Summary
In this study, we present data showing that Cdc42-dependent lumen formation by endothelial cells (ECs) in three-dimensional (3D) collagen matrices involves coordinated signaling by PKCε in conjunction with the Src-family kinases (SFKs) Src and Yes. Activated SFKs interact with Cdc42 in multiprotein signaling complexes that require PKCε during this process. Src and Yes are differentially expressed during EC lumen formation and siRNA suppression of either kinase, but not Fyn or Lyn, results in significant inhibition of EC lumen formation. Concurrent with Cdc42 activation, PKCε- and SFK-dependent signaling converge to activate p21-activated kinase (Pak)2 and Pak4 in steps that are also required for EC lumen formation. Pak2 and Pak4 further activate two Raf kinases, B-Raf and C-Raf, leading to ERK1 and ERK2 (ERK1/2) activation, which all seem to be necessary for EC lumen formation. This work reveals a multicomponent kinase signaling pathway downstream of integrin-matrix interactions and Cdc42 activation involving PKCε, Src, Yes, Pak2, Pak4, B-Raf, C-Raf and ERK1/2 to control EC lumen formation in 3D collagen matrices.
Keywords: Rho GTPases, PKCε, Raf, Lumen formation, Src, Cdc42, Extracellular matrix, Pak
Introduction
Vessel lumenization is a crucial step in developing a functional vascular system during vascular morphogenesis (Adams and Alitalo, 2007; Davis and Bayless, 2003; Davis et al., 2007; Egginton and Gerritsen, 2003; Holderfield and Hughes, 2008; Horowitz and Simons, 2008; Iruela-Arispe and Davis, 2009; Parker et al., 2004). Our previous work has shown that endothelial cell (EC) lumen formation in three-dimensional (3D) collagen matrices is regulated by the formation and coalescence of EC intracellular vacuoles, a process that is dependent on Cdc42 and Rac1 GTPases in response to α2β1-integrin–collagen-type-I interactions (Bayless and Davis, 2002; Bayless et al., 2000; Davis and Bayless, 2003; Davis et al., 2002; Davis and Camarillo, 1996; Davis and Senger, 2005; Koh et al., 2008). Regulation of EC intracellular vacuole formation and coalescence by Cdc42 GTPase has also been shown to be a major mechanism of vascular development in zebrafish, suggesting that Rho GTPases play a key role in EC vascular lumen formation in vivo (Kamei et al., 2006). Rho GTPases are well-known to control cytoskeletal structures and, thus, influence various cellular functions that are necessary for EC vascular morphogenic events (Davis and Bayless, 2003; Fryer and Field, 2005; Hall, 1998; Hall, 2005; Ridley, 2001; Schwartz, 2004). Among the diverse spectrum of RhoGTPase targets, p21-activated kinase (Pak) proteins are known as key downstream effectors that are involved in the regulation of cytoskeletal function (Bokoch, 2003), and we have shown that two members of the Pak family, Pak2 and Pak4, are required during EC lumen formation in 3D collagen matrices (Davis et al., 2007; Koh et al., 2008).
Because Rho GTPases are activated downstream of integrins, growth-factor receptors, cytokines and hormones, their activation can be regulated by Src (Robles et al., 2005; Tatin et al., 2006; Timpson et al., 2001). Src is a member of Src-family nonreceptor protein tyrosine kinases (SFKs). SFKs influence a broad range of cellular functions downstream of growth-factor receptors, integrins and other adhesion molecules (Abu-Ghazaleh et al., 2001; Eliceiri et al., 1999; Eliceiri et al., 2002; Kilarski et al., 2003; Parsons and Parsons, 2004; Playford and Schaller, 2004; Thomas and Brugge, 1997; Tsuda et al., 2002; Werdich and Penn, 2005; Werdich and Penn, 2006). SFKs are also known to be involved in protein kinase C (PKC)-mediated signaling pathways to regulate actin-cytoskeletal structures as well as cell invasion (Bruce-Staskal and Bouton, 2001; Nomura et al., 2007). We have previously shown that PKC plays a key role in EC lumen formation in 3D collagen matrices in response to phorbol ester (TPA) (Davis et al., 2007; Koh et al., 2008), raising a possible signaling mechanism involving both PKC and SFKs to regulate this process. Src has also previously been shown to control capillary-cord formation in 3D collagen matrices (Liu and Senger, 2004).
Previous studies have shown that SFKs regulate the activation of Pak2 along with Cdc42 or Rac1 (Renkema et al., 2002). It is important to examine Pak-dependent signaling pathways in more detail and their relationships to the formation of EC lumens and tubes (Koh et al., 2008). Previously, it was shown that Pak1 regulates angiogenesis and EC survival by activating C-Raf (Hood et al., 2003). C-Raf is a member of the Raf kinase family of serine/threonine kinases; this family is comprised of three isoforms, A-Raf, B-Raf and C-Raf. B-Raf and C-Raf can be directly activated by PKC, SFKs and other kinases (Fabian et al., 1993; Kolch et al., 1993; Marais et al., 1995; Ueffing et al., 1997). Raf kinases are a key component of the Raf-MEK-ERK mitogen-activated protein kinase (MAPK) pathway that regulates many cellular functions (Chong et al., 2003; Morrison and Cutler, 1997; Wellbrock et al., 2004). They are also activated by SFKs downstream of various angiogenic factors (Eliceiri et al., 1999; Eliceiri et al., 2002; Hood et al., 2003). However, the role of Raf kinases in EC lumen formation and the associated signaling pathways in relation to Rho GTPases, PKC and SFKs have not been elucidated.
In this work, using an in vitro EC-lumen-formation model in 3D collagen matrices, we analyzed how Cdc42, PKCε, SFKs, Pak2, Pak4 and Raf kinases coordinately regulate EC lumen formation in 3D collagen matrices, and evaluate their individual functions during this process. We show that SFKs play a key role in EC lumen formation in response to PKCε as well as through their association with Cdc42-dependent signaling. We identify Src and Yes as two key SFKs, which play critical roles during EC lumen formation and Pak2 and Pak4 which act downstream of PKCε, SFKs, as well as Cdc42. Paks in conjunction with SFKs lead to activation of B-Raf and C-Raf and in conjunction with downstream ERK1 and ERK2 (ERK1/2) phosphorylation control EC lumen formation in 3D collagen matrices. Thus, a coordinated signaling pathway involving Cdc42, PKCε, Src, Yes, B-Raf, C-Raf and ERK1/2 are required for ECs to form lumen and tube structures in a 3D-collagen-matrix environment.
Results
PKCε stimulates EC lumen formation in 3D collagen matrices
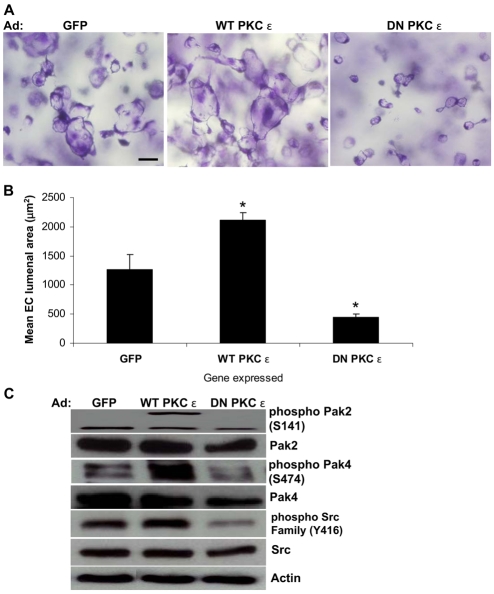
Previous work using chemical inhibitors and siRNA knockdown studies have identified a novel PKC isoform, PKCε, as a regulator of EC lumen formation (Koh et al., 2008). To further evaluate its functional relevance, we used recombinant adenoviruses carrying either wild-type (WT) PKCε or dominant-negative (DN) PKCε, which were confirmed to be expressed by western blotting (data not shown). ECs infected with control GFP, WT-PKCε or DN-PKCε virus were allowed to undergo lumen and tube morphogenesis. EC lumenogenesis was markedly impaired using ECs expressing DN PKCε, whereas ECs expressing WT PKCε showed significantly stimulated lumen formation compared with ECs infected with control GFP virus (Fig. 1A,B). These data suggest that activation of PKCε strongly enhances EC lumen formation in 3D collagen matrices.
Fig. 1.
PKCε stimulates EC lumen formation in 3D collagen matrices. (A) ECs infected with adenoviruses (Ad) expressing GFP, WT-PKCε or DN-PKCε were suspended within 3D collagen matrices for 24 hours. Scale bar: 50 μm. (B) Quantification of EC lumen formation at 24 hours. Data are shown as mean EC lumenal area ± s.d. (n=3). *P<0.05 compared with GFP control. (C) EC extracts were prepared at 24 hours for western blot analysis and probed for phospho-Pak2, phospho-Pak4, phospho-Src or Pak2, Pak4, Src and actin controls. The actin control blot was derived from cut lanes of the same gel and a single exposure.
We have previously shown that regulation of EC lumen formation by PKC downstream of phorbol-ester treatment is mediated by activation of two p21-activated kinases, Pak2 and Pak4 (Koh et al., 2008). Therefore, we investigated whether the influence of PKCε on EC lumen formation correlates with Pak2 and Pak4 activation. WT PKCε markedly induced Pak4 phosphorylation (Fig. 1C). Pak2 phosphorylation was also increased by WT PKCε, with an appearance of a protein band whose size is consistent with the expected size of dimeric Pak2 (Fig. 1C). It has been reported that phosphorylated Pak2 can dimerize through its kinase domain and that this dimerization process mediates trans-autophosphorylation of Pak2 to induce its full activation (Pirruccello et al., 2006). Expression of DN PKCε diminished phosphorylation of both Pak2 and Pak4 (Fig. 1C), indicating that PKCε regulates EC lumen formation by activating both Pak2 and Pak4.
