Abstract
Purpose
Prostate-specific antigen progression (PSA-P) is an indicator of progression in hormone-sensitive (HS) and castration-resistant (CR) prostate cancer (PC). We evaluated different definitions of PSA-P as predictors of overall survival (OS).
Patients and Methods
A total of 1,078 patients with HSPC who were on hormones (Southwest Oncology Group [SWOG] trial 9346 [S9346]) and 597 patients with CRPC who were treated with chemotherapy (SWOG trial 9916 [S9916]) were eligible for this analysis. PSA-P definitions tested included the following: PSA Working Group, Prostate Cancer Working Group (PCWG 2008), and other definitions. A time-varying approach analyzed associations between PSA-P at any time and OS. A landmark analysis examined the relationship between PSA-P status at 7 months for S9346, or 3 months for S9916, and subsequent OS.
Results
In the time-varying analysis, both working groups definitions were strongly associated with OS (P < .001) in both study settings. In patients enrolled onto S9346, both definitions predicted a 2.4-fold increased risk of death (ROD) and a greater than four-fold increased ROD if PSA-P occurred in the first 7 months. In S9916, they predicted a 40% increase in ROD and a two-fold increase in ROD if PSA-P occurred at 3 months. In landmark analyses of patients on S9346 by using the PCWG 2008 definition of PSA-P, median subsequent OS was 10 months versus 44 months in patients who did or did not have PSA-P by 7 months, respectively; in S9916, data were 11 months versus 18 months for patients who did or did not have PSA-P by 3 months, respectively.
Conclusion
PSA-P, defined as an increase of ≥ 25% greater than the nadir and an absolute increase of at least 2 or 5 ng/mL, predicts OS in HSPC and CRPC and may be a suitable end point for phase II studies in these settings.
INTRODUCTION
Progress in systemic therapy development for advanced prostate cancer (PC) has lagged behind other solid tumors. Contributing to this are difficulties in the objective assessment of antitumor effect in the context of bone-predominant metastasis and advanced age at diagnosis in which confounding comorbidities/competing causes of death exist.
Overall survival is considered the gold standard to assess the outcome of anticancer therapies in phase III trials in different stages of PC. To advance to phase III testing, agents must demonstrate clinically relevant activity. In castration-resistant PC (CRPC), the inability to objectively assess antitumor response has focused attention on the use of alternative end points to screen for activity. Prostate-specific antigen (PSA) has been recognized as a biomarker for diagnosis, prognosis, and monitoring of disease activity. The observation that systemic and local therapies impact PSA level has led to use of the PSA level in therapeutic decision making, both clinically and experimentally.1,2
We have previously reported in a secondary analysis of Southwest Oncology Group (SWOG) trial 9346 (S9346) that, in patients with metastatic, hormone-sensitive PC (HSPC), a PSA of ≤ 4 ng/mL after 7 months of androgen deprivation (AD) is a strong predictor of survival.3 In a secondary analysis of SWOG trial 9916 (S9916)4 in patients with CRPC, we reported that, at 3 months (after 4 courses of chemotherapy administered every 21 days), PSA decreases of 30% met surrogacy criteria for survival. That is, the significant survival benefit of docetaxel plus estramustine observed in this study could be fully explained by this PSA decline.5 Although PSA decline may prove a good surrogate for clinical benefit—thus, may prove valuable for screening for antitumor activity—it is likely that its surrogacy for different classes of agents and PC settings will need separate validation.
In HSPC and CRPC, PSA progression (PSA-P) heralds clinical progression6 and has been an accepted indicator of worsening disease. To date, no data exist regarding what constitutes an appropriate definition for PSA-P. Criteria have been generally consensus based according to best clinical judgment. During the past decade, the PSA Working Group (PSAWG 1999)2 and the Prostate Cancer Working Group (PCWG 2008)7 have published recommendations to streamline eligibility, conduct, and outcome measures for phase II trials. Recently, the PCWG 2008 recommended a shift from response to a time-to-event end point, specifically objective progression. Objective progression may be difficult to determine in the early stages of therapy, particularly in patients who have metastatic CRPC. In CRPC and HSPC, an early, easy, and reliable measure of progression would enhance therapeutic decisions to avoid unnecessary exposure to ineffective or no-longer effective therapy.
We hypothesized that, if well defined and outcome based, PSA-P could lead to improved therapeutic decisions in the clinic and in the context of experimental therapy. We, therefore, sought to determine the correlation of different definitions of PSA-P to overall survival and to compare the association of PSA-P versus PSA decline with overall survival by using data from two prospective SWOG intergroup, phase III trials.
