Summary
Directional motility is a complex process requiring the spatiotemporal integration of signals that regulate cytoskeletal changes, and the establishment of an anteroposterior or polarized cell axis. Focal adhesion kinase (FAK) promotes cell migration, but a molecular role for FAK in promoting cell polarity remains undefined. Here, using wound healing and Golgi-reorientation analyses, we show that fibroblast, endothelial and carcinoma polarity during cell migration requires FAK and is associated with a complex between FAK, p120RasGAP and p190RhoGAP (p190A), leading to p190A tyrosine phosphorylation. Fibronectin-integrin-mediated FAK activation and phosphorylation promote SH2-mediated binding of p120RasGAP to FAK and FAK-mediated p190A tyrosine phosphorylation. The association of p120RasGAP with FAK facilitates the formation of a FAK-p120RasGAP-p190A complex targeted to leading-edge focal adhesions by FAK. Knockdown of p120RasGAP, mutation of FAK Y397 or inhibition of FAK activity prevent the association of FAK with p190A and subsequent tyrosine phosphorylation of p190A, and result in the loss of cell polarity. Because reconstitution of FAK-null fibroblasts with FAK or a Pyk2-FAK chimera restore the normal decrease in RhoA GTP binding upon cell spreading on fibronectin, our studies support a model whereby FAK activity facilitates the recruitment and stabilization of a p120RasGAP-p190A complex at leading-edge focal adhesions connected to the transient inhibition of RhoA activity and the regulation of cell polarity.
Keywords: FAK, p190RhoGAP, p120RasGAP, Cell motility, Cell polarity
Introduction
Directional cell motility is important in physiological and pathological processes such as embryonic development, angiogenesis, wound repair, tumor invasion and metastasis (Ridley et al., 2003). The spatial and temporal formation of cell protrusions and focal adhesions (FAs) at the leading edge, reorientation of the Golgi and microtubule-organizing center, and coordination of FA turnover in trailing cell regions are required for efficient directional cell movement (Vicente-Manzanares et al., 2005). These processes are associated with the acquisition of anteroposterior polarized cell morphology. The best characterized network controlling cell polarity are the Par proteins, which are localized asymmetrically in cells and act in part by regulating small Rho-family GTPase activity to modulate cytoskeletal structures (Hall, 2005). Studies have connected Par3 and Par6 to changes in Rho-family GTPase activity within cellular protrusions (Goldstein and Macara, 2007).
Rho GTPases are held in balance by the opposing action of guanine nucleotide exchange factors (GEFs), which facilitate GTP loading, and GTPase-activating proteins (GAPs), which promote Rho inactivation by stimulating intrinsic GTP hydrolysis (Jaffe and Hall, 2005). The p190RhoGAP proteins, termed p190A (encoded by RASGRF1) and p190B, inhibit RhoA activity in cells (Bernards and Settleman, 2004). p190A tyrosine phosphorylation is associated with p190A activation (Rho inhibition) and binding to p120RasGAP (a GAP for Ras; encoded by RASA1) (McGlade et al., 1993). The p120RasGAP-p190A interaction is mediated by binding of the p120RasGAP Src homology (SH)2 domain to either phosphorylated p190A Y1087 or Y1105 (Hu and Settleman, 1997). The p120RasGAP-p190A complex is linked to changes in actin-cytoskeleton structures and directed cell movement (Arthur and Burridge, 2001; Kulkarni et al., 2000). Recent studies showed that Par6 regulates dendritic-spine polarity via p190A-associated RhoA inhibition (Zhang and Macara, 2008) and that p190A-null fibroblasts exhibit defects in directional motility (Jiang et al., 2008). Because serine phosphorylation of p190A is associated with reduced p190A activity and is required for polarized cell movement (Jiang et al., 2008), it is likely that both positive and negative p190A regulatory events contribute to directional motility via effects on RhoGTPase activity.
Src-family protein-tyrosine kinase (PTK) activation facilitates p190A tyrosine phosphorylation (Chang et al., 1995; Fincham et al., 1999) and is associated with RhoA inhibition during cell spreading on fibronectin (FN) (Arthur et al., 2000). Arg PTKs can also phosphorylate p190A (Hernandez et al., 2004) and Arg activity promotes p190A membrane recruitment to attenuate actomyosin contractility (Bradley et al., 2006; Peacock et al., 2007). Analyses of mouse embryonic fibroblasts (MEFs) that are null for Src-family or Arg PTKs showed that p190A tyrosine phosphorylation is reduced but not absent in these MEFs (Arthur et al., 2000; Hernandez et al., 2004); this indicate a potential role for another integrin-associated PTK in p190A phosphorylation upon cell binding to FN.
Focal adhesion kinase (FAK) is a cytoplasmic PTK recruited to and activated at sites of integrin-receptor binding to FN at FAs (Schlaepfer et al., 2004). FAK acts as an important regulator of FN-stimulated Src-PTK activation and cell motility (Mitra and Schlaepfer, 2006; Wu et al., 2008). FAK-null MEFs exhibit defects in directional motility, microtubule polarization, RhoGTPase regulation and FA turnover that are rescued by FAK re-expression (Mitra et al., 2005). Notably, inactivation of β1 integrin in keratinocytes is associated with reduced FAK phosphorylation, elevated RhoA activity and altered cell polarity (Raghavan et al., 2003). However, the FAK targets involved in promoting polarized cell motility remain undefined.
Herein, we show that FAK expression and activity are required for FN-stimulated p190A tyrosine phosphorylation and that this linkage between FAK and p190A is important in the regulation of cell polarity in MEFs, endothelial cells and colon carcinoma cells. Knockdown of p120RasGAP disrupts the linkage between FAK and p190A, and we found that the SH2-SH3-SH2 region of p120RasGAP binds FAK and p190A and might bridge both proteins. In Src-transformed MEFs, in which p190A tyrosine phosphorylation is elevated, FAK is required for cell polarization and the recruitment of p190A to leading-edge FAs. Because overexpression of phosphorylation-site-mutated (Y1087F and Y1105F) or GAP-inactive (R1283A) p190A proteins block cell polarity and do not localize to FAs, our studies support a role for FAK as a key mediator of p190A localization and tyrosine phosphorylation connected to the regulation of polarized cell motility.
Results
Cell polarity and p190A tyrosine phosphorylation require FAK
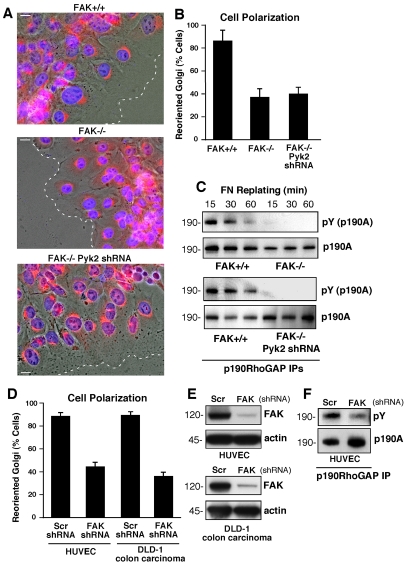
FAK–/– MEFs exhibit motility defects (Ilic et al., 1995) and FAK re-expression facilitates directionally persistent cell migration that is characterized by the formation of stable lamellipodia (Owen et al., 2007; Sieg et al., 1999; Tilghman et al., 2005; Wang et al., 2001). However, the targets of FAK action in promoting directional motility or cell polarity remain undefined. Expression of the FAK-related PTK proline-rich kinase 2 (Pyk2) is elevated in FAK–/– MEFs and we recently showed that Pyk2 knockdown reversed the morphological but not motility defects of FAK–/– MEFs (Lim Y. et al., 2008). To test the hypothesis that FAK–/– MEF motility defects arise in part because of an inability to establish an axis of cell polarity, scratch wound-healing assays with FAK+/+ MEFs, FAK–/– MEFs and FAK–/– MEFs transfected with Pyk2 short hairpin RNA (shRNA) (referred to hereafter as FAK–/– Pyk2-shRNA MEFs) were analyzed for Golgi-marker orientation in MEFs lining the wound edge after 4 hours (Fig. 1A). In unwounded cell populations, Golgi orientation within cells is randomly distributed. Upon creating a wound edge and allowing for initial cell movement within the denuded space, cells will establish an axis of polarity, with microtubules and Golgi markers facing the wound edge (Kodama et al., 2004; Ridley et al., 2003). Whereas >80% of FAK+/+ MEFs at the wound edge exhibited Golgi reorientation, less than 40% of FAK–/– or FAK–/– Pyk2-shRNA MEFs exhibited Golgi polarization towards the wound edge (Fig. 1A,B). These results show that FAK–/– MEFs exhibit polarity defects and that Pyk2 expression within FAK–/– MEFs is not causing this phenotype.
Fig. 1.