SFKs are involved in PKCε-induced EC lumen formation in 3D collagen matrices
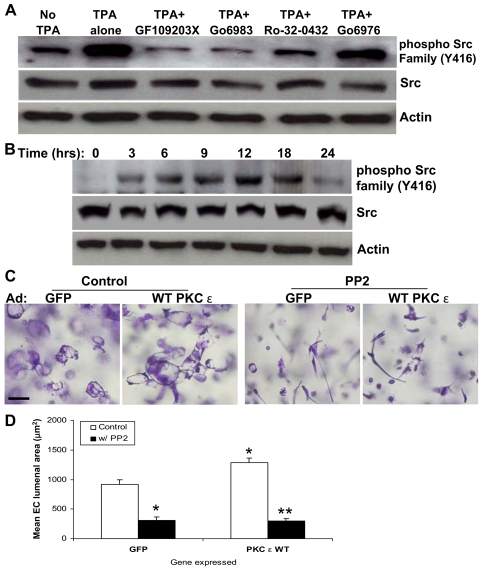
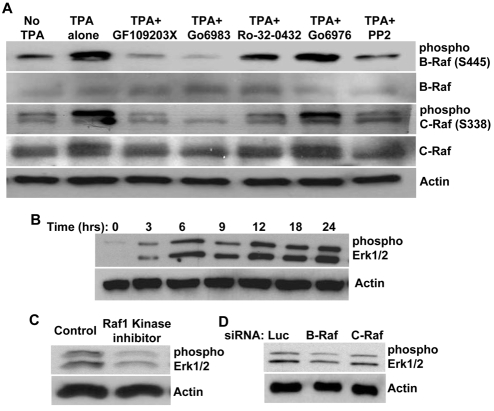
In an attempt to identify additional kinase targets that are involved in EC lumen formation, we examined the activation of SFKs and their functional importance during the process of lumen formation. Activity of SFKs is regulated by phosphorylation or dephosphorylation at different residues (Thomas and Brugge, 1997). The Y416 residue in the activation loop is known to be a key phosphorylation site that leads to full activation of SFKs (Roskoski, 2004; Roskoski, 2005; Thomas and Brugge, 1997). Expression of WT PKCε, which stimulates EC lumen formation, showed higher SFK phosphorylation compared with control GFP (Fig. 1C). By contrast, SFK phosphorylation was markedly diminished when DN PKCε was expressed (Fig. 1C). Moreover, when ECs were suspended in 3D collagen matrices in the presence of TPA to induce EC lumen formation by activating PKCε, phosphorylation of SFKs was elevated as well (Fig. 2A). PKC inhibitors that target PKCε (and which inhibit EC lumen formation) strongly reduce SFK phosphorylation, providing additional support for a collaborative role for these kinases in the molecular control of EC lumenogenesis (Fig. 2A). Furthermore, these data argue that PKCε is upstream of SFKs in this signaling cascade.
Fig. 2.
SFKs regulate EC lumen formation in 3D collagen matrices downstream of PKCε. (A) ECs were cultured in collagen matrices for 24 hours in the absence or presence of TPA and/or the PKC inhibitors GF109203X (2.5 μM), Go6983 (5 μM), Ro-32-0432 (5 μM) or Go6976 (5 μM). Lysates were prepared for western blot analysis and probed for phospho-Src or actin control. (B) Extracts of EC cultures in 3D collagen matrices were prepared at the indicated time points and probed for phospho-Src, actin and total Src. (C,D) ECs were infected with adenoviruses (Ad) expressing GFP or WT-PKCε and were suspended within 3D collagen matrices. Culture media contained either no additives or the Src inhibitor PP2 at 10 μM. Cultures were fixed after 24 hours and photographed (C) or quantitated for lumen formation (D). Scale bar: 50 μm. Data are shown as mean EC lumenal area ± s.d. (n=3). *P<0.05 compared with GFP control; **P<0.05 compared with PKCε-WT control.
To examine whether SFKs are activated during EC lumen and tube formation, we examined their phosphorylation levels over a time-course of this process. SFK phosphorylation was highly induced during EC lumen and tube formation (Fig. 2B), indicating that SFK activation is required for these events. Also, the stimulation of lumen formation that was observed following increased expression of PKCε was markedly blocked by the SFK inhibitor PP2 (Fig. 2C,D).
SFKs interact with Cdc42 in a PKCε-dependent manner to regulate EC lumen formation in 3D collagen matrices
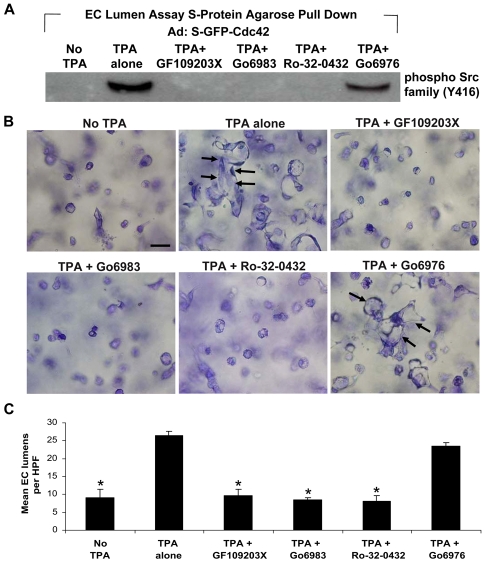
Because it has been shown that SFKs act downstream of integrins, and that SFKs can regulate activation of Rho GTPases by influencing RhoGDIs (DerMardirossian et al., 2006; Playford and Schaller, 2004), we next analyzed the relationship between SFKs and Cdc42 during EC lumen formation. To examine whether SFKs directly interact with Cdc42, we used a recombinant virus containing Cdc42 tagged with both GFP and S-tag, S-GFP-Cdc42 (Koh et al., 2008). ECs were infected with S-GFP-Cdc42 virus 24 hours before they were suspended within 3D collagen matrices. EC culture extracts were prepared at 16 hours during EC morphogenesis and lysates were incubated with S-protein agarose beads to capture the recombinant Cdc42 protein (Koh et al., 2008). Specificity of protein interactions through S-tag/S-protein agarose beads have been described previously in studies in which GFP-Cdc42 was used as a control (Koh et al., 2008). As shown in Fig. 3A, there was a strong association between Cdc42 and SFKs during EC lumen formation in 3D collagen matrices, and this interaction was dependent on PKCε. In the presence of PKC inhibitors that target PKCε (e.g. Go6983), and that block lumen formation, the association of Cdc42 with SFKs was strongly diminished (Fig. 3B,C). The addition of Go6976, which shows blocking selectivity for conventional PKC isoforms and not for novel isoforms such as PKCε, maintained the interaction between Cdc42 and SFKs, as this inhibitor did not show any inhibitory effect on this process (Fig. 3B,C). These data suggest that Cdc42 interacts with multi-protein complexes containing SFKs to regulate EC lumen formation in 3D collagen matrices and that the association of Cdc42 with these complexes is dependent on PKCε.
Fig. 3.
SFKs interact with Cdc42 in a PKCε-dependent manner to regulate EC lumen formation in 3D collagen matrices. ECs were treated with S-GFP-Cdc42 recombinant adenovirus prior to suspension in 3D collagen matrices in the absence or presence of TPA and/or GF109203X (5 μM), Go6983 (10 μM), Ro-32-0432 (10 μM) or Go6976 (10 μM). (A) Extracts were prepared at 16 hours and equal amounts of extracts were incubated with S-protein agarose beads and probed for phosphorylated SFKs to detect binding interactions. (B) Representative fields of EC cultures in the presence of the indicated chemical inhibitors. Scale bar: 50 μm. (C) Quantification of EC lumen formation at 24 hours. Data are shown as mean EC lumenal area ± s.d. (n=3). *P<0.01 compared with TPA control.
SFKs are required for EC lumen formation in 3D collagen matrices and are activated downstream of PKCε
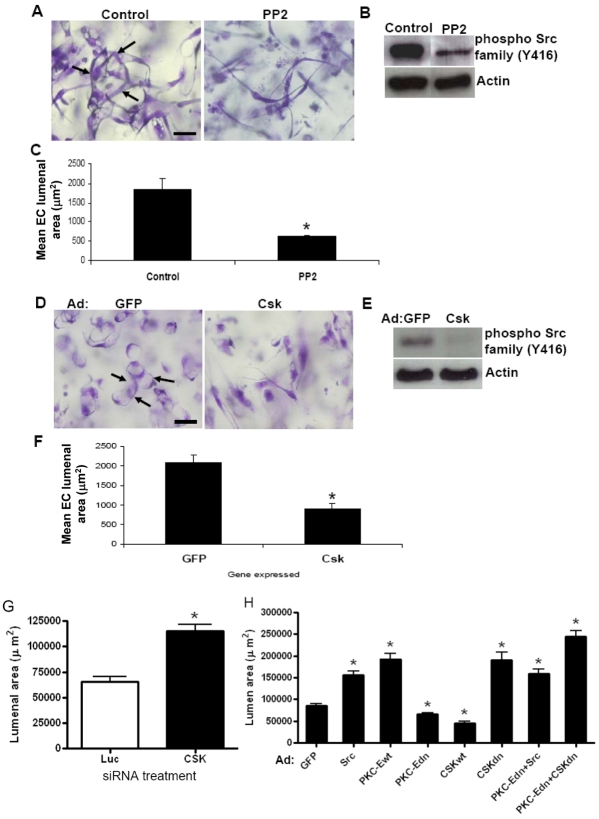
To further show the requirement of SFKs for EC lumen formation in 3D collagen matrices, general SFK activity was inhibited either by adding the chemical inhibitor PP2 or by expressing C-terminal Src kinase, Csk, which is known to negatively regulate SFKs (Howell and Cooper, 1994). The addition of PP2 to the EC-lumen-formation assay had a strong inhibitory effect on this process (Fig. 2; Fig. 4A,C). ECs expressing increased Csk levels were also strongly blocked in their ability to undergo lumen formation compared with ECs expressing control GFP protein (Fig. 4D,F). The presence of PP2 or expression of Csk also strongly reduced SFK activation as detected through phosphorylation (Fig. 4B,E), suggesting that SFK activation is required for EC lumen formation in 3D collagen matrices.
