Abstract
Purpose
Increased reactive oxygen species may exhaust the antioxidant capability of human defense systems, leading to oxidative stress and cancer development. Urinary F2-isoprostanes, secondary end products of lipid peroxidation, are more accurate markers of oxidative stress than other available biomarkers. No prospective study has investigated whether levels of 15-F2t-isoprostane (15-F2t-IsoP) and its metabolite 2,3-dinor-5,6-dihydro-15-F2t-IsoP (15-F2t-IsoPM) are related to breast cancer risk.
Patients and Methods
We conducted a nested case-control study within the Shanghai Women's Health Study, a population-based cohort study of 74,942 Chinese women between 40 and 70 years of age. Prediagnostic urinary 15-F2t-IsoP and 15-F2t-IsoPM were measured by gas chromatography mass spectrometry for 436 breast cancer cases and 852 individually matched controls.
Results
Urinary excretion of isoprostanes was not significantly different between cases and controls. However, among overweight women, levels of isoprostanes were positively associated with breast cancer risk, which became stronger with increasing body mass index (BMI). Among women with a BMI ≥ 29, the odds ratio (OR) increased to 10.27 (95% CI, 2.41 to 43.80) for the highest compared with the lowest tertile of 15-F2t-IsoPM (P for trend = .003; P for interaction = .0004). In contrast, 15-F2t-IsoP and 15-F2t-IsoPM were inversely associated with breast cancer risk among nonoverweight women. Among women with a BMI ≤ 23, breast cancer risk was reduced with increasing 15-F2t-IsoP levels in a dose-response manner (P for trend = .006), with an OR of 0.46 (95% CI, 0.26 to 0.80) for the highest tertile versus the lowest (P for interaction = .006).
Conclusion
Our results suggest that the role of oxidative stress in breast cancer development may depend on adiposity.
INTRODUCTION
Substantial evidence suggests estrogens play critical roles in the etiology of breast cancer.1,2 Nevertheless, the molecular basis for estrogen carcinogenesis is still unclear. One proposed mechanism is that excess estrogen exposure leads to elevated generation of reactive oxygen species (ROS; eg, 15-F2t-isoprostane [15-F2t-IsoP]) through estrogen-receptor3 and/or metabolic activation pathways.4,5 These ROS, along with ROS from external sources, such as smoking and dietary oxidants, may exhaust human antioxidant defense capability, leading to oxidative stress.6–10 Single-stranded DNA, present during breast cell division under estrogen stimulation, is particularly susceptible to damage caused by ROS.8
Accumulating evidence suggests that F2-isoprostanes (mainly 15-F2t-IsoP), secondary end products of lipid peroxidation of arachidonic acid, are more accurate markers of oxidative stress in humans than other available biomarkers.11–13 Unmetabolized 15-F2t-IsoP, however, may be artificially generated in vitro in fluids by autoxidation. Furthermore, the level may also be significantly affected by the local renal isoprostane production.14 After β-oxidation, 15-F2t-IsoP converts to 2,3-dinor-5,6-dihydro-15-F2t-IsoP (15-F2t-IsoPM), a metabolite not subject to autoxidation and renal production.15 Morrow et al14 developed a method with both high sensitivity and accuracy to measure 15-F2t-IsoPM using gas chromatography/negative ion chemical ionization mass spectrometry (GC/NICI MS). Previous epidemiologic studies have seldom considered 15-F2t-IsoPM, and most have used immunoassays to measure 15-F2t-IsoP and its metabolite because the GC/NICI MS method is lab-intensive and expensive. Validation studies, however, found merely moderate correlation coefficients, 0.6316 and 0.51,17 respectively, for 15-F2t-IsoP and 15-F2t-IsoPM between measurements assayed by GC/NICI MS and those determined by immunoassays. To our knowledge, only one case-control study reported that the level of 15-F2t-IsoP was increased among breast cancer cases compared with controls using an immunoassay.18 No study has prospectively investigated the etiologic role of 15-F2t-IsoP and its metabolite in the development of breast or other cancers. By using the GC/NICI MS assay, we prospectively investigated the associations of urinary 15-F2t-IsoP and 15-F2t-IsoPM with breast cancer risk in a nested case-control study conducted among Chinese women, a population with traditionally low risk for breast cancer.
Accumulative data indicate that ROS19–21 play a critical role in the regulation of multiple normal physiologies, including microorganism defense, cell signal transduction, cell growth, and cellular homeostasis,13 as well as induction of apoptosis and senescence, two key mechanisms for cancer prevention.13,22 Both urinary 15-F2t-IsoP and 15-F2t-IsoPM are detectable in healthy subjects, and normal levels have been defined in Western populations.11,14 However, higher levels of 15-F2t-IsoP have been linked to a number of diseases or conditions, such as smoking, type 2 diabetes, cardiovascular diseases, Alzheimer's disease, asthma, and other inflammatory diseases.11,13,23 These findings suggest that excessive production of ROS has detrimental effects, whereas basal level of ROS under physiologic conditions is critical in cancer prevention. Recently, several studies have found obese women to have a significantly higher level of 15-F2t-IsoP.13,24 Previously, we found that the associations between several risk factors and breast cancer differed by body mass index (BMI) level.25–27 We, therefore, hypothesized that the associations of levels of isoprostanes with breast cancer may vary by BMI status and evaluated this hypothesis in the current study.
PATIENTS AND METHODS
The Shanghai Women's Health Study
Detailed methods of the Shanghai Women's Health Study (SWHS) have been reported elsewhere.28 Briefly, 74,942 Chinese women aged 40 to 70 years were interviewed from March 1997 to May 2000, yielding a 92% participation rate. Trained interviewers elicited information on demographic characteristics, medical history, anthropometrics, usual dietary habits, physical activities, and other lifestyle factors. Two measurements were performed at the end of the in-person interview for weight, height, and circumferences of the waist and hips. A third measurement was conducted if the difference between the first two measurements was larger than 1 kg for weight, 1 cm for height, and 0.5 cm for circumferences. The study was approved by all relevant institutional review boards in China and the United States. All participants provided informed written consent.
