Abstract
First-degree family history of sporadic Alzheimer disease (AD) and the apolipoprotein E ε4 (APOE4) are risk factors for developing AD. Although the role of APOE4 in AD pathogenesis has been well studied, family history remains a rarely studied and poorly understood risk factor. Both putatively cause early brain changes before symptomatic disease, but the relative contribution of each to brain function is unknown. We examined 68 middle-aged participants with a parent diagnosed with AD [family history (+FH)] and 64 age- and education-matched controls without a first-degree family history of any dementia [no family history (−FH)]. All underwent cognitive testing, APOE genotyping, and a functional magnetic resonance imaging encoding task that required discrimination of novel items from previously learned items. A 2 × 2 factorial ANOVA (presence/absence of parental family history and presence/absence of the APOE4) was used to detect group effects. A greater response to novel items was detected in the mesial temporal lobe and fusiform gyrus bilaterally among persons without a first-degree family history of AD. In hippocampal areas, the −FH +ε4 group exhibited the greatest signal change, and the +FH +ε4 group exhibited the least. These findings indicate that FH of AD is an important predictor of hippocampal activation during encoding and that FH may modulate the effect of APOE4 in these middle-aged adults, suggesting that an as yet unspecified factor embodied in first-degree family history of AD is influencing the expression of APOE4 on brain function.
Keywords: Alzheimer disease, fMRI, memory formation, hippocampal function, imaging, dementia
Introduction
There is increasing evidence that the clinical syndrome of Alzheimer disease (AD) is preceded by a silent preclinical phase characterized by neuropathological change spanning over decades (Ohm et al., 1995; Braak et al., 1999; Corder et al., 2004). Both the apolipoprotein E (APOE) ε4 allele (APOE4 or ε4) and first-degree family history (Fratiglioni et al., 1993) increase the preclinical prevalence of neurofibrillary tangles and amyloid plaques, the main pathology in AD (Ghebremedhin et al., 1998, 2001; Corder et al., 2004). Additional evidence of preclinical AD comes from studies of cognitively normal APOE4 middle-aged adults who exhibit reduced cerebral metabolic rate of glucose (CMRgl) in many of the same regions affected by AD compared with noncarriers (Reiman et al., 1996, 2004; Small et al., 2000). In older adults, APOE4 has been associated with decreased mesial temporal lobe (MTL) volume (Plassman et al., 1997; Lemaitre et al., 2005) and cognitive decline (La Rue et al., 1995; Caselli et al., 1999, 2001; Small et al., 2000; Baxter et al., 2003; Blair et al., 2005). However, as a predictor of AD (Martinez et al., 1998; Green et al., 2002; Cupples et al., 2004; Pedersen et al., 2004), APOE4 accounts for <40% of AD cases, suggesting that other factors are involved in AD pathogenesis (Saunders et al., 1993; Slooter et al., 1998).
Although the influence of APOE genotype has been extensively studied with cognition and brain imaging studies, the risk factor of first-degree family history has not. Results of the REVEAL study (Cupples et al., 2004) highlight the importance of this risk factor, indicating that the risk of developing AD associated with a positive family history was additive to the risk associated with APOE4. Despite this, little is known about the biologic mechanisms of sporadic AD risk associated with a positive family history, and the relative contributions of family history and APOE genotype on AD risk are unknown. This relationship is important to disambiguate because APOE4 and family history co-occur, and in one study, 45% of adult offspring of AD patients carried the ε4 allele (Sager et al., 2005). An important question is whether the neural effects attributed to APOE are confounded by the influence of other AD risk factors, such as family history.
Functional magnetic resonance imaging (fMRI) is useful for detecting hemodynamically coupled neurocognitive brain activity during specific cognitive tasks. Most previous fMRI studies of at-risk subjects have stratified their participants by APOE4 status only. Other studies have examined family history and APOE4 together, but not as separable risk factors, defining the presence of both as high risk and absence of both as low risk (Smith et al., 1999, 2005; Fleisher et al., 2005). No studies to date have evaluated first-degree family history of AD as an explicit risk factor separate from APOE.
In this study, we compared the effects of first-degree family history (defined as having at least one biological parent with AD) and APOE genotype on neurocognitive function using an fMRI encoding paradigm in cognitively normal middle-aged adults. We hypothesized that a family history effect would be observed and that this effect would be independent of APOE genotype.
Materials and Methods
Subjects.
One hundred thirty-two subjects underwent fMRI scanning and cognitive testing (see Table 1). Sixty-eight participants (mean age, 54; SD, 6.4) had at least one parent with AD and were recruited from the Wisconsin Registry for Alzheimer's Prevention (Sager et al., 2005), a longitudinal registry of cognitively normal adults between the ages of 40 and 65 (at entry) who have at least one parent with sporadic AD (here this group is designated +FH). To verify the diagnosis of AD in the parent, parental medical records were obtained (including autopsy reports when available) and reviewed by a multidisciplinary diagnostic consensus panel. Typically, the clinical work-up and diagnosis in the parent were conducted at the University of Wisconsin Memory Clinics. The onset of AD in the parent was reported to be at age 73 on average. There were no families included with known autosomal-dominant mutations. In nine instances, the participants were siblings. All subjects in the +FH group underwent baseline neuropsychological evaluations and laboratory tests that included APOE genotyping using PCR and sequencing. Within the +FH group, 37 (54%) participants were ε4 positive (ε3/ε4, n = 26; ε4/ε4, n = 7; ε2/ε4, n = 4) and the remaining 31 were ε4 negative (ε3/ε3, n = 24; ε2/ε3, n = 6; ε2/ε2, n = 1).
Table 1.