Fig. 4.
SFKs are required for EC lumen formation in 3D collagen matrices and act downstream of PKC activation during this process. (A-C) ECs were suspended in collagen matrices for 24 hours in the absence or presence of the chemical inhibitor PP2 (10 μM). (A) Cultures were fixed for photography. Scale bar: 50 μm. Arrows indicate EC lumenal structures. (B) Extracts were made for western blot analysis and probed for phospho-Src or actin. (C) Quantification of EC lumen formation at 24 hours. (D-F) ECs infected with GFP- or Csk-expressing adenoviruses (Ad) were suspended in collagen matrices for 24 hours. (D) Cultures were fixed for photography. Scale bar: 50 μm. Arrows indicate EC lumenal structures. (E) Extracts were made for western blot analysis and probed for phospho-Src or actin. (F) Quantification of EC lumen formation at 24 hours. (G) ECs were treated with control luciferase versus a siRNA to Csk and then suspended in collagen matrices to undergo lumen formation. Cultures were fixed at 24 hours and quantitated for lumen formation. (H) ECs were transfected with the indicated adenoviral vectors and then suspended in collagen matrices. After fixation at 24 hours, cultures were quantitated for EC lumen formation. Data are shown as mean EC lumenal area ± s.d. (n=3). *P<0.01 compared with controls.
Additional experiments addressed the role of SFKs and their relationship to PKCε signaling in the lumen-formation cascade. Suppression of Csk by using siRNA caused significant increases in EC lumen formation (Fig. 4G), as did expression of a DN Csk mutant by using an adenoviral vector (Fig. 4H). Increased expression of WT Src or PKCε led to increases in EC lumenogenesis as well. By contrast, expression of DN PKCε or WT Csk blocked lumen formation (Fig. 4H). Interestingly, the inhibitory influence of DN PKCε was rescued and, thus, reversed by coexpression of either WT Src or DN Csk (Fig. 4H). These data suggest the crucial involvement of both PKCε and SFKs during EC lumen formation and that SFKs act downstream of PKCε in this signaling cascade.
Src and Yes play a key role in EC lumen formation in 3D collagen matrices
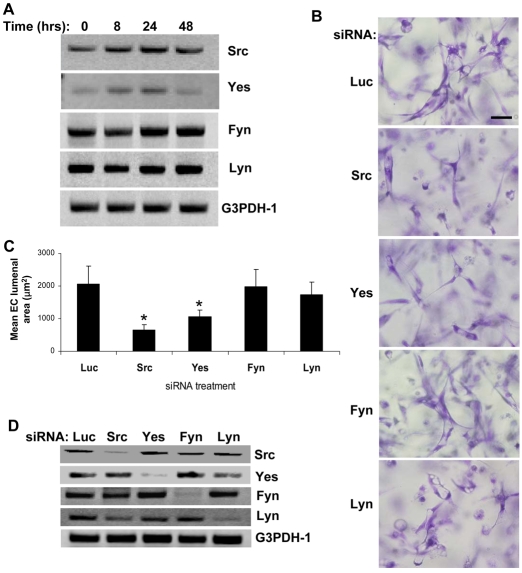
There are nine different SFKs in mammals – Src, Fyn, Yes, Yrk, Lyn, Hck, Fgr, Blk and Lck – each of which exhibits a wide range of expression patterns (Thomas and Brugge, 1997). To identify the relevant SFKs that are involved in lumen formation in collagen matrices, we examined the differential expression pattern of each SFK member during this morphogenic process. Our screening revealed that prominently expressed members of SFKs in human ECs include Src, Yes, Fyn and Lyn; this was determined using reverse transcriptase (RT)-PCR analysis (Fig. 5A) as well as western blot analysis (data not shown). The other SFKs were either not expressed or expressed at minimal levels in ECs undergoing lumen and tube formation (data not shown). The expression of Src and Yes mRNA was induced during the EC morphogenic process, whereas Fyn and Lyn showed a constant expression pattern throughout the process (Fig. 5A). Src and Yes have been previously shown to regulate vascular permeability in response to VEGF (Eliceiri et al., 1999), whereas Fyn and Lyn have been targeted for anti-angiogenic treatment on the basis of their role in apoptotic signaling pathways (Tang et al., 2007).
Fig. 5.
Src and Yes, play a key role in EC lumen formation in 3D collagen matrices. (A) Extracts of EC cultures in 3D collagen matrices were prepared at the indicated time points for RNA isolation. Semi-quantitative RT-PCR was performed for Src, Yes, Fyn, Lyn or G3PDH-1 control. (B) ECs were treated with the indicated siRNAs and were suspended within collagen matrices for 24 hours before fixation for photography. Scale bar: 50 μm. (C) Quantification of the EC-lumen-formation assay at 24 hours. Data are shown as the mean EC lumenal area ± s.d. (n=6). *P<0.01 compared with luciferase (Luc) control. (D) siRNA-transfected ECs were prepared for RNA isolation. Semi-quantitative RT-PCR was performed for Src, Yes, Fyn, Lyn or G3PDH-1 control.
To further examine the role of Src, Yes, Fyn and Lyn in EC lumen formation in 3D collagen matrices, we used siRNA suppression analysis. Suppression of Src or Yes, two members whose expression was differentially regulated during the EC morphogenic process, resulted in a significant reduction of EC lumen formation in 3D collagen matrices (Fig. 5B,C). siRNA suppression of Fyn or Lyn did not have any significant effect on EC lumen formation compared with control luciferase (Fig. 5B,C). Specificity of each siRNA and its ability to knock down corresponding Src-family members was confirmed by semi-quantitative RT-PCR (Fig. 5D). These data suggest that Src and Yes play important roles in EC lumen formation in 3D collagen matrices.
Pak2 and Pak4 serve as downstream targets of SFKs to regulate EC lumen formation in 3D collagen matrices
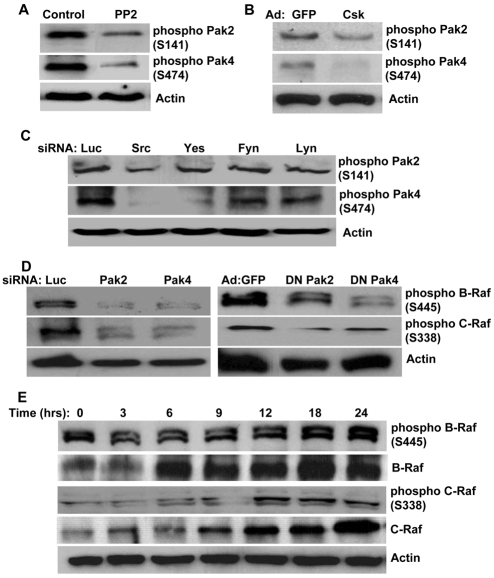
We next examined whether SFK-dependent EC lumen formation in 3D collagen matrices involves Pak2 and Pak4, two downstream targets of Cdc42 and PKCε that play a key role in EC lumen formation (Koh et al., 2008). It has been shown that Pak2 can be activated both by Cdc42 and SFKs (Renkema et al., 2002). Given that Cdc42 and SFKs are both required for EC lumen formation, it was vital to examine Pak2 and Pak4 activation by Cdc42 (Koh et al., 2008) and SFKs in the context of their regulatory roles on EC lumen formation. When ECs were treated with the Src-kinase inhibitor PP2, phosphorylation of both Pak2 and Pak4 were markedly reduced (Fig. 6A). Expression of Csk also diminished Pak2 and Pak4 phosphorylation (Fig. 6B). Overall phosphorylation levels of both Pak2 and Pak4 were also reduced with siRNA suppression of Src and Yes, but not of Fyn and Lyn, confirming the role of Src and Yes in the EC lumen formation process (Fig. 6C). These data suggest that SFKs regulate EC lumen formation in 3D collagen matrices by controlling Pak2 and Pak4 activation in conjunction with PKCε.
Fig. 6.
Pak2 and Pak4 act downstream of SFKs to coactivate Raf kinases that are involved in EC lumen formation in 3D collagen matrices. (A) ECs were resuspended in 3D collagen matrices in the absence or presence of PP2 (10 μM). Extracts were prepared at 24 hours for western blot analysis and probed for phospho-Pak2, phospho-Pak4 or actin. (B) ECs containing GFP- or Csk-expressing adenoviruses (Ad) were resuspended in 3D collagen matrices. Extracts were made at 24 hours for western blot analysis and probed for phospho-Pak2, phospho-Pak4 or actin. (C) ECs treated with the indicated siRNAs were resuspended in 3D collagen matrices. Extracts were made at 24 hours for western blot analysis and probed for phospho-Pak2, phospho-Pak4 or actin. (D) ECs treated with the indicated siRNAs or adenoviruses [GFP, DN Pak2 (T402A) or DN Pak4 (K350M)] were resuspended in 3D collagen matrices. Extracts were made at 24 hours for western blot analysis and probed for phospho-B-Raf, phospho-C-Raf or actin. (E) Extracts of EC cultures in 3D collagen matrices were prepared at the indicated time points and probed for phospho-B-Raf or phospho-C-Raf. Actin, B-Raf and C-Raf were used as loading controls.