FAK promotes cell polarity and is required for p190A tyrosine phosphorylation after FN plating. (A) FAK+/+, FAK–/– and FAK–/– Pyk2-shRNA MEFs were grown on FN-coated coverslips. Cells were wounded and allowed to migrate in the presence of serum for 4 hours. Cells were fixed, imaged in phase, and stained for Golgi (β-Cop, red) and nuclear (Hoechst, blue) markers. The position of the leading lamella (broken line) is indicated. Scale bars: 15 μm. (B) Golgi reorientation analyses, a square was drawn over cell nuclei at the wound edge and divided into quadrants. Reoriented Golgi was scored positive by β-Cop staining entirely within the quadrant facing the leading edge. In total, 100 cells were analyzed; data is percent of cells analyzed ± s.d. (C) p190A immunoprecipitations (IPs) were made from FAK–/–, FAK+/+ and FAK–/– Pyk2-shRNA lysates of serum-starved cells held in suspension for 1 hour and replated onto FN-coated dishes for the indicated times. p190A IPs were sequentially analyzed by anti-phosphotyrosine (pY) and anti-p190A immunoblotting. (D) HUVEC and DLD-1 carcinoma cells expressing scrambled (Scr) or anti-FAK shRNA were analyzed for Golgi reorientation after scratch wounding as above. In total, 100 cells lining the wound edge were scored (± s.d.). (E) FAK expression is reduced by anti-FAK compared with Scr shRNA as determined by immunoblotting. (F) p190A IPs from lysates from HUVECs expressing Scr or anti-FAK shRNA that were replated onto FN-coated dishes (30 minutes) and analyzed by anti-phosphotyrosine (pY) and anti-p190A immunoblotting.
Previous studies have identified p190A-mediated inhibition of RhoA as an important regulator of fibroblast spreading and polarity (Arthur and Burridge, 2001). This is mediated in part by FN-stimulated Src-PTK phosphorylation of p190A (Arthur et al., 2000). Because RhoA activity is elevated in FAK–/– compared with FAK+/+ MEFs (Ren et al., 2000), p190A tyrosine phosphorylation was analyzed after FN replating of FAK+/+, FAK–/– and FAK–/– Pyk2-shRNA MEFs (Fig. 1C). Notably, p190A was tyrosine phosphorylated within 15 minutes after the FN replating of FAK+/+ MEFs, but not in MEFs lacking FAK and/or Pyk2. Additionally, knockdown of FAK with FAK shRNA within human umbilical vein endothelial cells (HUVECs) and DLD-1 colon carcinoma cells suppressed Golgi polarization in scratch wound-healing assays (Fig. 1D,E) and was associated with reduced p190A tyrosine phosphorylation upon HUVEC binding to FN (Fig. 1F). These results support a role for FAK in promoting cell polarity in part via enhanced p190A tyrosine phosphorylation in multiple cell types.
Importance of p120RasGAP for cell polarity and for formation of the p190A-FAK complex
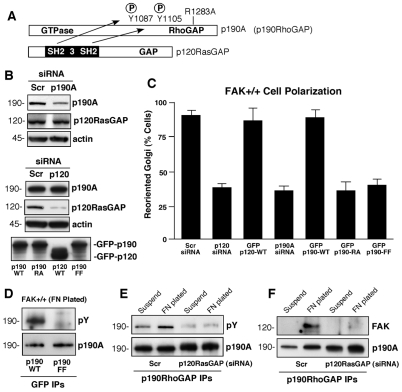
p190A tyrosine phosphorylation at Y1087 or Y1105 creates SH2-domain-binding sites and facilitates the association of p120RasGAP with p190A (Fig. 2A) (Hu and Settleman, 1997). Previous studies found that p120RasGAP–/– MEFs exhibit reduced p190A tyrosine phosphorylation and have defects in directional motility (Kulkarni et al., 2000; van der Geer et al., 1997). Because p190A–/– MEFs exhibit polarity defects (Jiang et al., 2008), transient siRNA-mediated knockdown of either p120RasGAP or p190A in MEFs (Fig. 2B) confirmed the importance of these proteins in promoting polarity as determined by Golgi-reorientation assays (Fig. 2C). Interestingly, overexpression of wild-type (WT) green fluorescent protein (GFP) fusion proteins of p120RasGAP or p190A had no effect on MEF polarization, whereas overexpression of GAP-inactive (R1283A) p190A or phosphorylation-site-mutated (Y1087F and Y1105F) p190A prevented MEF polarization (Fig. 2B,C). Because Y1087F and Y1105F mutations block p190A tyrosine phosphorylation after FN replating (Fig. 2D), these results support the notion that both intrinsic p190A GAP activity (needed for Rho inhibition) and p190A tyrosine phosphorylation are important in promoting cell polarity.
Fig. 2.
p120RasGAP promotes FN-stimulated p190A tyrosine phosphorylation, FAK association with p190A and cell polarity. (A) Schematic of the p190A and p120RasGAP proteins, and p120RasGAP SH2-mediated binding to phosphorylated Y1087 and Y1105 in p190A. R1283A (RA) mutation disrupts p190A GAP activity. (B) Scrambled (Scr)-, p190A- or p120RasGAP-siRNA transfection of MEFs and the associated protein levels after 48 hours. Actin levels were used as a control. Anti-GFP immunoblotting was used to confirm transient GFP–p190-WT, GFP–p190-RA, GFP–190-FF (Y1087F, Y1105F) and GFP-p120RasGAP expression. (C) Golgi reorientation after scratch wounding was performed with Scr p190A siRNA or p120RasGAP siRNA with siGlo co-transfection as a marker to detect transfected MEFs. Data is the percentage of 100 cells analyzed and is combined with similar analyses of MEFs transfected with GFP-p120RasGAP, GFP-p190A-WT, GFP-p190A-RA, or GFP-p190A-FF. Values are from 100 cells ± s.d. (D) Mutation of p190A Y1087F and Y1105F (p190A-FF) blocks FN-stimulated tyrosine phosphorylation as determined by transfection, anti-GFP-tag immunoprecipitation (IP), and sequential anti-pY and anti-HA immunoblotting. (E) p120RasGAP siRNA-knockdown disrupts p190A tyrosine phosphorylation. Lysates from MEFs in suspension or FN plated (30 minutes) were analyzed by anti-p190A IPs followed by anti-pY and anti-p190A immunoblotting. (F) p120RasGAP siRNA-knockdown disrupts FAK-p190A association upon FN plating. Lysates from MEFs in suspension or FN plated (30 minutes) were analyzed by anti-p190A IPs followed by anti-FAK and anti-p190A immunoblotting.
The binding of p120RasGAP to tyrosine-phosphorylated p190A protects p190A from dephosphorylation (Hu and Settleman, 1997). Accordingly, p120RasGAP siRNA knockdown in MEFs inhibited p190A tyrosine phosphorylation upon FN plating (Fig. 2E). Notably, FAK associated with p190A in an FN-adhesion-dependent manner (Fig. 2F), which is consistent with FAK-p190A association after thrombin stimulation of endothelial cells (Holinstat et al., 2006). Although recombinant FAK can phosphorylate p190A in vitro (Holinstat et al., 2006), p120RasGAP-knockdown prevented FAK association with p190A upon adhesion to FN (Fig. 2F). These results suggest that the FAK-p190A association might not be direct. Instead, our results support the notion that p120RasGAP facilitates the association between FAK and p190A, thus enabling FAK-enhanced p190A tyrosine phosphorylation.
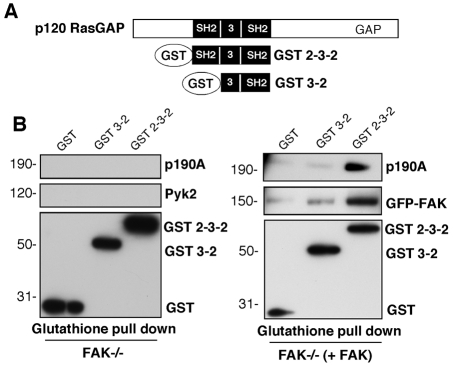
p120RasGAP might bridge FAK and p190A
p120RasGAP can bind to phosphorylated FAK in an SH2-dependent manner (Hecker et al., 2004). Because the p120RasGAP SH2 domains also bind to p190A (Hu and Settleman, 1997), we tested whether expression of the p120RasGAP SH2-SH3-SH2 (2-3-2) region is sufficient to facilitate a complex between FAK and p190A. Using a mammalian glutathione-S-transferase (GST) expression vector, the p120RasGAP 2-3-2 or 3-2 domains (Fig. 3A) were transiently expressed in FAK–/– MEFs with or without GFP-FAK coexpression (Fig. 3B). Glutathione-agarose pulldowns of FN-plated FAK–/– cells did not detect binding of endogenous Pyk2 or p190A to GST–2-3-2. However, upon FAK coexpression, both FAK and p190A associated with GST–2-3-2 and only weak binding of FAK to GST–3-2 was detected (Fig. 3B). These results indicate that either the N-terminal p120RasGAP SH2 domain binds to both FAK and p190A or that the 2-3-2 region of p120RasGAP acts as a bridge between FAK and p190A. The linkage hypothesis is supported by the fact that the N-terminal SH2 domain binds strongly to FAK (Hecker et al., 2004) and the C-terminal SH2 domain binds strongly to p190A (Hu and Settleman, 1997). Combined with the fact that p120RasGAP-knockdown prevents FAK-p190A association (Fig. 2F) and that the p120RasGAP 2-3-2 region binds synergistically to phosphorylated p190A (Bryant et al., 1995), our results support the notion that FAK facilitates the formation of a multi-protein p120RasGAP-p190A complex that is important in promoting cell polarity.
Fig. 3.
The p120RasGAP SH2-SH3-SH2 (2-3-2) region bridges FAK and p190A. (A) Schematic representation of the mammalian GST expression vectors of GST–2-3-2 and GST–3-2 of p120RasGAP. (B) Coexpression of GST–2-3-2 and FAK facilitate the formation of a complex with p190A. FAK–/– MEFs were transiently transfected with the indicated GST constructs (left) or GST constructs plus GFP-FAK (right), plated (30 minutes) onto FN-coated dishes and lysates were analyzed by glutathione-agarose bead pull down. Association of endogenous Pyk2, p190A or GFP-FAK was determined by immunoblotting.