Demographic, cognitive, performance, and medical information for each group
| Negative family history | Positive family history | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ϵ4 Pos | ϵ4 Neg | ϵ4 Pos | ϵ4 Neg | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F (4,128) | |
| n | 11 | 53 | 37 | 31 | |||||
| Age | 55.80 | 6.20 | 54.75 | 6.80 | 54.53 | 5.75 | 53.87 | 7.13 | NS |
| Education | 17.30 | 2.41 | 16.25 | 2.62 | 15.97 | 2.48 | 15.84 | 2.63 | NS |
| Gender (M/F) | 6/5 | 12/41 | 14/23 | 12/19 | 8.37*a | ||||
| Neuropsychological functioning | |||||||||
| WRAT-III reading subtest | 107.89 | 8.08 | 108.68 | 7.87 | 107.22 | 7.45 | 107.55 | 8.31 | NS |
| Trail making A (s) | 29.498 | 8.88 | 27.67 | 8.32 | 27.22 | 7.10 | 26.81 | 6.10 | NS |
| Trail making B (s) | 58.16 | 23.77 | 62.06 | 21.42 | 63.86 | 19.05 | 57.13 | 20.11 | NS |
| RAVL total | 48.45 | 6.11 | 49.75 | 7.15 | 52.44 | 7.08 | 51.97 | 8.29 | NS |
| RAVL delayed recall | 9.78 | 1.64 | 9.77 | 2.59 | 10.67 | 2.82 | 10.42 | 2.99 | NS |
| COWAT word generation | 45.44 | 11.58 | 43.98 | 9.90 | 46.92 | 9.35 | 44.35 | 10.32 | NS |
| Boston naming test | 57.22 | 3.63 | 56.06 | 7.77 | 57.36 | 1.93 | 55.81 | 8.10 | NS |
| Judgment of line orientation | 26.89 | 3.10 | 25.36 | 5.00 | 26.06 | 3.83 | 26.65 | 3.30 | NS |
| CES-D | 5.78 | 5.24 | 4.66 | 5.47 | 4.61 | 4.33 | 4.94 | 4.93 | NS |
| Performance in the scanner | |||||||||
| Reaction time NV | 0.79 | 0.15 | 0.84 | 0.11 | 0.93 | 0.20 | 0.92 | 0.17 | 11.20*b |
| Reaction time PL | 0.75 | 0.14 | 0.78 | 0.10 | 0.79 | 0.10 | 0.75 | 0.11 | NS |
| Accuracy (%) | 99.14 | 1.80 | 98.98 | 1.73 | 97.84 | 5.30 | 98.54 | 2.94 | NS |
| Labs | |||||||||
| Hemoglobin | 14.13 | 1.32 | 13.91 | 0.91 | 13.99 | 0.86 | 14.20 | 1.04 | NS |
| Systolic blood pressure | 127.44 | 17.39 | 131.25 | 15.66 | 130.73 | 16.85 | 134.39 | 18.73 | NS |
| Medications | ChiSq (3)a | ||||||||
| % Cholesterol lowering | 9% | 9% | 11% | 6% | NS | ||||
| % Antidepressant | 9% | 6% | 27% | 10% | 20.77* | ||||
| % Blood pressure lowering | 9% | 6% | 14% | 26% | 16.93* | ||||
| % NSAID | 36% | 43% | 46% | 39% | NS | ||||
| % Females on HRT | 20% | 15% | 35% | 21% | 9.7* | ||||
This table consists only of subjects who were included in the image analyses. ϵ4 Pos, ϵ4 Positive; ϵ4 Neg, ϵ4 negative; WRAT-III, Wide Range Achievement Test, 3rd Edition; RAVL, Rey Auditory Verbal Learning Test alternate form; COWAT, Controlled Oral Word Association Test; CES-D, Center for Epidemiological Studies Depression Scale; NSAID, nonsteroidal anti-inflammatory drug; HRT, hormone replacement therapy.
aχ2 Test with 3 degrees of freedom.
bThis is the F-test for the FH main effect; APOE status main effect and interaction were not significant.
*p < 0.05.
A group of 64 participants (mean age, 55; SD, 6.5) with no family history of AD (−FH) were recruited from the community and matched to the demographic characteristics of the +FH sample. Absence of first-degree family history of AD was determined through self-report of the participant through phone interview as well as on a detailed medical history questionnaire. To be included in the −FH group, both parents had to survive to at least age 70 and not carry a diagnosis of dementia or exhibit frank symptoms of dementia of any kind. Eleven (17%) of the controls were ε4 positive (ε3/ε4, n = 10; ε4/ε4, n = 1) and 53 were ε4 negative (ε2/ε3, n = 6; ε3/ε3, n = 47). The demographics of the ε4-positive and -negative subgroups are shown in Table 1 along with fMRI task performance, neuropsychological test scores, medication usage, blood pressure, and hemoglobin levels measured the day of the scan. Exclusions for this imaging study included MRI scanner incompatibility, history of major psychiatric disease (e.g., schizophrenia, substance dependence, current or recent major depression) or major medical conditions (e.g., history of neurological disorders including previous head trauma with loss of consciousness, cancer requiring chemotherapy or radiation, insulin-dependent diabetes), or abnormal structural MRI or neuropsychological testing as part of study participation. Most psychoactive drugs were excluded, although we did allow low-dose selective serotonin reuptake inhibitors if stable for >3 months.
The 132 subjects included in the statistical analysis were required to have useable behavioral and imaging data free from artifact or unacceptable motion (movement in the x, y, or z plane >3 mm). The data from an additional seven subjects were excluded and are not represented in Table 1. These subjects were excluded for neoplasm (one +FH male); cognitive impairment (one +FH male); excessive head motion (one +FH female); scanner error (one +FH female); no APOE result (one −FH male); and questionable or absent task performance data (one +FH male and one −FH female).
fMRI task.
The task has been described previously (Johnson et al., 2006) and shown to evoke an MTL response in healthy young and middle-aged adults. In that study, it was also reported that patients with mild cognitive impairment (MCI) exhibit a significantly reduced response on this task compared with age-matched controls despite comparable task performance. The task involved serial presentation of novel (NV) and previously learned (PL) line drawings obtained from a published normative set (Snodgrass and Vanderwart, 1980). The neurocognitive effect of interest was the cerebral response to NV versus PL items. PL items were acquired in two training sessions 40 and 15 min before the in-scanner task. The training set consisted of five items with similar frequency and image complexity as the novel items. The training items were presented repeatedly in pseudorandom manner for 15 exposures in each of the two training trials for a total of 30 exposures to each item. Participants were instructed to view the pictures and try to remember them.
During the fMRI scan, NV or PL items were presented every 3000 ms (2800 ms presentation; 200 ms interstimulus interval) for the duration of the scan, and the task was always to decide whether the present item was new or previously learned. There were no periods of rest or cross-hair fixation (see Discussion for rationale), and thus the participants remained in the same cognitive set for the duration of the task. The items were presented as trains of like-events that pseudorandomly varied in number, ranging from single items to five consecutive items of the same type. This variation reduced condition predictability while preserving blood oxygenation level-dependent (BOLD) efficiency (Liu et al., 2001). Two equivalent forms of the task were sequentially presented (counterbalanced) using the same PL items but different NV items (45 novel items presented in each form). The task duration for each run was 4 min and 42 s. Responses were made with a two-button MR-compatible response device held in the right hand. The software Presentation and a goggle system, set at 800 × 600, from Resonance Technology (Northridge, CA), were used to deliver the stimuli and log participant responses.
Scanning procedures.
The imaging exam was performed with a General Electric (Waukesha, WI) 3.0 tesla scanner. The sequence of the scan series was as follows: three-plane localizing scout, higher-order shimming (two iterations), field mapping, fMRI scans, T1-weighted inversion-prepared volume, and fast-recovery fast spin echo T2-weighted axial scans.