Raf kinases act downstream of Pak2 and Pak4 to regulate EC lumen formation in 3D collagen matrices
Pak2 and Pak4 appear to serve as key targets at which signals mediated by Cdc42, PKCε and SFKs converge to regulate EC lumen formation in 3D collagen matrices. To further dissect their signaling pathways, we examined downstream targets of Pak2 and Pak4 during EC lumen formation in 3D collagen matrices. C-Raf, a member of the Raf-kinase family, has been previously implicated in the regulation of angiogenesis and EC survival downstream of Pak1 and Src (Alavi et al., 2003; Eliceiri et al., 2002; Hood et al., 2003). B-Raf is also shown to play a crucial role in VEGF-induced angiogenesis and it is often mutated in various human cancers (Wan et al., 2004; Wellbrock et al., 2004). To examine whether both B-Raf and C-Raf are regulated by Pak2 and Pak4 to control EC lumen formation, we used antibodies that recognize a residue that is known to be phosphorylated by Pak proteins and which increases their activity. We have previously shown that suppression of Pak2 and Pak4 either by siRNA or expression of DN mutants significantly blocks EC lumen formation in 3D collagen matrices (Koh et al., 2008). To analyze whether these inhibitory effects are modulated by B-Raf and C-Raf activation, lysates were made 24 hours after ECs were suspended in 3D collagen matrices. Treatment of ECs with either Pak2 or Pak4 siRNA significantly reduced phosphorylation of both B-Raf and C-Raf (Fig. 6D). Expression of DN Pak2 (T402A) or Pak4 (K350M), which block lumen formation (Koh et al., 2008), also diminished B-Raf and C-Raf phosphorylation (Fig. 6D). Moreover, both B-Raf and C-Raf were highly activated during the EC-lumen-formation process in 3D collagen matrices (Fig. 6E). Interestingly, when total B-Raf and C-Raf proteins were examined, both of these proteins were induced during these events. Together, these data suggest that B-Raf and C-Raf represent key downstream targets of Pak2 and Pak4 that regulate EC lumen formation in 3D collagen matrices.
B-Raf and C-Raf are required for EC lumen formation in 3D collagen matrices
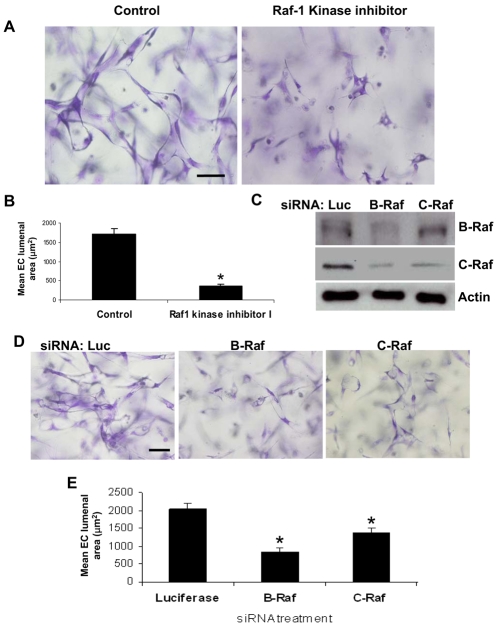
To examine the function of Raf kinases in EC lumen formation, we used a known Raf-kinase inhibitor (GW5074) (Lackey et al., 2000). The addition of this inhibitor resulted in significant blockade of lumen formation (Fig. 7A,B). To further address their functional role, we suppressed expression of B-Raf and C-Raf by siRNA treatment (Fig. 7C). siRNA suppression of either B-Raf or C-Raf significantly blocked EC lumen formation (Fig. 7D,E). Although suppression of either B-Raf or C-Raf resulted in statistically significant inhibition of EC lumen formation, B-Raf siRNA had a more dramatic effect than C-Raf siRNA (Fig. 7D,E). Western blot analysis showed that suppression of B-Raf led to a protein-level reduction of not only B-Raf but also C-Raf, whereas suppression of C-Raf did not have any effect on B-Raf protein levels, suggesting that regulation of B-Raf is linked to subsequent C-Raf stability or expression (Fig. 7C). Previous work has shown that B-Raf can compensate for C-Raf in vivo and that C-Raf can serve as an effector for B-Raf (Chong et al., 2003; Mikula et al., 2001; Wan et al., 2004). Our data from inhibitor and siRNA analysis reveal that both Raf isoforms play a role during the EC-lumen-formation process.
Fig. 7.
Inhibition of Raf kinases blocks EC lumen formation in 3D collagen matrices. (A,B) ECs were resuspended in 3D collagen matrices for 24 hours in the absence or presence of Raf1 kinase inhibitor GW5074 (5 μM). (A) Representative fields of EC lumen formation assay. Scale bar: 50 μm. (B) Quantification of EC lumen formation. (C-E) siRNA suppression of B-Raf or C-Raf inhibits EC lumen formation in 3D collagen matrices. (C) Lysates were prepared for western blot analysis and probed for phospho-B-Raf, phospho-C-Raf and actin control. ECs treated with the indicated siRNAs were resuspended in 3D collagen matrices for 24 hours. (D) Representative fields of EC-lumen-formation assay. Scale bar: 50 μm. (E) Quantification of EC-lumen-formation assay. Data are shown as the mean EC lumenal area ± s.d. (n=3). *P<0.01 compared with control.
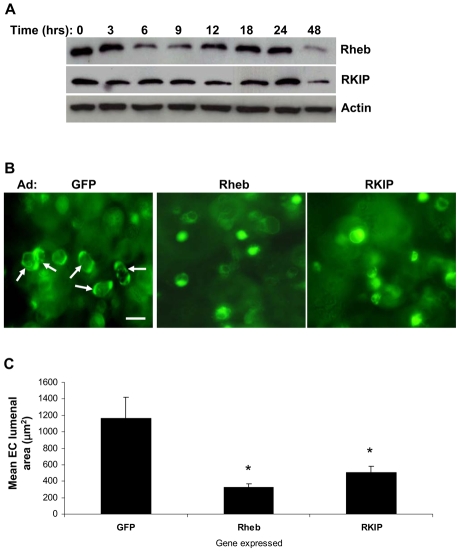
Rheb (Ras homolog enriched in brain) and RKIP (Raf kinase inhibitory protein) are two endogenous inhibitors of Raf kinases that act as negative modulators of Raf kinase signaling (Corbit et al., 2003; Karbowniczek et al., 2006; Klysik et al., 2008). Both Rheb and RKIP protein levels were examined during the lumen-formation process and were found to be differentially expressed (Fig. 8A). The level of both proteins decreased while ECs actively underwent vacuole and lumen formation (i.e. 3-18 hours) (Fig. 8A) (Koh et al., 2008). At a time when the EC-lumen-formation process is substantially completed (i.e. 24 hours), there was an increase in both Rheb and RKIP protein levels (Fig. 8A). Interestingly, these levels decreased again as EC lumen and tube formation became stabilized (48 hours) (Fig. 8A), indicating a complex expression pattern for these two Raf inhibitors. To further examine the potential role of Rheb and RKIP and their influence on Raf during these events, we generated adenoviral recombinant constructs expressing either Rheb or RKIP. ECs expressing increased Rheb or RKIP levels were markedly blocked in their ability to undergo EC lumen formation compared with ECs expressing control GFP protein (Fig. 8B,C). Expression of Rheb showed a greater inhibitory effect compared with that of RKIP. Rheb is known to affect the activity of both Raf isoforms because it disrupts heterodimerization of B-Raf and C-Raf (Im et al., 2002; Karbowniczek et al., 2006), whereas RKIP has been shown to selectively target the activity of C-Raf (Trakul et al., 2005). These data, together with that from our siRNA experiment, suggest that the activity of both B-Raf and C-Raf is crucial for EC lumen formation in 3D collagen matrices.
Fig. 8.
Increased expression of the Raf-kinase inhibitors Rheb or RKIP block EC lumen formation in 3D collagen matrices. (A) Extracts of EC cultures were prepared at the indicated time points and probed for Rheb, RKIP or actin. (B) Representative fields of the indicated cultures. Scale bar: 50 μm. (C) Quantification of EC lumen formation. Data are shown as the mean EC lumenal area ± s.d. (n=6). *P<0.01 compared with GFP control.
B-Raf and C-Raf mediate EC lumen formation downstream of PKCε, Paks and SFKs in 3D collagen matrices
Our data indicate that B-Raf and C-Raf are activated downstream of Pak2 and Pak4, which are regulated by PKCε and SFKs, as well as Cdc42 and Rac1. To further show that B-Raf and C-Raf lie downstream of Pak2 and Pak4 in response to PKCε and SFKs during the process of EC lumen formation, we examined phosphorylation of both B-Raf and C-Raf in the presence of PKC inhibitors or SFK inhibitors. B-Raf and C-Raf can undergo phosphorylation on various residues by different kinases (Chong et al., 2003; Thomas and Brugge, 1997). However, we focused our analysis on a residue that is phosphorylated by Paks, because Pak2 and Pak4 play a key role in EC lumen formation and this residue is conserved in both B-Raf and C-Raf (Li et al., 2001; Wellbrock et al., 2004). In the absence of TPA or in the presence of PKC inhibitors that block EC lumen formation by targeting PKCε, B-Raf and C-Raf phosphorylation on these crucial residues were dramatically reduced (Fig. 9A). Presence of the SFK inhibitor PP2 also diminished their phosphorylation compared with TPA alone or PKCα inhibitor, Go6976, which does not affect EC lumen formation in 3D collagen matrices (Fig. 9A). These data suggest that there is a linear signaling pathway involving PKCε–SFKs–Pak2–Pak4–B-Raf–C-Raf to regulate EC lumen formation in 3D collagen matrices.
Fig. 9.
B-Raf and C-Raf activation occur downstream of PKCε and SFKs during EC-lumen-formation events in 3D collagen matrices. (A) ECs were resuspended in 3D collagen matrices in the absence or presence of TPA, GF109203X (2.5 μM), Ro-32-0432 (5 μM), Go6983 (5 μM), Go6976 (5 μM) or PP2 (10 μM). Lysates were prepared at 24 hours for western blot analysis and probed for phospho-B-Raf, phospho-C-Raf, B-Raf, C-Raf or actin. (B-D) ERK1/2 proteins are phosphorylated during EC lumen formation in 3D collagen matrices. (B) Extracts of EC cultures were prepared at the indicated time points and probed for phospho-ERK1/2 or actin. (C) ECs were resuspended in 3D collagen matrices for 24 hours in the absence or presence of Raf-kinase inhibitor, GW5074 (5 μM). Lysates were prepared for western blot analysis and probed for phospho-ERK1/2 or actin. (D) ECs treated with the indicated siRNAs were resuspended in 3D collagen matrices. Lysates were prepared for western blot analysis and probed for phospho-ERK1/2 or actin.