Cohort follow-up and outcome ascertainment.
The SWHS participants were tracked for occurrence of cancer by follow-up surveys conducted every 2 years and annual linkage to records of the population-based Shanghai Cancer Registry and death certificates collected by the Shanghai Municipal Center for Disease Control and Prevention. Nearly all cohort members were successfully observed, with the response rates for the first in-person follow-up survey being 99.8% (2000 to 2002), for the second in-person follow-up survey being 98.7% (2002 to 2004), and for the third in-person follow-up survey being 96.7% (2004 to 2007). All cancer cases were verified by home visits and medical record review.
Sample collection, storage, and processing.
A spot urine sample was collected into a sterilized 100-mL cup containing 125 mg of ascorbic acid. The collected samples were kept in a portable, insulated bag with ice packs (at approximately 0° to 4°C).28 The urine samples were processed within 6 hours of collection and stored at −80°C.
Nested Case-Control Design
The nested case-control study was conducted among women who donated a urine sample (approximately 88% of the cohort) at baseline or during first follow-up (approximately 2 years later). Among them, 436 cases were identified during an average of 7.5 years of follow-up (more than 85% of these urine samples were collected at baseline). Two cancer-free controls were randomly selected and matched with each case on age at baseline (± 2 years), date at study enrollment (≤ 30 days), time (morning or afternoon) of urine collection, interval since last meal (≤ 2 hours), menopausal status (pre- or postmenopausal), and antibiotic use (yes/no) in the past week. Two controls were successfully matched with each of 416 cases, whereas 20 cases were matched with only one control each, yielding a total of 852 controls.
Quantification of Urinary F2-Isoprostanes and 5-F2t-IsoPM
Urinary excretion of 15-F2t-IsoP and its major metabolite of 15-F2t-IsoP, 2,3-dinor-5,6-dihydro-15-F2t-IsoP (2,3-dinor-5,6-dihydro-8-IsoPGF2α) were measured by GC/NICI MS. The method has been reported in detail previously.14,29,30 Briefly, GC/NICI MS was performed using an Agilent 5973 GC/MS instrument with an Agilent computer system (Santa Clara, CA). The column temperature was programmed from 190°C to 300°C at 15°C/min. The metabolite was chemically synthesized and converted to an18O2-labeled derivative for use as an internal standard.31 Final results were expressed after adjusting for creatinine concentrations (nanograms per milligram of creatinine). Precision of the assay was ± 4% and accuracy was 97%. The lower limit of sensitivity was approximately 20 picogram (pg).14
Statistical Analysis
Baseline covariates were compared between cases and controls to evaluate potential confounding factors (Table 1). The paired t test and Wilcoxon signed-rank test were used to compare levels of isoprostanes between cases and controls. Isoprostanes levels were categorized based on tertile distribution in controls. Conditional logistic regression was used to analyze the association between concentrations of 15-F2t-IsoP and 15-F2t-IsoPM and breast cancer risk. Potential confounding factors included in final regression models are listed in the footnotes of Tables 2 and 3. Stratified analyses by menopausal status and BMI at baseline were performed to evaluate whether breast cancer risk differed according to these factors. We used the WHO cut points for international classification of BMI (ie, BMI of 25 for overweight and 30 for obesity) as well as cut points for Asian populations (23 for overweight and 27.5 for obesity), recommended by WHO expert consultation.32 The sample size in the strata became smaller as BMI level increased, leading to unstable estimation of CIs for women with BMI ≥ 30. Thus we also used BMI of ≥ 29 as an additional cut point. Because this is a matched case-control design, stratum-specific odds ratios (ORs) were derived from conditional regression with the inclusion of terms for main effect along with two interaction terms. By adding these interaction terms, case-control pairs were not broken, and all of the subjects were included in the model building.27 P values of less than .05 (two-sided probability) were considered statistically significant. Tests for trend were performed by entering the categoric variables as continuous variable in the model. Statistical analyses were conducted using SAS statistical software (version 9.1; SAS Institute, Cary, NC).
Table 1.