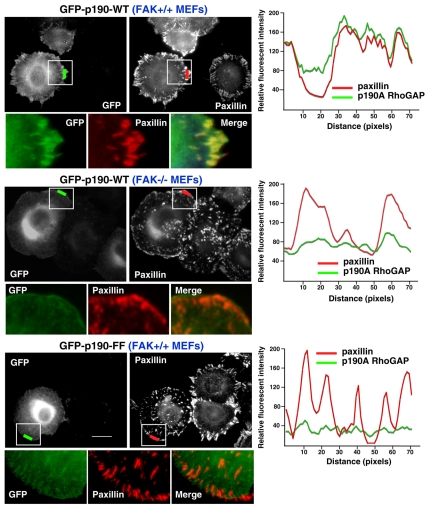
FAK is required for p190A localization to focal adhesions
Because FAK associates with p190A upon FN plating, we investigated where this complex is formed within cells. Previous studies have documented p190A localization to leading lamella in spreading cells (Bradley et al., 2006), and this localization is regulated in part by engagement of the heparan-sulfate proteoglycan syndecan-4 and FN-binding integrins (Bass et al., 2008). To determine p190A localization independent of antibody binding, GFP-190A was transfected into MEFs and the cells were plated onto FN for 30 minutes, the time of maximal FAK-dependent p190A tyrosine phosphorylation (Fig. 4). A strong co-distribution of GFP-p190A and paxillin staining at leading-edge FAs was observed in FAK+/+ MEFs but not FAK–/– MEFs. This result supports the importance of FAK in the recruitment of p190A to FAs. Importantly, expression of GFP–190A-FF exhibited a perinuclear distribution and did not colocalize with paxillin at FAs upon FN plating (Fig. 4). These results show that FAK expression and p190A tyrosine phosphorylation are important in the recruitment of p190A to FAs upon cell adhesion to FN.
Fig. 4.
FAK and p190A tyrosine phosphorylation promote p190A FA localization upon FN plating. FAK+/+ or FAK–/– MEFs were transfected with GFP–p190-WT or GFP–p190-FF, serum starved, plated onto FN (10 μg/ml, 30 minutes), and analyzed for GFP and paxillin colocalization. Fluorescence-intensity profiles represent the area marked by the colored lines (green p190A, red paxillin). Insets, enlargement of the area of interest shown for GFP-p190A, paxillin and the merged images. GFP–p190-WT and paxillin show colocalization at peripheral FAs in FAK+/+ but not in FAK–/– MEFs as determined by fluorescent-intensity overlap. The GFP–p190-FF fluorescent-intensity profile does not overlap with paxillin at FAs. Scale bar: 20 μm.
FAK inhibition blocks MEF polarization and p190A tyrosine phosphorylation
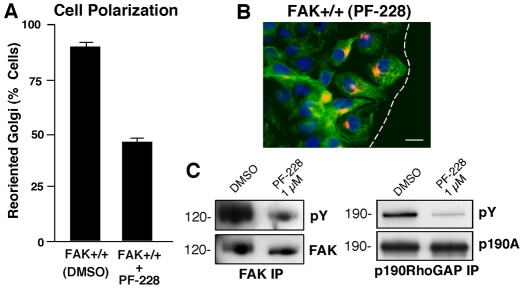
To determine the role of FAK activity in promoting directional motility, scratch wound-healing assays were performed in the presence of a FAK-specific ATP-competitive inhibitor (PF-228) (Slack-Davis et al., 2007). At 1 μM, PF-228 inhibited Golgi reorientation (Fig. 5A) without affecting overall lamellipodial spreading at the wound edge (Fig. 5B). PF-228 inhibited both FAK and p190A tyrosine phosphorylation upon FN plating of MEFs (Fig. 5C). Importantly, when used at 1 μM, PF-228 is highly specific for FAK (Src and Abl are not inhibited at this concentration) and PF-228 blocks MEF motility, but not proliferation (Slack-Davis et al., 2007). Our results support the importance of FAK activity in promoting p190A tyrosine phosphorylation and cell polarity, and in contributing to efficient directional cell migration.
Fig. 5.
Pharmacological FAK inhibition blocks MEF polarization and p190A tyrosine phosphorylation. (A) Golgi-reorientation analyses of scratch-wounded FAK+/+ MEFs performed in the presence of DMSO or 1 μM PF-228 (FAK inhibitor). Data is the percentage of 100 cells with Golgi that were reoriented towards the wound edge (± s.d.). (B) Representative image from a wound healing assay. MEFs were stained with β-Cop antibody (red), anti-tubulin antibody (green) and Hoechst nuclear dye (blue). The position of leading lamella (broken white line) is shown. Scale bar: 30 μm. (C) FAK and p190A IPs from FN-plated MEFs (30 minutes) in the presence of DMSO or PF-228 (1 μM) were sequentially analyzed by anti-pY followed by anti-FAK or anti-p190A immunoblotting, respectively.
Src-transformed FAK–/– MEFs exhibit polarity defects
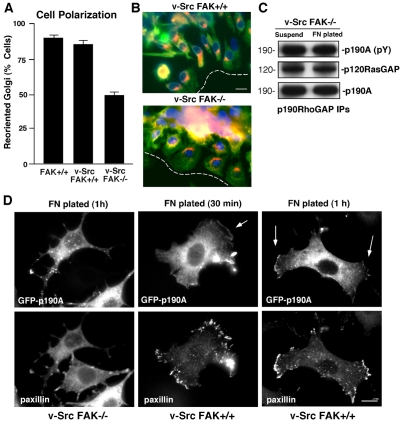
Transformation of MEFs by activated Src promotes p190A tyrosine phosphorylation, p120RasGAP binding to p190A and cytoskeletal changes associated with RhoA inhibition (Fincham et al., 1999). Src transformation of FAK–/– MEFs increases cell motility on FN (Moissoglu and Gelman, 2003) and p190A tyrosine phosphorylation (Hsia et al., 2003). However, Src-transformed FAK–/– (Src FAK–/–) MEFs did not respond equivalently to normal FAK-reconstituted MEFs in wound-healing assays, and time-lapse analyses revealed a lack of directionality associated with Src FAK–/– MEF motility (Hsia et al., 2003). Interestingly, Golgi polarization was deficient in Src FAK–/– compared with Src FAK+/+ MEFs (Fig. 6A,B) under conditions in which p190A was tyrosine phosphorylated and associated with p120RasGAP in Src FAK–/– MEFs (Fig. 6C). These results support the notion that Src-mediated p190A phosphorylation and complex formation with p120RasGAP is not sufficient to promote cell polarity in the absence of FAK.
Fig. 6.
FAK is required for Src-transformed MEF polarity independent of p190A tyrosine phosphorylation. (A) Scratch-wound-healing Golgi-reorientation analyses performed with normal FAK+/+ or Src-transformed FAK+/+ and FAK–/– MEFs. Data is the percentage of 100 cells with Golgi that were reoriented towards the wound edge (± s.d.). Representative images from a wound healing assay. Src FAK+/+ and Src FAK–/– MEFs were stained with β-Cop antibody (red), anti-tubulin antibody (green) and Hoechst nuclear dye (blue). The position of leading lamella (broken white line) is shown. Scale bar: 30 μm. (C) Src promotes adhesion-independent and FAK-independent p190A tyrosine phosphorylation. p190A IPs from suspended or FN-replated Src FAK–/– MEF lysates analyzed for p190A tyrosine phosphorylation and p120RasGAP association by anti-pY, anti-p120RasGAP and anti-p190A immunoblotting. (D) FAK facilitates p190A membrane and FA localization upon Src MEF adhesion to FN. Src FAK–/– or Src FAK+/+ MEFs were transfected with GFP–p190A-WT and plated onto FN-coated coverslips for 30 minutes or 1 hour as indicated. Cells were imaged for GFP-p190A and co-stained for paxillin localization (rhodamine). Arrows show the localization of p190A at membrane projections at 30 minutes and at peripheral FAs at 1 hour in Src FAK+/+ MEFs. GFP-p190A exhibits a punctate distribution in Src FAK–/– MEFs. Scale bar: 10 μm.
FAK localizes p190A to cell protrusions in Src-transformed MEFs
Leading- and/or trailing-edge cell protrusions are common sites of cell-polarity-signal generation (Goldstein and Macara, 2007; Ridley et al., 2003). To determine whether FAK influences p190A localization in Src-transformed MEFs, GFP-p190A was transiently expressed and cells were plated onto FN-coated slides for 30 minutes or 1 hour (Fig. 6D). In Src-transformed FAK–/– (Src FAK–/–) MEFs, GFP-p190A exhibited a punctate and uneven distribution and was associated with the generation of multiple large filopodia-like projections. In Src FAK+/+ MEFs, GFP-p190A was detected at larger lamellipodia-like cell protrusions within 30 minutes (Fig. 6D, arrow) and, at 1 hour, GFP-p190A colocalized with paxillin at peripheral FAs (Fig. 6D, arrows). Taken together, these results show that FAK expression and activity are key events in coordinating FA recruitment and p190A tyrosine phosphorylation, which are involved in promoting cell polarity.