Echo-planar imaging.
A T2* gradient-echo echo-planar imaging (EPI) sequence was used. The EPI parameters were as follows: echo time (TE), 30 ms; repetition time (TR), 2000 ms; flip angle, 90°; acquisition matrix, 64 × 64 voxels; field of view (FOV), 240 mm. Thirty sagittal slices of brain were acquired within each TR. Voxel resolution was 3.75 × 3.75 × 5 mm (4-mm-thick slices with a 1 mm skip). A time course of 141 temporal volume images was collected, of which the initial three volumes were discarded.
To correct for EPI image distortions, three-dimensional (3D) field maps (coplanar with the fMRI slices) were acquired on each subject by measuring the phase of non-EPI gradient-echo images at two echo times (7 and 10 ms). Static field inhomogeneity correction was achieved with locally developed software using a previously published algorithm (Jenkinson, 2003).
Image processing and statistics.
The 4D time series was motion corrected (using Statistical Parametric Mapping software SPM2). The field map from each subject was then applied to the time series. This was followed by spatial normalization into the Montreal standard atlas space using an echoplanar template, resampling to 2 mm isotropic voxels, and spatial smoothing to 8 mm. To derive single-subject activations, the conditions were convolved with a canonical hemodynamic response function. High-frequency signal filtering (cutoff, 0.0078 Hz) was used. Nonsphericity caused by temporal autocorrelation was estimated with an autoregressive (AR1) model. The contrast NV > PL was computed for each subject and subsequently entered into a second-level random-effects 2 × 2 factorial ANOVA to examine the group interactions and main effects of family history and APOE4 status. The omnibus F statistic was computed, and subsequent analyses were restricted only to regions in which this was significant pFWE < 0.05, corresponding to an F statistic of 8.88. This procedure reduces the risk of false positive errors from the thousands of voxelwise tests that are performed by reducing the search region to those voxels in which at least one of the four groups was significantly different from zero. We then tested the group t contrasts of −FH > +FH and its opposite (both comprising the main effect of FH factor); −ε4 < +ε4 and −ε4 > +ε4 (comprising the main effect of ε4 factor); and the interaction between factors. We also tested the effect of FH on only ε4-positive carriers. All of these contrasts were corrected for multiple comparisons using the false discovery rate (FDR) method (Genovese et al., 2002), pFDR < 0.005, and a cluster threshold of at least 100 contiguous 2 × 2 × 2 mm voxels (>0.8 cm3). Similar ANCOVA models were subsequently tested for reaction time and gender to determine their influence on the findings.
Anatomic imaging and voxel-based morphometry analysis.
Axial T1- and T2-weighted images were acquired after the functional runs. A 3D inversion recovery prepared fast gradient-echo pulse sequence provided high-resolution T1-weighted structural images with the following parameters: inversion time, 600 ms; fast gradient-echo read-out with TR, 9 ms; TE, 1.8 ms; flip angle, 20°; acquisition matrix, 256 × 192 × 124 (interpolated to 256 × 256 × 124); field of view, 240 mm; slice thickness, 1.2 mm (124 slices); ±16 kHz receiver bandwidth.
A fast-recovery fast-spin echo 2D T2-weighted axial sequence was also acquired with the same start and stop locations as the T1-weighted images. The parameters were as follows: field of view, 240 mm; matrix, 256 × 256; TR, 9000 ms; TE, 93 ms; flip angle, 90°. Seventy slices were acquired; slice thickness equaled 1.7 mm with 0.3 mm skip. An experienced neuroradiologist examined all images before the analysis for clinical evidence of any neurovascular disease or structural abnormality that would exclude the subjects from the analysis.
The T1 volume was subsequently used for VBM to determine whether there were volumetric differences between groups that might account for any observed fMRI differences. The VBM procedure used a standard approach (Good et al., 2001) that included optimized normalization to standard atlas space (and resampled at 2 mm isotropic voxels), modulation of the normalized image by the Jacobian determinants to preserve volume information, followed by spatial smoothing to 12 mm. The Gaussian smoothing function differed from that of the functional images because the intrinsic smoothness of the high-resolution structural scans was less than that of the functional scans. A comparable 2 × 2 statistical design was used to determine whether there were gray matter differences at a threshold of pFDR < 0.05 corrected for multiple comparisons.
Results
Neuropsychological and behavioral findings
Table 1 includes demographic, neuropsychological, behavioral, and laboratory data. The groups did not differ on age, education, cognitive test performance, or fMRI task accuracy. Reaction time to PL items was equivalent between groups; however, reaction time to NV items was slightly slower (∼100 ms on average) in the +FH subjects; this main effect reached statistical significance, and NV reaction time was treated as a covariate in a follow-up ANCOVA. χ2 analyses of medication usage indicated that the groups were equivalent on the proportion taking nonsteroidal anti-inflammatory and cholesterol-lowering drugs. However, use of hormone replacement therapy in women, antihypertensives, and antidepressants were higher in one or both of the +FH groups. We therefore computed follow-up analyses to determine whether medications might have affected the overall findings. Gender proportion was different between subgroups (χ2 = 8.37; p = 0.03) and was treated as a covariate in a follow-up ANCOVA analysis.
Imaging results
Family history main effect
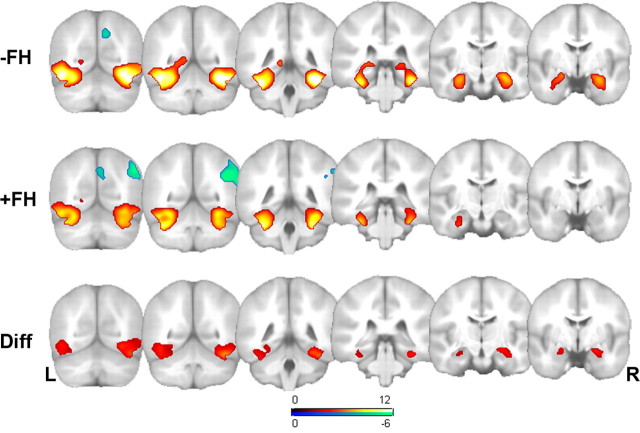
Although not part of the planned comparisons, the results from the −FH and +FH groups are presented separately in the first two rows of Figure 1 and Table 2 to show qualitatively where each group activated. These maps were masked with the omnibus F as described above and further constrained to pFWE < 0.05 (t = 4.11; 100 voxel extent threshold). The reverse contrasts are also reported in Figure 1. In the −FH group, there was a single region in the precuneus more active during PL > NV (x, y, z: 10, 68, 40; t = 5.30, pFWE = 0.001; 253 voxels). In the +FH group there were two clusters involving the right lateral parietal lobe (44, −54, 32; t = 6.61; pFWE < 0.001; 1137 voxels) and right precuneus (6, −74, 34; t = 5.73; pFWE < 0.001; 589 voxels) in which the PL > NV contrast was significant.