Comparison of Breast Cancer Cases and Controls by Selected Baseline Demographic and Risk Factors in a Nested Case-Control Study Within the Shanghai Women's Health Study, 1997 to 2006
| Patient Characteristic | Cases(n = 436) | Controls(n = 852) | P* |
|---|---|---|---|
| Age, years | .87 | ||
| Mean | 53.3 | 53.4 | |
| SD | 8.9 | 8.9 | |
| Income, % | |||
| Low | 28.4 | 29.8 | |
| Middle | 38.5 | 38.5 | |
| High | 33.0 | 31.7 | .84 |
| Education, % | |||
| Elementary school or less | 15.4 | 23.1 | |
| Middle or high school | 68.7 | 65.6 | |
| Middle or high school | 15.9 | 11.3 | < .01 |
| Breast cancer in first-degree relative, % | 4.6 | 1.6 | < .01 |
| Ever had breast fibroadenoma, % | 7.8 | 4.6 | .02 |
| Age at menarche, years | .06 | ||
| Mean | 14.8 | 15.0 | |
| SD | 1.8 | 1.7 | |
| Age at first live birth, years† | < .01 | ||
| Mean | 26.1 | 25.5 | |
| SD | 4.2 | 4.1 | |
| Months of breastfeeding† | < .01 | ||
| Mean | 13.8 | 16.4 | |
| SD | 15.8 | 17.6 | |
| Postmenopausal, % | 51.8 | 52.4 | .84 |
| Age at menopause, years | .22 | ||
| Mean | 49.3 | 48.8 | |
| SD | 4.8 | 3.9 | |
| Use of hormone replacement therapy, % | 6.0 | 3.5 | .04 |
| Physically active past 5 years, % | 35.3 | 33.2 | .45 |
| BMI, kg/m2 | .39 | ||
| Mean | 24.3 | 24.2 | |
| SD | 3.4 | 3.4 | |
| Waist-to-hip ratio | .91 | ||
| Mean | 0.82 | 0.82 | |
| SD | 0.06 | 0.05 | |
| Ever smoke regularly, % | 1.1 | 2.7 | .07 |
| Ever exposure to passive smoking, % | 79.8 | 82.9 | .40 |
| Ever drink alcohol regularly, % | 1.8 | 3.0 | .20 |
| Ever drink tea regularly, % | 32.1 | 28.4 | .17 |
| Use of ginseng regularly, % | 30.0 | 26.3 | .15 |
| Dietary factors | |||
| Daily energy intake, kcal | .14 | ||
| Mean | 1,668.9 | 1,701.1 | |
| SD | 357.6 | 402.6 | |
| Daily intake of fish | .84 | ||
| Mean | 51.3 | 51.8 | |
| SD | 42.0 | 47.9 | |
| Daily intake of red meat, g | .02 | ||
| Mean | 48.0 | 52.6 | |
| SD | 31.7 | 37.4 | |
| Daily intake of vegetables | .68 | ||
| Mean | 297.8 | 301.9 | |
| SD | 161.1 | 170.7 | |
| Daily intake of fruits | .79 | ||
| Mean | 265.5 | 268.2 | |
| SD | 170.9 | 173.0 | |
| Daily intake of isoflavones, mg | 29.7 | 32.3 | .04 |
| Mean | 20.3 | 24.2 | |
| SD |
Abbreviation: SD, standard deviation.
For χ2 test (categorical variables) or t test (continuous variables).
Among parous women only.
Table 2.
ORs and 95% CIs for Risk of Breast Cancer Associated With Urinary Excretion of 15-F2t-IsoP and 15-F2t-IsoPM and Stratified by Menopausal Status in a Nested Case-Control Study Within the Shanghai Women's Health Study, 1997 to 2006
| Factor | No. of Cases | No. of Controls | Urinary Excretion Rate of Isoprostanes by Tertile* |
|||||
|---|---|---|---|---|---|---|---|---|
| T1 (low), OR | T2 |
T3 |
P for Trend | |||||
| OR | 95% CI | OR | 95% CI | |||||
| All subjects | ||||||||
| 15-F2t-IsoP | ||||||||
| Model 1 | 434 | 851 | 1.00 | 0.96 | 0.71 to 1.30 | 0.84 | 0.61 to 1.15 | .27 |
| Model 2 | 1.00 | 1.00 | 0.72 to 1.38 | 0.91 | 0.64 to 1.28 | .58 | ||
| 15-F2t-IsoPM | ||||||||
| Model 1 | 410 | 803 | 1.00 | 1.03 | 0.77 to 1.40 | 0.82 | 0.59 to 1.15 | .27 |
| Model 2 | 1.00 | 1.10 | 0.80 to 1.52 | 0.98 | 0.68 to 1.41 | .95 | ||
| Premenopausal women† | ||||||||
| 15-F2t-IsoP | ||||||||
| Model 1 | 209 | 405 | 1.00 | 1.03 | 0.67 to 1.59 | 0.59 | 0.36 to 0.95 | .03 |
| Model 2 | 1.00 | 1.07 | 0.67 to 1.69 | 0.58 | 0.35 to 0.98 | .04 | ||
| 15-F2t-IsoPM | ||||||||
| Model 1 | 198 | 384 | 1.00 | 0.93 | 0.63 to 1.38 | 0.66 | 0.40 to 1.08 | .13 |
| Model 2 | 1.00 | 0.96 | 0.63 to 1.44 | 0.68 | 0.41 to 1.14 | .30 | ||
| Postmenopausal women† | ||||||||
| 15-F2t-IsoP | ||||||||
| Model 1 | 225 | 445 | 1.00 | 0.89 | 0.58 to 1.36 | 1.12 | 0.73 to 1.72 | .61 |
| Model 2 | 1.00 | 0.89 | 0.56 to 1.41 | 1.33 | 0.83 to 2.13 | .23 | ||
| 15-F2t-IsoPM | ||||||||
| Model 1 | 212 | 418 | 1.00 | 1.25 | 0.78 to 2.02 | 1.04 | 0.64 to 1.68 | .99 |
| Model 2 | 1.00 | 1.45 | 0.85 to 2.47 | 1.47 | 0.86 to 2.53 | .19 | ||
NOTE. Model 1, conditional logistic regression model adjusting for age only. Model 2, conditional logistic regression model adjusting for age, education, age at menarche (continuous), age at first live birth (continuous), months of breastfeeding (continuous), history of breast fibroadenoma (yes/no), first-degree family cancer history (yes/no), ever smoker (never/ever), total intake of red meat and isoflavones, and use of hormone replacement therapy.
Abbreviations: 15-F2t-IsoP, 15-F2t-isoprostane; 15-F2t-IsoPM, 2,3-dinor-5,6-dihydro-15-F2t-IsoP; OR, odds ratio.
Thirty-third and 66th percentiles were 1.32 and 1.99 for 15-F2t-IsoP, respectively, and 0.44 and 0.66 for 15-F2t-IsoPM.
P for interactions were .03 and < .01 for 15-F2t-IsoP, 0.40 and 0.12 for 15-F2t-IsoPM in age-adjusted and full-adjusted models, respectively.
Table 3.