Pyk2 that is targeted to FAs can substitute for FAK in promoting MEF polarity, p190A tyrosine phosphorylation and RhoA regulation
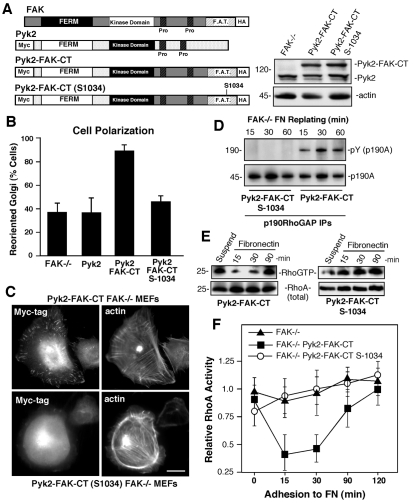
We previously showed that FAK reconstitution of FAK–/– MEFs restored normal RhoA regulation and cell motility upon FN plating (Ren et al., 2000). Although FAK-related Pyk2 expression is elevated in FAK–/– MEFs, Pyk2 remains localized perinuclearly upon FN plating (Klingbeil et al., 2001). We previously determined that, if Pyk2 was targeted to FAs by fusion of the N-terminal and kinase regions to the FAK C-terminal domain (Pyk2-FAK-CT), that this could also rescue FAK–/– motility defects (Klingbeil et al., 2001). FAK-CT encompasses the FA-targeting (FAT) region, and mutation of L1034 in the FAK FAT domain to serine (S1034) disrupts paxillin binding (Fig. 7A) and association with FAs (Sieg et al., 1999).
Fig. 7.
Pyk2 that is targeted to FAs can substitute for FAK in promoting MEF polarity, p190A tyrosine phosphorylation and RhoA regulation. (A) Schematic representation of HA-FAK (murine), Myc-Pyk2 (human), Pyk2–FAK-CT (Pyk2 residues 1-682 including the kinase domain fused to FAK residues 680-1052) and Pyk2–FAK-CT-S1034 [containing a point mutation within the FAK FA-targeting (FAT) domain that disrupts paxillin binding]. Expression levels of endogenous Pyk2 and Pyk2–FAK-CT constructs within FAK–/– MEFs determined by anti-Pyk2 and anti-actin immunoblotting are shown on the right. (B) Scratch-wound-healing Golgi-reorientation analyses in FAK–/– MEFs and FAK–/– MEFs expressing Pyk2, Pyk2–FAK-CT or Pyk2–FAK-CT-S1034. Data is the percentage of 100 cells with Golgi that are reoriented towards the wound edge (± s.d.). (C) Disruption of Pyk2–FAK-CT FA localization that is associated with S1034 mutation. Indirect immunofluorescent localization of Pyk2–FAK-CT and Pyk2–FAK-CT-S1034 by anti-Myc-tag (fluorescein) and actin (phalloidin) staining of FN-plated (45 minutes) MEFs is shown. Scale bar: 10 μm. (D) The S1034 mutation disrupts Pyk2–FAK-CT-facilitated p190A tyrosine phosphorylation. p190A IPs, made from Pyk2–FAK-CT and Pyk2–FAK-CT-S1034 FAK–/– MEFs that were replated onto FN-coated dishes for the indicated times, were sequentially analyzed by anti-pY and anti-p190A blotting. (E) Pyk2–FAK-CT expression results in less Rho GTPase activation upon FN plating. GTP-bound RhoA levels were determined by affinity pulldown assays from lysates of suspended and FN-replated FAK–/– Pyk2–FAK-CT and FAK–/– Pyk2–FAK-CT-S1034 MEFs followed by blotting for total RhoA levels. (F) Quantitation of FN-associated RhoA-GTP binding. Data is plotted as relative RhoA-GTP levels in suspended FAK–/– MEFs and is the mean of two independent experiments. Error bars show ± s.d.
FAK–/– MEFs stably expressing equal levels of Myc-tagged Pyk2, Pyk2–FAK-CT or Pyk2–FAK-CT-S1034 were evaluated for Golgi reorientation in wound healing assays (Fig. 7B). Only Pyk2–FAK-CT reversed the cell-polarity defects of FAK–/– MEFs and this parallels the ability of Pyk2–FAK-CT to promote FAK–/– MEF motility. Pyk2–FAK-CT was recruited to FAs upon FN plating, whereas Pyk2–FAK-CT-S1034 was cytoplasmically distributed within FAK–/– MEFs (Fig. 7C). Pyk2–FAK-CT also promoted p190A tyrosine phosphorylation upon FN plating (Fig. 7D) and this was associated with low levels of RhoA GTP binding in the early stages of FN-stimulated cell spreading (Fig. 7E,F). By contrast, Pyk2–FAK-CT-S1034 expression did not promote p190A tyrosine phosphorylation (Fig. 7D) nor did it lead to RhoA inhibition upon FN plating of FAK–/– MEFs (Fig. 7E,F). Together, these results support the conclusion that the presence of FAK or Pyk2–FAK-CT at FAs is required for FN-stimulated p190A tyrosine phosphorylation leading to the regulation of RhoA GTPase activity, and that this is associated with signals that lead to MEF polarization.
Genetic support for the importance of FAK kinase activity and Y397 phosphorylation in promoting cell polarity
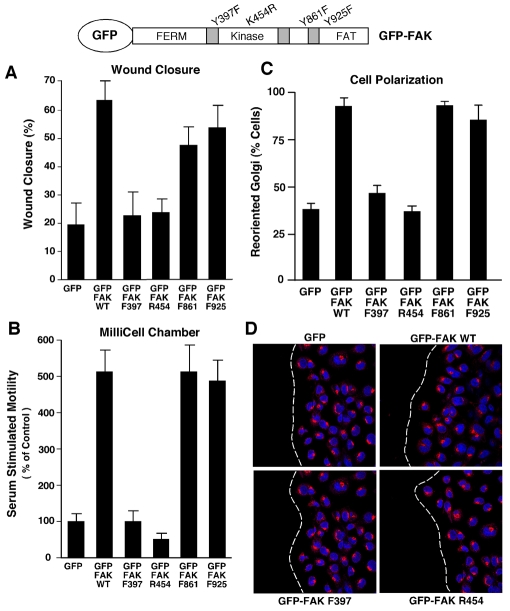
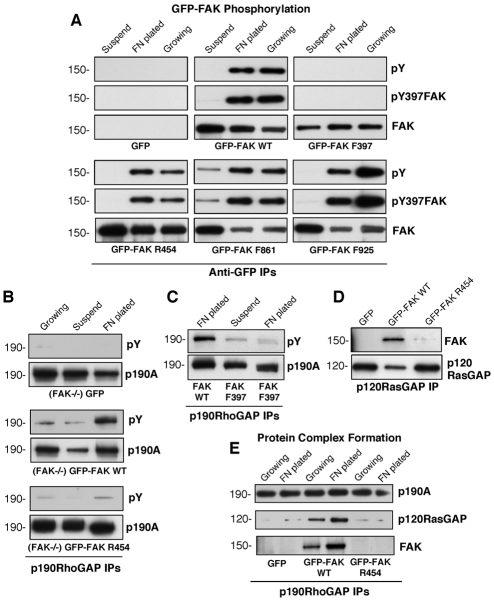
Few studies to date have reported the stable re-expression of various FAK mutants in FAK–/– MEFs. To identify the required signaling features of FAK that facilitate Golgi reorientation, GFP fusions of WT and various FAK point mutants were used to create stable GFP-FAK-reconstituted MEFs (Fig. 8). The panel of FAK mutants include: F397, a mutation of Y397 to phenylalanine (F), which eliminates the major FAK phosphorylation site and prevents binding of SH2-domain-containing proteins to this site; R454, a mutation of K454 to arginine (R), which creates a kinase-defective mutant; F861, a mutation of Y861 to F, which prevents phosphorylation; and F925, a mutation of Y925 to F, which prevents phosphorylation and binding of SH2-domain-containing proteins to this site (Schlaepfer et al., 2007). All GFP-FAK constructs were correctly targeted to FAs in the reconstituted MEFs (data not shown).
Fig. 8.
FAK Y397 phosphorylation and kinase activity are required for directional MEF motility and polarization. Schematic representation of mutations within GFP-FAK constructs used to reconstitute FAK–/– MEFs (top panel). (A) WT- and mutant-FAK-reconstituted MEFs were grown to confluence, serum starved for 16 hours, scratch-wounded using a pipette tip and allowed to migrate for 20 hours in the presence of serum. The percentage of wound closure was calculated from three independent experiments, presented ± s.d. (B) Directional motility of WT- and mutant-FAK-reconstituted MEFs was measured using FN-coated (10 μg/ml) Boyden-chamber (top and bottom) motility over 4 hours. Serum-containing media was added to the lower chamber. The number of motile cells (membrane underside) from three independent experiments is expressed as the percentage of FAK–/– GFP control (normalized to 100, ± s.d.). (C) Golgi-reorientation analyses of GFP-expressing and GFP-FAK-reconstituted FAK–/– MEFs. Data is the percentage of cells that had reoriented Golgi towards the wound edge (100 cells ± s.d.). (D) Representative images of scratch-wounded GFP-expressing or indicated GFP-FAK-reconstituted MEFs after 4 hours as analyzed by β-Cop Golgi (rhodamine; red) and Hoechst nuclear (blue) staining. The position of the leading lamella (broken white line) is shown.