PATIENTS AND METHODS
Study Design
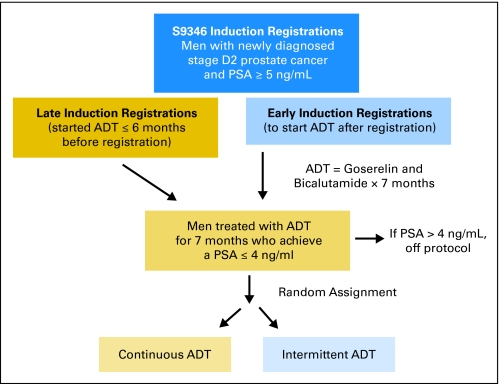
The designs of S9346 and S9916 have been described in detail previously.3,4 Briefly, S9346 is a recently closed, randomized, phase III, intergroup trial (INT 0162) of SWOG; Cancer and Leukemia Group B; Eastern Cooperative Oncology Group; European Organisation for Research and Treatment of Cancer; and National Cancer Institute of Canada, in which the primary objective is to assess whether survival with intermittent androgen-deprivation therapy (ADT) is not inferior to survival with continuous ADT in patients who have newly diagnosed, metastatic HSPC. Key eligibility requirements included stage M1 (D2) PC and a minimum pretreatment PSA of 5 ng/mL. A study schema for S9346 is shown in Figure 1. Step 1 of the treatment regimen consists of a 7-month induction course with goserelin and bicalutamide. Patients who had started ADT within 6 months before registration and who were otherwise eligible were allowed to enroll and were categorized as late induction, whereas those who began ADT immediately after registration were early-induction patients. Patients whose PSA levels decreased to 4 ng/mL or less, with stable or declining trend, at months 6 and 7 of induction treatment were randomly assigned in step 2 to intermittent or continuous ADT. Patients whose PSA levels did not decrease to 4 ng/mL or less at the end of the induction phase of the trial were removed from protocol but were observed for progression and survival. PSA levels were obtained at months 1, 4, 6, and 7 of the induction period; this was followed by monthly assessments after random assignment. For those patients not randomly assigned, PSA was assessed every 6 months after the end of induction and as clinically indicated.
Fig 1.
S9346 (Intergroup 0162) study schema. PSA, prostate-specific antigen; ADT, androgen-deprivation therapy.
Patients continue to be treated on S9346, but the study's Data and Safety Monitoring committee has given permission for these results to be reported, because no information about randomized treatment assignment and corresponding survival is being reported and because the data generated will not prospectively influence trial conduct or assessment of outcomes.
S9916 was a randomized, intergroup, phase III trial that demonstrated a 20% improvement in survival among patients with CRPC who were treated with docetaxel and estramustine (D/E) compared with patients who were treated with mitoxantrone and prednisone (M/P).4 To be eligible, patients must have had stage N1 or M1 CRPC that progressed by either measurable or bone disease or by an increasing PSA level. Patients were randomly assigned to the D/E or the M/P arm and were treated for a maximum of 12 cycles that were administered every 3 weeks. PSA levels were obtained every 3 weeks after random assignment. For both studies, all patients signed institutional review board–approved consent forms.
Statistical Analysis
The objective of this analysis is to determine the correlation of PSA-P to subsequent survival and to evaluate several definitions for PSA-P to determine the one most strongly correlated, either in men with HSPC who are treated with ADT or in men with CRPC who are treated with chemotherapy. Five definitions of PSA-P were explored: any increase above the nadir (ie, rising trend); an increase by ≥ 25% and ≥ 5 ng/mL (ie, 25% + 5); an increase by ≥ 50% and ≥ 5 ng/mL (ie, 50% + 5); an increase by ≥ 25% and ≥ 2 ng/mL (ie, PCWG 2008; 25% + 2); and an increase by ≥ 25% and ≥ 5 ng/mL for patients who did not achieve a 50% decrease in PSA, or an increase by ≥ 50% and ≥ 5 ng/mL for patients who achieved a 50% decrease in PSA (PSAWG 1999). Nadir was defined as the minimum PSA level achieved while on protocol treatment or, for those patients whose PSA did not decrease while on study, baseline (ie, pretreatment) PSA. For all definitions, PSA-P must have occurred at least 7 days after nadir and must have been confirmed by a second, increased, PSA measurement at least 7 days later. The nadir and two increasing PSA measurements were not required to be consecutive. The PSA-P date was recorded as the date of the first increased measurement. Details of the statistical methods are described in the Appendix (online only).
RESULTS
Patient Characteristics
Of the 1,373 patients from S9346 eligible for this analysis, baseline covariates were missing for 295 (21%); GS was missing in 184 (13%), bone pain was missing in 130 (9%), and PS was missing in 16 (1%; Table 1). The remaining 1,078 patients were included in this analysis. Of these, 1,029 had adequate follow-up; were alive at the end of induction therapy; and, therefore, could be included in the landmark analysis. With a median follow-up of 4.4 years in this subset of patients on S9346, 702 patients have died, and the median survival was 3.3 years (95% CI, 3.0 to 3.5 years).