ORs and 95% CIs for Risk of Breast Cancer Associated With Urinary Excretion of 15-F2t-IsoP and 15-F2t-IsoPM, Stratified by BMI, in a Nested Case-Control Study Within the Shanghai Women's Health Study, 1997 to 2006
| BMI and Isoprostanes | No. of Cases | No. of Controls | Urinary Excretion Rate of Isoprostanes by Tertile* |
|||||
|---|---|---|---|---|---|---|---|---|
| T1 (low), OR | T2 |
T3 |
P for Trend | |||||
| OR | 95% CI | OR | 95% CI | |||||
| BMI less than 23† | ||||||||
| 15-F2t-IsoP | 158 | 293 | 1.00 | 0.66 | 0.39 to 1.13 | 0.46 | 0.26 to 0.80 | .006 |
| 15-F2t-IsoPM | 149 | 279 | 1.00 | 0.97 | 0.56 to 1.67 | 0.79 | 0.44 to 1.43 | .49 |
| BMI less than 25† | ||||||||
| 15-F2t-IsoP | 268 | 520 | 1.00 | 0.84 | 0.56 to 1.26 | 0.71 | 0.46 to 1.10 | .12 |
| 15-F2t-IsoPM | 253 | 494 | 1.00 | 0.83 | 0.55 to 1.25 | 0.87 | 0.56 to 1.38 | .60 |
| BMI ≥ 25† | ||||||||
| 15-F2t-IsoP | 166 | 331 | 1.00 | 1.36 | 0.82 to 2.26 | 1.36 | 0.80 to 2.33 | .25 |
| 15-F2t-IsoPM | 157 | 309 | 1.00 | 1.73 | 1.00 to 2.99 | 1.15 | 0.64 to 2.07 | .67 |
| BMI ≥ 27.5† | ||||||||
| 15-F2t-IsoP | 69 | 154 | 1.00 | 2.07 | 0.95 to 4.52 | 1.32 | 0.59 to 2.95 | .49 |
| 15-F2t-IsoPM | 65/ | 146 | 1.00 | 4.13 | 1.57 to 10.86 | 2.59 | 0.93 to 7.20 | .14 |
| BMI ≥ 29† | ||||||||
| 15-F2t-IsoP | 42 | 81 | 1.00 | 2.39 | 0.85 to 6.73 | 1.53 | 0.52 to 4.51 | .48 |
| 15-F2t-IsoPM | 40 | 77 | 1.00 | 10.20 | 2.35 to 44.29 | 10.27 | 2.41 to 43.80 | .003 |
| BMI ≥ 30† | ||||||||
| 15-F2t-IsoP | 28 | 52 | 1.00 | 2.95 | 0.77 to 11.27 | 2.06 | 0.52 to 8.23 | .41 |
| 15-F2t-IsoPM | 28 | 48 | 1.00 | 13.62 | 1.38 to 134.08 | 23.47 | 2.46 to 223.69 | .003 |
NOTE. Conditional logistic regression model adjusting for age, education, age at menarche (continuous), age at first live birth (continuous), months of breastfeeding (continuous), history of breast fibroadenoma (yes/no), first-degree family cancer history (yes/no), ever smoker (never/ever), total intake of red meat and isoflavones, and use of hormone replacement therapy.
Abbreviations: 15-F2t-IsoP, 15-F2t-isoprostane; 15-F2t-IsoPM, 2,3-dinor-5,6-dihydro-15-F2t-IsoP; BMI, body mass index; OR, odds ratio.
Thirty-third and 66th percentiles were 1.32 and 1.99 for 15-F2t-IsoP, and 0.44 and 0.66 for 15-F2t-IsoPM.
P for interactions were .006, .13, .13, .22, and .24 for 15-F2t-IsoP and .63, .10, .008, .0004, and .001 for 15-F2t-IsoPM using BMI cut points of 23, 25, 27.5, 29, and 30, respectively.
RESULTS
The median age of cases at diagnosis was 53.3 years. Approximately half of the cases and controls were postmenopausal at baseline (Table 1). Compared with controls, cases were more likely to have higher education, earlier age at menarche, later age at first live birth, a family history of breast cancer, and a history of breast fibroadenoma. Cases were also more likely to take hormone replacement therapy than controls, although the overall rate was low. Controls were more likely to smoke cigarettes, have a longer breastfeeding period, and have a higher intake of red meat and soy isoflavones compared with cases.
The correlation coefficient between urinary 15-F2t-IsoP and 15-F2t-IsoPM was 0.31 in all subjects (0.37 among breast cancer cases and 0.27 among controls; P < .01 for all). The urinary excretion levels (mean ± standard deviation) for 15-F2t-IsoP were 1.95 ± 1.51 for cases and 1.99 ± 2.16 for healthy controls. The corresponding levels for F2t-IsoPM were 0.69 ± 0.67 and 0.71 ± 0.73, respectively. The differences between cases and controls were statistically insignificant for both markers.
Breast cancer risk was not associated with urinary excretions of isoprostanes overall (Table 2). However, among premenopausal women, urinary 15-F2t-IsoP and 15-F2t-IsoPM were associated with a reduced risk of breast cancer. The inverse association with 15-F2t-IsoP was statistically significant in both age-adjusted and multivariate models, with an OR of 0.58 (95% CI, 0.36 to 0.95) for the highest tertile versus the lowest (P for trend = .04) in the multivariate model. In contrast, among postmenopausal women, both biomarkers were associated with an increased risk of breast cancer, although none of the associations was statistically significant. The tests for multiplicative interaction with menopausal status were statistically significant for 15-F2t-IsoP in both age-adjusted (P for interaction = .03) and multivariate (P for interaction = .008) models.
