary afferents we determined the effect of IB4-saporin, a selective toxin for IB4+/Ret+-nociceptors (Vulchanova et al., 2001, Gerke and Plenderleith, 2004, Nishiguchi et al., 2004, Joseph et al., 2008). Pretreatment with IB4-saporin almost completely eliminated MCP-1 hyperalgesia, supporting the idea that MCP-1 is producing hyperalgesia by acting on just one subset, the Ret+/IB4+-subpopulation of sensory neurons. To confirm the specificity of IB4-saporin for Ret+/IB4+-sensory afferents we also tested the effect of IB4-saporin on NGF-induced hyperalgesia. IB4-saporin had no effect on NGF-hyperalgesia. On the other hand, Trk A antisense attenuated NGF-hyperalgesia but had no effect on MCP-1 hyperalgesia. Taken together, these data strongly support the suggestion that MCP-1 induces hyperalgesia by acting on Ret-expressing, IB4-binding nociceptors.
That MCP-1 induced mechanical hyperalgesia is mediated by IB4+/Ret+-nociceptors does not necessarily mean that MCP-1 sensitizes IB4+/Ret+ -sensory terminals directly. As CCR2 expression has been reported to be low in naïve animals (Bhangoo et al., 2007, Menetski et al., 2007), it is more likely that MCP-1 acts on a CCR2-expressing cell type, e.g. keratinocytes or a resident subset of monocytes/macrophages that – in turn – releases a soluble factor that selectively sensitizes the sensory terminals of IB4+/Ret+-nociceptors (Menetski et al., 2007). However, the situation might change under inflammatory conditions, when also IB4+/Ret+-sensory afferents up-regulate their expression of CCR2 (Bhangoo et al., 2007, Jung et al., 2008).
We have recently shown that the extracellular matrix molecule versican is one of the molecules rendering Ret-expressing sensory afferents IB4(+)-positive (Bogen et al., 2005). Versican carries several chondroitin sulfate glycosaminoglycan side chains that have been shown, in vitro, to bind chemokines such as MCP-1 and that are known to attract mononuclear leukocytes (Hirose et al., 2001, Kawashima et al., 2002), which can also contribute to pain (White et al., 2005a). We therefore speculated that the chondroitin sulfate glycosaminoglycan chains on versican also immobilize the MCP-1 that is released by the peripheral terminals of Ret+/IB4+-sensory afferents under inflammatory conditions. In a first test of this hypothesis we intrathecally administered antisense ODN for versican mRNA and analyzed if this treatment affects MCP-1 hyperalgesia. That the antisense ODN we used to downregulate the expression of versican in sensory neurons is effective has been demonstrated previously (Bogen et al., 2008). Indeed, spinal administration of antisense targeting versican attenuates MCP-1 hyperalgesia, but not NGF hyperalgesia, supporting the idea that versican might function as a low affinity receptor for MCP-1.
That a knockdown of the expression of versican attenuates MCP-1 hyperalgesia might be due to a downregulation of the versican level in the periphery, at the central synapses in the spinal dorsal horn, where versican might stabilize synaptic connections between the central terminals of Ret+/IB4+-sensory afferents and secondary projection neurons or both (Bogen et al., 2005, Yamagata and Sanes, 2005). To test the hypothesis that the inhibition of MCP-1 hyperalgesia is caused by a peripheral attenuation of the versican expression we tested the effect of intradermal administered ADAMTS-4, a matrix metalloprotease that has already been shown to degrade, beside others, versican (Sandy et al., 2001, Westling et al., 2004). Indeed, intradermal administration of ADAMTS-4 has the same effect on MCP-1 hyperalgesia as intrathecal antisense to versican, further substantiating our idea that MCP-1 hyperalgesia is dependent on the presence of versican at the peripheral terminals of Ret+/IB4+-fibers. Finally, to test the suggestion that it is versicans sulfated glycosaminoglycan chains that are retaining MCP-1 at the cell surface of Ret+/IB4+-nociceptors we analyzed the effect of chondroitinase ABC on MCP-1 hyperalgesia. Pretreatment with chondroitinase ABC almost completely eliminated MCP-1 hyperalgesia without affecting that induced by NGF, again supporting the suggestion that versican might serve as a low-affinity receptor for MCP-1.
