Abstract
The classic grasping network has been well studied but thus far the focus has been on cortical regions in the control of grasping. Sub-cortically, specific nuclei of the basal ganglia have been shown to be important in different aspects of precision grip force control but these findings have not been well integrated. In this review we outline the evidence to support the hypothesis that key basal ganglia nuclei are involved in parameterizing specific properties of precision grip force. We review literature from different areas of human and animal work that converges to build a case for basal ganglia involvement in the control of precision gripping. Following on from literature showing anatomical connectivity between the basal ganglia nuclei and key nodes in the cortical grasping network, we suggest a conceptual framework for how the basal ganglia could function within the grasping network, particularly as it relates to the control of precision grip force.
Keywords: grasping, network, gripping, sub-cortical, imaging
1. Introduction
The ability to grip an object is an important skill in everyday life impacting such actions as writing, grooming, and holding a drink. The ability to skillfully manipulate objects around the environment is not possible unless appropriate forces are developed and then adjusted to the demands of the object being gripped and the task at hand. The consequences of a poor grip in healthy adults can range from mild (e.g. sloppy handwriting) to serious (e.g. spilling hot coffee). The brain circuitry that underlies the control of gripping an object is not yet fully understood.
The ability to grip is itself part of a larger construct of grasping, which includes reaching toward an object and pre-shaping the hand appropriately depending on the visual properties of the object (Cattaneo et al., 2005; Jeannerod et al., 1995; Santello and Soechting, 1998; Wang and Stelmach, 1998). While there are many ways to reach out to an object or hold it, the act of exerting force against the object is a common action across all grasping tasks. Control of grip force during grasping differs from other aspects of grasping and movement because it is defined by the interaction of the hand with the object. Thus, precision grip control requires precise and fine manipulation of the forces applied to an object. The forces developed are, at least initially, based on the visual properties of the object and prior experience, and thus the visuomotor transformation process plays an important part in precision grip force control.
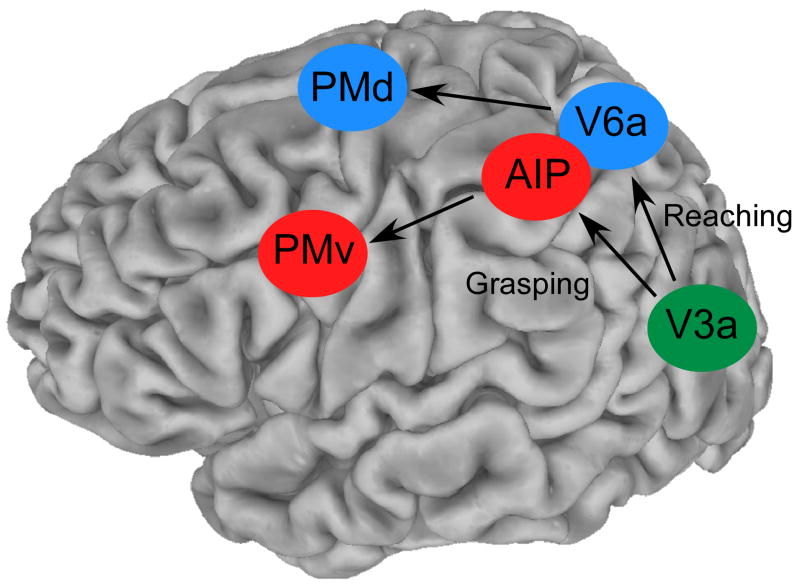
Standard neuroscience teaching often defines the visuomotor transformations required for reaching and grasping an object as involving two different pathways from the primary visual cortex to the premotor areas (Figure 1): a dorsolateral circuit consisting of the anterior intraparietal (AIP) area connected to the rostral part of the ventral premotor cortex (PMv; area F5), and a dorsomedial circuit consisting of the anterior portion of the occipito-parietal sulcus (area V6A) and the caudal dorsal premotor cortex (PMd; area F2). Within this classification, it is the dorsolateral circuit which has been linked to the grasping and manipulation of prehension, whereas the dorsomedial circuit has been linked to the reaching component of grasping. However, within the grasping circuit, the role of subcortical structures, particularly the basal ganglia, in such a classification has not been well defined. In a recent review entitled “The Neuroscience of Grasping”, Castiello (2005) argued that the classic grasping circuit is overly simplistic and does not include other regions such as cerebellar and subcortical areas. In this review, we focus on recent evidence to support the role of the basal ganglia in precision gripping.
Figure 1.
The classic reaching and grasping network showing cortical regions involved in grasping and reaching. The dorsomedial circuit (blue) connects area V3A in the visual cortex, area V6A in the parietal-occipital sulcus, and the dorsal premotor cortex (PMd). The dorsolateral circuit (red) connects area V3A, the anterior intraparietal area (AIP), and the ventral premotor cortex (PMv).
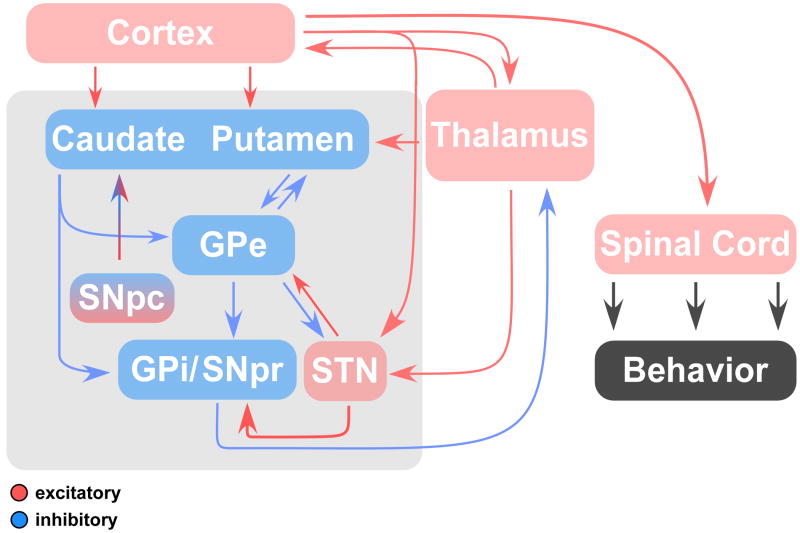
The basal ganglia (BG) are a set of interconnected subcortical nuclei in a circuit composed of excitatory and inhibitory neurotransmitters that are hypothesized to regulate cognitive, limbic, and sensorimotor processes (Alexander et al., 1990; Bhatia and Marsden, 1994; DeLong and Wichmann, 2007; Parent and Hazrati, 1995; Utter and Basso, 2008). The five principle nuclei of the basal ganglia are the putamen, caudate nucleus, globus pallidus, substantia nigra, and subthalamic nucleus (STN) (Figure 2). They have major projections to the thalamus, cerebral cortex, and certain brain stem nuclei, and receive major input from several regions including the cerebral cortex, thalamus, and cerebellum. Over the past several decades, models of basal ganglia circuitry have been developed in animals that describe how neurotransmitters change the inhibitory and excitatory properties of specific basal ganglia nuclei (Alexander et al. 1986). The role of the basal ganglia in the control of limb movements has also been well documented (Anderson and Turner, 1991; Mushiake and Strick, 1995; Turner et al., 2003; Vaillancourt et al., 2004b). However, there is emerging evidence from work in healthy individuals, as well as in individuals with diseases affecting the basal ganglia, that the basal ganglia also play an important role in the control of precision grip force. Indeed, it has been suggested that a pallidothalamic pathway directly innervates the hand representation in primary motor cortex (Holsapple et al., 1991; Nambu et al., 1988) suggesting that the basal ganglia can directly influence the hand representation of the primary motor cortex.