Figure 1.
Statistical parametric maps of the signal change to novel versus previously learned items, and the reverse, in the negative family history (−FH) group (top) (n = 64), and the positive family history (+FH) (middle) group (n = 68) collapsed across APOE status. The bottom row contains the results of the SPM{t} contrast of the difference (Diff) between FH groups indicating greater activity in the mesial and ventral temporal lobe in the −FH group (there were no significant voxels in the reverse direction). The statistical maps are overlaid on the same atlas brain in Montreal Neurological Institute (MNI) space. All maps are constrained to voxels in which the global F statistic was significant (pFWE < 0.05). Left is on the left of the image. The single-group contrasts (top two rows are thresholded at pFWE < 0.05; t = 4.11); the between-group contrast (on which inferences are based) is thresholded at pFDR < 0.005; t = 3.10. The positive (hot colors) and negative (blue) t-maps are shown for the single-group maps. See Table 2 for a summary of the statistical results and voxel coordinates of maxima. L, Left; R, right.
Table 2.
fMRI results: main effect of family history (−FH > +FH)
| Cluster p (cor) | Cluster size | Voxel p (FDR-cor) | Voxel t | x, y, z (MNI) | VBM t | Location |
|---|---|---|---|---|---|---|
| <0.001 | 2626 | <0.001 | 7.46 | 42, −52, −22 | −0.27 | Right fusiform |
| <0.001 | 5.29 | 34, −22, −18 | 2.03 | Right hippocampus | ||
| <0.001 | 5.14 | 24, −6, −16 | 2.50 | Right amygdala | ||
| <0.001 | 1850 | <0.001 | 5.12 | −42, −58, −10 | −2.56 | Left fusiform |
| <0.001 | 4.55 | −26, −20, −16 | 1.87 | Left hippocampus | ||
| <0.001 | 4.12 | −20, −4, −16 | 2.24 | Left amygdala |
The maxima and submaxima of bilateral clusters are shown. FDR-cor, FDR correction for multiple comparisons; MNI, Montreal Neurological Institute. For comparison, the VBM statistics are provided at the same locations as the fMRI maxima.
The main effect of FH is shown in the third row of Figure 1 and in Table 1. There was a robust main effect of first-degree FH in which −FH activated more than +FH in the ventral and mesial temporal lobe including the hippocampus bilaterally (right more than left). The contrast +FH > −FH revealed no significant voxels.
APOE4 status main effect and interaction
There was no main effect of APOE4 status, meaning that no voxels survived the analysis (in either direction). An F test for the interaction of family history and APOE was not significant at the prespecified threshold.
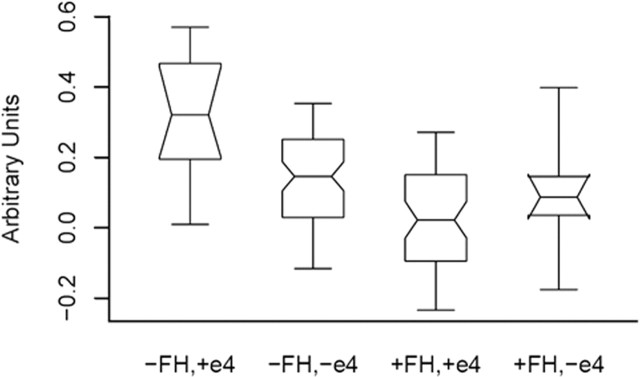
To further describe the response in our hypothesized areas of interest, we selected the locations of maximal activity in the right and left hippocampi at 34, −22, −18 (right), and −26, −20, and −16 (left) and plotted the signal values. The result of the plot for the right hippocampus location is shown in Figure 2. The −FH, +ε4 group exhibited the greatest signal change, followed by the −FH, −ε4 group. The +FH, +ε4 group activated the least. Although this interaction was not significant at the prespecified FDR corrected threshold and voxel extent threshold, it is significant at an uncorrected threshold for the right hippocampus (F(3,128) = 12.6; p = 0.001) as well as the left (F(3,128) = 4.2; p = 0.043) and is presented here descriptively.
Figure 2.
Notched box plot of signal change in the right hippocampal region (at MNI location 34–22-18) for each of the four groups. The plot contains the mean of each group, the 95% confidence interval about the mean represented by the notch in each box, and the 5th, 25th, 75th, and 95th percentiles, representing the range of variability in fMRI signal change at this location.
The effect of FH in ε4 carriers
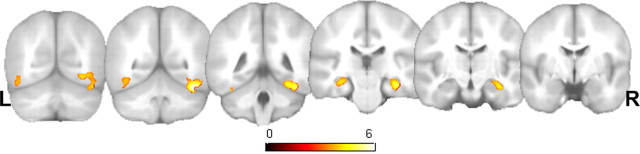
To address our hypothesis that the ε4 effect would be influenced by family history, we examined the contrast of −FH, +ε4 versus +FH, +ε4. This contrast resulted in significantly greater signal change in the −FH, ε4+ group in the right ventral temporal lobe and hippocampus as well as the left hippocampus (Fig. 3, Table 3). There were no significant findings in the reverse contrast.
Figure 3.
The influence of FH on ε4 carriers only. Statistical parametric map coronal montage of the contrast −FH, +ε4 > +FH, + ε4. The map is thresholded at pFDR < 0.005. The right (R) and left (L) hippocampi as well as the ventral temporal lobes are significantly different between groups. There were no differences in the reverse direction. Left is on the left side of the image. See Table 3 for a summary of the statistics and voxel coordinates of maxima.
Table 3.
fMRI results: effect of family history on APOE ϵ4 carriers
| Cluster p (cor) | Cluster size | Voxel p (FDR-cor) | Voxel t | x, y, z (MNI) | VBM t | Location |
|---|---|---|---|---|---|---|
| <0.001 | 1451 | <0.001 | 6.66 | 42, −50, −22 | −0.34 | Right fusiform |
| <0.001 | 5.43 | 34, −20, −20 | 1.89 | Right hippocampus | ||
| 0.138 | 130 | 0.001 | 4.26 | −32, −26, −14 | 2.01 | Left hippocampus |
| 0.01 | 290 | 0.002 | 3.99 | −40, −52, −14 | −2.84 | Left fusiform |
This t-contrast tests for voxels in which the −FH, +ϵ4 group exhibits more activity than the +FH, +ϵ4 group. There were no significant results in the reverse contrast. cor, Corrected; FDR-cor, FDR correction for multiple comparisons.