Table 1.
Patient Characteristics
| Characteristic | Trial |
|
|---|---|---|
| S9346 (HSPC) | S9916 (CRPC) | |
| No. of patients in analysis | 1,078 | 597 |
| Age, years | ||
| Median | 69 | 71 |
| Range | 38-94 | 44-88 |
| Baseline PSA, ng/mL | ||
| Median | 89.5 | 96.7 |
| Range | 5.0-48,670 | 0.1-10,820 |
| Ethnicity, % | ||
| White | 63 | 85 |
| Black | 15 | 13 |
| Other | 3 | 2 |
| Unknown* | 19 | 0 |
| Presence of bone pain, %† | 40 | 34 |
| Performance status of 2-3, % | 8 | 9 |
| Randomly assigned to D/E arm, % | — | 50 |
| Gleason score, % | ||
| < 7 | 17 | — |
| 7 | 31 | — |
| > 7 | 52 | — |
| Prior prostatectomy, %‡ | 20 | 34 |
| Follow-up, years | ||
| Median | 4.4 | 3.5 |
| Range | 0.4 to 12.3 | 0.3 to 6.1 |
| PSA value, total on study | ||
| Median | 12 | 10 |
| Range | 2 to 121 | 2 to 57 |
| PSA value, by landmark time§ | ||
| Median | 5 | 4 |
| Range | 2 to 18 | 1 to 16 |
Abbreviations: HSPC, hormone-sensitive prostate cancer; CRPC, castration-resistant prostate cancer; D/E, docetaxel and estramustine; PSA, prostate-specific antigen.
The European Organisation for Research and Treatment of Cancer does not collect data pertaining to patient race/ethnicity.
For S9346, any grade bone pain was included; for S9916, only grades ≥ 2 were included.
N = 1,065, as prior prostatectomy status was missing for 13 patients.
For S9346, the landmark time was 7 months after starting androgen-deprivation therapy. For S9916, the landmark time was 3 months after starting chemotherapy.
Of the 611 patients from S9916 who were eligible for this analysis, 14 (2%) were missing baseline covariates. The remaining 597 patients were included in this analysis. Of these, 582 survived at least 3 months after random assignment and, therefore, could be included in the landmark analysis. With a median follow-up of 3.5 years in this subset of patients on S9916, 551 patients have died, and the median survival was 1.5 years (95% CI, 1.4 to 1.7 years).
Table 2 lists the five definitions of PSA-P explored and the number of events under each definition per trial. With a median follow-up of 4.4 years, 669 (62%) of patients on S9346 who were included in this analysis have had an increase in PSA above the nadir that was confirmed at least 7 days later (ie, rising trend). According to this definition of PSA-P, median PSA progression-free survival (PFS) is 14.5 months. According to the PCWG 2008 definition (ie, 25% + 2), PSA-P occurred in 528 (49%) of patients on S9346, and the median PSA PFS was 3 years. According to the PSAWG 1999 definition (ie, 50% + 5 if PSA declined by ≥ 50%, or 25% + 5 if not), PSA-P occurred in 440 patients (41%), and the current estimate of median PSA PFS is greater than 5 years. The results for these two PSA-P definitions were similar to the results seen for the 25% + 5 and 50% + 5 definitions.
Table 2.
PSA-Progression Definitions
| PSA-Progression Definition* | Trial |
|||||||
|---|---|---|---|---|---|---|---|---|
| S9346 (HSPC) |
S9916 (CRPC) |
|||||||
| Events |
PSA PFS (months)† |
Events |
PSA PFS (months)† |
|||||
| No. | % | Median | 95% CI | No. | % | Median | 95% CI | |
| Rising trend increase | 669 | 62 | 14.5 | 12.3 to 16.6 | 458 | 77 | 5.7 | 5.0 to 6.2 |
| 50% + 5‡ | 435 | 40 | 64.1 | NA§ | 346 | 58 | 11.7 | 10.8 to 12.6 |
| 25% + 5‡ | 440 | 41 | 61.5 | NA§ | 396 | 66 | 9.5 | 8.4 to 10.5 |
| PSAWG 1999 (25% + 5; or, if PSA declined by ≥ 50%, 50% + 5)‡ | 435 | 40 | 64.1 | NA§ | 380 | 64 | 10.2 | 9.2 to 11.3 |
| PCWG 2008 (25% + 2)‡ | 528 | 49 | 36.1 | 30.5 to 44.2 | 415 | 70 | 8.1 | 7.0 to 9.5 |
Abbreviations: PSA, prostate-specific antigen; PFS, progression-free survival; HSPC, hormone-sensitive prostate cancer; CRPC, castration-resistant prostate cancer; PSAWG, Prostate-Specific Antigen Working Group; PCWG, Prostate Cancer Working Group.