If all versican does is to concentrate released MCP-1 locally to attract monocytes/macrophages intradermal MCP-1 should produce mechanical hyperalgesia, no matter if versican is present at the peripheral sensory terminals or not. However, many chemokines including MCP-1 have to form dimers or higher order oligomers to induce signal transduction (Zhang and Rollins, 1995, Proudfoot et al., 2003) and it has been shown that the assembly of chemokines into biologically active complexes is coupled to the binding to sulfated glycosaminoglycans either at the cell surface or within the extracellular matrix (Hoogewerf et al., 1997, Crown et al., 2006).
Taken together, our data support the suggestion that the MCP-1 that is upregulated in Ret+/IB4+-nociceptors under inflammatory conditions is retained by versicans sulfated glycosaminoglycan side chains to create the chemotactic gradient that attracts CCR2 expressing monocytes/macrophages and to allow the formation of biologically active MCP-1 dimers. The high concentration of biologically active MCP-1 surrounding the peripheral terminals of Ret+/IB4+-nociceptors contributes to the mechanical hyperalgesia under inflammatory conditions either directly, by activating IB4+/Ret+-sensory terminals or indirectly, by activating a CCR2 expressing cell-type that – in turn – activates IB4+/Ret+-nociceptors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, Li B, Monahan PE, Chan DM, Ripsch MS, White FA. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Molecular pain. 2007;3:38. doi: 10.1186/1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen O, Dreger M, Gillen C, Schroder W, Hucho F. Identification of versican as an isolectin B4-binding glycoprotein from mammalian spinal cord tissue. Febs J. 2005;272:1090–1102. doi: 10.1111/j.1742-4658.2005.04543.x. [DOI] [PubMed] [Google Scholar]
- Bogen O, Joseph EK, Chen X, Levine JD. GDNF hyperalgesia is mediated by PLCgamma, MAPK/ERK, PI3K, CDK5 and Src family kinase signaling and dependent on the IB4-binding protein versican. Eur J Neurosci. 2008 doi: 10.1111/j.1460-9568.2008.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Braunewell KH, Martini R, LeBaron R, Kresse H, Faissner A, Schmitz B, Schachner M. Up-regulation of a chondroitin sulphate epitope during regeneration of mouse sciatic nerve: evidence that the immunoreactive molecules are related to the chondroitin sulphate proteoglycans decorin and versican. Eur J Neurosci. 1995;7:792–804. doi: 10.1111/j.1460-9568.1995.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Crown SE, Yu Y, Sweeney MD, Leary JA, Handel TM. Heterodimerization of CCR2 chemokines and regulation by glycosaminoglycan binding. J Biol Chem. 2006;281:25438–25446. doi: 10.1074/jbc.M601518200. [DOI] [PubMed] [Google Scholar]
- Dina OA, Hucho T, Yeh J, Malik-Hall M, Reichling DB, Levine JD. Primary afferent second messenger cascades interact with specific integrin subunits in producing inflammatory hyperalgesia. Pain. 2005;115:191–203. doi: 10.1016/j.pain.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Dina OA, Khasar SG, Alessandri-Haber N, Green PG, Messing RO, Levine JD. Alcohol-induced stress in painful alcoholic neuropathy. Eur J Neurosci. 2008;27:83–92. doi: 10.1111/j.1460-9568.2007.05987.x. [DOI] [PubMed] [Google Scholar]
- Dina OA, Parada CA, Yeh J, Chen X, McCarter GC, Levine JD. Integrin signaling in inflammatory and neuropathic pain in the rat. Eur J Neurosci. 2004;19:634–642. doi: 10.1111/j.1460-9568.2004.03169.x. [DOI] [PubMed] [Google Scholar]
- Gerke MB, Plenderleith MB. Ultrastructural analysis of the central terminals of primary sensory neurones labelled by transganglionic transport of bandeiraea simplicifolia I-isolectin B4. Neuroscience. 2004;127:165–175. doi: 10.1016/j.neuroscience.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Hirose J, Kawashima H, Yoshie O, Tashiro K, Miyasaka M. Versican interacts with chemokines and modulates cellular responses. J Biol Chem. 2001;276:5228–5234. doi: 10.1074/jbc.M007542200. [DOI] [PubMed] [Google Scholar]
- Hoogewerf AJ, Kuschert GS, Proudfoot AE, Borlat F, Clark-Lewis I, Power CA, Wells TN. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry. 1997;36:13570–13578. doi: 10.1021/bi971125s. [DOI] [PubMed] [Google Scholar]