To determine what signaling features of FAK were required for the rescue of FAK–/– MEF motility, wound closure (Fig. 8A) and serum-stimulated chamber (Fig. 8B) cell-migration assays were performed. GFP-expressing, GFP–FAK-F397-reconstituted and GFP–FAK-R454-reconstituted MEFs exhibited motility defects in both scratch-wound and chamber assays, at levels 2.5- to 5-fold less than GFP–FAK-WT-reconstituted MEFs (Fig. 8A,B). GFP-expressing, GFP–FAK-F397-reconstituted and GFP–FAK-R454-reconstituted MEFs also showed defective Golgi reorientation, with >50% of cells at the wound edge not being correctly polarized (Fig. 8C,D). By contrast, mutation of FAK Y861 and Y925 phosphorylation sites had only minor inhibitory effects in wound-healing cell movement and no significant differences in chamber-motility or cell-polarization analyses compared with FAK-WT-reconstituted MEFs (Fig. 8A-D). In cell-motility assays, there was no statistical difference between FAK–/– MEFs reconstituted with GFP–FAK-WT, GFP–FAK-F861 or GFP–FAK-F925 and FAK+/+ MEFs (data not shown). These results show that FAK activity and site-specific FAK phosphorylation at Y397 are required for efficient cell movement and polarity.
FAK activity is required for p190A tyrosine phosphorylation and the formation of a p190A-p120RasGAP-FAK complex
FAK phosphorylation is regulated in part by integrins and growth-factor stimuli (Mitra and Schlaepfer, 2006). To determine whether GFP-FAK regulation occurs normally in the reconstituted MEFs, WT and point-mutated GFP-FAK constructs were isolated from lysates prepared from adherent growing, suspended and FN-replated MEFs (Fig. 9A). GFP–FAK-WT was phosphorylated in FN-plated and growing MEFs but not in suspended cells. This is identical to endogenous FAK regulation. GFP–FAK-F861 and GFP–FAK-F925 showed similar regulation as WT FAK, whereas FAK-F397 was not detectably tyrosine phosphorylated. Interestingly, kinase-inactive GFP–FAK-R454 was phosphorylated at Y397 upon FN plating and in growing MEFs, and was dephosphorylated upon cell suspension (Fig. 9A). These results support previous studies showing that kinase-inactive FAK is a substrate for other tyrosine kinases (Wu et al., 2008).
Fig. 9.
FAK activity facilitates p190A tyrosine phosphorylation and complex formation with p120RasGAP and p190A. (A) Regulation of GFP-FAK phosphorylation in reconstituted FAK–/– MEFs. Lysates were made from starved cells held in suspension (1 hour), cells that were replated onto FN-coated dishes (45 minutes) and from growing cells. Anti-GFP IPs were sequentially analyzed by anti-pY, FAK pY397 phospho-specific and total FAK blotting. (B) p190A tyrosine phosphorylation is dependent upon FAK activity. p190A IPs were made from growing, suspended or FN-plated (30 minutes) FAK–/– MEFs expressing GFP or reconstituted with GFP–FAK-WT or GFP–FAK-R454 and analyzed by anti-pY followed by anti-p190A immunoblotting. (C) Mutation of FAK Y397 blocks p190A tyrosine phosphorylation. p190A IPs from suspended or FN-plated FAK–/– MEFs expressing GFP–FAK-WT or GFP–FAK-F397 were analyzed by anti-pY followed by anti-p190A immunoblotting. (D) FAK activity is required for p120RasGAP association. p120RasGAP IPs from FN-plated FAK–/– MEFs expressing GFP or reconstituted with GFP–FAK-WT or GFP–FAK-R454 were analyzed for FAK association by anti-FAK followed by anti-p120RasGAP immunoblotting. (E) p190A-p120RasGAP-FAK association is dependent upon FN-stimulated FAK activity. p190A IPs from growing or FN-plated FAK–/– MEFs expressing GFP or reconstituted with GFP–FAK-WT or GFP–FAK-R454 were analyzed for p120RasGAP and FAK association by sequential immunoblotting.
To determine a molecular connection between FAK re-expression and the re-establishment of FAK–/– MEF polarity, p190A immunoprecipitations (IPs) were performed with lysates from GFP-expressing or GFP-FAK-reconstituted MEFs (Fig. 9B). Enhanced p190A tyrosine phosphorylation was observed in GFP–FAK-WT MEFs plated on FN and this was dependent upon intrinsic FAK activity (Fig. 9B) and the integrity of the FAK Y397 phosphorylation site (Fig. 9C). Because equivalent p190A tyrosine phosphorylation to WT FAK was detected in lysates of F861 and F925 FAK-reconstituted MEFs (data not shown), these results establish a connection between FAK re-expression, FAK activity, p190A tyrosine phosphorylation and cell polarity.
To test the linkage between FAK and p190A, p120RasGAP coimmunoprecipitation assays were performed (Fig. 9D). Whereas WT FAK associated with p120RasGAP upon FN stimulation of cells, R454 FAK did not form a complex with p120RasGAP. Because R454 FAK is phosphorylated at the major Y397 site upon FN plating of MEFs (Fig. 9A), this result indicates that intrinsic FAK activity that is separate from FAK Y397 phosphorylation is required for association with p120RasGAP. F397 FAK also did not associate with p120RasGAP (data not shown). When p190A IPs were made from the same lysates, only WT FAK associated with p120RasGAP and p190A in a complex (Fig. 9E). R454 kinase-inactive FAK did not associate with p190A, and this is consistent with the lack of p190A tyrosine phosphorylation in R454 MEFs (Fig. 9B) and the lack of polarity of R454-FAK MEFs (Fig. 8C,D). Together, our studies show that FAK expression and activity facilitate the formation of a FAK-p120RasGAP-p190A complex that promotes p190A tyrosine phosphorylation downstream of integrins and acts to regulate cell polarity.
Discussion
Proper cell movement is a complex process that requires spatiotemporal integration of signals that regulate cytoskeletal changes as well as FA formation and turnover (Ridley et al., 2003). FAK is a cytoplasmic PTK that integrates and regulates motility and survival signals within cells (Lim S. T. et al., 2008). Although previous studies have linked FAK expression to increased directional persistence of cell movement (Gu et al., 1999; Li et al., 2002; Owen et al., 2007; Wang et al., 2001), the molecular mechanism(s) of FAK action in promoting cell polarity are not known. Here, we show that FAK generates polarity cues through the formation of a complex with p120RasGAP and p190RhoGAP (p190A) wherein p190A is tyrosine phosphorylated. Integrin-associated p190A tyrosine phosphorylation does not occur in the absence of FAK, and we found that FAK re-expression or an FA-associated Pyk2–FAK-CT chimera is sufficient to promote p190A tyrosine phosphorylation, rescue the polarity defects of FAK–/– MEFs and restore normal RhoA GTPase regulation upon cell engagement with FN. Our results support a model (Fig. 10) whereby integrin-mediated FAK activation and FAK Y397 phosphorylation promote the SH2-mediated binding of p120RasGAP to FAK, and subsequent p190A tyrosine phosphorylation. The association of p120RasGAP with p190A facilitates and most probably stabilizes the formation of a FAK-p120RasGAP-p190A complex at leading-edge FAs. Notably, knockdown of p120RasGAP, mutation of FAK Y397 or inhibition of FAK activity prevent FAK association with p190A and tyrosine phosphorylation of p190A, and result in the loss of cell polarity. Additionally, we found that FAK facilitates p190A localization to leading-edge-associated FAs.
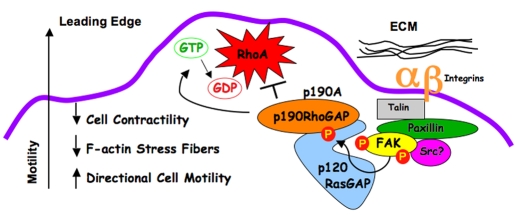
Fig. 10.
Model of the spatial and temporal regulation of p190A by FAK. FAK is activated by integrin clustering and is localized to leading-edge FAs in migrating MEFs via binding to the integrin-associated proteins paxillin and talin. FAK Y397 phosphorylation promotes the SH2-mediated binding of p120RasGAP to FAK and the SH2-SH3-SH2 region of p120RasGAP facilitates a bridge between FAK and p190A. Increased p190A tyrosine phosphorylation is dependent upon FN-stimulated FAK activity that can either phosphorylate p190A directly (Holinstat et al., 2006) or promote p190A tyrosine phosphorylation via Src activation (Wu et al., 2008). In cells with activated Src, cell polarity and recruitment of p190A to leading-edge paxillin-containing FAs is dependent upon FAK expression. Increased GAP activity of tyrosine-phosphorylated p190A that is localized to FAs acts to inhibit RhoA GTPase activity, and this is associated with decreased actin contractility at leading-edge lamella and enables directional or polarized cell movement.
Because we found that overexpression of p190A-RA (lacking GAP activity) or p190A-FF (Y1087F, Y1105F) block MEF polarization, and that Pyk2–FAK-CT expression decreases FN-associated RhoA GTP levels in FAK–/– MEFs, our studies support the notion that FAK acts to recruit the p120RasGAP-p190A complex to FAs, thereby facilitating RhoA inhibition at cell protrusions. Previous studies have linked p190A tyrosine phosphorylation to its activation and RhoA inhibition (Bernards and Settleman, 2004). Our results with p190A-FF support the importance of p190A tyrosine phosphorylation in connection with the regulation of cell polarity and are also consistent with alternative models of Rho regulation involving paxillin tyrosine phosphorylation (Tsubouchi et al., 2002). Although recent studies have shown that RhoA is activated at lamellipodial projections (Pertz et al., 2006), the regulation of RhoA is probably cyclical in migrating cells, because cells actively form and remodel FAs within cell projections.