Figure 2.
Basal ganglia circuitry and their relationship to the cortex and thalamus. Excitatory connections are shown in red, inhibitory connections in blue (modified from DeLong and Wichmann (2007)).
In the review that follows, we first review literature from different areas of human and animal work that converges to build a case for basal ganglia involvement in the control of precision gripping. Then, we review the literature that establishes anatomical connectivity between the basal ganglia nuclei and key nodes in the grasping network and suggest a conceptual framework for how the basal ganglia could function within the grasping network, particularly as it relates to the control of precision grip force.
2. The basal ganglia are involved in the control of precision grip force
A review of the literature from both human and animal work is provided. We first discuss evidence linking the basal ganglia to the control of precision gripping in healthy individuals. Next we examine evidence from lesion studies of the basal ganglia in both animals and humans. Then we review precision gripping deficits in humans with movement disorders affecting the basal ganglia.
2.1.Evidence from neuroimaging in neurologically intact individuals
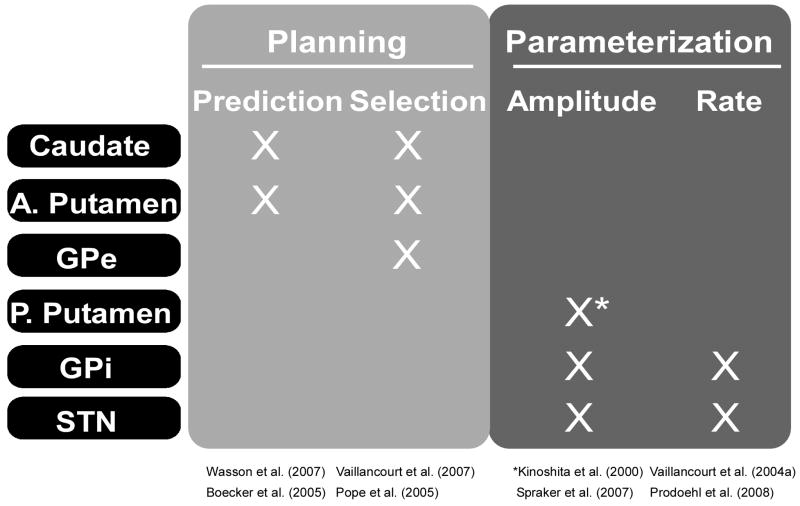
Transforming visual cues about an object into an appropriate grip force to apply to an object requires both planning to select an appropriate initial grip force, and then careful modulation of the applied grip force following interaction with the object. The basal ganglia are well suited to meeting both of those needs. Advances in neuroimaging techniques have recently allowed investigation of how specific nuclei of the basal ganglia function during precision grip force control. These studies in healthy individuals have suggested that while all BG nuclei show increased activation with precision grip force tasks, the more anteriorly located basal ganglia nuclei (caudate, posterior putamen, anterior putamen, and external portion of the globus pallidus (GPe)) show a scaled blood-oxygenation-level-dependent (BOLD) response in relation to the planning aspects of precision gripping such as selecting which force to apply or predicting an appropriate force level for a given task. In contrast, the more posteriorly located nuclei (STN and internal portion of the globus pallidus (GPi)) show a BOLD response that scales with dynamic parameters of grip force output such as force amplitude and rate of force generation (Figure 3).
Figure 3.
A summary of studies which have shown specific grip force processes and the basal ganglia nuclei that have reported BOLD activation that scales when the grip force process is manipulated. It is important to point out that all basal ganglia nuclei are generally active during grip force tasks, but that specific nuclei have BOLD activation that scales with specific neural processes. While SNpr is not included in this summary, this does not mean that SNpr is not involved in precision grip control. Due to the technical difficulties inherent in separating BOLD signal changes in different portions of the substantia nigra, there has not yet been any strong evidence to support scaling of the BOLD signal in SNpr with different grip force processes, although this evidence may emerge as imaging and processing technology advances.
Planning to apply precision grip force requires, among other things, predicting how much force would be required to grip the object and selection of an appropriate initial level of force. Specific basal ganglia nuclei have been implicated as being important in each of these planning steps. Vaillancourt et al. (2007) asked subjects to produce precision grip force that was either at a consistent force amplitude level or was deliberately varied by the subject to select different force amplitudes. Using fMRI to examine activation in the basal ganglia, they found increased activation in caudate, putamen, GPe, GPi, and STN. However, only the anterior basal ganglia nuclei (caudate, anterior putamen and GPe) show increased activation related to the selection component of the task whereas activation in the posterior nuclei was specifically involved in the regulation of basic aspects of dynamic force pulse production. Pope and colleagues (2005) used a slightly different task to Vaillancourt et al., but also found a specific role for anterior basal ganglia nuclei in precision grip force control. They used fMRI to examine brain activation during four self-paced pinch grip force tasks that varied in force amplitude and relative timing. They found a clear pattern of activation across all tasks compared to rest that included activation in the basal ganglia. The magnitude of activation in putamen and caudate was highest for the two conditions where the task required switching between two force amplitude levels compared to the two tasks where the timing varied but force amplitude was the same. This switching between different force amplitudes may be analogous to the selection of different force amplitudes.
Memory of previous gripping experiences allows adjustment of force output in a predictive, feed-forward manner (Gordon et al., 1993; Johansson et al., 1992). Prediction therefore is an important element in grip control. Wasson et al. (2007) investigated how predictability influenced both the amplitude and time-varying response of the BOLD signal in the cortex, basal ganglia, and cerebellum during a precision grip force control task. Within the basal ganglia, both putamen and caudate scaled in activation with increases in the predictability of the grip force necessary to achieve the target. Boecker et al. (2005) used positron emission tomography (PET) to examine predictive motor control in a task which required coordinated grip and pull forces. During the task, subjects were required to exert a grip force on an object which was coordinated with a pull force exerted on that object which acted through the elbow. This task therefore required the subject to predict the consequences on grip of their own pulling behavior and adjust the grip force appropriately. Examining the interaction effect of grip force and pull force while factoring out activity related to grip or pull force alone revealed activation in the ipsilateral posterior cerebellum, the anterior cingulate cortex, the ipsilateral lingual gyrus, and the ipsilateral caudate nucleus. Taken together, these studies offer direct support to the role of anterior basal ganglia nuclei in the planning aspects of grip force control.
Following the initial selection and prediction of an appropriate grip force, there are many gripping parameters that must be controlled in order for the grip to be successful. These include the rate at which grip force is developed so that there is appropriate coordination of the grip and load forces, the amplitude of applied grip force so that it is scaled appropriately to the weight of the object being lifted, and the duration over which grip force is applied. Specific basal ganglia nuclei have been implicated in the control of each of these parameters. Vaillancourt et al. (2004a) used fMRI during a visually guided precision grip task to investigate brain regions that scaled in activation volume with the rate of change of force development and the duration of force. Subjects produced force at different rates and durations to a target of set amplitude. They found that only activation in the STN and GPi scaled parametrically with the rate of change of force production. In contrast, the putamen and GPe increased in activation with the duration but not the rate of grip force contraction. These findings were subsequently replicated by Prodoehl et al. (2008) and extended to include the control of auditory guided precision grip force control.