All definitions are based on increases above the prostate-specific antigen nadir.
Kaplan-Meier estimates, in months.
50% + 5 indicates an increase of prostate-specific antigen by ≥ 50% and an absolute increase of ≥ 5 ng/mL; 25% + 5 indicates an increase by ≥ 25% and ≥ 5 ng/mL; 25% + 2 indicates an increase by ≥ 25% and ≥ 2 ng/mL.
95% confidence intervals not estimable when too few prostate-specific antigen progression events were observed.
Not surprisingly, according to all definitions of PSA-P, events occurred more often and PSA PFS times were shorter in patients on S9916 compared with patients on S9346. With a median follow-up of 3.5 years, 458 (77%) of the 582 patients who were observed on S9916 had a rising-trend PSA-P. Median PSA PFS was 5.7 months in this population. Even at the most restrictive definition (ie, 50% + 5), PSA-P occurred in 346 (58%) of these patients, and the median PSA PFS was approximately 1 year.
Table 3 lists the results of both univariate and multivariate survival analyses of the relationship between PSA-P and survival in patients with HSPC (ie, on S9346) and CRPC (ie, on S9916). These models included PSA-P as a time-varying predictor of survival. For S9346, according to all definitions, PSA-P was highly significantly (P < .0001) associated with subsequent survival. Hazard ratio (HR) estimates were smallest for PSA-P when it was defined as a rising trend (adjusted HR, 1.97; 95% CI, 1.67 to 2.32) and were largest according to the PCWG 2008 definition (adjusted HR, 2, 49; 95% CI, 2.13 to 2.91). According to the latter definition, the partial r2 associated with PSA progression was 17.2%. Results according to the 25% + 5 and 50% + 5 definitions were similar to those of the PCWG 2008 definition. Because only 19 patients on S9346 did not achieve a ≥ 50% PSA decline on study, results according to the PSAWG 1999 definition (in which progression was defined differently for patients who did not have a ≥ 50% PSA decline) are identical to those of the 50% + 5 definition.
Table 3.
Time-Varying Univariate and Multivariate Survival Analyses of the Relationship Between PSA Progression and Survival in Patients With Metastatic Hormone-Naïve (Trial S9346) and Hormone-Refractory (Trial S9916) Prostate Cancer
| Data by Analysis Type | Analysis by Trial |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S9346 |
S9916 |
|||||||||
| HR | 95% CI | P | Partial r2 (%)*† | Full r2 (%)*‡ | HR | 95% CI | P | Partial r2 (%)*† | Full r2 (%)*‡ | |
| Rising trend | ||||||||||
| Univariate | 2.22 | 1.89 to 2.61 | < .0001 | 13.2 | — | 1.21 | 0.99 to 1.49 | .06 | 0.6 | — |
| Multivariate§ | 1.97 | 1.67 to 2.32 | < .0001 | 9.3 | 25.5 | 1.21 | 0.98 to 1.48 | .07 | 0.6 | 6.2 |
| 25% + 5‖ | ||||||||||
| Univariate | 2.84 | 2.44 to 3.30 | < .0001 | 22.1 | — | 1.42 | 1.18 to 1.70 | .0002 | 2.6 | — |
| Multivariate§ | 2.44 | 2.09 to 2.85 | < .0001 | 16.0 | 30.9 | 1.41 | 1.17 to 1.69 | .0003 | 2.4 | 7.9 |
| 50% + 5‖ | ||||||||||
| Univariate | 2.78 | 2.39 to 3.23 | < .0001 | 21.3 | — | 1.20 | 1.00 to 1.43 | .05 | 0.7 | — |
| Multivariate§ | 2.39 | 2.05 to 2.80 | < .0001 | 15.3 | 30.4 | 1.22 | 1.02 to 1.45 | .03 | 0.8 | 6.4 |
| PCWG 2008 (25% + 2)‖ | ||||||||||
| Univariate | 2.82 | 2.42 to 3.28 | < .0001 | 22.4 | — | 1.38 | 1.15 to 1.67 | .001 | 2.1 | — |
| Multivariate§ | 2.49 | 2.13 to 2.91 | < .0001 | 17.2 | 31.9 | 1.38 | 1.14 to 1.67 | .001 | 2.0 | 7.5 |
| PSAWG 1999 (25% + 5; or, if PSA declined by ≥ 50%, 50% + 5)‖ | ||||||||||
| Univariate | 2.78 | 2.39 to 3.23 | < .0001 | 21.3 | — | 1.37 | 1.14 to 1.64 | .0006 | 2.1 | — |
| Multivariate§ | 2.39 | 2.05 to 2.80 | < .0001 | 15.3 | 30.4 | 1.37 | 1.15 to 1.65 | .0006 | 2.2 | 7.7 |
Abbreviations: PSA, prostate-specific antigen; HR, hazard ratio; PCWG, Prostate Cancer Working Group; PSAWG, Prostate-Specific Antigen Working Group.