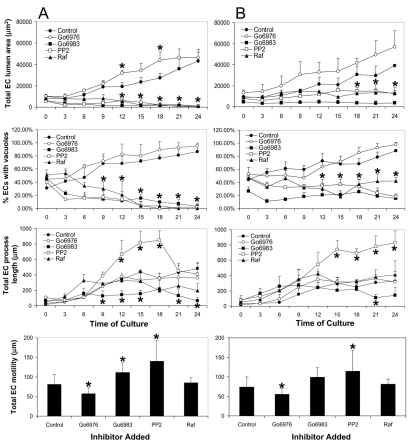
One issue that is raised by this data is how these different signaling molecules temporally function in relation to the complex processes of intracellular vacuolation and coalescence, lumen expansion, EC process extension, and EC motility that characterize EC tubulogenesis in 3D collagen matrices. To address this issue, we performed time-lapse experiments over a 24-hour period in the presence or absence of PKCε, Src and Raf inhibitors at different doses. As shown in Fig. 10, both intracellular vacuolation and lumen expansion were markedly suppressed by Go6983, PP2 and GW5074, which block PKCε, Src and Raf kinases, respectively. Interestingly, Go6976, which selectively blocks PKCα and PKC β (PKCα/β) isoforms, accelerates lumen expansion, suggesting that these PKC isoforms might be inhibitory to EC lumen formation. EC motility was increased by Src blockade and by novel and atypical PKC-isoform blockade, whereas PKCα/β blockade decreased motility (Fig. 10). In these cases, motility responses were inversely correlated with lumen formation. Thus, although EC motility is required for tube formation, there appears to be complex relationships between motility and lumen formation that need to be investigated further. EC process extension, which increases over time, is stimulated by Src blockade and inhibited by novel and atypical PKC blockade (Fig. 10). Overall, it appears that this PKCε–SFKs–Pak2–Pak4–B-Raf–C-Raf-kinase cascade appears to act proximally in the lumen-formation process such that both EC vacuolation and lumen formation are strongly inhibited by the blockade of each of the kinases in this pathway.
Fig. 10.
Temporal analysis of the influence of PKC, Src and Raf kinases on EC lumen and tube formation in 3D collagen matrices. EC cultures were established in 3D collagen matrices and the indicated kinase inhibitors were added at either 10 μM (A panels) or 2.5 μM (B panels). Time-lapse movies were made by acquiring images every 10 minutes over a 24-hour period as described (Koh et al., 2008). Four independent parameters were assessed, including measurements of total EC lumen area per field (first row), the percentage of ECs with intracellular vacuoles (second row), total EC process length per field (third row) and total EC motility per field (fourth row). Fields were acquired at a magnification of 150×. Quantitation of lumen area, process lengths and EC motility used MetaMorph software as described (Koh et al., 2008). Images were obtained from three independent cultures and from at least three different fields for each indicated value at each time point. Statistical significance relative to control cultures was set at P<0.05 and is indicated by an asterisk.
B-Raf and C-Raf regulate EC lumen formation in 3D collagen matrices through ERK1/2
Raf kinases are key components of the MAPK pathway (Leicht et al., 2007; Morrison and Cutler, 1997; Wellbrock et al., 2004). To examine whether B-Raf and C-Raf regulate EC lumen formation in 3D collagen matrices through the MAPK pathway, we analyzed the phosphorylation and functions of ERK1/2 during this process. Following an early initial induction in their phosphorylation level, ERK1/2 activation levels remained fairly constant (Fig. 9B). Because ERK1/2 is known as a major downstream target of Raf kinases, its involvement in EC lumen formation downstream of Raf kinases was examined. Inhibition of Raf-kinase activity by the Raf inhibitor GW5074 resulted in a reduction of ERK1/2 phosphorylation (Fig. 9C), which accompanied its ability to block EC lumen formation (Fig. 7A,B). Suppression of B-Raf or C-Raf by siRNA also showed a modest reduction in ERK1/2 phosphorylation (Fig. 9D), indicating that ERK1/2 plays a role downstream of B-Raf and C-Raf in EC lumen formation.
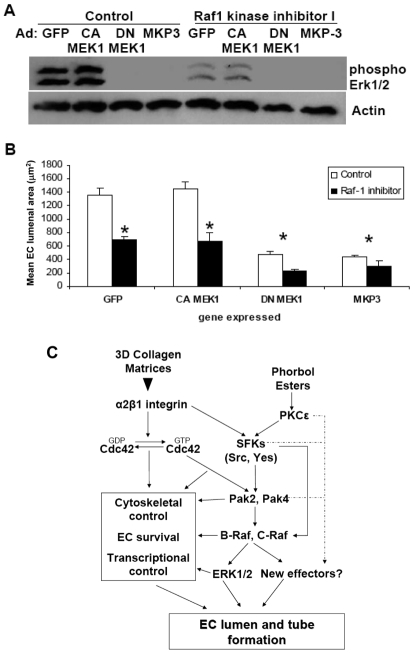
To further evaluate the function of the MAPK pathway in EC lumen formation, we used recombinant viruses that express constitutively active (CA) MEK1, DN MEK1, or MKP3, a phosphatase that selectively dephosphorylates ERK1/2 (Arkell et al., 2008; Keyse, 2008). Expression of DN MEK1 or MKP3 significantly impaired EC lumen formation and strongly inhibited ERK1/2 phosphorylation compared with GFP control (Fig. 11A,B). Expression of CA MEK1 induced the phosphorylation of ERK1/2 but did not show enhanced EC lumen formation (Fig. 11B). To further analyze the MAP signaling pathway in EC lumen formation, we next examined whether the expression of CA MEK1 could rescue the EC-lumen-formation defect resulting from Raf-kinase-inhibitor addition, because ERK1/2 acts downstream of Raf kinases. When ECs expressing control GFP, CA MEK1, DN MEK1 or MKP3 virus were suspended in 3D collagen matrices in the presence of GW5074, blockade of EC lumen formation was not rescued by the expression of CA MEK1 (Fig. 11B). When the inhibitor was added to ECs expressing DN MEK1 or MKP3 virus, we observed decreased EC survival (supplementary material Fig. S1), showing the dual involvement of ERK1/2 and Raf kinases in supporting EC survival during morphogenic events. This data is consistent with the known ability of Raf kinases to inactivate kinases such as the pro-apoptotic kinase ASK-1 independently of ERK1/2 activation (Alavi et al., 2003). These data together indicate that, although ERK1/2 is required for EC lumen formation, it is not sufficient for these events and, thus, works in conjunction with the other identified kinases in the pathway (Fig. 11C).
Fig. 11.
Raf kinases regulate EC lumen formation in 3D collagen matrices through ERK1/2 and possibly other targets. ECs infected with GFP, CA MEK1, DN MEK1, or MKP-3 adenovirus were resuspended in 3D collagen matrices in the absence or presence of the Raf kinase inhibitor GW5074 (5 μM). (A) Extracts were made at 24 hours for western blot analysis and probed for phospho-ERK1/2 or actin. (B) Quantification of EC lumen formation at 24 hours. Data are shown as the mean EC lumenal area ± s.d. (n=3). *P<0.01 compared with GFP control. (C) Schematic diagram illustrating the mechanisms underlying Cdc42-dependent EC lumen and tube formation signaling pathways in 3D collagen matrices. Pak2 and Pak4 also serve as downstream effectors of PKCε, which activates SFKs and controls their interaction with Cdc42. Pak2 and Pak4 activation leads to phosphorylation of B-Raf and C-Raf as well as ERK1/2, which together control EC lumen formation. In addition, these kinases might directly activate new effectors to regulate EC lumen formation (shown as broken lines). Their coordinated influence appears to control events such as cytoskeletal rearrangements, EC survival and transcriptional events that are necessary to both form and maintain tube structures.
Discussion
PKCε, Src and Yes control EC lumen formation in 3D collagen matrices
Activation of PKC has been implicated in the regulation of angiogenesis both in vivo and in vitro (Davis and Camarillo, 1996; Montesano and Orci, 1985; Montesano et al., 1987; Morris et al., 1988). Our previous work identified PKCε as a key PKC isoform mediating TPA-induced lumen formation in 3D collagen matrices (Koh et al., 2008). Here we show that increased expression of PKCε markedly stimulates EC lumen formation. PKCε has been shown to promote cell survival and anchorage-independent growth (Ding et al., 2002; Okhrimenko et al., 2005). Various human cancers show increased expression of PKCε, indicating that this PKC isoform plays a key role in developing tumors as well as in other pathological conditions that are often accompanied by angiogenesis (Basu and Weixel, 1995; Gubina et al., 1998). PKCε has also been shown to regulate the trafficking of integrins, thereby influencing cell motility and adhesion (Ivaska et al., 2005; Ivaska et al., 2002), which are necessary functions regulating EC vascular morphogenesis.