Our results linking p190A to cell polarity are consistent with Par6 regulation of dendritic-spine polarity via p190A-associated RhoA inhibition (Zhang and Macara, 2008) and p190A–/– MEFs exhibiting directional-motility defects (Jiang et al., 2008). Our studies connecting FAK to p190A tyrosine phosphorylation in MEFs, HUVECs and carcinoma cells are consistent with findings from FAK-null keratinocytes (Schober et al., 2007) and support the conclusion that FAK-p190A is a conserved signaling linkage in multiple cell types. Interestingly, studies from caveolin-1-null cells have proposed a model wherein caveolin-1 stimulates RhoA GTP loading and directional motility through inactivation of the Src-p190RhoGAP pathway (Grande-Garcia et al., 2007). Because β1 integrin and FAK are localized to leading lamellipodia, whereas caveolin-1 is enriched at the posterior region of migrating cells (Beardsley et al., 2005), these findings support a general model of spatially restricted p190A regulation, with p190A tyrosine phosphorylation and Rho inhibition occurring at leading lamellipodia and caveolin-1 inactivation of Src at the cell posterior.
Our results also add FAK to the list of PTKs, such as Src (Arthur et al., 2000; Chang et al., 1995; Fincham et al., 1999) and Arg (Bradley et al., 2006; Hernandez et al., 2004; Peacock et al., 2007), that are linked to p190A tyrosine phosphorylation. Although recombinant FAK can phosphorylate p190A in vitro (Holinstat et al., 2006), FN-stimulated FAK also directly phosphorylates Src within the kinase domain, leading to Src activation (Wu et al., 2008). Thus, it is possible that FAK indirectly regulates p190A tyrosine phosphorylation through the activation of Src-family PTKs. This is consistent with inhibition of MEF polarity with PP2 (pharmacological Src inhibitor) treatment (A.T. and D.D.S., unpublished). Moreover, FAK-Src signaling has been linked to Rac1 GTPase activation at leading lamellipodia, promoting keratinocyte polarization (Choma et al., 2007), and, in regions of Rac GTPase activation in lamellipodia, there can be corresponding inhibition of RhoA (Hall, 2005).
Our study also provides new data into the function of Y861 and Y925 FAK phosphorylation sites. Although FAK Y861 phosphorylation has been linked to αvβ5-integrin coupling to FAK (Eliceiri et al., 2002) and in the modulation of p130Cas binding to FAK (Eliceiri et al., 2002), phosphorylation of this site was not essential for directional MEF motility. It is possible that FAK Y861 phosphorylation functions downstream of particular integrins or in different contexts; for example, promoting survival signaling in response to hyperosmotic stress (Lunn et al., 2007). Additionally, FAK Y925 phosphorylation promotes Grb2 adaptor-protein binding and this is one pathway linking FAK to the ERK–mitogen-activated-protein (MAP)-kinase pathway (Schlaepfer et al., 2004). FAK Y925 phosphorylation promotes tumor-associated angiogenesis signaling (Mitra et al., 2006b) and is important in promoting breast-cancer-cell extravasation events (Earley and Plopper, 2008). Because we found that constitutive activation of the ERK–MAP-kinase cascade did not rescue motility defects of FAK–/– MEFs (Schlaepfer et al., 2007), we conclude that FAK Y925 phosphorylation and signaling to ERK–MAP-kinase is a route through which FAK can enhance gene-expression events (Mitra et al., 2006a; Mitra et al., 2006b) and that the effects of FAK on promoting cell motility might be locally confined to FAs.
Finally, we demonstrate that FAK was required for the proper polarization of Src FAK–/– MEFs that exhibit elevated p190A tyrosine phosphorylation and constitutive p190A-p120RasGAP complex formation. We found that FAK targets p190A to leading-edge FAs in migrating cells and we hypothesize that this will facilitate RhoA inhibition that is associated with a directional-motility cue. Recent studies by our group also characterized FAK association with a GEF for RhoA (p190RhoGEF, Rgnef) at FA sites, after FN plating of MEFs (Lim Y. et al., 2008). Similarly, FAK expression was required for Rgnef localization at FAs approximately 1 hour after FN plating. Importantly, the timing of FAK-Rgnef association was 1 hour after FN plating as opposed to FAK-p120RasGAP-p190A complexes, which are detected earlier (15-45 minutes) during FN-stimulated cell spreading. Taken together, time-dependent formation of p120RasGAP-p190A or Rgnef complexes with FAK support the notion that FAK might serve as a temporal and spatial platform for either RhoA inhibition or RhoA activation connected to cytoskeletal changes and polarized cell migration.
Materials and Methods
Cells and constructs
FAK–/–, FAK+/+, Src FAK–/– and Src FAK+/+ MEFs are p53-null and were maintained as described (Hsia et al., 2003; Lim Y. et al., 2008). FAK–/– MEFs expressing Myc-tagged Pyk2, Pyk2–FAK-CT or Pyk2–FAK-CT-S1034 were created as described (Klingbeil et al., 2001). GFP-FAK-reconstituted FAK–/– MEFs (WT, R454, F397, F861 and F925 FAK) were created as described (Schlaepfer et al., 2007). FAK–/– Pyk2-shRNA MEFs were created as described (Lim Y. et al., 2008). DLD-1 human colorectal cancer cells were obtained from ATCC (Manassas, VA) and were maintained in MEF media (DMEM with 10% FBS) as described (Lim Y. et al., 2008). HUVECs were obtained and maintained as described (Mitra et al., 2006a).
Human FAK and scrambled (Scr) shRNA were used as described (Mitra et al., 2006a). The expression vectors for rat hemagglutinin (HA)-tagged p190A-WT and HA–p190A-FF (Y1087F, Y1105F) were a generous gift from Jeff Settleman (Massachusetts General Hospital Cancer Center, Charlestown, MA); GFP-tagged p190A-WT and GAP-inactive GFP–p190A-RA (R1283A) were from Keith Burridge (University of North Carolina, Chapel Hill, NC); and GFP-p120RasGAP was from Tony Koleske (Yale University, New Haven, CT). GFP-p190A-FF was generated by PCR using HA-p190A-FF as template using the forward 5′-AAAGCCTCCTGTGCATTTTG-3′ and reverse 5′-AAAAGGATCCTCAAGAAGACAACTG-3′ primers to amplify a 1376-bp fragment, BglII and BamHI digestion, and sub-cloning into pEGFP-C1-p190A. GST fusions of the p120RasGAP 2-3-2 (residues 172-467) and p120RasGAP 3-2 (residues 272-467) were generated by PCR cloning using forward primers 5′-AAAAGATCTCGAGGATCCCATACGACGTCCCATGGTATCATGGAAAACTT-3′ and 5′-AAAAGATCTCGAGGATACCCATACGACGTCCCAGACTACGCTGAAGATAGAAGACGTGTA-3′, respectively (underlined residues indicate hemagglutinin tag). The same reverse primer, 5′-TTTGGAATTCCTATGCATCCTTTGTTTTACG-3′, was used for both constructs. PCR products were digested with BglII and BamHI and ligated into BamHI sites within the pEBG mammalian expression vector. All constructs were verified by DNA sequencing.
Antibodies and reagents
Monoclonal antibodies (mAbs) to phosphotyrosine (clone 4G10), FAK (clone 4.47) and p120RasGAP (clone B4F8) were from Millipore (Billerica, MA). Paxillin (clone M107) and p190RhoGAP (clone 30) mAbs were from BD Biosciences (San Jose, CA). HA-tag (clone 16B12), Myc-tag (clone 9E10) and GFP (clone B34) mAbs were from Covance (Princeton, NJ). β-actin (clone AC-17) and α-tubulin (clone DM1A) mAbs, and purified bovine plasma FN were from Sigma-Aldrich (St Louis, MO). Rabbit FAK pY-397 (44-625G) mAb and Alexa-Fluor-350-conjugated phalloidin were from Invitrogen (Carlsbad, CA). Polyclonal antibodies to β-Cop were from Calbiochem-EMD (Gibbstown, NJ). Rabbit polyclonal anti-RhoA (sc-179) was from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibodies to GST were from Pierce-Thermo Scientific (Rockford, IL). The PF-228 FAK inhibitor was a generous gift from Pfizer (La Jolla, CA). Mouse p190A RhoGAP ON-TARGETplus SMART pool siRNA (L-042292-01), p120RasGAP ON-TARGETplus SMART pool siRNA (L-052335-01), ON-TARGETplus Si Control (Scr) siRNA (D-001810-01) and siGLO green transfection indicator (D-001630-01) were obtained from Dharmacon-Thermo Scientific (Lafayette, CO). The ON-TARGETplus non-targeting siRNA pools (Scr control) are designed, modified and microarray-confirmed to have minimal targeting of known genes in human, mouse and rat cells according to the manufacturer. siRNA (100 pmol) + siGLO (100 pmol) was used to transfect (Lipofectamine 2000) FAK+/+ MEFs. After 48 hours, p190A- and p120RasGAP-knockdown was confirmed by blotting.
Cell lysis, immunoprecipitation, immunoblotting and pulldown assays
Cells were washed in ice-cold PBS and solubilized in modified RIPA lysis buffer containing 1% Triton X-100, 0.5% sodium deoxycholate and 0.1% SDS as described (Schlaepfer et al., 2007). For immunoprecipitation, 2.5 μg of antibody was incubated with lysates (each sample containing 500-1000 μg of total protein) for 3 hours at 4°C and collected by binding to protein G plus- or protein A-agarose beads, and washed in lysis buffer without SDS and sodium deoxycholate. SDS-PAGE, antibody blotting and sequential membrane reprobing was performed as previously described (Lim Y. et al., 2008). Rho activity was measured by pulldown assays using GST-Rhotekin Rho-binding domain (GST-RBD) as described (Ren et al., 2000). Clarified RIPA cell lysates were incubated with GST-RBD (20 μg) beads at 4°C for 45 minutes and beads were washed four times with Tris pH 7.5 buffer containing 1% Triton X-100, 150 mM NaCl, 10 mM MgCl2, 10 μg/ml leupeptin, 10 μg/ml aprotinin and 0.1 mM PMSF. Bound Rho proteins were detected by polyclonal RhoA blotting. GST pulldown assays were performed as described by adding glutathione agarose beads to lysates and then processing samples identically to immunoprecipitation (Hsia et al., 2003).