In addition to the rate at which grip force is controlled, there must be appropriate coordination between the applied grip force and the load force. Ehrsson and colleagues (2003) examined the coordination of thumb-finger grasp in a grip-load force task using fMRI. They found an extensive distribution of activation which included bilateral putamen when comparing the grip-load force task to the baseline rest condition. They concluded that fronto-parietal areas located in both hemispheres and subcortical motor structures work in concert in the control of skillful manipulatory actions.
Another important parameter in precision grip force that must be controlled is the peak grip force exerted. When attempting to lift an object, the size of the object itself influences the peak grip force exerted on the object such that larger objects are lifted with higher peak grip forces (Gordon et al., 1991). Kinoshita et al. (2000) used positron emission tomography (PET) to examine functional brain areas involved when subjects used a precision grip to lift small objects of different weights. (Note: recording of grip and lift forces were performed in separate experimental sessions to PET scanning in this study). They found that in addition to the primary and secondary sensorimotor cortical areas expected to be activated during a grip and lift task, there was activation in the inferior parietal cortex, contralateral posterior putamen, thalamus and cerebellum. Although activation in the primary motor cortex scaled across all three object weights, activation in the putamen increased only when the heaviest weight was compared to the lowest weight suggesting some scaling of basal ganglia activation with object weight. Spraker et al. (2007) specifically investigated activation scaling in the basal ganglia associated with changes across a large range of grip force amplitudes. They showed that specific nuclei of the basal ganglia and thalamus increased in percent signal change with the amplitude of grip force. Subjects produced pinch grip force to targets ranging from 5% to 80% of their maximum voluntary contraction. Within the basal ganglia they found that the GPi and STN had a positively scaled increase in percent signal change when producing force contractions of increasing force amplitude whereas GPe, putamen, and caudate did not.
In summary, there is evidence in the literature to support the hypothesis that anteriorly located basal ganglia nuclei (caudate, anterior putamen, and GPe) are involved in planning aspects of precision gripping, whereas posteriorly located nuclei (posterior putamen, GPi and STN) are involved in scaling dynamic parameters of grip force output (e.g. rate and amplitude). Based on the model presented in Figure 3, one might ask the following question: how do planning signals pass to the thalamus if the output nucleus (e.g. GPi) does not specifically regulate planning of grip force output? In addressing this question, it is important to emphasize that even though activation in posterior basal ganglia nuclei does not scale when the planning aspects of grip tasks are manipulated, there is activation present in posterior basal ganglia nuclei (e.g. GPi) when grip force contractions are compared to a resting baseline state (Vaillancourt et al. 2007). By the same token, even though the activation in anterior basal ganglia nuclei does not scale with the rate and amplitude of grip force, there is greater activation in anterior basal ganglia nuclei during a grip force task than at a baseline resting state (Spraker et al. 2007). Thus, as shown in Figure 2, we suggest that neural signals are sent from the basal ganglia to the thalamus via GPi or the substantia nigra pars reticulata (SNpr), and these two output nuclei do not necessarily have to modify the neural signals in the same way as previous basal ganglia structures. Next, we review the findings from studies which have examined behavioral changes following damage to the basal ganglia.
2.2.Evidence from lesion studies
Animal work has focused on lesioning specific sub cortical regions and examining the behavioral consequences on grip force control. These studies have demonstrated that specific lesions within the basal ganglia have direct consequences on grip force behavior. For example, Dunnett et al. (1998) examined grip strength in rats following unilateral lesioning of the nigrostriatal bundle. In their task, the rat held on to a bar and resisted attempts to be pulled away. The applied force at which the rat released the bar was measured as grip strength. Two weeks after lesioning, the animals showed significantly increased peak grip force on the grip force release task in the contralateral limb compared to control rats. The authors interpreted the increase in grip force following lesion as either being a homologue to the rigidity seen in human Parkinson’s disease or as impairment in the ability to release applied force following lesioning. The increase in contralateral limb grip strength following nigrostriatal lesioning was later replicated by Jeyasingham et al. (2001) and the results extended to include increases in grip strength following lesions isolated to the neostriatum. When comparing grip strength changes between rats with nigrostriatal lesions and rats with striatal lesioning, striatal lesioning did not produce as much change in grip strength as nigrostriatal lesioning. These two animal lesion studies provide a direct link between structural changes in the basal ganglia and changes in grip force behavior.
Individuals who have suffered a stroke centered in the BG offer a unique opportunity to study the isolated effects of BG damage on motor control. Specifically related to grip force, one task which is expected to show deficits in individuals with BG lesions is the ability to scale applied grip force to the size of the object. In healthy individuals, the grip force exerted to lift an object normally increases as object size increases (Gordon et al., 1991) suggesting that the programming of grip force is dependent on the integration of visual and tactile information. Dubrowski et al. (2005) examined grip force control in a single case study design of an individual with unilateral basal ganglia damage following ischemic stroke which was centered on the left putamen, but extended to include the anterior internal capsule, the head of the ventral caudate and the GPe. They examined grasping of three objects which differed in size but not mass. They found that, unlike healthy individuals, the individual with chronic stroke produced significantly higher grip forces and was unable to appropriately scale the applied force to the size of the object when using the contralesional hand. In addition, the individual showed an impaired ability to scale the peak rate of grip force production to object size when using the contralesional hand, but not when using the ipsilesional hand. These findings suggest that the BG nuclei are important in the appropriate scaling of motor output to the demands of the task and in the initial selection of gripping parameters based on visual properties of the object.
2.3.Evidence from studies of patients with movement disorders affecting the basal ganglia
There are many movement disorders which are thought to arise as a result of basal ganglia malfunction. Each of these movement disorders has a widely differing clinical presentation and yet the most common disorders have precision gripping deficits associated with them.
2.3.1. Parkinson’s disease
Parkinson’s disease (PD) affects basal ganglia functioning due to striatal dopamine depletion. Similar to individuals with focal BG lesions, individuals with PD have been shown to produce higher peak grip forces compared to controls when the load required for the task is unknown (Fellows et al., 1998; Wenzelburger et al., 2002) or even when patients have some a priori knowledge of the upcoming load (Nowak and Hermsdorfer, 2006). In patients with deep brain stimulation (DBS) of the subthalamic nucleus (STN), this excessive grip force has been shown to be either improved (Nowak et al., 2005; Wenzelburger et al., 2002) or worsened (Fellows et al., 2006) when stimulation is turned on.