Analysis of possible confounds
The four cells of the 2 × 2 design differed in gender composition (ranging from 45 to 77% women) (Table 1); therefore, the same 2 × 2 model was set up as an ANCOVA with gender as the covariate to determine its influence on the results. Using a contrast that weighted the covariate column with 1, there were no regions in which gender significantly influenced the result even at a liberal uncorrected threshold of p < 0.001. Furthermore, the main effect of FH and the APOE4 by FH effect in the presence of the covariate were each highly similar in magnitude and spatial pattern to the original model, leading us to conclude that gender composition did not have a significant effect on these results.
The −FH subjects had slightly faster reaction times to NV items. To determine whether this was influencing the result, a separate ANCOVA was conducted with NV reaction time as a covariate. As with gender, the pattern of findings remained highly significant (pFDR < 0.01) in the presence of the reaction time covariate, leading us to conclude that reaction time was not accounting for our results.
Common medications used by participants are described in Table 1. The +FH, +ε4 group were taking more antidepressants (10 subjects or 27% of the group) and the +FH, −ε4 group were taking more antihypertensives (8 subjects; 26%). However, scores on a depression scale and mean systolic blood pressure were similar across all groups, suggesting these factors were likely not affecting the results. Nevertheless, we conducted two additional analyses to test these possible effects. In the first analysis, we randomly excluded 8 of 10 subjects in the +FH, +ε4 group who were taking antidepressants (reducing the proportion to 7%) to equate the proportion taking this class of drug across all four groups. The analysis revealed the same general pattern: there was a substantial main effect of family history in the mesial and ventral temporal lobes (FDR, p < 0.05), and there continued to be no effect of APOE. The second analysis randomly excluded six of eight subjects in the +FH, −ε4 group who were taking blood-pressure-lowering medications (reducing the proportion on blood-pressure-lowering medication in that group from 26 to 8%). The general pattern of findings was again highly similar: the family history main effect was quite robust (surpassing pFDR < 0.05 threshold), whereas the ε4 main effect across groups remained nonsignificant.
The effect of atrophy
To ensure the fMRI effect was not attributable to regional differences in brain atrophy, a voxel-based analysis of gray matter volume was performed. Using the same statistical design and threshold of pFDR < 0.05, there were no significant differences in gray matter volume anywhere in the brain. For comparison with the fMRI results, the VBM statistics are shown in Tables 2 and 3 at each of the fMRI maxima.
Discussion
In this study, MTL activation was reduced in people with parental family history of AD independent of APOE genotype. This bilateral main effect occurred in the absence of meaningful differences in neuropsychological test results, task accuracy, and regional differences in brain volume, and the results were not attributable to group differences in gender composition, task reaction time, or medication usage. The interaction between family history and APOE was not significant at a prespecified stringent threshold. However, more specific to our hypothesis, a direct contrast of ε4 carriers with and without family history of AD indicated that −FH ε4 carriers exhibited significantly more signal change in the mesial and ventral temporal lobe. Previous fMRI studies do not provide a consensus on whether AD-vulnerable regions, such as the MTL, show a diminished response associated with risk factors (Small et al., 1999; Smith et al., 1999, 2005; Machulda et al., 2003; Johnson et al., 2004; Trivedi et al., 2006) or a paradoxical increased response (Bookheimer et al., 2000; Bondi et al., 2005; Dickerson et al., 2005; Fleisher et al., 2005). It is intriguing that the −FH ε4 carriers in this study exhibited the greatest signal change in the MTL. This aspect of our results replicates previous studies that have reported an increased response (Bookheimer et al., 2000; Bondi et al., 2005) and suggests that modeling FH may help explain discrepancies in the literature on whether APOE confers an increased or decreased fMRI response in people at risk for AD.
The ventral temporal lobe (fusiform gyrus) also exhibited a significant main effect of family history. The region is involved in object identification and has been shown in several studies to be more responsive to novel items in nonhuman primates (Ringo, 1996) and in humans (Detre et al., 1998; Rand-Giovannetti et al., 2006). The region has been found to be one of several regions that are atrophic in MCI subjects who later convert to AD (Chetelat et al., 2005). In AD patients undergoing a face-encoding task, the fusiform was less active than controls but improved with 10 weeks of cholinesterase-inhibitor treatment (Kircher et al., 2005).
The current findings in persons with risk of AD are consistent with positron emission tomography studies supporting the concept that reduced glucose metabolism may lead to reduced neuronal activation in the MTL during fMRI tasks. CMRgl is coupled to regional cerebral blood flow (rCBF) (Sokoloff, 1984a,b), and both CMRgl (Mosconi et al., 2004) and rCBF (Heeger and Ress, 2002; Logothetis and Wandell, 2004) are coupled to the neural response. More research is needed to determine the relationship between CMRgl in patient groups and task-related changes in the fMRI BOLD signal. In the present analyses, the canonical hemodynamic response function (HRF) was used to estimate the neural response. Others have shown that the HRF is reduced in older adults (D'Esposito et al., 1999) and delayed in persons with dementia (Rombouts et al., 2005). Although the middle-aged subjects in this report were cognitively normal, differences in the shape of the HRF as a function of group may have contributed to the effects observed here. The structural integrity of the MTL may also affect fMRI signal. MTL volumetric APOE4 effects have been observed in older adults (Moffat et al., 2000; Lemaitre et al., 2005), and trends have been reported in middle-aged adults at risk for AD (Reiman et al., 1998). Our VBM result in the MTL also suggests a nonsignificant trend. Combined ROI analyses of MTL structure and function (Dickerson et al., 2004, 2005) may help further delineate the effect of atrophy on MTL fMRI signal change.
The findings of the present study suggest that an unknown factor or factors embodied in a family history of AD may be influencing the expression of ε4 on brain function. This possibility should not be surprising, given the accumulating evidence that the risk of developing AD can be influenced by many factors (Cupples et al., 2004) including newly discovered gene–gene interactions (Borroni et al., 2004; Bernardini et al., 2005; Dunckley et al., 2005; Lambert et al., 2005; Papassotiropoulos et al., 2005) and gene–race interactions (Green et al., 2002; Gureje et al., 2006), as well as interactions with other medical (Evans et al., 2004), and lifestyle/environmental factors (Mutter et al., 2004; Bird, 2005; Salerno-Kennedy and Cashman, 2005), all of which contribute to disease pathogenesis in poorly understood ways. More work is needed to understand the factors in family history that confer AD risk and their influence on brain structure and function.