Cell-migration assays
Cells were serum starved in 0.5% FBS overnight at subconfluent densities. Millicell chambers with 8-μm pores (Millipore) were coated on both sides with 10 μg/ml FN for 2 hours at room temperature. Membranes were washed with PBS and air dried for 2 hours. Cells were suspended by limited EDTA-trypsin treatment, trypsin inactivated by 0.25 mg/ml soybean trypsin inhibitor in DMEM, suspended in Migration medium (0.5% BSA in DMEM) and counted. Approximately 5×104 cells in 0.3 ml (held in suspension for 1 hour) were added to each Millicell chamber and incubated for 4 hours at 37°C. Cells were fixed and stained with crystal violet and only cells on the lower membrane surface were enumerated. Each data point represents three individual chambers from at least two independent experiments. Scratch-wound assay was performed as described (Hsia et al., 2003). One-way analysis of variance (ANOVA) was used to determine significance between groups.
Golgi reorientation
Cells were plated onto FN-coated (10 μg/ml) glass slides until confluent, starved for 18 hours in DMEM plus 0.5% FBS, wounded with a pipette tip, washed with PBS, incubated in growth media for 4 hours, fixed with 4% formaldehyde-PBS (10 minutes), and then permeabilized with PBS containing 0.01% Triton X-100 and 0.05% SDS for 5 minutes. Blocking was performed (0.05% BSA in PBS) at room temperature (RT) for 1 hour and cells were incubated with polyclonal β-Cop antibody (1:100) overnight at 4°C. After PBS washing, cells were incubated with rhodamine goat-anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) and Hoechst 33342 (Invitrogen) at 10 μg/ml for 1 hour at RT. Images were captured at 60× using an inverted microscope (IX51; Olympus). To measure Golgi orientation of cells at the wounded edge, a square was drawn over the nucleus and divided into quadrants. Quadrant A was assigned to the area of the cell between the nucleus and the leading edge. A properly reoriented Golgi was indicated by β-Cop staining entirely within quadrant A. Analyses were performed on 100 cells per experiment to determine the percentage of cells with reoriented Golgi.
Immunofluorescent staining
Cells were plated onto 10 μg/ml FN-coated glass coverslips in the absence of serum for the indicated times. Cells were fixed in 3.7% paraformaldehyde for 15 minutes and permeabilized with 0.1% Triton X-100 for 10 minutes. Blocking was performed with 100 μg/ml ChromPure donkey IgG in PBS (Jackson ImmunoResearch Laboratories) at RT for 1 hour. Paxillin (1:100) or tubulin (1:100) antibodies were diluted in PBS and incubated overnight at 4°C. After three washes in PBS, coverslips were incubated for 45 minutes in PBS containing rhodamine-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories) and fluorescein-conjugated phalloidin (Invitrogen-Molecular Probes, Carlsbad, CA) to visualize actin stress fibers. In cells expressing GFP or GFP fusion proteins, actin was visualized with Alexa-Fluor-350-conjugated phalloidin (blue). After washing, coverslips were mounted in Vectashield (Vector Laboratories, Burlingame, CA). Images from wide-UV (excitation, 330-385 nm; dichroic mirror, 400 nm; emission, 420 nm), wide-blue (excitation, 450-480 nm; dichroic mirror, 500 nm; emission, 515 nm) excitations and wide-green (excitation, 510-550 nm; dichroic mirror, 570 nm; emission, 590 nm) were sequentially captured at 60× (UPLFL objective, 1.25 NA; Olympus) using a MAC5000 controller and LEP shutter (Ludl Electronics, Hawthorne, NY), a monochrome charge-coupled camera (ORCA ER; Hamamatsu, Japan), an inverted microscope (IX51; Olympus) and Openlab software (Improvision, Waltham, MA). Images were pseudo-colored using ImageJ 1.38, and overlaid and merged using Photoshop CS3 (Adobe, San Jose, CA). Colocalization calculations at peripheral FAs were performed using ImageJ 1.38 as described (Humphries et al., 2007). Images from wide-blue and wide-green excitations were merged, a threshold was set to restrict FAs and the relative fluorescent signal intensity per pixel was calculated.
We thank Jeff Settleman, Keith Burridge and Tony Koleske for their generous gifts of reagents used in this study. A.T. and S.-T.L. were supported in part by American Heart Association postdoctoral fellowships 0825166F and 0725169Y, respectively. This work was supported by NIH grants to D.S. (GM087400 and HL093156). D.S. is an Established Investigator of the AHA (0540115N). Deposited in PMC for release after 12 months.
References
- Arthur, W. T. and Burridge, K. (2001). RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell 12, 2711-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur, W. T., Petch, L. A. and Burridge, K. (2000). Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10, 719-722. [DOI] [PubMed] [Google Scholar]
- Bass, M. D., Morgan, M. R., Roach, K. A., Settleman, J., Goryachev, A. B. and Humphries, M. J. (2008). p190RhoGAP is the convergence point of adhesion signals from alpha 5 beta 1 integrin and syndecan-4. J. Cell Biol. 181, 1013-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley, A., Fang, K., Mertz, H., Castranova, V., Friend, S. and Liu, J. (2005). Loss of caveolin-1 polarity impedes endothelial cell polarization and directional movement. J. Biol. Chem. 280, 3541-3547. [DOI] [PubMed] [Google Scholar]
- Bernards, A. and Settleman, J. (2004). GAP control: regulating the regulators of small GTPases. Trends Cell Biol. 14, 377-385. [DOI] [PubMed] [Google Scholar]
- Bradley, W. D., Hernandez, S. E., Settleman, J. and Koleske, A. J. (2006). Integrin signaling through Arg activates p190RhoGAP by promoting its binding to p120RasGAP and recruitment to the membrane. Mol. Biol. Cell 17, 4827-4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, S. S., Briggs, S., Smithgall, T. E., Martin, G. A., McCormick, F., Chang, J. H., Parsons, S. J. and Jove, R. (1995). Two SH2 domains of p120 Ras GTPase-activating protein bind synergistically to tyrosine phosphorylated p190 Rho GTPase-activating protein. J. Biol. Chem. 270, 17947-17952. [DOI] [PubMed] [Google Scholar]
- Chang, J. H., Gill, S., Settleman, J. and Parsons, S. J. (1995). c-Src regulates the simultaneous rearrangement of actin cytoskeleton, p190RhoGAP, and p120RasGAP following epidermal growth factor stimulation. J. Cell Biol. 130, 355-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choma, D. P., Milano, V., Pumiglia, K. M. and DiPersio, C. M. (2007). Integrin alpha3beta1-dependent activation of FAK/Src regulates Rac1-mediated keratinocyte polarization on laminin-5. J. Invest. Dermatol. 127, 31-40. [DOI] [PubMed] [Google Scholar]
- Earley, S. and Plopper, G. E. (2008). Phosphorylation of focal adhesion kinase promotes extravasation of breast cancer cells. Biochem. Biophys. Res. Commun. 366, 476-482. [DOI] [PubMed] [Google Scholar]
- Eliceiri, B. P., Puente, X. S., Hood, J. D., Stupack, D. G., Schlaepfer, D. D., Huang, X. Z., Sheppard, D. and Cheresh, D. A. (2002). Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J. Cell Biol. 157, 149-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham, V. J., Chudleigh, A. and Frame, M. C. (1999). Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal disruption during transformation. J. Cell Sci. 112, 947-956. [DOI] [PubMed] [Google Scholar]
- Goldstein, B. and Macara, I. G. (2007). The PAR proteins: fundamental players in animal cell polarization. Dev. Cell 13, 609-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande-Garcia, A., Echarri, A., de Rooij, J., Alderson, N. B., Waterman-Storer, C. M., Valdivielso, J. M. and del Pozo, M. A. (2007). Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J. Cell Biol. 177, 683-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, J., Tamura, M., Pankov, R., Danen, E. H., Takino, T., Matsumoto, K. and Yamada, K. M. (1999). Shc and FAK differentially regulate cell motility and directionality modulated by PTEN. J. Cell Biol. 146, 389-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, A. (2005). Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans. 33, 891-895. [DOI] [PubMed] [Google Scholar]
- Hecker, T. P., Ding, Q., Rege, T. A., Hanks, S. K. and Gladson, C. L. (2004). Overexpression of FAK promotes Ras activity through the formation of a FAK/p120RasGAP complex in malignant astrocytoma cells. Oncogene 23, 3962-3971. [DOI] [PubMed] [Google Scholar]
- Hernandez, S. E., Settleman, J. and Koleske, A. J. (2004). Adhesion-dependent regulation of p190RhoGAP in the developing brain by the Abl-related gene tyrosine kinase. Curr. Biol. 14, 691-696. [DOI] [PubMed] [Google Scholar]
- Holinstat, M., Knezevic, N., Broman, M., Samarel, A. M., Malik, A. B. and Mehta, D. (2006). Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J. Biol. Chem. 281, 2296-2305. [DOI] [PubMed] [Google Scholar]