The r2 coefficient of explained randomness was calculated using the method of O'Quigley et al.8
For univariate analyses, this is the crude r2. For multivariate analyses, this is the partial r2 for prostate-specific antigen progression after adjustment for baseline characteristics.
The r2 for the full multivariate model, including all baseline characteristics.
For S9346, model adjusted for age, ethnicity, site of patient registration (Europe v United States/Canada), pretreatment PSA level, bone pain, Gleason sum, and late induction registration. For S9916, model adjusted for treatment arm, age, ethnicity, pretreatment PSA level, SWOG PS, bone pain, prior prostatectomy, and progression of measurable or bone disease.
25% + 5 indicates an increase of prostate-specific antigen by ≥ 25% and ≥ 5 ng/mL; 50% + 5 indicates an increase by ≥ 50% and ≥ 5 ng/mL; 25% + 2 indicates an increase by ≥ 25% and ≥ 2 ng/mL.
For all S9916 analyses, tests for interaction between treatment arm and PSA-P were performed and were nonsignificant (P > .20). This suggests that treatment type does not affect the association between PSA-P and survival, so the results are reported in Table 3 as pooled across the treatment arm. According to all definitions, HR and r2 estimates (both crude and adjusted) for PSA-P were much smaller in patients on S9916 than in patients on S9346. For S9916, in all instances except when defined as a rising trend (P = .07), PSA-P was significantly associated (P < .05) with subsequent survival. This was seen most strongly when the 25% + 5 definition was applied (adjusted HR, 1.41; 95% CI, 1.17 to 1.69), but it was similar for the PCWG 2008 (ie, 25% + 2) definition. The partial r2 associated with PSA-P according to the 25% + 5 definition was 2.4%. Again, the PCWG 2008 and PSAWG 1999 definitions produced similar results.
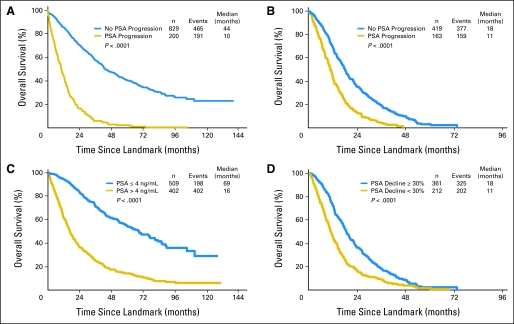
In Figures 2A and 2B, the subset of patients who survived to the landmark times of 7 months in S9346 (with HSPC) and 3 months in S9916 (with CRPC) are stratified by PSA-P status at that time. The PCWG 2008 (ie, 25% + 2) definition of PSA-P was used for these plots because of its high correlation with survival and because of the benefit of earlier-identified progression with this definition. This classification yields highly statistically significant survival differences (P < .0001). In S9346, median postlandmark survival rates were 10 months and 44 months, respectively, among patients whose PSA had and had not progressed at the landmark time, respectively; in S9916, median survival times beyond the landmark time were 18 months and 11 months, respectively. As listed in Table 4, this corresponds in S9346 to an adjusted HR of 4.39 (95% CI, 3.65 to 5.30); in S9916, the adjusted HR was 2.06 (95% CI, 1.69 to 2.51).
Fig 2.
Overall survival in (A) Southwest Oncology Group (SWOG) study 9346 (S9346) by prostate-specific antigen progression (PSA-P) at 7 months as per the Prostate Cancer Working Group (PCWG 2008) definition; in (B) S9916 by PSA-P at 3 months as per the PCWG 2008 definition; in (C) S9346 by PSA level at 7 months; and in (D) S9916 by PSA decline at 3 months.
Table 4.
Landmark Analyses of PSA Progression and PSA Response in Trials S9346 and S9916
| PSA Data by Analysis Type | Analyses by Trial |
|||||||
|---|---|---|---|---|---|---|---|---|
| S9346 (HSPC) |
S9916 (CRPC) |
|||||||
| HR | 95% CI* | Partial r2†‡ (%) | Full r2†§ (%) | HR | 95% CI* | Partial r2†‡ (%) | Full r2†§ (%) | |
| PSA progression by landmark time (PCWG2008 [25% + 2])‖¶ | ||||||||
| Univariate | 4.99 | 4.17 to 6.00 | 31.5 | — | 2.06 | 1.70 to 2.49 | 8.9 | — |
| Multivariate# | 4.39 | 3.65 to 5.30 | 26.7 | 38.8 | 2.06 | 1.69 to 2.51 | 8.3 | 13.0 |
| Lack of PSA response by landmark time** | ||||||||
| Univariate | 3.76 | 3.16 to 4.46 | 33.5 | — | 1.76 | 1.47 to 2.10 | 6.8 | — |
| Multivariate# | 3.58 | 3.00 to 4.27 | 30.0 | 41.6 | 1.87 | 1.52 to 2.28 | 6.6 | 10.9 |
Abbreviations: PSA, prostate-specific antigen; HSPC, hormone-sensitive prostate cancer; CRPC, castration-resistant prostate cancer; HR, hazard ratio; PCWG, Prostate Cancer Working Group.