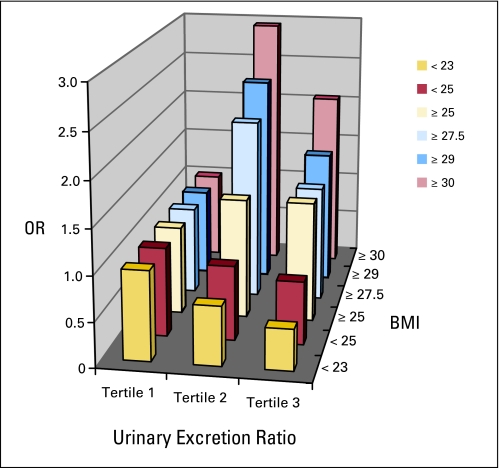
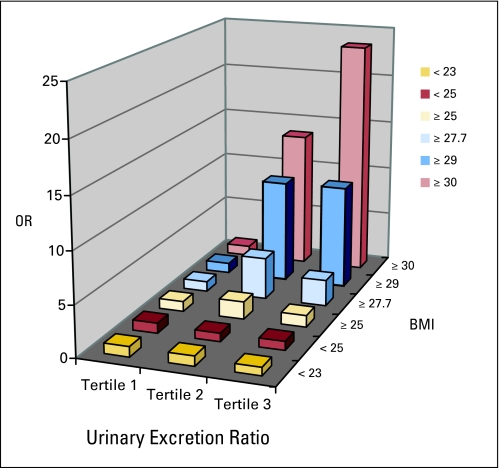
Levels of 15-F2t-IsoP and 15-F2t-IsoPM were inversely associated with breast cancer risk among women with a BMI less than 25, although the associations for 15-F2t-IsoPM were not statistically significant (Table 3; Figs 1 and 2). Among women with a BMI less than 23, 15-F2t-IsoP was inversely associated with breast cancer risk in a dose-response manner (P for trend = .006) with an OR of 0.46 (95% CI, 0.26 to 0.80) for the highest tertile versus the lowest. The test for interaction with BMI (< 23 v ≥ 23) was significant (P for interaction = .006). The reduction in risk among women with a low BMI appeared in both pre- and postmenopausal women, but was only significant in premenopausal women (data not shown). Conversely, 15-F2t-IsoP and 15-F2t-IsoPM were positively associated with breast cancer risk among women with a BMI ≥ 25. The magnitude of the associations became stronger among women with a higher BMI. In particular, 15-F2t-IsoPM was associated with a two- to four-fold increased risk among women with a BMI ≥ 27.5; for women with a BMI ≥ 29, the ORs increased to 10.20 (95% CI, 2.35 to 44.29) for the middle tertile and 10.27 (95% CI, 2.41 to 43.80) for the highest tertile compared with the lowest tertile (P for trend = .003) with a significant interaction with BMI (BMI < 29 v BMI ≥ 29; P for interaction = .0004). The corresponding ORs further increased to 13.62 (95% CI, 1.38 to 134.08) and 23.47 (95% CI, 2.46 to 223.69; P for interaction = .001) among those with a BMI of ≥ 30. In sensitivity analysis excluding cases diagnosed within 3 years from urine collection, the corresponding ORs for 15-F2t-IsoPM were 8.68 (95% CI, 0.75 to 103.49) and 10.60 (95% CI, 0.96 to 116.56) in the middle and highest tertile, respectively. The elevated risks were observed in both pre- and postmenopausal women (data not shown). In the same sensitivity analysis, the inverse association for 15-F2t-IsoP among women with BMI less than 23 became more pronounced.
Fig 1.
Odds ratios for risk of breast cancer associated with tertile urinary excretion of 15-F2t-isoprostane, stratified by body mass index (BMI), in a nested case-control study within the Shanghai Women's Health Study, 1997 to 2006.
Fig 2.
Odds ratios for risk of breast cancer associated with tertile urinary excretion of 2,3-dinor-5,6-dihydro-15-F2t-IsoP, stratified by body mass index (BMI), in a nested case-control study within the Shanghai Women's Health Study, 1997 to 2006.
DISCUSSION
In this nested case-control study, we prospectively evaluated urinary excretion of 15-F2t-IsoP and its metabolite (15-F2t-IsoPM) in relation to subsequent breast cancer risk. To our knowledge, no prospective study has evaluated the associations of isoprostanes or other lipid peroxidation biomarkers with breast cancer risk. Two small case-control studies found that plasma levels of malonaldehyde, an indirect measure of lipid peroxidation,33 were elevated among patients with breast cancer as compared with controls,34,35 consistent with that found in a recent case-control study using urinary 15-F2t-IsoP.18 Another small study observed that normal breast tissues from patients with cancer had an increased level of malonaldehyde adducts than that found in breast tissues of noncancer controls.36 None of the previous case-control studies have examined the potential modifying effect of BMI.
In the current study, an increased risk of breast cancer associated with urine levels of isoprostanes, particularly 15-F2t-IsoPM, was mainly observed among women with a high BMI, and the association became stronger with increasing BMI level. Except for one recent study conducted among older adult men, previous studies consistently found that levels of 15-F2t-IsoP were significantly correlated with BMI.13,37,38 Women with a BMI greater than 28 had a higher level of 15-F2t-IsoP regardless of fat distribution,13 whereas weight loss was associated with a decreased level of 15-F2t-IsoP.13,39 Because the same cut points of isoprostanes were used in each stratum of the nonoverweight, overweight, or obese women, our findings indicate that some obese women could have normal levels of isoprostanes. Our data further show that obese women were at a substantially increased risk for breast cancer only when they had increased levels of isoprostanes. The association for 15-F2t-IsoPM is much stronger than 15-F2t-IsoP, suggesting that F2t-IsoPM may be a relatively more accurate and specific biomarker of lipid peroxidation. Alternatively, F2t-IsoPM, including activity of β-oxidation, may be more directly involved in the pathogenesis of breast cancer.21 In vitro addition of 15-F2t-IsoP led to a two- to four-fold increase in DNA and cell proliferation,21 indicating that ROS play a critical role in carcinogenesis. If our findings are confirmed in further studies, it may point a way to preventive strategy by reducing 15-F2t-IsoP and F2t-IsoPM levels among postmenopausal women with a high BMI through weight loss or other antioxidant approaches.