- Jeon SM, Lee KM, Park ES, Jeon YH, Cho HJ. Monocyte chemoattractant protein-1 immunoreactivity in sensory ganglia and hindpaw after adjuvant injection. Neuroreport. 2008;19:183–186. doi: 10.1097/WNR.0b013e3282f3c781. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Bogen O, Alessandri-Haber N, Levine JD. PLC-beta 3 signals upstream of PKC epsilon in acute and chronic inflammatory hyperalgesia. Pain. 2007;132:67–73. doi: 10.1016/j.pain.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Chen X, Bogen O, Levine JD. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J Pain. 2008;9:463–472. doi: 10.1016/j.jpain.2008.01.335. [DOI] [PubMed] [Google Scholar]
- Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima H, Atarashi K, Hirose M, Hirose J, Yamada S, Sugahara K, Miyasaka M. Oversulfated chondroitin/dermatan sulfates containing GlcAbeta1/IdoAalpha1-3GalNAc(4,6-O-disulfate) interact with L- and P-selectin and chemokines. J Biol Chem. 2002;277:12921–12930. doi: 10.1074/jbc.M200396200. [DOI] [PubMed] [Google Scholar]
- Kawashima H, Hirose M, Hirose J, Nagakubo D, Plaas AH, Miyasaka M. Binding of a large chondroitin sulfate/dermatan sulfate proteoglycan, versican, to L-selectin, P-selectin, and CD44. J Biol Chem. 2000;275:35448–35456. doi: 10.1074/jbc.M003387200. [DOI] [PubMed] [Google Scholar]
- Kuschert GS, Coulin F, Power CA, Proudfoot AE, Hubbard RE, Hoogewerf AJ, Wells TN. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38:12959–12968. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neurosci. 2005;21:3387–3394. doi: 10.1111/j.1460-9568.2005.04173.x. [DOI] [PubMed] [Google Scholar]
- Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, Demartino JA, Macintyre DE, Abbadie C. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience. 2007;149:706–714. doi: 10.1016/j.neuroscience.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Nishiguchi J, Sasaki K, Seki S, Chancellor MB, Erickson KA, de Groat WC, Kumon H, Yoshimura N. Effects of isolectin B4-conjugated saporin, a targeting cytotoxin, on bladder overactivity induced by bladder irritation. Eur J Neurosci. 2004;20:474–482. doi: 10.1111/j.1460-9568.2004.03508.x. [DOI] [PubMed] [Google Scholar]
- Proudfoot AE, Handel TM, Johnson Z, Lau EK, LiWang P, Clark-Lewis I, Borlat F, Wells TN, Kosco-Vilbois MH. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A. 2003;100:1885–1890. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Wan Y, Wang X. CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons. J Neurosci Res. 2005;82:51–62. doi: 10.1002/jnr.20612. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Furie MB. A soluble gradient of endogenous monocyte chemoattractant protein-1 promotes the transendothelial migration of monocytes in vitro. J Immunol. 1995;155:3610–3618. [PubMed] [Google Scholar]
- Rollins BJ. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol Med Today. 1996;2:198–204. doi: 10.1016/1357-4310(96)88772-7. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, Lemire JM, Fischer JW, Wight TN, Clowes AW. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276:13372–13378. doi: 10.1074/jbc.M009737200. [DOI] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci Res. 2004;48:463–469. doi: 10.1016/j.neures.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Olson TH, Stone LS, Riedl MS, Elde R, Honda CN. Cytotoxic targeting of isolectin IB4-binding sensory neurons. Neuroscience. 2001;108:143–155. doi: 10.1016/s0306-4522(01)00377-3. [DOI] [PubMed] [Google Scholar]
- Westling J, Gottschall PE, Thompson VP, Cockburn A, Perides G, Zimmermann DR, Sandy JD. ADAMTS4 (aggrecanase-1) cleaves human brain versican V2 at Glu405-Gln406 to generate glial hyaluronate binding protein. Biochem J. 2004;377:787–795. doi: 10.1042/BJ20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov. 2005a;4:834–844. doi: 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, Lamotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A. 2005b;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Versican in the developing brain: lamina-specific expression in interneuronal subsets and role in presynaptic maturation. J Neurosci. 2005;25:8457–8467. doi: 10.1523/JNEUROSCI.1976-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rollins BJ. A dominant negative inhibitor indicates that monocyte chemoattractant protein 1 functions as a dimer. Molecular and cellular biology. 1995;15:4851–4855. doi: 10.1128/mcb.15.9.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng PS, Vais D, Lapierre D, Liang YY, Lee V, Yang BL, Yang BB. PG-M/versican binds to P-selectin glycoprotein ligand-1 and mediates leukocyte aggregation. J Cell Sci. 2004;117:5887–5895. doi: 10.1242/jcs.01516. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]