The present study examined the relative cerebral response to novel items versus previously learned items using an easily performed task based on a well known paradigm (Stern et al., 1996; Tulving et al., 1996; Detre et al., 1998; Saykin et al., 1999; Johnson et al., 2006). This approach assumes that the MTL, a region vulnerable to AD pathology, is involved in encoding novel episodic information (Tulving et al., 1994). The fMRI paradigm for this study consisted of variable-length epochs, and transitions between conditions were not announced to the participant. The participants thus remained in a discriminatory (new vs old) cognitive set for the duration of the task. The variable-length design had many of the advantages of boxcar-style designs in which a steady cognitive state is assumed, including greater efficiency for detecting the BOLD signal (Liu et al., 2001), as well as greater statistical power (Henson, 2003). The variability in epoch length also reduced cognitive predictability. We did not use a low-level baseline control task such as cross-hair fixation. Rather, we used previously encoded items as the baseline condition. Stark and Squire (2001) found that MTL signal change was significantly greater when an active task was used as a baseline rather than rest. Furthermore, the posterior cingulate and hippocampus exhibit correlated activity during passive fMRI tasks such as cross-hair fixation relative to more goal-directed tasks (Greicius and Menon, 2004; Greicius et al., 2004). Thus, the assumption that rest or other low-level baseline is psychologically and physiologically neutral is likely not true for regions such as the hippocampus, and for this reason, we avoided a resting baseline. Figure 1 indicates that the medial parietal lobe was more active to the PL baseline than NV items, consistent with previous research on recognition (Buckner et al., 2005; Wagner et al., 2005). However, the groups did not differ from each other in this region. We have not attempted to characterize this region in great detail because the PL condition is limited to only five items that repeat, making it less than ideal for studying recognition effects. Other studies in our laboratory are ongoing to investigate this region in greater detail.
There are limitations to this study. First, we relied on a consensus diagnostic conference to review parental medical records. Although consensus conference diagnoses were based on established clinical diagnostic criteria for AD, only 10% of the family history cohort had parents with autopsy-proven AD. As a result, our family history cohort may have been contaminated with subjects whose parents did not have AD. Second, the control group may have contained persons whose parents may eventually develop AD. We tried to minimize this by requiring parents to have survived to at least the age of 70 without evidence of dementia. Nevertheless, we cannot exclude the possibility that the control cohort may have contained persons whose parents may eventually develop AD. Both of these possibilities would have had the effect of diminishing the robustness of our findings and illustrate the need for diagnostic biomarkers in AD research. Another potential limitation is the unequal sample sizes in the ANOVA. The cell sizes were proportionate to the populations from which they were drawn; however, the −FH +ε4 group only contained 11 subjects, rendering some of our analyses vulnerable to both low statistical power to detect effects (although effects were indeed observed) and undue influence from outliers. We have shown the range of the data in Figure 2. These box plots indicate that the within-group range of signal change was equivalent across groups, and our inspection of the data revealed no outliers in the group of 11. This group was small in size because only ∼15% of the normal population are ε4 positive, whereas approximately half of people with a first-degree relative with AD are ε4 positive (Sager et al., 2005).
Conclusions
We found that family history of AD was an important predictor of MTL activation independent of APOE genotype in cognitively normal people at risk for AD. This finding may help reconcile the conflicting literature on the fMRI response in preclinical AD. Our findings underscore the importance of evaluating other factors, such as family history, that may interact with APOE genotype in conferring risk of AD. To date, the factor or factors that impart AD risk to family history are poorly understood (Bertram et al., 2005; Tanzi and Bertram, 2005). A recent study found that monozygotic twins have a concordance rate for AD of 59%, highlighting the fact that genetics alone do not completely account for sporadic AD and that other variables are involved in AD pathogenesis (Gatz et al., 2005).
This study has implications for future research. First, our findings need to be replicated and expanded on. Imaging studies that measure neurofibrillary tangles or amyloid deposition (Klunk et al., 2004), glucose uptake (Reiman et al., 2004), or resting perfusion (Johnson et al., 2005) are required to better delineate the neurobiology of AD risk conferred by a family history of AD. Second, although persons with risk factors for AD have always been important volunteers for AD research, a systematic study of the biological and neurocognitive changes occurring in middle-aged first-degree relatives is needed to characterize the preclinical course of disease. Finally, longitudinal cohort studies are needed to determine the genetic, environmental, and lifestyle factors that contribute to the risk of AD associated with family history and to determine whether the findings we report are predictive of subsequent cognitive decline and eventual development of MCI or AD.
Footnotes
This work was supported by National Institutes of Health Grant R01 AG21155 (S.C.J.). We thank Tim Hess, Dr. Andrew Alexander, and Dr. Howard Rowley for their assistance in accomplishing this work.
References
- Baxter LC, Caselli RJ, Johnson SC, Reiman E, Osborne D (2003). Apolipoprotein E epsilon 4 affects new learning in cognitively normal individuals at risk for Alzheimer's disease. Neurobiol Aging 24:947–952. [DOI] [PubMed] [Google Scholar]
- Bernardini S, Bellincampi L, Ballerini S, Federici G, Iori R, Trequattrini A, Ciappi F, Baldinetti F, Bossu P, Caltagirone C, Spalletta G (2005). Glutathione S-transferase P1 *C allelic variant increases susceptibility for late-onset Alzheimer disease: association study and relationship with apolipoprotein E epsilon4 allele. Clin Chem 51:944–951. [DOI] [PubMed] [Google Scholar]