Increased PKCε-induced phosphorylation of SFKs suggests that SFKs are regulated by PKCε to control EC lumen formation in 3D collagen matrices. Studies have shown that SFKs act downstream of various signal-transduction pathways such as integrins, growth-factor receptors, Rho GTPases and PKC to regulate angiogenesis, vascular permeability, actin-cytoskeleton organization, capillary morphogenesis, cell proliferation and endothelial remodeling (Abu-Ghazaleh et al., 2001; Amos et al., 2005; Basu and Weixel, 1995; Bruce-Staskal and Bouton, 2001; Eliceiri et al., 1999; Eliceiri et al., 2002; Friedlander et al., 1995; Liu and Senger, 2004; Nomura et al., 2007; Robles et al., 2005; Tatin et al., 2006). Given that SFKs exhibit such versatile functions in response to diverse cellular factors, including PKC, Rho GTPases and integrins, it has led us to examine their role during EC lumen formation in 3D collagen matrices. Our study found that the expression of SFKs is highly induced during EC morphogenesis and there is a strong association between SFKs and Cdc42 in a PKCε-dependent manner during EC lumen formation. Inhibition of SFK activity by either PP2 or increased Csk expression impaired EC lumen formation and, furthermore, we demonstrate that Src and Yes, but not Fyn and Lyn, control this process. The opposite experiment was performed whereby siRNA suppression of Csk or increased expression of a DN Csk protein led to marked increases in lumen formation. The inhibitory influence of DN-PKCε expression was overcome and reversed by increased expression of either WT Src or DN Csk, showing that SFKs are activated downstream of PKCε. Downstream of the Cdc42, PKCε and SFK signals, Pak2 and Pak4 represent common platforms at which these signaling events converge to control EC lumen formation in 3D collagen matrices. Stimulation and inhibition of lumen formation by WT PKCε and DN PKCε, respectively, directly correlates with the levels of activated Pak2 and Pak4. Furthermore, activation of both Paks is controlled by SFKs, suggesting that they work together during EC lumen formation and appear to be activated downstream of SFK activation.
Accumulating data reveals how work using in vitro morphogenesis systems directly correlate with findings demonstrated in vivo with regards to lumen and tube formation (Davis et al., 2007; Egginton and Gerritsen, 2003; Holderfield and Hughes, 2008; Iruela-Arispe and Davis, 2009). Two key examples are those showing the role of intracellular vacuole formation and coalescence in vitro using human ECs, and in vivo lumenogenesis during zebrafish vascular development (Kamei et al., 2006), as well as the demonstration that the cerebral cavernous malformation protein, CCM2, is required for EC lumen formation in vitro as well as lumen formation and patency of the developing mouse vasculature, including the first branchial arch artery and intersomitic arteries (Whitehead et al., 2009).
Pak- and Src-dependent Raf activation regulates EC lumen formation
Further molecular examination downstream of Pak2 and Pak4 revealed B-Raf and C-Raf as targets, the activities of which are regulated by Pak2 and Pak4 to mediate EC lumen formation in 3D collagen matrices. Raf kinases have drawn much attention as a promising target for anti-angiogenic therapy as they control many crucial cellular functions by integrating signals that regulate vascular events (Alavi et al., 2003; Hood et al., 2003; Leicht et al., 2007; Morrison and Cutler, 1997; Wellbrock et al., 2004). The effect of Raf kinases is often carried out by their involvement in a conserved signaling pathway, the Raf-MEK-ERK MAPK pathway (Leicht et al., 2007; Roberts and Der, 2007; Wan et al., 2004; Zebisch et al., 2007). The MAPK pathway also regulates other crucial cellular functions such as survival, cytoskeletal organization, proliferation and transcriptional regulation (Chong et al., 2003; Lefloch et al., 2008; Leicht et al., 2007; Wellbrock et al., 2004), as well as playing a role during epithelial tube morphogenesis (O'Brien et al., 2004). It is clear that overlapping signaling pathways control tubulogenesis in endothelial and epithelial cells, although there are unique features of each that are becoming increasingly apparent (Davis et al., 2007; Iruela-Arispe and Davis, 2009; Lubarsky and Krasnow, 2003; O'Brien et al., 2004).
Here, we show that both B-Raf and C-Raf play a role to regulate EC lumen formation. Suppression of their activity either by siRNA, chemical inhibitors or increased expression of negative regulators (i.e. Rheb and Rkip) resulted in marked inhibition of EC lumen formation. In support of these findings, studies using genetic knockout mice have shown that, although knockout of either B-Raf or C-Raf causes embryonic lethality (Wojnowski et al., 1997), MAPK signaling in C-Raf-knockout mice can be rescued by B-Raf (Chong et al., 2003). Because both B-Raf and C-Raf kinases appear to be required for EC lumen formation, we then examined the role of the MAPK signaling during these events. Suppression of MEK activity either by expression of a DN mutant or by increased expression of the ERK1/2 phosphatase MKP3 blocked EC lumen formation in 3D collagen matrices, indicating that ERK1/2 activity is also required. Interestingly, the Raf-ERK pathway is typically associated with cell-proliferation signaling; however, there is no evidence for EC proliferation during the tube-formation process in 3D collagen matrices in this system.
Protein-kinase cascades downstream of Cdc42-dependent signaling control EC lumen formation in 3D collagen matrices
Data presented in this study provide new insights into signaling pathways regulating the crucial EC-lumen-formation step during vascular morphogenesis in a collagen-matrix environment (Fig. 10C). EC–collagen-matrix interactions result in integrin-dependent signaling, leading to activation of Cdc42 as well as its downstream effectors, Pak2 and Pak4 (Koh et al., 2008). Activation of Pak2 and Pak4 are also regulated by SFKs, especially Src and Yes, as well as PKCε. Similar to Pak2 and Pak4, activated SFKs associate with Cdc42 in multiprotein signaling complexes (Koh et al., 2008) to control the lumen-formation process. These data indicate that, during EC lumen formation in 3D collagen matrices, Pak2 and Pak4 serve as common targets that integrate signals from Cdc42, SFKs and PKCε to induce Raf activation and EC lumenogenesis. Overall, it appears that EC lumenogenesis requires coordinated signaling events leading to cytoskeletal changes (Cdc42, SFKs, Paks), prosurvival signals (Raf kinases) and transcriptional controls (ERK1/2) that are necessary for EC lumen and tube formation. These latter controls through ERK1/2 are probably responsible in part for the marked changes in gene expression that accompany EC lumen and tube formation (Bell et al., 2001).
Materials and Methods
Reagents
GF109203X, Go6983, Ro-32-0432, Go6976, PP2 and Raf-1-kinase inhibitor were purchased from Calbiochem (La Jolla, CA). 12-O-tetradecanoyl-phorbol-13-acetate (TPA) and a polyclonal antibody against phospho-Pak2 (Ser141) were obtained from Sigma-Aldrich (St Louis, MO). Polyclonal antibodies targeting phospho-Pak4 (Ser474), B-Raf, phospho-B-Raf (Ser445), C-Raf, phospho-C-Raf (Ser338), phospho-Src (Y416), Rheb, RKIP and phospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204) were obtained from Cell Signaling Technology (Danvers, MA). A monoclonal antibody against actin (CP01) was obtained from Calbiochem. WT PKCε, DN PKCε, CA MEK1, DN MEK1 and MKP3 adenoviruses were purchased from Seven Hills Bioreagents (Cincinnati, OH), WT Src, WT Csk and DN Csk were purchased from Cell Biolabs (San Diego, CA) and amplified as previously described (Bayless and Davis, 2002).
EC lumen and tube formation in 3D collagen matrices
Human umbilical vein ECs (HUVECs) were purchased from Clonetics (San Diego, CA) and were cultured (passage 2-5) as previously described (Davis and Camarillo, 1996). For the lumen-formation assay, ECs were suspended within 3.75 mg/ml of collagen-type-I matrices and allowed to undergo EC morphogenesis as described previously (Davis and Camarillo, 1996). Cultures were fixed at the indicated time points with 3% glutaraldehyde for 30 minutes. In some cases, cultures were stained with 0.1% Toluidine Blue in 30% methanol and destained prior to photography and visualization. Some 3D collagen gels were also extracted to examine protein expression. Extracts were run on SDS-PAGE gels, transferred to membranes, probed and developed. Adenovirus infection of ECs was carried out as previously described (Bayless and Davis, 2002).
Transfection of ECs with siRNAs
siGENOME SMARTpool human Src, Yes, Fyn, Lyn, B-Raf and C-Raf were obtained from Dharmacon (Lafayette, CO) and prepared as previously described (Saunders et al., 2005). Luciferase GL2 duplex was used as a control. EC transfection with siRNAs was carried out in growth media with 1% serum. Details of our siRNA-transfection protocol have been described previously (Saunders et al., 2005).
EC-lumen-formation pulldown assay
Generation of S-GFP-Cdc42 adenovirus has been described previously (Koh et al., 2008). ECs were infected with S-GFP-Cdc42 adenovirus and the EC-lumen-formation assay was set up as described (Koh et al., 2008). EC cultures were extracted at the indicated time points and bound Cdc42-associated proteins were detected by western blot analysis.
RT-PCR
Total RNA was extracted from EC cultures at the indicated time points or from siRNA-treated (luciferase, Src, Yes, Fyn and Lyn) ECs using the Totally RNA Isolation kit obtained from Ambion (Austin, TX) according to the manufacturer's instructions. RNA (1 μg) was reverse transcribed using AccuScript High Fidelity 1st strand cDNA synthesis kit (Stratagene). RT-PCR amplification was performed using the primers: Src up (5′-TGTATTGCCAAGTACAACTTC-3′), Src dn (5′-CAAAGTACACCTCCTCGTC-3′), Yes up (5′-CAAGTGTGAGCCATTATG-3′), Yes dn (5′-AAATACCATTCTTCTGCC-3′), Fyn up (5′-ACGAGAAGGAGGAACAGGAG-3′), Fyn dn (5′-GTATCCACCATTGTCAAGTTTG-3′), Lyn up (5′-AGGCCAGTTCCAGAATCTC-3′), Lyn dn (5′-GCACAGGGTCAAAGTCTC-3′), G3PDH-1 up (5′-GCCAAAAGGGTCATCATCTC-3′) and G3PDH-1 dn (5′-GTAGAGGCAGGGATGATGTTC-3′).
Generation of Rheb and RKIP adenoviruses
Rheb and RKIP were amplified from human cDNA clone (Origene) using the primers: Rheb up (5′-AGCTCGAGGCCACCATGCCGCAGTCCAAGTCCCGGAAG-3′), Rheb dn (5′-AGTCTAGATCACATCACCGAGCATGAAGACTTGCC-3′), RKIP up (5′-AGCTCGAGGCCACCATGCCGGTGGACCTCAGCAAG-3′) and RKIP dn (5′-AGTCTAGACTACTTCCCAGACAGCTGCTC-3′) (Sigma Genosys, The Woodlands, TX). Standard restriction digestion cloning was performed to clone Rheb and RKIP into pAdTrack-CMV. Recombination and virus production were carried out as previously described (Bayless and Davis, 2002).