In relation to the control of precision grip force, individuals with PD show increased variability in grip force output when maintaining a consistent grip force compared to healthy individuals (Vaillancourt et al., 2001). While it appears that patients retain the ability to scale grip force according to load (Fellows et al., 1998; Nowak and Hermsdorfer, 2006), individuals with PD show dyscoordination of the temporal coupling between grip and load force (Alberts et al., 1998; Fellows et al., 1998; Ingvarsson et al., 1997; Nowak and Hermsdorfer, 2002). This is particularly evident in more complicated tasks requiring multi-finger coordination. For example, Santello and colleagues (2004) examined the control of multi-digit grasping in PD by changing an object’s center of mass either predictably or unpredictably. They found clear differences between controls and individuals with PD in the temporal evolution of fingertip forces as a function of the object’s center of mass location. When on medication, PD subjects were able to modulate forces to the object’s center of mass location to a greater extent compared to when off medication. Control subjects employed a wider range of forces when the object’s center of mass location could be predicted than when it could not be. In contrast, predictability did not enhance overall force modulation in individuals with PD when off medication, suggesting that they did not take advantage of the a priori knowledge of the object’s center of mass to plan the next set of grip forces.
A higher than necessary grip force is not unique to individuals with BG lesions or PD. This phenomena is also seen in healthy individuals when cutaneous afferents of the hand are subjected to local anesthesia (Nowak et al., 2001; Witney et al., 2004) and in individuals with peripheral sensory neuropathy (Hermsdorfer et al., 2004; Nowak et al., 2004) suggesting that sensory afferent information from the digits may also play an important role in scaling applied grip force to expected object load. Indeed, individuals with PD have been shown to have deficits in sensory processing (Demirci et al., 1997; Jobst et al., 1997; Klockgether et al., 1995; Schneider et al., 1987) which might be related to their grip force scaling deficits. Higher than expected grip forces and disturbances in grip and load force coordination have also been shown in patients with cerebellar dysfunction (Fellows et al., 2001; Serrien and Wiesendanger, 1999a; Serrien and Wiesendanger, 1999b), and it has been suggested that the integrity of sensorimotor cortical areas, the cerebellum, and the basal ganglia is necessary for the optimal regulation of grasping forces during manipulative tasks (Wiesendanger and Serrien, 2001).
In summary, lesioning the basal ganglia and PD can result in higher grip forces, an impairment in releasing objects, a reduced ability to scale forces, and increased variability in force production. Each of these deficits could be characterized as an alteration in the ability to program and regulate grip force in the face of rigidity. However, while it is true that that the presence of rigidity could lead to difficulties in releasing objects and could also result in higher grip forces, it is not clear that rigidity per se would cause problems in programming and regulating grip force beyond simply overgripping. In addition, STN DBS has been shown to improve limb rigidity (Shapiro et al., 2007) and yet it can impair grip force control (Fellows et al., 2006). As such, limb rigidity probably does not explain all the gripping impairments seen following focal BG lesions or in PD.
2.3.2. Dystonia
Dystonia describes a movement disorder characterized by abnormal, involuntary sustained twisting movements and abnormal postures of affected body parts (Fahn et al., 1987). Evidence thus far has suggested that dystonia is related to an abnormality in basal ganglia function (Bhatia and Marsden, 1994; Sheehy and Marsden, 1982). Patients with dystonia have been shown to have difficulty in different aspects of precision gripping. Similar to individuals with PD, individuals with focal hand dystonia have been shown to program higher than normal grip forces (Nowak and Hermsdorfer, 2006; Odergren et al., 1996; Serrien et al., 2000). Patients with writer’s cramp have also shown an impaired ability to regulate precision grip force in both the symptomatic and asymptomatic hands, although their ability to anticipate an upcoming load perturbation appears to be intact (Serrien et al. 2000).
2.3.3. Huntington’s disease
Huntington’s disease (HD) is an inherited neurodegenerative disorder of a progressive nature, with known basal ganglia pathology, particularly affecting the striatum. Similar to PD and dystonia, HD is associated with elevated grip force levels (Schwarz et al., 2001; Serrien et al., 2001; Serrien et al., 2002a) and slowness of grip force development (Serrien et al., 2001). Grip and load force coupling is also disturbed in HD, and these disturbances increase with task complexity (Serrien et al., 2002a). Individuals with HD also show increased grip force variability, both in magnitude (Reilmann et al., 2001; Schwarz et al., 2001) and timing relative to the load (Gordon et al., 2000; Schwarz et al., 2001). This increased variability in static grip force has been shown to be correlated with motor measures of clinical impairment (Gordon et al., 2000), and to worsen over a three year follow-up period (Reilmann et al., 2001). When a precision grip is unexpectedly perturbed in patients with HD, they show a significant lag in their response (Fellows et al., 1997; Serrien et al., 2001). This delayed response to loading in HD has been interpreted as being due to attenuated afferent responses by a damaged striatum. Under this hypothesis, the threshold for eliciting a corrected response would be reached significantly later than normal, thus explaining the delayed grip change responses seen in individuals with HD.
2.3.4. Gilles de la Tourette syndrome
Gilles de la Tourette syndrome (TS) is an inherited neuropsychiatric disorder with onset in childhood, characterized by the presence of multiple physical tics and at least one vocal tic. The pathophysiology of TS has been linked to BG and thalamocortical circuit dysfunction (Singer, 1997). Individuals with TS have been shown to overscale their motor output during unimanual and bimanual grip and lift tasks compared to controls, and to have more difficulty coordinating grip and load forces during bimanual grip and lift tasks than during unimanual tasks (Serrien et al., 2002b). Functional MRI during a grip and lift task in individuals with TS showed underactivation in the basal ganglia and thalamus compared to controls (Serrien et al., 2002b).
In summary, basal ganglia dysfunction associated with Parkinson’s disease, Huntington’s disease, dystonia and Gilles de la Tourette syndrome is associated with specific grip force deficits. The common deficit in the presence of basal ganglia dysfunction across both animal and human studies is an overproduction of grip force which is consistent with a role of specific nuclei of the basal ganglia in the parameterization of grip force (Figure 3).
3. Conceptual framework for how the basal ganglia function within the classic grasping circuit
The control of grasping requires precise coordination of fingertip forces (Blakemore et al., 1998; Flanagan and Wing, 1997; Johansson and Westling, 1984; Johansson and Westling, 1988; Wing et al., 1997). The motor commands for grasp and object manipulation must be finely matched to the properties of the object and the goal of the task. Specific nuclei of the basal ganglia may assist the cortex in the planning and parameterization of grip force, via the thalamus. Such parameters would include grip force amplitude and rate of grip force development (Figure 3).
There appears to be two anatomically segregated parieto-frontal circuits which control reaching and grasping: a dorsolateral circuit controlling the grasp component, consisting of an anterior intraparietal (AIP) area connected to the rostral part of the ventral premotor cortex (PMv), and a dorsomedial circuit controlling the reaching component, consisting of the anterior portion of the occipito-parietal sulcus (area V6A) and the caudal dorsal premotor cortex (PMd) (Galletti et al., 2003; Tanne-Gariepy et al., 2002). Application of force to an object falls under the “grasp” part of this network (i.e. the dorsolateral circuit). However, it should be noted that there may not be a strict dichotomy between circuitry controlling the reach and grasp components. In an analysis of the changes in effective connectivity in the occipito-parieto-frontal network during a visually guided reach and grasp task, Grol et al. (2007) suggest that contributions to the dorsolateral and dorsomedial circuits are a function of the degree of on-line control required by the task related in part to the object size and width. Using dynamic causal modeling, they showed that during the prehension of small objects, effective connectivity of the dorsolateral circuit was increased whereas grasping large objects increased inter-regional coupling within the dorsomedial circuit.