- Bertram L, Hiltunen M, Parkinson M, Ingelsson M, Lange C, Ramasamy K, Mullin K, Menon R, Sampson AJ, Hsiao MY, Elliott KJ, Velicelebi G, Moscarillo T, Hyman BT, Wagner SL, Becker KD, Blacker D, Tanzi RE (2005). Family-based association between Alzheimer's disease and variants in UBQLN1. N Engl J Med 352:884–894. [DOI] [PubMed] [Google Scholar]
- Bird TD (2005). Genetic factors in Alzheimer's disease. N Engl J Med 352:862–864. [DOI] [PubMed] [Google Scholar]
- Blair CK, Folsom AR, Knopman DS, Bray MS, Mosley TH, Boerwinkle E (2005). APOE genotype and cognitive decline in a middle-aged cohort. Neurology 64:268–276. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG (2005). fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology 64:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW (2000). Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med 343:450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni B, Archetti S, Agosti C, Akkawi N, Brambilla C, Caimi L, Caltagirone C, Di Luca M, Padovani A (2004). Intronic CYP46 polymorphism along with ApoE genotype in sporadic Alzheimer disease: from risk factors to disease modulators. Neurobiol Aging 25:747–751. [DOI] [PubMed] [Google Scholar]
- Braak E, Griffing K, Arai K, Bohl J, Bratzke H, Braak H (1999). Neuropathology of Alzheimer's disease: what is new since A. Alzheimer? Eur Arch Psychiatry Clin Neurosci 249:Suppl 3, 14–22. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA (2005). Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25:7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Graff-Radford NR, Reiman EM, Weaver A, Osborne D, Lucas J, Uecker A, Thibodeau SN (1999). Preclinical memory decline in cognitively normal apolipoprotein E-epsilon4 homozygotes. Neurology 53:201–207. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Osborne D, Reiman EM, Hentz JG, Barbieri CJ, Saunders AM, Hardy J, Graff-Radford NR, Hall GR, Alexander GE (2001). Preclinical cognitive decline in late middle-aged asymptomatic apolipoprotein E-e4/4 homozygotes: a replication study. J Neurol Sci 189:93–98. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Landeau B, Eustache F, Mezenge F, Viader F, de la Sayette V, Desgranges B, Baron JC (2005). Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. NeuroImage 27:934–946. [DOI] [PubMed] [Google Scholar]
- Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H (2004). The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann NY Acad Sci 1019:24–28. [DOI] [PubMed] [Google Scholar]
- Cupples LA, Farrer LA, Sadovnick AD, Relkin N, Whitehouse P, Green RC (2004). Estimating risk curves for first-degree relatives of patients with Alzheimer's disease: the REVEAL study. Genet Med 6:192–196. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Zarahn E, Aguirre GK, Rympa B (1999). The effect of normal aging on the coupling of neural activity to the BOLD hemodynamic response. NeuroImage 10:6–14. [DOI] [PubMed] [Google Scholar]
- Detre JA, Maccotta L, King D, Alsop DC, Glosser G, D'Esposito M, Zarahn E, Aguirre GK, French JA (1998). Functional MRI lateralization of memory in temporal lobe epilepsy. Neurology 50:926–932. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA (2004). Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol 56:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA (2005). Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunckley T, Beach TG, Ramsey KE, Grover A, Mastroeni D, Walker DG, Lafleur BJ, Coon KD, Brown KM, Caselli R, Kukull W, Higdon R, McKeel D, Morris JC, Hulette C, Schmechel D, Reiman EM, Rogers J, Stephan DA (2006). Gene expression correlates of neurofibrillary tangles in Alzheimer's disease. Neurobiol Aging in press. [DOI] [PMC free article] [PubMed]
- Evans RM, Hui S, Perkins A, Lahiri DK, Poirier J, Farlow MR (2004). Cholesterol and APOE genotype interact to influence Alzheimer disease progression. Neurology 62:1869–1871. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Houston WS, Eyler LT, Frye S, Jenkins C, Thal LJ, Bondi MW (2005). Identification of Alzheimer disease risk by functional magnetic resonance imaging. Arch Neurol 62:1881–1888. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Ahlbom A, Viitanen M, Winblad B (1993). Risk factors for late-onset Alzheimer's disease: a population-based, case-control study. Ann Neurol 33:258–266. [DOI] [PubMed] [Google Scholar]
- Gatz M, Fratiglioni L, Johansson B, Berg S, Mortimer JA, Reynolds CA, Fiske A, Pedersen NL (2005). Complete ascertainment of dementia in the Swedish Twin Registry: the HARMONY study. Neurobiol Aging 26:439–447. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 15:870–878. [DOI] [PubMed] [Google Scholar]
- Ghebremedhin E, Schultz C, Braak E, Braak H (1998). High frequency of apolipoprotein E epsilon4 allele in young individuals with very mild Alzheimer's disease-related neurofibrillary changes. Exp Neurol 153:152–155. [DOI] [PubMed] [Google Scholar]
- Ghebremedhin E, Schultz C, Thal DR, Rub U, Ohm TG, Braak E, Braak H (2001). Gender and age modify the association between APOE and AD-related neuropathology. Neurology 56:1696–1701. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14:21–36. [DOI] [PubMed] [Google Scholar]
- Green RC, Cupples LA, Go R, Benke KS, Edeki T, Griffith PA, Williams M, Hipps Y, Graff-Radford N, Bachman D, Farrer LA (2002). Risk of dementia among white and African Am relatives of patients with Alzheimer disease. JAMA 287:329–336. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Menon V (2004). Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci 16:1484–1492. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V (2004). Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 101:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureje O, Ogunniyi A, Baiyewu O, Price B, Unverzagt FW, Evans RM, Smith-Gamble V, Lane KA, Gao S, Hall KS, Hendrie HC, Murrell JR (2006). APOE epsilon4 is not associated with Alzheimer's disease in elderly Nigerians. Ann Neurol 59:182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger DJ, Ress D (2002). What does fMRI tell us about neuronal activity? Nat Rev Neurosci 3:142–151. [DOI] [PubMed] [Google Scholar]
- Henson RN (2003). Analysis of fMRI time series. In: Human brain function (Frackowiak RSJ, Friston KJ, Frith CD, Dolan R, Price CJ, Zeki S, Ashburner J, Penny WD, eds) Ed 2 New York: Academic.