All P values < .0001.
The r2 coefficient of explained randomness was calculated using the method of O'Quigley et al.8
For univariate analyses, this is the crude r2. For multivariate analyses, this is the partial r2 for prostate-specific antigen progression after adjustment for baseline characteristics.
The r2 for the full multivariate model, including all baseline characteristics.
In trial S9346, the landmark time was the end of the 7-month induction period; in trial S9916, it was 3 months after random assignment.
25% + 2 indicates an increase of prostate-specific antigen by ≥ 25% and ≥ 2 ng/mL.
For S9346, model adjusted for age, ethnicity, site of patient registration (Europe v United States/Canada), pretreatment PSA level, bone pain, Gleason sum, and late induction registration. For S9916, model adjusted for treatment arm, age, ethnicity, pretreatment PSA level, SWOG PS, bone pain, prior prostatectomy, and progression of measurable or bone disease.
Prostate-specific antigen response was defined in trial S9346 as normalization to ≤ 4 ng/mL at months 6 and 7 (end of induction therapy) and in trial S9916 as a decline of ≥ 30% during the first 3 months on study.
PSA Response and Survival
Figures 2C and 2D show Kaplan-Meier plots of survival stratified by PSA response end points that were highly correlated with survival in previous analyses.3,5 For S9346, 1,015 patients were included in this analysis; the median postlandmark survival rates were 69 months for patients whose PSA decreased to ≤ 4 ng/mL and 16 months for patients whose PSA did not decrease to ≤ 4 ng/mL. For S9916, 573 patients were included; the median survival rates were beyond the landmark time of 18 and 11 months, respectively. Within each study, PSA-P and response yielded similarly large survival differences, although the adjusted HRs for PSA-P were somewhat larger than those for lack of response (Table 4).
DISCUSSION
The attractiveness of PSA as an intermediate end point for disease and/or treatment outcomes in different stages of PC is obvious. PSA level is easily measurable, is reproducible, and could be substituted as the end point of interest in therapeutic trials. This offers the potential to obtain the same conclusions regarding treatment but eliminates the need for longer follow-up for the end point of interest, and it overcomes difficulties in testing new agents.
It has been recognized that, in metastatic PC, PSA-P heralds clinical progression. Thus, therapeutic decisions have been influenced by an increasing PSA. The first attempt to standardize the definition of PSA-P was proposed by the PSAWG 19992; PSA-P was defined as a 25% increase and an absolute increase of 5 ng/mL or greater. Two thresholds were proposed for PSA-P on the basis of achievement of a 50% PSA decline or not. For patients who achieved a 50% PSA decline, PSA-P was defined as a 50% increase in PSA greater than the nadir and an absolute increase of 5 ng/mL. To minimize patient exposure to ineffective therapy, particularly when patients achieve or start with a low PSA, the PCWG 2008 recommendation was to maintain the 25% PSA increase above the nadir or baseline, whichever is lower, but to reduce the absolute increase to 2 ng/mL or greater. This definition has the appeal of applying to all patients, regardless of magnitude of prior PSA decline.
To date, all PSA-P definitions have been consensus- or experience-based definitions, and none have been formally evaluated in the context of large, prospective, randomized trials in any metastatic disease setting. Both consensus criteria were intended for patients who had CRPC; however, no PSA-P definitions have been proposed for HSPC. With data from two SWOG intergroup, randomized trials in metastatic HSPC and CRPC, we assessed the association of several PSA-P definitions. This analysis demonstrates that PSA-P is a significant predictor of survival in patients who have newly diagnosed, metastatic HSPC treated with continuous ADT as well as in those who have CRPC treated with chemotherapy. We found that a confirmed increase of at least 25% with an absolute increase of ≥ 2 or 5 ng/mL was the most powerful predictor of survival in both populations. However, the PCWG 2008 definition has clinical appeal, because patients are identified with progression relatively earlier. We also found that having two criteria for PSA-P, depending on whether a PSA response occurred or not, is not necessary, which thereby simplifies the definition. Our results, therefore, do not support any modifications to the PCWG 2008 definition of PSA-P.