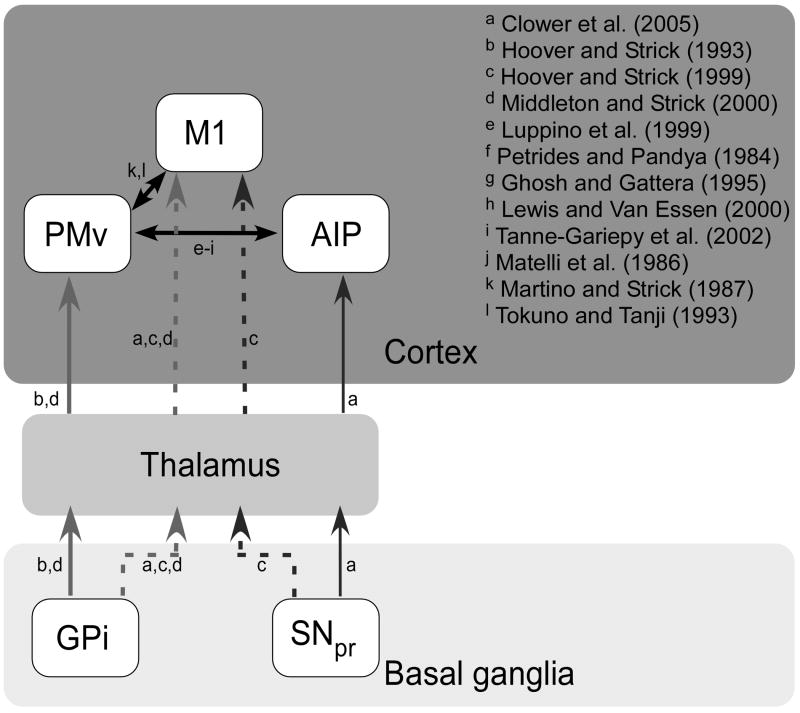
The output nuclei of the basal ganglia have shown clear anatomical connectivity with the two key nodes in the dorsolateral circuit controlling grasping, namely the anterior intraparietal area (AIP) and the ventral premotor cortex (PMv) (Figure 4). AIP contains neurons that respond to three dimensional shape, size, and orientation of an object (Murata et al., 2000) and this region may be involved in the reactive online adjustment of grip force during object manipulation (Dafotakis et al., 2008). Consistent activation has been shown in area AIP in humans using fMRI when performing visually guided grasping (Frey et al., 2005), and disruption of AIP in monkeys by injection of muscimol leads to deficits in hand preshaping (Gallese et al., 1994). Area AIP in monkeys has been shown to be selectively and reciprocally connected to area F5, the rostral part of PMv (Ghosh and Gattera, 1995; Lewis and Van Essen, 2000; Luppino et al., 1999; Matelli et al., 1986; Petrides and Pandya, 1984; Tanne-Gariepy et al., 2002), while PMv has been shown to be connected to the primary motor cortex (Martino and Strick, 1987; Tokuno and Tanji, 1993). Reversible inactivation of neurons in the rostral part of area F5 in monkeys, the homologue of human PMv, produces impairments in hand shaping preceding grasping, and impaired scaling of hand posture to object size and shape (Fogassi et al., 2001). In humans, PMv has also been linked to the predictive scaling of precision grip force (Dafotakis et al., 2008).
Figure 4.
Schematic diagram of the anatomical connectivity between output nuclei of the basal ganglia and key nodes in the cortical grasping network thought to control precision gripping.
Connections to the dorsolateral circuit from the basal ganglia come through both PMv and AIP (Figure 4). PMv is the target of output from both the dentate and the internal segment of the globus pallidus (GPi) via the thalamus (Hoover and Strick, 1993; Middleton and Strick, 2000), and AIP is the target of both basal ganglia and cerebellar output via the thalamus (Clower et al., 2005). Specifically, AIP receives a broadly distributed as well as a focal projection from the dentate nucleus of the cerebellum which forms an output channel via the thalamus to AIP. Within the basal ganglia, AIP receives a projection, via the thalamus, from neurons located in the caudal two thirds of the substantia nigra pars reticulata (Clower et al., 2005). Therefore, as well as output connections to the motor cortex (Akkal et al., 2007; Clower et al., 2005; Hoover and Strick, 1999; Middleton and Strick, 2000), prefrontal cortex (Middleton and Strick, 1994; Middleton and Strick, 2002), and temporal lobe (Middleton and Strick, 1996), the output of the basal ganglia clearly targets the parietal lobe.
AIP has been shown to be the target of output from SNpr via the thalamus (Clower et al., 2005) whereas PMv has been shown to be the target of output from GPi (Hoover and Strick, 1993). Of the two basal ganglia output nuclei which have targeted output to key nodes in the grasping network, SNpr has been implicated more in the control of saccadic eye movements (Hikosaka and Wurtz, 1983) whereas GPi has been more implicated in the control of limb movements (Horak and Anderson, 1984). Based on this, and the anatomical connection between GPi and PMv, we suggest that basal ganglia output through the GPi is ideally suited to directly influence grasping by helping in the planning and parameterization of grip force.
In addition to the coordination and parameterization of gripping forces, successful grasping also relies on integrating sensory information about the object to maintain appropriate grasp. The basal ganglia have been heavily implicated in the process of sensorimotor integration (Abbruzzese and Berardelli 2003; Boecker et al. 1999; Kaji and Murase 2001; Maschke et al. 2003; Murase et al. 2006; Nagy et al. 2005). Tunik and colleagues (2005) used transcranial magnetic stimulation to induce virtual lesions of AIP and found profound effects on grasping. They suggested AIP could be conceived of as a node responsible for integrating the efference copy of a motor command with incoming sensory input, either for detecting or correcting errors between the signals.
In addition to the importance of sensorimotor integration during grasping, inappropriate grip responses must also be inhibited. The basal ganglia have been implicated in the inhibition of undesirable motor programs (Mink, 1996). Inhibition of inappropriate responses must happen rapidly given the potentially disastrous consequences of a premature release of grip force. Take for example a situation in which a container holding a hot drink starts to break. The initial response to drop it in order to prevent a burn of the hand must be delayed until the arm is far enough away from the body to prevent injury to other parts of the body. The subthalamic nucleus has been implicated in suppressing an initiated go response in a stop signal response inhibition task (Aron and Poldrack, 2006). It has been suggested that stop signal inhibition could be implemented via a hyperdirect pathway between the inferior frontal cortex and the STN (Aron et al., 2007). A hyperdirect pathway between the STN and one of the main nodes in the grasping network would be extremely useful, although this possibility has not yet been explored.
This review has focused exclusively on the role of the basal ganglia in precision gripping. However, the contribution of other subcortical brain regions to the control of precision gripping should not be ignored. As discussed earlier in the review, the cerebellum is one region which may also assist in the regulation of grasping forces during manipulative tasks. It has been suggested that one possible role of the cerebellum may be related to the anticipatory and reactive aspects of disturbances in grip behavior (Serrien and Wiesendanger, 1999b). What the potential division of labor is between the basal ganglia and cerebellum remains unclear. One possibility is that activation in different neural pathways is associated with different classes of movement (e.g. internally versus externally guided movement generation), or specific task demands. Addressing how the cerebellum and basal ganglia function together to assist in the control of different aspects of precision gripping is an area rich for future exploration.