- Jenkinson M (2003). Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med 49:193–197. [DOI] [PubMed] [Google Scholar]
- Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N (2005). Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology 234:851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Baxter L, Susskind-Wilder L, Connor DJ, Sabbagh MN, Caselli RJ (2004). Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia 42:980–989. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Moritz CH, Meyerand ME, Rowley HA, Alexander AL, Hansen KW, Gleason CE, Carlsson CM, Ries ML, Asthana S, Chen K, Reiman EM, Alexander GE (2006). Activation of brain regions vulnerable to Alzheimer's disease: the effect of mild cognitive impairment. Neurobiol Aging in press. [DOI] [PMC free article] [PubMed]
- Kircher TT, Erb M, Grodd W, Leube DT (2005). Cortical activation during cholinesterase-inhibitor treatment in Alzheimer disease: preliminary findings from a pharmaco-fMRI study. Am J Geriatr Psychiatry 13:1006–1013. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B (2004). Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 55:306–319. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Mann D, Richard F, Tian J, Shi J, Thaker U, Merrot S, Harris J, Frigard B, Iwatsubo T, Lendon C, Amouyel P (2005). Is there a relation between APOE expression and brain amyloid load in Alzheimer's disease? J Neurol Neurosurg Psychiatry 76:928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rue A, R OH, Matsuyama SS, Jarvik LF (1995). Cognitive changes in young-old adults: effect of family history of dementia. J Clin Exp Neuropsychol 17:65–70. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Crivello F, Dufouil C, Grassiot B, Tzourio C, Alperovitch A, Mazoyer B (2005). No epsilon4 gene dose effect on hippocampal atrophy in a large MRI database of healthy elderly subjects. NeuroImage 24:1205–1213. [DOI] [PubMed] [Google Scholar]
- Liu TT, Frank LR, Wong EC, Buxton RB (2001). Detection power, estimation efficiency, and predictability in event-related fMRI. NeuroImage 13:759–773. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA (2004). Interpreting the BOLD signal. Annu Rev Physiol 66:735–769. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O'Brien PC, Petersen RC, Boeve BF, Knopman D, Tang-Wai DF, Ivnik RJ, Smith GE, Tangalos EG, Jack Jr CR (2003). Comparison of memory fMRI response among normal, MCI, and Alzheimer's patients. Neurology 61:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Campion D, Brice A, Hannequin D, Dubois B, Didierjean O, Michon A, Thomas-Anterion C, Puel M, Frebourg T, Agid Y, Clerget-Darpoux F (1998). Apolipoprotein E epsilon4 allele and familial aggregation of Alzheimer disease. Arch Neurol 55:810–816. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Szekely CA, Zonderman AB, Kabani NJ, Resnick SM (2000). Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology 55:134–136. [DOI] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Rusinek H, Convit A, de Leon MJ (2004). Magnetic resonance and PET studies in the early diagnosis of Alzheimer's disease. Expert Rev Neurother 4:831–849. [DOI] [PubMed] [Google Scholar]
- Mutter J, Naumann J, Sadaghiani C, Schneider R, Walach H (2004). Alzheimer disease: mercury as pathogenetic factor and apolipoprotein E as a moderator. Neuro Endocrinol Lett 25:331–339. [PubMed] [Google Scholar]
- Ohm TG, Muller H, Braak H, Bohl J (1995). Close-meshed prevalence rates of different stages as a tool to uncover the rate of Alzheimer's disease-related neurofibrillary changes. Neuroscience 64:209–217. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Wollmer MA, Tsolaki M, Brunner F, Molyva D, Lutjohann D, Nitsch RM, Hock C (2005). A cluster of cholesterol-related genes confers susceptibility for Alzheimer's disease. J Clin Psychiatry 66:940–947. [PubMed] [Google Scholar]
- Pedersen NL, Gatz M, Berg S, Johansson B (2004). How heritable is Alzheimer's disease late in life? Findings from Swedish twins. Ann Neurol 55:180–185. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Welsh-Bohmer KA, Bigler ED, Johnson SC, Anderson CV, Helms MJ, Saunders AM, Breitner JC (1997). Apolipoprotein E epsilon 4 allele and hippocampal volume in twins with normal cognition. Neurology 48:985–989. [DOI] [PubMed] [Google Scholar]
- Rand-Giovannetti E, Chua EF, Driscoll AE, Schacter DL, Albert MS, Sperling RA (2006). Hippocampal and neocortical activation during repetitive encoding in older persons. Neurobiol Aging 27:173–182. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D (1996). Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med 334:752–758. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Uecker A, Caselli RJ, Lewis S, Bandy D, de Leon MJ, De Santi S, Convit A, Osborne D, Weaver A, Thibodeau SN (1998). Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer's disease. Ann Neurol 44:288–291. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J (2004). Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci USA 101:284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringo JL (1996). Stimulus specific adaptation in inferior temporal and medial temporal cortex of the monkey. Behav Brain Res 76:191–197. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Goekoop R, Stam CJ, Barkhof F, Scheltens P (2005). Delayed rather than decreased BOLD response as a marker for early Alzheimer's disease. NeuroImage 26:1078–1085. [DOI] [PubMed] [Google Scholar]
- Sager MA, Hermann B, La Rue A (2005). Middle-aged children of persons with Alzheimer's disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer's Prevention. J Geriatr Psychiatry Neurol 18:245–249. [DOI] [PubMed] [Google Scholar]
- Salerno-Kennedy R, Cashman KD (2005). Relationship between dementia and nutrition-related factors and disorders: an overview. Int J Vitam Nutr Res 75:83–95. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George Hyslop PH, Pericak Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ (1993). Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology 43:1467–1472. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Johnson SC, Flashman LA, McAllister TW, Sparling MB, Darcey TM, Moritz CH, Guerin SJ, Weaver JB, Mamourian A (1999). Functional differentiation of medial temporal and frontal regions involved in processing novel and familiar words: an fMRI study. Brain 122:1963–1971. [DOI] [PubMed] [Google Scholar]
- Slooter AJ, Cruts M, Kalmijn S, Hofman A, Breteler MM, Van Broeckhoven C, van Duijn CM (1998). Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: the Rotterdam Study. Arch Neurol 55:964–968. [DOI] [PubMed] [Google Scholar]
- Small G, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY, Lavretsky H, Miller K, Siddarth P, Rasgon NL, Mazziotta JC, Saxena S, Wu HM, Mega MS, Cummings JL, Saunders AM, Pericak-Vance MA, Roses AD, Barrio JR, Phelps ME (2000). Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. Proc Natl Acad Sci USA 97:6037–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small S, Perera GM, DeLaPaz R, Mayeux R, Stern Y (1999). Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer's disease. Ann Neurol 45:466–472. [DOI] [PubMed] [Google Scholar]
- Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, Avison MJ (1999). Altered brain activation in cognitively intact individuals at high risk for Alzheimer's disease. Neurology 53:1391–1396. [DOI] [PubMed] [Google Scholar]
- Smith CD, Kryscio RJ, Schmitt FA, Lovell MA, Blonder LX, Rayens WS, Andersen AH (2005). Longitudinal functional alterations in asymptomatic women at risk for Alzheimer's disease. J Neuroimaging 15:271–277. [DOI] [PubMed] [Google Scholar]
- Snodgrass J, Vanderwart M (1980). A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn] 6:174–215. [DOI] [PubMed] [Google Scholar]
- Sokoloff L (1984a). Modeling metabolic processes in the brain in vivo. Ann Neurol 15:Suppl, S1–S11. [DOI] [PubMed] [Google Scholar]
- Sokoloff L (1984b). Metabolic probes for localization of functional activity in the central nervous system. Int J Neurol 18:40–48. [PubMed] [Google Scholar]
- Stark CE, Squire LR (2001). When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci USA 98:12760–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR (1996). The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA 93:8660–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L (2005). Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell 120:545–555. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Torgerson BM, Sager MA, Hermann BP, Asthana S, Johnson SC (2006). Reduced hippocampal activation during episodic encoding in middle-aged individuals at genetic risk of Alzheimer's disease: a cross-sectional study. BMC Med 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Kapur S, Habib R, Houle S (1994). Novelty encoding networks in the human brain: positron emission tomography data. NeuroReport 5:2525–2528. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Craik FE, Habib R, Houle S (1996). Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex 6:71–79. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL (2005). Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9:445–453. [DOI] [PubMed] [Google Scholar]