Our observation of an inverse association with 15-F2t-IsoP and F2t-IsoPM levels among premenopausal women or those with a low BMI are consistent with the fact that several protective factors for breast cancer risk, such as physical activity,40,41 parity (normal pregnancy),13,42 and preeclampsia,13 were associated with significantly elevated levels of lipid peroxidation.43,44 Compared with postmenopausal women, 15-F2t-IsoP was lower among healthy premenopausal women.13 Under normal physiological conditions, 1% to 2% of the oxygen consumed in mitochondria is converted to superoxide anion (O· 2-) and, in turn, ROS,45,46 which are necessary to trigger p53 activation, directly mediate apoptosis19,47 and induce senescence.22 In addition, 15-F2t-IsoP was found to stimulate high glucose-induced synthesis of transforming growth factor β1,48,49 an important tumor suppressor.50
Our findings of a null overall association and differing associations with oxidative stress by BMI level may provide one possible explanation for the results from clinical trials.51,52 These studies found α-tocopherol supplementation provided no overall benefit for total mortality and for incidence and mortality of major cardiovascular diseases or cancer, including breast cancer.52 None of these studies evaluated the potential modifying effect of BMI. Also consistent with these findings, several recent clinical trials found that the antioxidant vitamin E reduced 15-F2t-IsoP levels in several disease conditions in which levels of isoprostanes were increased, but no reduction in isoprostanes was found with vitamin E supplementation in healthy subjects.13
It is worth noting that the obesity rate was much lower in our study population than that in the United States.28 We also found that levels of both isoprostanes, particularly 15-F2t-IsoPM, in our study population were substantially higher than levels (mean ± 2 standard deviations) of urinary 15-F2t-IsoP (1.6 ± 0.6 ng/mg creatinine) and 15-F2t-IsoPM (0.39 0.18 ng/mg creatinine) in healthy subjects in the United States.11,14 In comparison, the age-adjusted incidence rates of breast cancer were more than three times higher in the United States than in Shanghai, China.53 These ecologic data, therefore, supported our findings. The reasons for the higher levels of 15-F2t-IsoP and F2t-IsoPM among healthy women in China than the United States are unclear, although underlining differences in exposure profiles may play a role. For instance, the standard metabolic equivalent (MET), a summary estimate of physical activity energy expenditure, was 13 MET-hours/d in our study cohort54 compared with approximately 7 MET-hours/d among healthy, middle-aged Canadian women55 when a broad spectrum of light, moderate, and vigorous activities was evaluated.
The present study has several notable strengths. Levels of 15-F2t-IsoP were measured together with its major metabolite15-F2t-IsoPM using a newly developed, more sensitive method. The parent population-based cohort study had remarkably high rates for baseline participation and follow-up, which minimized selection bias. To evaluate whether incipient patients with breast cancer may have contributed to the elevated associations observed among women with a high BMI, we conducted sensitivity analyses by excluding women who were diagnosed with breast cancer within 3 years from urine collection and found similar results. Still, we cannot exclude the possibility that 15-F2t-IsoP and 15-F2t-IsoPM are not etiologic factors but serve only as biomarkers for cancer progression and/or early detection, due to the relatively short cohort follow-up time (average of 7.5 years). In addition, reliability studies found that at a group level, 15-F2t-IsoP measured in one spot urine did not significantly differ from that measured using multiple urines or 24-hour urine collection in 1 day.13 Previous studies generated inconsistent results on the interday variation13,56 whereas our unpublished data conducted in the study population suggest that the major contributor to intraperson variation is seasonal fluctuation. Therefore, cases and controls were matched on urine collection date. Because interday variation is random, any residual interday variation may lead to nondifferential misclassification, which usually biases the result to the null. To the extent that residual interday variation levels exist in our data, the true associations of isoprostanes levels with breast cancer risk could be stronger than those we observed. Finally, the sample size of this study is sufficiently large for stratified analyses. The number of women with a BMI of ≥ 30 is small in our study. Despite this, we still found a significant result.
Future studies are necessary to confirm our findings, particularly among obese women. Our findings, if validated, may lead to a new path of inquiry in the early detection and/or prevention of breast cancer and other diseases related to oxidative stress.
Acknowledgment
Jason Morrow, MD, our beloved colleague, long-term collaborator, and key coinvestigator of the project, died shortly after he read the manuscript. Without him, our research work would have been impossible. This article is dedicated to his memory. We thank the Shanghai residents who participated in the study and the research staff of the Shanghai Women's Health Study for their dedication and contributions to the study; and Brandy Sue Venuti for technical assistance in the preparation of this manuscript.