Although we have focused on precision gripping in this review, different handgrip configurations should not be ignored. The power grip for instance provides an important function during many everyday tasks. Different task requirements exist for different grip configurations: a power grip requires grasp stability during the production of high force levels whereas a precision grip is useful for the careful manipulation of small objects. Single cell recordings in non human primates has suggested that different grip configurations are controlled by different neural mechanisms (Lemon, 1993). Recent work by Kuhtz-Buschbeck et al. (2008) in humans examined cortical and sub-cortical brain activation during precision and power gripping using light force loads both with and without visual feedback present. Of the brain regions found to be active during the gripping tasks (which included primary sensorimotor cortex, dorsolateral premotor cortex, SMA and cerebellum), no region was specifically more activated during the precision gripping task than during the power gripping task. There was a relative increase in task related activation in the contralateral primary motor cortex and ipsilateral cerebellar hemisphere during the power grip relative to the precision grip. Basal ganglia activation was present during the precision and power gripping tasks performed without visual feedback, and activation was located within the ipsilateral globus pallidus and the contralateral putamen. Based on the study by Kuhtz-Buschbeck and colleagues, we would anticipate that the framework presented in Figure 4 would also exist for power grip tasks. Whether the planning and parameterization model presented in Figure 3 would also exist for power grip tasks remains to be determined.
In conclusion, the literature reviewed suggests that the basal ganglia are involved in the control of precision gripping. Specific basal ganglia nuclei have been shown to be actively involved in the planning and parameterizing of different aspects of grip force with anterior nuclei regulating planning aspects of grip force control and posterior nuclei regulating specific dynamic parameters of grip force. Additionally, deficits in grip force control are a known consequence of pathology involving the basal ganglia, and there is a clear anatomical link between the output nuclei of the basal ganglia and specific nodes of the cortical grasping network. We therefore suggest that the established cortical grasping network be expanded to include the basal ganglia.
Acknowledgments
This research was supported in part by grants from the National Institutes of Health (R01-NS-52318, R01-NS-28127, R01-NS-40902, R01-NS-58487).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J Neurosci. 2007;27:10659–73. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts JL, Tresilian JR, Stelmach GE. The co-ordination and phasing of a bilateral prehension task. The influence of Parkinson’s disease. Brain. 1998;121(Pt 4):725–42. doi: 10.1093/brain/121.4.725. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–46. [PubMed] [Google Scholar]
- Anderson ME, Turner RS. A quantitative analysis of pallidal discharge during targeted reaching movement in the monkey. Exp Brain Res. 1991;86:623–32. doi: 10.1007/BF00230536. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–52. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–33. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117:859–876. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Goodbody SJ, Wolpert DM. Predicting the consequences of our own actions: the role of sensorimotor context estimation. J Neurosci. 1998;18:7511–8. doi: 10.1523/JNEUROSCI.18-18-07511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker H, Lee A, Muhlau M, Ceballos-Baumann A, Ritzl A, Spilker ME, Marquart C, Hermsdorfer J. Force level independent representations of predictive grip force-load force coupling: a PET activation study. Neuroimage. 2005;25:243–52. doi: 10.1016/j.neuroimage.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Castiello U. The neuroscience of grasping. Nat Rev Neurosci. 2005;6:726–36. doi: 10.1038/nrn1744. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Voss M, Brochier T, Prabhu G, Wolpert DM, Lemon RN. A cortico-cortical mechanism mediating object-driven grasp in humans. Proc Natl Acad Sci U S A. 2005;102:898–903. doi: 10.1073/pnas.0409182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clower DM, Dum RP, Strick PL. Basal ganglia and cerebellar inputs to ‘AIP’. Cereb Cortex. 2005;15:913–20. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- Dafotakis M, Sparing R, Eickhoff SB, Fink GR, Nowak DA. On the role of the ventral premotor cortex and anterior intraparietal area for predictive and reactive scaling of grip force. Brain Res. 2008 doi: 10.1016/j.brainres.2008.06.027. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–4. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Demirci M, Grill S, McShane L, Hallett M. A mismatch between kinesthetic and visual perception in Parkinson’s disease. Ann Neurol. 1997;41:781–8. doi: 10.1002/ana.410410614. [DOI] [PubMed] [Google Scholar]
- Dubrowski A, Roy EA, Black SE, Carnahan H. Unilateral basal ganglia damage causes contralesional force control deficits: a case study. Neuropsychologia. 2005;43:1379–84. doi: 10.1016/j.neuropsychologia.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Torres EM, Annett LE. A lateralised grip strength test to evaluate unilateral nigrostriatal lesions in rats. Neurosci Lett. 1998;246:1–4. doi: 10.1016/s0304-3940(98)00194-3. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Johansson RS, Forssberg H. Evidence for the involvement of the posterior parietal cortex in coordination of fingertip forces for grasp stability in manipulation. J Neurophysiol. 2003;90:2978–86. doi: 10.1152/jn.00958.2002. [DOI] [PubMed] [Google Scholar]
- Fahn S, Marsden CD, Calne DB. Classification and investigation of dystonia. In: Marsden CD, Fahn S, editors. Movement Disorders 2. Butterworth; London: 1987. pp. 332–358. [Google Scholar]
- Fellows S, Schwarz M, Schaffrath C, Domges F, Noth J. Disturbances of precision grip in Huntington’s disease. Neurosci Lett. 1997;226:103–6. doi: 10.1016/s0304-3940(97)00264-4. [DOI] [PubMed] [Google Scholar]
- Fellows SJ, Ernst J, Schwarz M, Topper R, Noth J. Precision grip deficits in cerebellar disorders in man. Clin Neurophysiol. 2001;112:1793–802. doi: 10.1016/s1388-2457(01)00623-x. [DOI] [PubMed] [Google Scholar]
- Fellows SJ, Kronenburger M, Allert N, Coenen VA, Fromm C, Noth J, Weiss PH. The effect of subthalamic nucleus deep brain stimulation on precision grip abnormalities in Parkinson’s disease. Parkinsonism Relat Disord. 2006;12:149–54. doi: 10.1016/j.parkreldis.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Fellows SJ, Noth J, Schwarz M. Precision grip and Parkinson’s disease. Brain. 1998;121(Pt 9):1771–84. doi: 10.1093/brain/121.9.1771. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Wing AM. The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J Neurosci. 1997;17:1519–28. doi: 10.1523/JNEUROSCI.17-04-01519.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G. Cortical mechanism for the visual guidance of hand grasping movements in the monkey: A reversible inactivation study. Brain. 2001;124:571–86. doi: 10.1093/brain/124.3.571. [DOI] [PubMed] [Google Scholar]