Our previously reported analyses assessed the impact on survival of achievement of a 7-month PSA of ≤ 4 ng/mL in patients with HSPC3 and that of a 3-month 30% PSA decline in patients with CRPC5 in the context of the same phase III trials. In both analyses, the intermediate PSA response end point was highly predictive of survival. This study reproduces those results in the subset of patients who could also be evaluated for PSA-P. In addition, we have found that PSA-P at the same landmark times was similarly highly predictive of survival in both patient populations. In both settings, PSA-P and response provide complementary information, and their utility is not mutually exclusive, as practically all patients in both settings will develop disease progression. The strength of these results is in the fact that this simply defined PSA-P end point (ie, 25% + 2 or 5) identifies patient subgroups with such dramatic outcome differences. In addition, it does so in these two diverse disease settings, two different treatment modalities, and two different chemotherapeutic agents; thus, it has broader applicability.
Although our analysis may be somewhat limited by the number of patients excluded as a result of missing baseline covariates, these missing patients do not appear to have any difference in outcomes from the rest of the study population. Approximately 10% of patients were also excluded from both data sets because of missing longitudinal PSA data. Survival among these patients was somewhat worse than in the overall study populations. If they were more likely to have PSA-P, then their inclusion in the analysis might have been expected to yield even larger survival differences by PSA-P status. Finally, incomplete PSA data among patients included in this analysis most likely resulted in some PSA-P events being missed. As a result, the findings presented here may represent an underestimate of the true strength of association between PSA-P and overall survival in both HSPC and CRPC.
This study finds that PSA-P is much more highly correlated with survival in patients with HSPC who are treated with ADT than in patients with CRPC who are treated with chemotherapy. This finding certainly is influenced, in part, by the natural history and the efficacy of treatment modality in each setting. The time-varying analyses found that patients in both of these disease settings were at significantly increased risk of death after PSA-P, regardless of when that progression occurred.
In conclusion, these results suggest that a simple measure of PSA-P is strongly associated with overall survival in metastatic HSPC and CRPC. PSA-P and PSA decline provide clinically meaningful information early in the treatment course of patients with metastatic PC. The strengths of our findings stem from analyses performed on two large groups of patients from two phase III trials, in which patients were treated and were observed uniformly for response and survival. Because this was a secondary analysis, these findings need to be validated prospectively. Because the PCWG 2008 definition identifies progression earlier, yet has a strong correlation with survival, this may be the most desirable definition for identification of patients with worse prognosis. PSA-P, defined as an increase of ≥ 25% above the nadir and an absolute increase of at least 2 or 5 ng/mL, be a suitable intermediate end point, particularly in the context of phase II studies in these settings.
Acknowledgment
Supported in part by Public Health Service Cooperative Agreement Grants No. CA32102, CA38926, CA27057, CA20319, CA68183, CA42777, CA14028, CA58882, CA46441, CA35192, CA46282, CA128567, CA45907, CA46113, CA58416, CA04919, CA58861, CA76132, CA58686, CA12644, CA35261, CA35431, CA22433, CA46368, CA63848, CA67575, CA76447, CA46136, CA86780, CA35281, CA45560, CA63844, CA37981, CA67663, CA11083, CA35178, CA95860, CA16385, CA12213, CA35119, CA35090, CA63845, CA74647, CA45461, CA45377, CA45808, CA76462, CA35128, CA35262, CA45807, CA35176, CA76426, CA58658 CA63850, CA13612, CA58723, and CA74811. CA60138 to Cancer and Leukemia Group B; CA25224 to North Central Cancer Treatment Group; and CA21076 and CA21115 to Eastern Cooperative Oncology Group, awarded by the National Cancer Institute, Department of Health and Human Services; by AstraZeneca Pharmaceuticals and Sanofi-aventis US Inc; and by Grants No. 2U10CA11488-28 through 5Y10CA011488-38 to the European Organisation for Reasearch and Treatment of Cancer from the National Cancer Institute.
Appendix
Statistical methods.
To be eligible for this analysis, patients had to be eligible for either Southwest Oncology Group (SWOG) study 9346 (S9346) or SWOG study 9916 (S9916) and had to have a baseline prostate-specific antigen (PSA) and at least two subsequent PSA values collected after starting protocol treatment, at least one of which must have been collected while the patient was still on protocol treatment. For those on S9346, only patients not randomly assigned (ie, PSA not normalized at 7 months) and those randomly assigned to the continuous therapy arm were included in the analysis, because those on the intermittent arm experienced PSA increase during the off-treatment period, which is not indicative of androgen-independent progression. To ensure adequate follow-up in terms of both PSA and survival, patients had to be registered to S9346 at least 2 years before the database was frozen for this analysis. This led to the inclusion of 1,373 patients from S9346 and 611 from S9916.