Footnotes
Supported by Grant No. R01CA106591 from the National Institutes of Health (Q.D.) as well as Grants No. R01CA70867 from the National Institutes of Health (W.Z.) and N02 CP1101066 from the National Institutes of Health Intramural Program (X.O.S.) for the parent study.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Qi Dai, Xiao-Ou Shu, Wei Zheng
Financial support: Qi Dai, Wei Zheng
Administrative support: Qi Dai, Yu-Tang Gao, Yongbing Xiang, Wei Zheng
Provision of study materials or patients: Yu-Tang Gao, Xiao-Ou Shu, Gong Yang, Yongbing Xiang, Wei Zheng
Collection and assembly of data: Qi Dai, Yu-Tang Gao, Xiao-Ou Shu, Gong Yang, Ginger Milne, Qiuyin Cai, Hui Cai, Honglan Li, Yongbing Xiang, Wei Zheng
Data analysis and interpretation: Qi Dai, Xiao-Ou Shu, Wanqing Wen, Hui Cai, Wong-Ho Chow, Wei Zheng
Manuscript writing: Qi Dai, Xiao-Ou Shu, Wong-Ho Chow
Final approval of manuscript: Qi Dai, Xiao-Ou Shu, Qiuyin Cai, Nathaniel Rothman, Wong-Ho Chow, Wei Zheng
REFERENCES
- 1.Henderson BE, Pike MC, Bernstein L, et al. Breast cancer. In: Schottenfeld D, Parkin DM, editors. Cancer Epidemiology and Prevention. ed 2. New York, NY: Oxford University Press; 1996. pp. 1022–1039. [Google Scholar]
- 2.Hankinson SE, Willett WC, Manson JE, et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90:1292–1299. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 3.Mobley JA, Brueggemeier RW. Estrogen receptor-mediated regulation of oxidative stress and DNA damage in breast cancer. Carcinogenesis. 2004;25:3–9. doi: 10.1093/carcin/bgg175. [DOI] [PubMed] [Google Scholar]
- 4.Bhat HK, Calaf G, Hei TK, et al. Critical role of oxidative stress in estrogen-induced carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:3913–3918. doi: 10.1073/pnas.0437929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: Review and perspec-tives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr. 2000:67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- 7.Cavalieri E, Frenkel K, Liehr JG, et al. Estrogens as endogenous genotoxic agents: DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000:75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 8.Jefcoate CR, Liehr JG, Santen RJ, et al. Tissue-specific synthesis and oxidative metabolism of estrogens. J Natl Cancer Inst Monogr. 2000:95–112. doi: 10.1093/oxfordjournals.jncimonographs.a024248. [DOI] [PubMed] [Google Scholar]
- 9.Emerit I. Reactive oxygen species, chromosome mutation, and cancer: Possible role of clastogenic factors in carcinogenesis. Free Radic Biol Med. 1994;16:99–109. doi: 10.1016/0891-5849(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 10.Loeb LA. Endogenous carcinogenesis: Molecular oncology into the twenty-first century—Presidential address. Cancer Res. 1989;49:5489–5496. [PubMed] [Google Scholar]
- 11.Morrow JD. The isoprostanes: Their quantification as an index of oxidant stress status in vivo. Drug Metab Rev. 2000;32:377–385. doi: 10.1081/dmr-100102340. [DOI] [PubMed] [Google Scholar]
- 12.Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of oxidative stress study II: Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Basu S. F2-isoprostanes in human health and diseases: From molecular mechanisms to clinical implications. Antioxid Redox Signal. 2008;10:1405–1434. doi: 10.1089/ars.2007.1956. [DOI] [PubMed] [Google Scholar]
- 14.Morrow JD, Zackert WE, Yang JP, et al. Quantification of the major urinary metabolite of 15-F2t-isoprostane (8-iso-PGF2alpha) by a stable isotope dilution mass spectrometric assay. Anal Biochem. 1999;269:326–331. doi: 10.1006/abio.1999.4008. [DOI] [PubMed] [Google Scholar]
- 15.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 16.Proudfoot J, Barden A, Mori TA, et al. Measurement of urinary F(2)-isoprostanes as markers of in vivo lipid peroxidation-A comparison of enzyme immunoassay with gas chromatography/mass spectrometry. Anal Biochem. 1999;272:209–215. doi: 10.1006/abio.1999.4187. [DOI] [PubMed] [Google Scholar]
- 17.Il'yasova D, Morrow JD, Ivanova A, et al. Epidemiological marker for oxidant status: Comparison of the ELISA and the gas chromatography/mass spectrometry assay for urine 2,3-dinor-5,6-dihydro-15-F2t-isoprostane. Ann Epidemiol. 2004;14:793–797. doi: 10.1016/j.annepidem.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Rossner P, Jr, Gammon MD, Terry MB, et al. Relationship between urinary 15-F2t-isoprostane and 8-oxodeoxyguanosine levels and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:639–644. doi: 10.1158/1055-9965.EPI-05-0554. [DOI] [PubMed] [Google Scholar]
- 19.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 20.Morrow JD. The isoprostanes: Unique products of arachidonate peroxidation—Their role as mediators of oxidant stress. Curr Pharm Des. 2006;12:895–902. doi: 10.2174/138161206776055985. [DOI] [PubMed] [Google Scholar]
- 21.Comporti M, Signorini C, Arezzini B, et al. F2-isoprostanes are not just markers of oxidative stress. Free Radic Biol Med. 2008;44:247–256. doi: 10.1016/j.freeradbiomed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Nemoto S, Finkel T. Ageing and the mystery at Arles. Nature. 2004;429:149–152. doi: 10.1038/429149a. [DOI] [PubMed] [Google Scholar]
- 23.Montine KS, Quinn JF, Zhang J, et al. Isoprostanes and related products of lipid peroxidation in neurodegenerative diseases. Chem Phys Lipids. 2004;128:117–124. doi: 10.1016/j.chemphyslip.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006;30:400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 25.Dai Q, Shu XO, Jin F, et al. Population-based case-control study of soy food intake and breast cancer risk in Shanghai. Br J Cancer. 2001;85:372–378. doi: 10.1054/bjoc.2001.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai Q, Shu XO, Jin F, et al. Consumption of animal foods, cooking methods, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:801–808. [PubMed] [Google Scholar]