- Frey SH, Vinton D, Norlund R, Grafton ST. Cortical topography of human anterior intraparietal cortex active during visually guided grasping. Brain Res Cogn Brain Res. 2005;23:397–405. doi: 10.1016/j.cogbrainres.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Gallese V, Murata A, Kaseda M, Niki N, Sakata H. Deficit of hand preshaping after muscimol injection in monkey parietal cortex. Neuroreport. 1994;5:1525–9. doi: 10.1097/00001756-199407000-00029. [DOI] [PubMed] [Google Scholar]
- Galletti C, Kutz DF, Gamberini M, Breveglieri R, Fattori P. Role of the medial parieto-occipital cortex in the control of reaching and grasping movements. Exp Brain Res. 2003;153:158–70. doi: 10.1007/s00221-003-1589-z. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Gattera R. A comparison of the ipsilateral cortical projections to the dorsal and ventral subdivisions of the macaque premotor cortex. Somatosens Mot Res. 1995;12:359–78. doi: 10.3109/08990229509093668. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Forssberg H, Johansson RS, Westling G. Visual size cues in the programming of manipulative forces during precision grip. Exp Brain Res. 1991;83:477–82. doi: 10.1007/BF00229824. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Quinn L, Reilmann R, Marder K. Coordination of prehensile forces during precision grip in Huntington’s disease. Exp Neurol. 2000;163:136–48. doi: 10.1006/exnr.2000.7348. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Westling G, Cole KJ, Johansson RS. Memory representations underlying motor commands used during manipulation of common and novel objects. J Neurophysiol. 1993;69:1789–96. doi: 10.1152/jn.1993.69.6.1789. [DOI] [PubMed] [Google Scholar]
- Grol MJ, Majdandzic J, Stephan KE, Verhagen L, Dijkerman HC, Bekkering H, Verstraten FA, Toni I. Parieto-frontal connectivity during visually guided grasping. J Neurosci. 2007;27:11877–87. doi: 10.1523/JNEUROSCI.3923-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermsdorfer J, Hagl E, Nowak DA. Deficits of anticipatory grip force control after damage to peripheral and central sensorimotor systems. Hum Mov Sci. 2004;23:643–62. doi: 10.1016/j.humov.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata I. Relation of visual and auditory responses to saccades. J Neurophys. 1983;49:1230–1253. doi: 10.1152/jn.1983.49.5.1230. [DOI] [PubMed] [Google Scholar]
- Holsapple JW, Preston JB, Strick PL. The origin of thalamic inputs to the “hand” representation in the primary motor cortex. J Neurosci. 1991;11:2644–54. doi: 10.1523/JNEUROSCI.11-09-02644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993;259:819–821. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. Journal of Neuroscience. 1999;19:1446–63. doi: 10.1523/JNEUROSCI.19-04-01446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB, Anderson ME. Influence of globus pallidus on arm movements in monkeys I. Effects of kainic acid-induced lesions. J Neurophysiol. 1984;52:290–304. doi: 10.1152/jn.1984.52.2.290. [DOI] [PubMed] [Google Scholar]
- Ingvarsson PE, Gordon AM, Forssberg H. Coordination of manipulative forces in Parkinson’s disease. Experimental Neurology. 1997;145:489–501. doi: 10.1006/exnr.1997.6480. [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci. 1995;18:314–20. [PubMed] [Google Scholar]
- Jeyasingham RA, Baird AL, Meldrum A, Dunnett SB. Differential effects of unilateral striatal and nigrostriatal lesions on grip strength, skilled paw reaching and drug-induced rotation in the rat. Brain Res Bull. 2001;55:541–8. doi: 10.1016/s0361-9230(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Jobst EE, Melnick ME, Byl NN, Dowling GA, Aminoff MJ. Sensory perception in Parkinson disease. Arch Neurol. 1997;54:450–4. doi: 10.1001/archneur.1997.00550160080020. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Riso R, Hager C, Backstrom L. Somatosensory control of precision grip during unpredictable pulling loads. I. Changes in load force amplitude. Exp Brain Res. 1992;89:181–91. doi: 10.1007/BF00229015. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Experimental Brain Research. 1984;56:550–564. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Programmed and triggered actions to rapid load changes during precision grip. Exp Brain Res. 1988;71:72–86. doi: 10.1007/BF00247523. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Oku N, Hashikawa K, Nishimura T. Functional brain areas used for the lifting of objects using a precision grip: a PET study. Brain Res. 2000;857:119–30. doi: 10.1016/s0006-8993(99)02416-6. [DOI] [PubMed] [Google Scholar]
- Klockgether T, Borutta M, Rapp H, Spieker S, Dichgans J. A defect of kinesthesia in Parkinson’s disease. Mov Disord. 1995;10:460–5. doi: 10.1002/mds.870100410. [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, Gilster R, Wolff S, Ulmer S, Siebner H, Jansen O. Brain activity is similar during precision and power gripping with light force: an fMRI study. Neuroimage. 2008;40:1469–81. doi: 10.1016/j.neuroimage.2008.01.037. [DOI] [PubMed] [Google Scholar]
- Lemon RN. The G. L. Brown Prize Lecture. Cortical control of the primate hand. Exp Physiol. 1993;78:263–301. doi: 10.1113/expphysiol.1993.sp003686. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428:112–37. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Luppino G, Murata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4) Exp Brain Res. 1999;128:181–7. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- Martino AM, Strick PL. Corticospinal projections originate from the arcuate premotor area. Brain Research. 1987;404:307–312. doi: 10.1016/0006-8993(87)91384-9. [DOI] [PubMed] [Google Scholar]
- Matelli M, Camarda R, Glickstein M, Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the macaque monkey. J Comp Neurol. 1986;251:281–98. doi: 10.1002/cne.902510302. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. The temporal lobe is a target of output from the basal ganglia. Proc Natl Acad Sci U S A. 1996;93:8683–7. doi: 10.1073/pnas.93.16.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–50. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal-ganglia ‘projections’ to the prefrontal cortex of the primate. Cereb Cortex. 2002;12:926–35. doi: 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol. 2000;83:2580–601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Strick PL. Pallidal neuron activity during sequential arm movements. Journal of Neurophysiology. 1995;74:2754–2758. doi: 10.1152/jn.1995.74.6.2754. [DOI] [PubMed] [Google Scholar]
