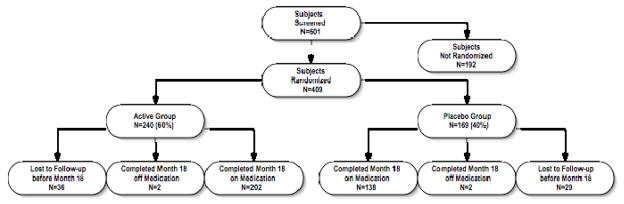
Figure 1.
Participant flow. The reasons for screening failures were MMSE score out of range (n=47), excluded or unstable medications (n=38), unstable medical condition (n=29), abnormal screening laboratory results (n=29);,withdrawn consent (n=12), abnormal MRI findings (n=10), caregiver issues (n=10) and diagnostic uncertainty (n=4); the reason was unknown in 13 cases. The reasons for dropping out of the study were caregiver unwillingness to continue (active 13, placebo 13), travel difficulties (active 5, placebo 7), non-compliance with study medication (active 4, placebo 6), decisions to try alternate therapy (active 6, placebo 3), concern about potential adverse effects (active 3, placebo 2), frequency of assessments (active 1, placebo 4), safety concerns (active 1, placebo 3), length of protocol (active 1, placebo 3), concern regarding placebo assignment (active 2, placebo 2), protocol violations (active 1, placebo 1), and unspecified (active 18, placebo 14); participants were allowed to specify multiple reasons. All subjects in the active treatment arm were included in the primary analysis; three subjects in the placebo group were excluded because of missing data.