Microscopy/imaging and statistical analysis
Visualization and image acquisition of EC lumen and tube-formation assays were done using an inverted microscope (CKX41; Olympus) as previously described (Saunders et al., 2006). Image analysis was done using MetaMorph software.
Statistical analysis of EC lumen and tube formation was performed using SPSS 11.0 software (SPSS). Statistical significances were accessed by paired-samples t-test or a one-way ANOVA with a Dunnett's test.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/11/1812/DC1
The authors would like to thank Kristine Malotte for excellent technical assistance. This work was supported by NIH grants HL59373 and HL79460 to G.E.D. Deposited in PMC for release after 12 months.
References
- Abu-Ghazaleh, R., Kabir, J., Jia, H., Lobo, M. and Zachary, I. (2001). Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem. J. 360, 255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, R. H. and Alitalo, K. (2007). Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell. Biol. 8, 464-478. [DOI] [PubMed] [Google Scholar]
- Alavi, A., Hood, J. D., Frausto, R., Stupack, D. G. and Cheresh, D. A. (2003). Role of Raf in vascular protection from distinct apoptotic stimuli. Science 301, 94-96. [DOI] [PubMed] [Google Scholar]
- Amos, S., Martin, P. M., Polar, G. A., Parsons, S. J. and Hussaini, I. M. (2005). Phorbol 12-myristate 13-acetate induces epidermal growth factor receptor transactivation via protein kinase Cdelta/c-Src pathways in glioblastoma cells. J. Biol. Chem. 280, 7729-7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkell, R. S., Dickinson, R. J., Squires, M., Hayat, S., Keyse, S. M. and Cook, S. J. (2008). DUSP6/MKP-3 inactivates ERK1/2 but fails to bind and inactivate ERK5. Cell. Signal. 20, 836-843. [DOI] [PubMed] [Google Scholar]
- Basu, A. and Weixel, K. M. (1995). Comparison of protein kinase C activity and isoform expression in cisplatin-sensitive and -resistant ovarian carcinoma cells. Int. J. Cancer 62, 457-460. [DOI] [PubMed] [Google Scholar]
- Bayless, K. J. and Davis, G. E. (2002). The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J. Cell Sci. 115, 1123-1136. [DOI] [PubMed] [Google Scholar]
- Bayless, K. J., Salazar, R. and Davis, G. E. (2000). RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the alpha(v)beta(3) and alpha(5)beta(1) integrins. Am. J. Pathol. 156, 1673-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, S. E., Mavila, A., Salazar, R., Bayless, K. J., Kanagala, S., Maxwell, S. A. and Davis, G. E. (2001). Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J. Cell Sci. 114, 2755-2773. [DOI] [PubMed] [Google Scholar]
- Bokoch, G. M. (2003). Biology of the p21-activated kinases. Annu. Rev. Biochem. 72, 743-781. [DOI] [PubMed] [Google Scholar]
- Bruce-Staskal, P. J. and Bouton, A. H. (2001). PKC-dependent activation of FAK and src induces tyrosine phosphorylation of Cas and formation of Cas-Crk complexes. Exp. Cell Res. 264, 296-306. [DOI] [PubMed] [Google Scholar]
- Chong, H., Vikis, H. G. and Guan, K. L. (2003). Mechanisms of regulating the Raf kinase family. Cell. Signal. 15, 463-469. [DOI] [PubMed] [Google Scholar]
- Corbit, K. C., Trakul, N., Eves, E. M., Diaz, B., Marshall, M. and Rosner, M. R. (2003). Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. J. Biol. Chem. 278, 13061-13068. [DOI] [PubMed] [Google Scholar]
- Davis, G. E. and Camarillo, C. W. (1996). An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp. Cell Res. 224, 39-51. [DOI] [PubMed] [Google Scholar]
- Davis, G. E. and Bayless, K. J. (2003). An integrin and Rho GTPase-dependent pinocytic vacuole mechanism controls capillary lumen formation in collagen and fibrin matrices. Microcirculation 10, 27-44. [DOI] [PubMed] [Google Scholar]
- Davis, G. E. and Senger, D. R. (2005). Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 97, 1093-1107. [DOI] [PubMed] [Google Scholar]
- Davis, G. E., Bayless, K. J. and Mavila, A. (2002). Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat. Rec. 268, 252-275. [DOI] [PubMed] [Google Scholar]
- Davis, G. E., Koh, W. and Stratman, A. N. (2007). Mechanisms controlling human endothelial lumen formation and tube assembly in three-dimensional extracellular matrices. Birth Defects Res. C Embryo Today 81, 270-285. [DOI] [PubMed] [Google Scholar]
- DerMardirossian, C., Rocklin, G., Seo, J. Y. and Bokoch, G. M. (2006). Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Mol. Biol. Cell 17, 4760-4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, L., Wang, H., Lang, W. and Xiao, L. (2002). Protein kinase C-epsilon promotes survival of lung cancer cells by suppressing apoptosis through dysregulation of the mitochondrial caspase pathway. J. Biol. Chem. 277, 35305-35313. [DOI] [PubMed] [Google Scholar]
- Egginton, S. and Gerritsen, M. (2003). Lumen formation: in vivo versus in vitro observations. Microcirculation 10, 45-61. [DOI] [PubMed] [Google Scholar]
- Eliceiri, B. P., Paul, R., Schwartzberg, P. L., Hood, J. D., Leng, J. and Cheresh, D. A. (1999). Selective requirement for Src kinases during VEGF-Induced angiogenesis and vascular permeability. Mol. Cell 4, 915-924. [DOI] [PubMed] [Google Scholar]
- Eliceiri, B. P., Puente, X. S., Hood, J. D., Stupack, D. G., Schlaepfer, D. D., Huang, X. Z., Sheppard, D. and Cheresh, D. A. (2002). Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J. Cell Biol. 157, 149-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian, J. R., Daar, I. O. and Morrison, D. K. (1993). Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol. Cell. Biol. 13, 7170-7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander, M., Brooks, P. C., Shaffer, R. W., Kincaid, C. M., Varner, J. A. and Cheresh, D. A. (1995). Definition of two angiogenic pathways by distinct alpha v integrins. Science 270, 1500-1502. [DOI] [PubMed] [Google Scholar]
- Fryer, B. H. and Field, J. (2005). Rho, Rac, Pak and angiogenesis: old roles and newly identified responsibilities in endothelial cells. Cancer Lett. 229, 13-23. [DOI] [PubMed] [Google Scholar]
- Gubina, E., Rinaudo, M. S., Szallasi, Z., Blumberg, P. M. and Mufson, R. A. (1998). Overexpression of protein kinase C isoform epsilon but not delta in human interleukin-3-dependent cells suppresses apoptosis and induces bcl-2 expression. Blood 91, 823-829. [PubMed] [Google Scholar]
- Hall, A. (1998). Rho GTPases and the actin cytoskeleton. Science 279, 509-514. [DOI] [PubMed] [Google Scholar]
- Hall, A. (2005). Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans. 33, 891-895. [DOI] [PubMed] [Google Scholar]
- Holderfield, M. T. and Hughes, C. C. (2008). Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ. Res. 102, 637-652. [DOI] [PubMed] [Google Scholar]
- Hood, J. D., Frausto, R., Kiosses, W. B., Schwartz, M. A. and Cheresh, D. A. (2003). Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J. Cell Biol. 162, 933-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz, A. and Simons, M. (2008). Branching morphogenesis. Circ. Res. 103, 784-795. [DOI] [PubMed] [Google Scholar]
- Howell, B. W. and Cooper, J. A. (1994). Csk suppression of Src involves movement of Csk to sites of Src activity. Mol. Cell. Biol. 14, 5402-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im, E., von Lintig, F. C., Chen, J., Zhuang, S., Qui, W., Chowdhury, S., Worley, P. F., Boss, G. R. and Pilz, R. B. (2002). Rheb is in a high activation state and inhibits B-Raf kinase in mammalian cells. Oncogene 21, 6356-6365. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe, M. L. and Davis, G. E. (2009). Cellular and molecular mechanisms of vascular lumen formation. Dev. Cell 16, 222-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska, J., Whelan, R. D., Watson, R. and Parker, P. J. (2002). PKC epsilon controls the traffic of beta1 integrins in motile cells. EMBO J. 21, 3608-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska, J., Vuoriluoto, K., Huovinen, T., Izawa, I., Inagaki, M. and Parker, P. J. (2005). PKCepsilon-mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J. 24, 3834-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei, M., Saunders, W. B., Bayless, K. J., Dye, L., Davis, G. E. and Weinstein, B. M. (2006). Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 442, 453-456. [DOI] [PubMed] [Google Scholar]
- Karbowniczek, M., Robertson, G. P. and Henske, E. P. (2006). Rheb inhibits C-raf activity and B-raf/C-raf heterodimerization. J. Biol. Chem. 281, 25447-25456. [DOI] [PubMed] [Google Scholar]
- Keyse, S. M. (2008). Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 27, 253-261. [DOI] [PubMed] [Google Scholar]
- Kilarski, W. W., Jura, N. and Gerwins, P. (2003). Inactivation of Src family kinases inhibits angiogenesis in vivo: implications for a mechanism involving organization of the actin cytoskeleton. Exp. Cell Res. 291, 70-82. [DOI] [PubMed] [Google Scholar]