- Nambu A, Yoshida S, Jinnai K. Projection on the motor cortex of thalamic neurons with pallidal input in the monkey. Exp Brain Res. 1988;71:658–62. doi: 10.1007/BF00248759. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Glasauer S, Hermsdorfer J. How predictive is grip force control in the complete absence of somatosensory feedback? Brain. 2004;127:182–92. doi: 10.1093/brain/awh016. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J. Coordination of grip and load forces during vertical point-to-point movements with a grasped object in Parkinson’s disease. Behav Neurosci. 2002;116:837–50. [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J. Predictive and reactive control of grasping forces: on the role of the basal ganglia and sensory feedback. Exp Brain Res. 2006;173:650–60. doi: 10.1007/s00221-006-0409-7. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J, Glasauer S, Philipp J, Meyer L, Mai N. The effects of digital anaesthesia on predictive grip force adjustments during vertical movements of a grasped object. Eur J Neurosci. 2001;14:756–62. doi: 10.1046/j.0953-816x.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Topka H, Tisch S, Hariz M, Limousin P, Rothwell JC. The beneficial effects of subthalamic nucleus stimulation on manipulative finger force control in Parkinson’s disease. Exp Neurol. 2005;193:427–36. doi: 10.1016/j.expneurol.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Odergren T, Iwasaki N, Borg J, Forssberg H. Impaired sensory-motor integration during grasping in writer’s cramp. Brain. 1996;119(Pt 2):569–83. doi: 10.1093/brain/119.2.569. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–16. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Pope P, Wing AM, Praamstra P, Miall RC. Force related activations in rhythmic sequence production. Neuroimage. 2005;27:909–18. doi: 10.1016/j.neuroimage.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Yu H, Wasson P, Corcos DM, Vaillancourt DE. Effects of Visual and Auditory Feedback on Sensorimotor Circuits in the Basal Ganglia. J Neurophysiol. 2008 doi: 10.1152/jn.01108.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilmann R, Kirsten F, Quinn L, Henningsen H, Marder K, Gordon AM. Objective assessment of progression in Huntington’s disease: a 3-year follow-up study. Neurology. 2001;57:920–4. doi: 10.1212/wnl.57.5.920. [DOI] [PubMed] [Google Scholar]
- Santello M, Muratori L, Gordon AM. Control of multidigit grasping in Parkinson’s disease: effect of object property predictability. Exp Neurol. 2004;187:517–28. doi: 10.1016/j.expneurol.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Santello M, Soechting JF. Gradual molding of the hand to object contours. J Neurophysiol. 1998;79:1307–20. doi: 10.1152/jn.1998.79.3.1307. [DOI] [PubMed] [Google Scholar]
- Schneider J, Diamond S, Markham C. Parkinson’s disease: sensory and motor problems in arms and hands. Neurology. 1987;37:951–956. doi: 10.1212/wnl.37.6.951. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Fellows SJ, Schaffrath C, Noth J. Deficits in sensorimotor control during precise hand movements in Huntington’s disease. Clin Neurophysiol. 2001;112:95–106. doi: 10.1016/s1388-2457(00)00497-1. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Burgunder JM, Wiesendanger M. Disturbed sensorimotor processing during control of precision grip in patients with writer’s cramp. Mov Disord. 2000;15:965–72. doi: 10.1002/1531-8257(200009)15:5<965::aid-mds1030>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Burgunder JM, Wiesendanger M. Grip force scaling and sequencing of events during a manipulative task in Huntington’s disease. Neuropsychologia. 2001;39:734–41. doi: 10.1016/s0028-3932(00)00153-6. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Burgunder JM, Wiesendanger M. Control of manipulative forces during unimanual and bimanual tasks in patients with Huntington’s disease. Exp Brain Res. 2002a;143:328–34. doi: 10.1007/s00221-001-0992-6. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Nirkko AC, Loher TJ, Lovblad KO, Burgunder JM, Wiesendanger M. Movement control of manipulative tasks in patients with Gilles de la Tourette syndrome. Brain. 2002b;125:290–300. doi: 10.1093/brain/awf024. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Wiesendanger M. Grip-load force coordination in cerebellar patients. Exp Brain Res. 1999a;128:76–80. doi: 10.1007/s002210050820. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Wiesendanger M. Role of the cerebellum in tuning anticipatory and reactive grip force responses. J Cogn Neurosci. 1999b;11:672–81. doi: 10.1162/089892999563634. [DOI] [PubMed] [Google Scholar]
- Shapiro MB, Vaillancourt DE, Sturman MM, Metman LV, Bakay RA, Corcos DM. Effects of STN DBS on rigidity in Parkinson’s disease. IEEE Trans Neural Syst Rehabil Eng. 2007;15:173–81. doi: 10.1109/TNSRE.2007.896997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy MP, Marsden CD. Writers’ cramp-a focal dystonia. Brain. 1982;105(Pt 3):461–80. doi: 10.1093/brain/105.3.461. [DOI] [PubMed] [Google Scholar]
- Singer HS. Neurobiology of Tourette syndrome. Neurol Clin. 1997;15:357–79. doi: 10.1016/s0733-8619(05)70318-2. [DOI] [PubMed] [Google Scholar]
- Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual Basal Ganglia nuclei in force amplitude generation. J Neurophysiol. 2007;98:821–34. doi: 10.1152/jn.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanne-Gariepy J, Rouiller EM, Boussaoud D. Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res. 2002;145:91–103. doi: 10.1007/s00221-002-1078-9. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Tanji J. Input organization of distal and proximal forelimb areas in the monkey primary motor cortex: a retrograde double labeling study. J Comp Neurol. 1993;333:199–209. doi: 10.1002/cne.903330206. [DOI] [PubMed] [Google Scholar]
- Tunik E, Frey SH, Grafton ST. Virtual lesions of the anterior intraparietal area disrupt goal-dependent on-line adjustments of grasp. Nat Neurosci. 2005;8:505–11. doi: 10.1038/nn1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RS, Desmurget M, Grethe J, Crutcher MD, Grafton ST. Motor subcircuits mediating the control of movement extent and speed. J Neurophysiol. 2003;90:3958–66. doi: 10.1152/jn.00323.2003. [DOI] [PubMed] [Google Scholar]
- Utter AA, Basso MA. The basal ganglia: an overview of circuits and function. Neurosci Biobehav Rev. 2008;32:333–42. doi: 10.1016/j.neubiorev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Mayka MA, Thulborn KR, Corcos DM. Subthalamic nucleus and internal globus pallidus scale with the rate of change of force production in humans. Neuroimage. 2004a;23:175–186. doi: 10.1016/j.neuroimage.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Prodoehl J, Verhagen Metman L, Bakay RA, Corcos DM. Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson’s disease. Brain. 2004b;127:491–504. doi: 10.1093/brain/awh057. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Slifkin AB, Newell KM. Intermittency in the visual control of force in Parkinson’s disease. Exp Brain Res. 2001;138:118–27. doi: 10.1007/s002210100699. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Yu H, Mayka MA, Corcos DM. Role of the basal ganglia and frontal cortex in selecting and producing internally guided force pulses. Neuroimage. 2007;36:793–803. doi: 10.1016/j.neuroimage.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Stelmach GE. Coordination among the body segments during reach-to-grasp action involving the trunk. Exp Brain Res. 1998;123:346–50. doi: 10.1007/s002210050578. [DOI] [PubMed] [Google Scholar]
- Wasson P, Prodoehl J, Yu H, Corcos DM, Vaillancourt DE. Prediction and the basal ganglia. Society for Neuroscience; San Diego, CA: 2007. [Google Scholar]
- Wenzelburger R, Zhang BR, Poepping M, Schrader B, Muller D, Kopper F, Fietzek U, Mehdorn HM, Deuschl G, Krack P. Dyskinesias and grip control in Parkinson’s disease are normalized by chronic stimulation of the subthalamic nucleus. Ann Neurol. 2002;52:240–3. doi: 10.1002/ana.10254. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M, Serrien DJ. Neurological problems affecting hand dexterity. Brain Res Brain Res Rev. 2001;36:161–8. doi: 10.1016/s0165-0173(01)00091-1. [DOI] [PubMed] [Google Scholar]
- Wing AM, Flanagan JR, Richardson J. Anticipatory postural adjustments in stance and grip. Exp Brain Res. 1997;116:122–30. doi: 10.1007/pl00005732. [DOI] [PubMed] [Google Scholar]
- Witney AG, Wing A, Thonnard JL, Smith AM. The cutaneous contribution to adaptive precision grip. Trends Neurosci. 2004;27:637–43. doi: 10.1016/j.tins.2004.08.006. [DOI] [PubMed] [Google Scholar]