- Hsia, D. A., Mitra, S. K., Hauck, C. R., Streblow, D. N., Nelson, J. A., Ilic, D., Huang, S., Li, E., Nemerow, G. R., Leng, J. et al. (2003). Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 160, 753-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, K. Q. and Settleman, J. (1997). Tandem SH2 binding sites mediate the RasGAP-RhoGAP interaction: a conformational mechanism for SH3 domain regulation. EMBO J. 16, 473-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries, J. D., Wang, P., Streuli, C., Geiger, B., Humphries, M. J. and Ballestrem, C. (2007). Vinculin controls focal adhesion formation by direct interactions with talin and actin. J. Cell Biol. 179, 1043-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic, D., Furuta, Y., Kanazawa, S., Takeda, N., Sobue, K., Nakatsuji, N., Nomura, S., Fujimoto, J., Okada, M., Yamamoto, T. et al. (1995). Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377, 539-544. [DOI] [PubMed] [Google Scholar]
- Jaffe, A. B. and Hall, A. (2005). Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247-269. [DOI] [PubMed] [Google Scholar]
- Jiang, W., Betson, M., Mulloy, R., Foster, R., Levay, M., Ligeti, E. and Settleman, J. (2008). p190A RhoGAP is a glycogen synthase kinase-3beta substrate required for polarized cell migration. J. Biol. Chem. 283, 20978-20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingbeil, C. K., Hauck, C. R., Hsia, D. A., Jones, K. C., Reider, S. R. and Schlaepfer, D. D. (2001). Targeting Pyk2 to beta 1-integrin-containing focal contacts rescues fibronectin-stimulated signaling and haptotactic motility defects of focal adhesion kinase-null cells. J. Cell Biol. 152, 97-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama, A., Lechler, T. and Fuchs, E. (2004). Coordinating cytoskeletal tracks to polarize cellular movements. J. Cell Biol. 167, 203-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni, S. V., Gish, G., van der Geer, P., Henkemeyer, M. and Pawson, T. (2000). Role of p120 Ras-GAP in directed cell movement. J. Cell Biol. 149, 457-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., Butler, P., Wang, Y., Hu, Y., Han, D. C., Usami, S., Guan, J. L. and Chien, S. (2002). The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc. Natl. Acad. Sci. USA 99, 3546-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, S. T., Mikolon, D., Stupack, D. G. and Schlaepfer, D. D. (2008a). FERM control of FAK function: Implications for cancer therapy. Cell Cycle 7, 2306-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, Y., Lim, S. T., Tomar, A., Gardel, M., Bernard-Trifilo, J. A., Chen, X. L., Uryu, S. A., Canete-Soler, R., Zhai, J., Lin, H. et al. (2008b). PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J. Cell Biol. 180, 187-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn, J. A., Jacamo, R. and Rozengurt, E. (2007). Preferential phosphorylation of focal adhesion kinase tyrosine 861 is critical for mediating an anti-apoptotic response to hyperosmotic stress. J. Biol. Chem. 282, 10370-10379. [DOI] [PubMed] [Google Scholar]
- McGlade, J., Brunkhorst, B., Anderson, D., Mbamalu, G., Settleman, J., Dedhar, S., Rozakis-Adcock, M., Chen, L. B. and Pawson, T. (1993). The N-terminal region of GAP regulates cytoskeletal structure and cell adhesion. EMBO J. 12, 3073-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra, S. K. and Schlaepfer, D. D. (2006). Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18, 516-523. [DOI] [PubMed] [Google Scholar]
- Mitra, S. K., Hanson, D. A. and Schlaepfer, D. D. (2005). Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell. Biol. 6, 56-68. [DOI] [PubMed] [Google Scholar]
- Mitra, S. K., Lim, S. T., Chi, A. and Schlaepfer, D. D. (2006a). Intrinsic focal adhesion kinase activity controls orthotopic breast carcinoma metastasis via the regulation of urokinase plasminogen activator expression in a syngeneic tumor model. Oncogene 25, 4429-4440. [DOI] [PubMed] [Google Scholar]
- Mitra, S. K., Mikolon, D., Molina, J. E., Hsia, D. A., Hanson, D. A., Chi, A., Lim, S. T., Bernard-Trifilo, J. A., Ilic, D., Stupack, D. G. et al. (2006b). Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene 25, 5969-5984. [DOI] [PubMed] [Google Scholar]
- Moissoglu, K. and Gelman, I. H. (2003). v-Src rescues actin-based cytoskeletal architecture and cell motility, and induces enhanced anchorage-independence during oncogenic transformation of focal adhesion kinase-null fibroblasts. J. Biol. Chem. 278, 47946-47959. [DOI] [PubMed] [Google Scholar]
- Owen, K. A., Pixley, F. J., Thomas, K. S., Vicente-Manzanares, M., Ray, B. J., Horwitz, A. F., Parsons, J. T., Beggs, H. E., Stanley, E. R. and Bouton, A. H. (2007). Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J. Cell Biol. 179, 1275-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock, J. G., Miller, A. L., Bradley, W. D., Rodriguez, O. C., Webb, D. J. and Koleske, A. J. (2007). The Abl-related gene tyrosine kinase acts through p190RhoGAP to inhibit actomyosin contractility and regulate focal adhesion dynamics upon adhesion to fibronectin. Mol. Biol. Cell 18, 3860-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertz, O., Hodgson, L., Klemke, R. L. and Hahn, K. M. (2006). Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 440, 1069-1072. [DOI] [PubMed] [Google Scholar]
- Raghavan, S., Vaezi, A. and Fuchs, E. (2003). A role for alphabeta1 integrins in focal adhesion function and polarized cytoskeletal dynamics. Dev. Cell 5, 415-427. [DOI] [PubMed] [Google Scholar]
- Ren, X., Kiosses, W. B., Sieg, D. J., Otey, C. A., Schlaepfer, D. D. and Schwartz, M. A. (2000). Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 113, 3673-3678. [DOI] [PubMed] [Google Scholar]
- Ridley, A. J., Schwartz, M. A., Burridge, K., Firtel, R. A., Ginsberg, M. H., Borisy, G., Parsons, J. T. and Horwitz, A. R. (2003). Cell migration: integrating signals from front to back. Science 302, 1704-1709. [DOI] [PubMed] [Google Scholar]
- Schlaepfer, D. D., Mitra, S. K. and Ilic, D. (2004). Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim. Biophys. Acta 1692, 77-102. [DOI] [PubMed] [Google Scholar]
- Schlaepfer, D. D., Hou, S., Lim, S. T., Tomar, A., Yu, H., Lim, Y., Hanson, D. A., Uryu, S. A., Molina, J. and Mitra, S. K. (2007). Tumor necrosis factor-alpha stimulates focal adhesion kinase activity required for mitogen-activated kinase-associated interleukin 6 expression. J. Biol. Chem. 282, 17450-17459. [DOI] [PubMed] [Google Scholar]
- Schober, M., Raghavan, S., Nikolova, M., Polak, L., Pasolli, H. A., Beggs, H. E., Reichardt, L. F. and Fuchs, E. (2007). Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J. Cell Biol. 176, 667-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg, D. J., Hauck, C. R. and Schlaepfer, D. D. (1999). Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 112, 2677-2691. [DOI] [PubMed] [Google Scholar]
- Slack-Davis, J. K., Martin, K. H., Tilghman, R. W., Iwanicki, M., Ung, E. J., Autry, C., Luzzio, M. J., Cooper, B., Kath, J. C., Roberts, W. G. et al. (2007). Cellular characterization of a novel focal adhesion kinase inhibitor. J. Biol. Chem. 282, 14845-14852. [DOI] [PubMed] [Google Scholar]
- Tilghman, R. W., Slack-Davis, J. K., Sergina, N., Martin, K. H., Iwanicki, M., Hershey, E. D., Beggs, H. E., Reichardt, L. F. and Parsons, J. T. (2005). Focal adhesion kinase is required for the spatial organization of the leading edge in migrating cells. J. Cell Sci. 118, 2613-2623. [DOI] [PubMed] [Google Scholar]
- Tsubouchi, A., Sakakura, J., Yagi, R., Mazaki, Y., Schaefer, E., Yano, H. and Sabe, H. (2002). Localized suppression of RhoA activity by Tyr31/118-phosphorylated paxillin in cell adhesion and migration. J. Cell Biol. 159, 673-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Geer, P., Henkemeyer, M., Jacks, T. and Pawson, T. (1997). Aberrant Ras regulation and reduced p190 tyrosine phosphorylation in cells lacking p120-Gap. Mol. Cell. Biol. 17, 1840-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares, M., Webb, D. J. and Horwitz, A. R. (2005). Cell migration at a glance. J. Cell Sci. 118, 4917-4919. [DOI] [PubMed] [Google Scholar]
- Wang, H. B., Dembo, M., Hanks, S. K. and Wang, Y. (2001). Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc. Natl. Acad. Sci. USA 98, 11295-11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L., Bernard-Trifilo, J. A., Lim, Y., Lim, S. T., Mitra, S. K., Uryu, S., Chen, M., Pallen, C. J., Cheung, N. K., Mikolon, D. et al. (2008). Distinct FAK-Src activation events promote alpha5beta1 and alpha4beta1 integrin-stimulated neuroblastoma cell motility. Oncogene 27, 1439-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. and Macara, I. G. (2008). The PAR-6 polarity protein regulates dendritic spine morphogenesis through p190 RhoGAP and the Rho GTPase. Dev. Cell 14, 216-226. [DOI] [PMC free article] [PubMed] [Google Scholar]