- Klysik, J., Theroux, S. J., Sedivy, J. M., Moffit, J. S. and Boekelheide, K. (2008). Signaling crossroads: the function of Raf kinase inhibitory protein in cancer, the central nervous system and reproduction. Cell. Signal. 20, 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh, W., Mahan, R. D. and Davis, G. E. (2008). Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J. Cell Sci. 121, 989-1001. [DOI] [PubMed] [Google Scholar]
- Kolch, W., Heidecker, G., Kochs, G., Hummel, R., Vahidi, H., Mischak, H., Finkenzeller, G., Marme, D. and Rapp, U. R. (1993). Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature 364, 249-252. [DOI] [PubMed] [Google Scholar]
- Lackey, K., Cory, M., Davis, R., Frye, S. V., Harris, P. A., Hunter, R. N., Jung, D. K., McDonald, O. B., McNutt, R. W., Peel, M. R. et al. (2000). The discovery of potent cRaf1 kinase inhibitors. Bioorg. Med. Chem. Lett. 10, 223-226. [DOI] [PubMed] [Google Scholar]
- Lefloch, R., Pouyssegur, J. and Lenormand, P. (2008). Single and combined silencing of ERK1 and ERK2 reveals their positive contribution to growth signaling depending on their expression levels. Mol. Cell. Biol. 28, 511-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leicht, D. T., Balan, V., Kaplun, A., Singh-Gupta, V., Kaplun, L., Dobson, M. and Tzivion, G. (2007). Raf kinases: function, regulation and role in human cancer. Biochim. Biophys. Acta 1773, 1196-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., Chong, H. and Guan, K. L. (2001). Function of the Rho family GTPases in Ras-stimulated Raf activation. J. Biol. Chem. 276, 34728-34737. [DOI] [PubMed] [Google Scholar]
- Liu, Y. and Senger, D. R. (2004). Matrix-specific activation of Src and Rho initiates capillary morphogenesis of endothelial cells. FASEB J. 18, 457-468. [DOI] [PubMed] [Google Scholar]
- Lubarsky, B. and Krasnow, M. A. (2003). Tube morphogenesis: making and shaping biological tubes. Cell 112, 19-28. [DOI] [PubMed] [Google Scholar]
- Marais, R., Light, Y., Paterson, H. F. and Marshall, C. J. (1995). Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 14, 3136-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikula, M., Schreiber, M., Husak, Z., Kucerova, L., Ruth, J., Wieser, R., Zatloukal, K., Beug, H., Wagner, E. F. and Baccarini, M. (2001). Embryonic lethality and fetal liver apoptosis in mice lacking the c-raf-1 gene. EMBO J. 20, 1952-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano, R. and Orci, L. (1985). Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell 42, 469-477. [DOI] [PubMed] [Google Scholar]
- Montesano, R., Pepper, M. S., Vassalli, J. D. and Orci, L. (1987). Phorbol ester induces cultured endothelial cells to invade a fibrin matrix in the presence of fibrinolytic inhibitors. J. Cell Physiol. 132, 509-516. [DOI] [PubMed] [Google Scholar]
- Morris, P. B., Hida, T., Blackshear, P. J., Klintworth, G. K. and Swain, J. L. (1988). Tumor-promoting phorbol esters induce angiogenesis in vivo. Am. J. Physiol. 254, C318-C322. [DOI] [PubMed] [Google Scholar]
- Morrison, D. K. and Cutler, R. E. (1997). The complexity of Raf-1 regulation. Curr. Opin. Cell Biol. 9, 174-179. [DOI] [PubMed] [Google Scholar]
- Nomura, N., Nomura, M., Sugiyama, K. and Hamada, J. (2007). Src regulates phorbol 12-myristate 13-acetate-activated PKC-induced migration via Cas/Crk/Rac1 signaling pathway in glioblastoma cells. Int. J. Mol. Med. 20, 511-519. [PubMed] [Google Scholar]
- O'Brien, L. E., Tang, K., Kats, E. S., Schutz-Geschwender, A., Lipschutz, J. H. and Mostov, K. E. (2004). ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Dev. Cell 7, 21-32. [DOI] [PubMed] [Google Scholar]
- Okhrimenko, H., Lu, W., Xiang, C., Hamburger, N., Kazimirsky, G. and Brodie, C. (2005). Protein kinase C-epsilon regulates the apoptosis and survival of glioma cells. Cancer Res. 65, 7301-7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, L. H., Schmidt, M., Jin, S. W., Gray, A. M., Beis, D., Pham, T., Frantz, G., Palmieri, S., Hillan, K., Stainier, D. Y. et al. (2004). The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature 428, 754-758. [DOI] [PubMed] [Google Scholar]
- Parsons, S. J. and Parsons, J. T. (2004). Src family kinases, key regulators of signal transduction. Oncogene 23, 7906-7909. [DOI] [PubMed] [Google Scholar]
- Pirruccello, M., Sondermann, H., Pelton, J. G., Pellicena, P., Hoelz, A., Chernoff, J., Wemmer, D. E. and Kuriyan, J. (2006). A dimeric kinase assembly underlying autophosphorylation in the p21 activated kinases. J. Mol. Biol. 361, 312-326. [DOI] [PubMed] [Google Scholar]
- Playford, M. P. and Schaller, M. D. (2004). The interplay between Src and integrins in normal and tumor biology. Oncogene 23, 7928-7946. [DOI] [PubMed] [Google Scholar]
- Renkema, G. H., Pulkkinen, K. and Saksela, K. (2002). Cdc42/Rac1-mediated activation primes PAK2 for superactivation by tyrosine phosphorylation. Mol. Cell. Biol. 22, 6719-6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A. J. (2001). Rho proteins: linking signaling with membrane trafficking. Traffic 2, 303-310. [DOI] [PubMed] [Google Scholar]
- Roberts, P. J. and Der, C. J. (2007). Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26, 3291-3310. [DOI] [PubMed] [Google Scholar]
- Robles, E., Woo, S. and Gomez, T. M. (2005). Src-dependent tyrosine phosphorylation at the tips of growth cone filopodia promotes extension. J. Neurosci. 25, 7669-7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski, R., Jr (2004). Src protein-tyrosine kinase structure and regulation. Biochem. Biophys. Res. Commun. 324, 1155-1164. [DOI] [PubMed] [Google Scholar]
- Roskoski, R., Jr (2005). Src kinase regulation by phosphorylation and dephosphorylation. Biochem. Biophys. Res. Commun. 331, 1-14. [DOI] [PubMed] [Google Scholar]
- Saunders, W. B., Bayless, K. J. and Davis, G. E. (2005). MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J. Cell Sci. 118, 2325-2340. [DOI] [PubMed] [Google Scholar]
- Schwartz, M. (2004). Rho signalling at a glance. J. Cell Sci. 117, 5457-5458. [DOI] [PubMed] [Google Scholar]
- Tang, X., Feng, Y. and Ye, K. (2007). Src-family tyrosine kinase fyn phosphorylates phosphatidylinositol 3-kinase enhancer-activating Akt, preventing its apoptotic cleavage and promoting cell survival. Cell Death Differ. 14, 368-377. [DOI] [PubMed] [Google Scholar]
- Tatin, F., Varon, C., Genot, E. and Moreau, V. (2006). A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J. Cell Sci. 119, 769-781. [DOI] [PubMed] [Google Scholar]
- Thomas, S. M. and Brugge, J. S. (1997). Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13, 513-609. [DOI] [PubMed] [Google Scholar]
- Timpson, P., Jones, G. E., Frame, M. C. and Brunton, V. G. (2001). Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr. Biol. 11, 1836-1846. [DOI] [PubMed] [Google Scholar]
- Trakul, N., Menard, R. E., Schade, G. R., Qian, Z. and Rosner, M. R. (2005). Raf kinase inhibitory protein regulates Raf-1 but not B-Raf kinase activation. J. Biol. Chem. 280, 24931-24940. [DOI] [PubMed] [Google Scholar]
- Tsuda, S., Ohtsuru, A., Yamashita, S., Kanetake, H. and Kanda, S. (2002). Role of c-Fyn in FGF-2-mediated tube-like structure formation by murine brain capillary endothelial cells. Biochem. Biophys. Res. Commun. 290, 1354-1360. [DOI] [PubMed] [Google Scholar]
- Ueffing, M., Lovric, J., Philipp, A., Mischak, H. and Kolch, W. (1997). Protein kinase C-epsilon associates with the Raf-1 kinase and induces the production of growth factors that stimulate Raf-1 activity. Oncogene 15, 2921-2927. [DOI] [PubMed] [Google Scholar]
- Wan, P. T., Garnett, M. J., Roe, S. M., Lee, S., Niculescu-Duvaz, D., Good, V. M., Jones, C. M., Marshall, C. J., Springer, C. J., Barford, D. et al. (2004). Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116, 855-867. [DOI] [PubMed] [Google Scholar]
- Wellbrock, C., Karasarides, M. and Marais, R. (2004). The RAF proteins take centre stage. Nat. Rev. Mol. Cell. Biol. 5, 875-885. [DOI] [PubMed] [Google Scholar]
- Werdich, X. Q. and Penn, J. S. (2005). Src, Fyn and Yes play differential roles in VEGF-mediated endothelial cell events. Angiogenesis 8, 315-326. [DOI] [PubMed] [Google Scholar]
- Werdich, X. Q. and Penn, J. S. (2006). Specific involvement of SRC family kinase activation in the pathogenesis of retinal neovascularization. Invest. Ophthalmol. Vis. Sci. 47, 5047-5056. [DOI] [PubMed] [Google Scholar]
- Whitehead, K. J., Chan, A. C., Navankasattusas, S., Koh, W., London, N. R., Ling, J., Mayo, A. H., Drakos, S. G., Marchuk, D. A., Davis, G. E. and Li, D. Y. (2009). The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat. Med. 15, 177-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnowski, L., Zimmer, A. M., Beck, T. W., Hahn, H., Bernal, R., Rapp, U. R. and Zimmer, A. (1997). Endothelial apoptosis in Braf-deficient mice. Nat. Genet. 16, 293-297. [DOI] [PubMed] [Google Scholar]
- Zebisch, A., Czernilofsky, A. P., Keri, G., Smigelskaite, J., Sill, H. and Troppmair, J. (2007). Signaling through RAS-RAF-MEK-ERK: from basics to bedside. Curr. Med. Chem. 14, 601-623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.