- 27.Dai Q, Franke AA, Yu H, et al. Urinary phytoestrogen excretion and breast cancer risk: Evaluating potential effect modifiers endogenous estrogens and anthropometrics. Cancer Epidemiol Biomarkers Prev. 2003;12:497–502. [PubMed] [Google Scholar]
- 28.Zheng W, Chow WH, Yang G, et al. The Shanghai Women's Health Study: Rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162:1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 29.Morrow JD, Roberts LJ. Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 30.Milne GL, Sanchez SC, Musiek ES, et al. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 31.Taber DF, Herr RJ, Gleave DM. Diastereoselective synthesis of an isoprostane: (+/−)-8-epi-PGF(2)(alpha) ethyl ester. J Org Chem. 1997;62:194–198. doi: 10.1021/jo9616365. [DOI] [PubMed] [Google Scholar]
- 32.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 33.Moore K, Roberts LJ. Measurement of lipid peroxidation. Free Radic Res. 1998;28:659–671. doi: 10.3109/10715769809065821. [DOI] [PubMed] [Google Scholar]
- 34.Ray G, Batra S, Shukla NK, et al. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res Treat. 2000;59:163–170. doi: 10.1023/a:1006357330486. [DOI] [PubMed] [Google Scholar]
- 35.Gönenç A, Ozkan Y, Torun M, et al. Plasma malondialdehyde (MDA) levels in breast and lung cancer patients. J Clin Pharm Ther. 2001;26:141–144. doi: 10.1046/j.1365-2710.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang M, Dhingra K, Hittelman WN, et al. Lipid peroxidation-induced putative malondialdehyde-DNA adducts in human breast tissues. Cancer Epidemiol Biomarkers Prev. 1996;5:705–710. [PubMed] [Google Scholar]
- 37.Keaney JF, Jr, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 38.Davì G, Guagnano MT, Ciabattoni G, et al. Platelet activation in obese women: Role of inflammation and oxidant stress. JAMA. 2002;288:2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 39.Bougoulia M, Triantos A, Koliakos G. Plasma interleukin-6 levels, glutathione peroxidase and isoprostane in obese women before and after weight loss: Association with cardiovascular risk factors. Hormones (Athens) 2006;5:192–199. doi: 10.14310/horm.2002.11182. [DOI] [PubMed] [Google Scholar]
- 40.McAnulty SR, McAnulty LS, Nieman DC, et al. Effect of alpha-tocopherol supplementation on plasma homocysteine and oxidative stress in highly trained athletes before and after exhaustive exercise. J Nutr Biochem. 2005;16:530–537. doi: 10.1016/j.jnutbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Steensberg A, Morrow J, Toft AD, et al. Prolonged exercise, lymphocyte apoptosis and F2-isoprostanes. Eur J Appl Physiol. 2002;87:38–42. doi: 10.1007/s00421-002-0584-6. [DOI] [PubMed] [Google Scholar]
- 42.Schraag S, Mandach U, Schweer H, et al. Metabolic changes, hypothalamo-pituitary-adrenal axis and oxidative stress after short-term starvation in healthy pregnant women. J Perinat Med. 2007;35:289–294. doi: 10.1515/JPM.2007.076. [DOI] [PubMed] [Google Scholar]
- 43.Gago-Dominguez M, Castelao JE, Pike MC, et al. Role of lipid peroxidation in the epidemiology and prevention of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2829–2839. doi: 10.1158/1055-9965.EPI-05-0015. [DOI] [PubMed] [Google Scholar]
- 44.Gago-Dominguez M, Jiang X, Esteban CJ. Lipid peroxidation and the protective effect of physical exercise on breast cancer. Med Hypotheses. 2007;68:1138–1143. doi: 10.1016/j.mehy.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 45.Thompson PA, Ambrosone C. Molecular epidemiology of genetic polymorphisms in estrogen metabolizing enzymes in human breast cancer. J Natl Cancer Inst Monogr. 2000:125–134. doi: 10.1093/oxfordjournals.jncimonographs.a024235. [DOI] [PubMed] [Google Scholar]
- 46.Gómez-Zubeldia MA, Hinchado G, Arbues JJ, et al. Influence of estradiol on oxidative stress in the castrated rat uterus. Gynecol Oncol. 2001;80:227–232. doi: 10.1006/gyno.2000.6057. [DOI] [PubMed] [Google Scholar]
- 47.Liu B, Chen Y, St Clair DK. ROS and p53: A versatile partnership. Free Radic Biol Med. 2008;44:1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montero A, Munger KA, Khan RZ, et al. F(2)-isoprostanes mediate high glucose-induced TGF-beta synthesis and glomerular proteinuria in experimental type I diabetes. Kidney Int. 2000;58:1963–1972. doi: 10.1111/j.1523-1755.2000.00368.x. [DOI] [PubMed] [Google Scholar]
- 49.McGowan TA, Dunn SR, Falkner B, et al. Stimulation of urinary TGF-beta and isoprostanes in response to hyperglycemia in humans. Clin J Am Soc Nephrol. 2006;1:263–268. doi: 10.2215/CJN.00990905. [DOI] [PubMed] [Google Scholar]
- 50.Chang CF, Westbrook R, Ma J, et al. Transforming growth factor-beta signaling in breast cancer. Front Biosci. 2007;12:4393–4401. doi: 10.2741/2396. [DOI] [PubMed] [Google Scholar]
- 51.Vitamin supplements. Obstet Gynecol. 2006;107:174–176. doi: 10.1097/01.AOG.0000195214.24453.df. [DOI] [PubMed] [Google Scholar]
- 52.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women's Health Study—A randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 53.Parkin DM, Whelen SL, Ferlay J, et al. Cancer Incidence in Five Continents. Lyon, France: International Agency for Research on Cancer; 1997. [Google Scholar]
- 54.Matthews CE, Jurj AL, Shu XO, et al. Influence of exercise, walking, cycling, and overall nonexercise physical activity on mortality in Chinese women. Am J Epidemiol. 2007;165:1343–1350. doi: 10.1093/aje/kwm088. [DOI] [PubMed] [Google Scholar]
- 55.Weller I, Corey P. The impact of excluding non-leisure energy expenditure on the relation between physical activity and mortality in women. Epidemiology. 1998;9:632–635. [PubMed] [Google Scholar]
- 56.Richelle M, Turini ME, Guidoux R, et al. Urinary isoprostane excretion is not confounded by the lipid content of the diet. FEBS Lett. 1999;459:259–262. doi: 10.1016/s0014-5793(99)01259-4. [DOI] [PubMed] [Google Scholar]