The association between PSA-P and survival was assessed by using multivariate proportional hazards regression. Multivariate models were constructed from covariates that were significantly associated with PSA-P or survival in univariate analyses. For S9346, this model was adjusted for age (in decades), ethnicity (African American v other), site of patient registration (Europe v United States/Canada), pretreatment PSA level (in 50-ng/mL increments), presence of bone pain, Gleason sum (GS; < 7 v 7 v > 7), and late induction registration. For S9916, the model was adjusted for treatment assignment (docetaxel and estramustine compared with mitoxantrone and prednisone), age (in decades), ethnicity (African American v other), pretreatment PSA level (in 50-ng/mL increments), SWOG performance status (PS; 2-3 v 0-1), presence of bone pain, prior prostatectomy, and type of progression at study entry (increasing PSA only v progression of measurable or bone disease).
Primary analyses included PSA progression (PSA-P) in all models as a time-varying covariate. This approach accounts for patient development of PSA-P at different times; therefore, exposure time to this risk factor varies by patient. To allow a comparison between previously identified PSA response end points and PSA-P as they relate to subsequent survival, landmark analyses were also performed. In this approach, patients who survived to a specified time were classified by their PSA-P status at that time, and subsequent survival was compared in the resulting subgroups. Landmark times were 7 months (the end of induction therapy) for S9346 and 3 months (after four courses of chemotherapy) for S9916, as previous analyses demonstrated these time points to be clinically meaningful.3,5
Finally, landmark analyses also were performed with PSA response variables previously found to be strong predictors of survival in S93463 and S9916.5 The response end point used in S9346 was PSA decline to ≤ 4 ng/mL at months 6 and 7 (the end of induction therapy), and in S9916 a PSA decline of ≥ 30% during the first 3 months on treatment. Slightly smaller subsets of the patients were eligible for this portion of the analysis. The objective of these analyses was to explore how strongly PSA-P predicts survival compared with these known response predictors.
From all models, the r2 coefficient of explained randomness was calculated by using the method of O'Quigley et al (Stat Med 24:479-489, 2005). This r2 value estimates the fraction of information about overall survival that is explained by the model, and it ranges from 0% to 100%. For univariate analyses of the PSA end points, the crude r2 was calculated. For multivariate analyses, the partial r2 that described the fraction of survival information explained by the PSA end point after adjustment for baseline covariates was calculated.
Footnotes
Presented in part at the 44th annual meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00002651.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: E. David Crawford, Sanofi-aventis Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Maha Hussain, Bryan Goldman, Cathy Tangen
Administrative support: Bryan Goldman, Cathy Tangen
Provision of study materials or patients: Maha Hussain, Celestia S. Higano, Daniel P. Petrylak, George Wilding, Atif M. Akdas, Eric J. Small, Bryan J. Donnelly, Patrick A. Burch, Robert S. DiPaola, E. David Crawford
Collection and assembly of data: Bryan Goldman, Cathy Tangen
Data analysis and interpretation: Maha Hussain, Bryan Goldman, Cathy Tangen
Manuscript writing: Maha Hussain, Bryan Goldman, Cathy Tangen, Celestia S. Higano, Daniel P. Petrylak, Atif M. Akdas, Eric J. Small, Bryan J. Donnelly, Patrick A. Burch, Robert S. DiPaola, E. David Crawford
Final approval of manuscript: Maha Hussain, Bryan Goldman, Cathy Tangen, Celestia S. Higano, Daniel P. Petrylak, George Wilding, Atif M. Akdas, Eric J. Small, Bryan J. Donnelly, Subramanian Kanaga Sundram, Patrick A. Burch, Robert S. DiPaola, E. David Crawford
REFERENCES
- 1.Kelly WK, Scher HI, Mazumdar M, et al. Prostate-specific antigen as a measure of disease outcome in metastatic hormone-refractory prostate cancer. J Clin Oncol. 1993;11:607–615. doi: 10.1200/JCO.1993.11.4.607. [DOI] [PubMed] [Google Scholar]
- 2.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: Recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 3.Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: Data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24:3984–3990. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.Petrylak DP, Ankerst DP, Jiang CS, et al. Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99-16. J Natl Cancer Inst. 2006;98:516–521. doi: 10.1093/jnci/djj129. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberger M, Crawford ED, Mcleod D, et al. The prognostic significance of prostate specific antigen (PSA) in stage D2 prostate cancer (PC) interim evaluation of intergroup study 0105. J Clin Oncol. 1995;14:235a. abstr 613. [Google Scholar]
- 7.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Quigley J, Xu R, Stare J. Explained randomness in proportional hazards methods. Stat Med. 2005;24:479–489. doi: 10.1002/sim.1946. [DOI] [PubMed] [Google Scholar]