Abstract
Purpose
To determine the relative frequency with which Kaposi's sarcoma–associated herpesvirus/HHV-8 (KSHV) DNA is detected in peripheral-blood mononuclear cells (PBMCs) and in plasma of patients with AIDS-KS and AIDS-associated non-Hodgkin's lymphoma (NHL; AIDS-NHL); to determine whether the presence of viral DNA in plasma reflects lysis of tumor cells or reflects the presence of viremia (ie, virion-encapsidated DNA); and to determine the effect of lymphoma therapy on KSHV DNA.
Patients and Methods
Samples were obtained from patients enrolled in AIDS Malignancy Consortium clinical trials and from healthy donors. Real time PCR was used to quantify KSHV DNA in peripheral blood mononuclear cells (PBMC) and plasma. DNase digestion and fragment size determination studies were used to characterize the DNA detected.
Results
In patients with AIDS-KS, KSHV DNA was detected in PBMC (54%) and in plasma (62%). In patients with AIDS-NHL, KSHV DNA was detected in PBMC (19%) and in plasma (22%). Median copy numbers also differed. KSHV DNA in plasma appeared to be encapsidated. In six patients with AIDS-NHL who were treated with chemotherapy (with or without rituximab), KSHV copy number declined in PBMC and in plasma.
Conclusion
KSHV DNA is sometimes detected in PBMC or in plasma of patients with AIDS-NHL without KS. Among patients with KSHV DNA detected in PBMC or in plasma, copy number does not distinguish between patients with AIDS-NHL and AIDS-KS. KSHV DNA in plasma likely reflects viremia and not simply lysis of tumor or other KSHV-infected cells. KSHV DNA copy number in PBMC and in plasma declined with lymphoma-directed cytotoxic chemotherapy in each of the six patients studied.
INTRODUCTION
Kaposi's sarcoma (KS)–associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), is a gammaherpesvirus associated with all types of KS.1 In lesions, viral nucleic acids and proteins are detected in spindle cells and monocytes.2–7 Viral DNA is detected in peripheral-blood mononuclear cells (PBMCs) and in cell-free blood (ie, plasma or serum).8,9 As with other gammaherpesviruses, lymphocytes harbor latent virus in vivo.
To better characterize KSHV infection and the effects of treatment, we studied PBMCs and plasma specimens from patients with AIDS-associated non-Hodgkin's lymphoma (AIDS-NHL) undergoing chemotherapy. For comparison, we also studied specimens from patients with AIDS-associated KS (AIDS-KS) and healthy blood donors.
PATIENTS AND METHODS
Specimens, Specimen Handling, DNA Extraction
Blood specimens were obtained from patients with untreated, aggressive, B-cell AIDS-NHL enrolled in an AIDS Malignancy Consortium trial.10 Pretreatment blood specimens from patients with AIDS-KS entered on two AIDS Malignancy Consortium trials11,12 and from healthy blood donors (screened to eliminate HIV-seropositive donors and those with evidence of other infection that would preclude blood donation) were also studied. Specimen collections were approved by institutional review boards at each participating AIDS Malignancy Consortium site and by the Cancer Treatment Evaluation Program of the National Cancer Institute when specimen collection was associated with a therapeutic trial.
Blood was collected into heparin tubes, was transported at ambient temperature (usually by overnight express shipping), and was processed within 30 hours of being drawn. Plasma was separated by centrifugation. PBMCs were separated by density gradient centrifugation. DNA was isolated by using the QIAGEN Blood Kit (QIAGEN Inc, Valencia, CA).
Polymerase Chain Reaction Primers, Probe, and Standard Curves
KSHV DNA copy numbers in plasma and in PBMCs were measured by using real-time polymerase chain reaction (PCR) with primers and a probe that targeted the K8 region (Table 1). The size distributions of KSHV DNA fragments were studied by using overlapping sets of real-time PCR primers and a common probe that targeted the LANA region (Table 1). Standard curves (with duplicate serial 10-fold dilutions of plasmid DNA that included a target sequence from 105 to 10 copies) were run in parallel with each analysis. Each of the primer sets amplified a panel of KSHV primary effusion lymphoma cell lines (ie, BC-1, BCBL-1 and JSC-1). Real-time primers and a probe for β actin were used as controls in some experiments. For all assays, fluorogenic PCR reactions were set up in a volume of 50 μL.
Table 1.
Primers and Probes
| KSHV Region | Primer | Primer | Probe |
|---|---|---|---|
| K8 | 5′-TCCAACTCGCAGATCCAAGAG-3′ | 5′-CGACCTGCG CCCTGTTT-3′ | 5′-FAM-AAGTTTGAAGAGGAACGCTTATGCACTAAGGC-TAMRA-3′ |
| 5′-TTGTGTCTAGTCCTACTTTACCGG-3′ | |||
| 5′-GGCAATGCAGGAGATGGAGAAT-3′ | |||
| LANA | 5′-TTGTGTCTAGTCCTACTTTACCGG-3′ | 5′-GACGACTTGGAGGGAGGCT-3′ | 5′-FAM-TCCCATTCCTTCACCCGCTCCCGCA-TAMRA-3′ |
| 5′-GGTTGGCGTGGCGGAGTA-3 | |||
| 5′-GGATGCTTCTTCTGCAATCTCC-3′ |
Abbreviation: KSHV, Kaposi's sarcoma herpesvirus.
DNase Protection Assay
Plasma specimens were treated with DNase I (150 U/μL; Invitrogen, Carlsbad, CA) and were incubated at 37°C for approximately 3 minutes. Real-time, quantitative PCR for KSHV (K8 primers described above) and β-actin DNA yielded estimates of copy number before and after DNase digestion. Controls for virion DNA and viral DNA released from latently infected cells were constructed by the addition of purified virion DNA or infected-cell DNA to plasma from healthy donors and from patients with AIDS-NHL without detectable KSHV DNA.
Purified Virus
Virions for use as controls in DNase protection assays were prepared from JSC-1 cells.13 TPA was added to achieve a final concentration of 20 ng/mL in culture. After 3 days, fresh media and additional TPA were added to the culture. After 6 days in culture, cells were centrifuged at 1,200 rpm for 10 minutes at 4°C. Supernatants were separated, were centrifuged again under the same conditions, and then were filtered through a 0.22-μm filter. The filter flow-through was pelleted for 1 hour at 4°C at 15,000 rpm. The pellet was resuspended at 4°C overnight.
Biostatistics
The confidence intervals for proportions were calculated by using the modified Wald method (www.graphpad.com/quickcalcs). Scatterplots, boxplots, Spearman rank correlation, linear regression, and Wilcoxon matched pairs tests were calculated by using GraphPad Prism software (version 5.00 for Windows; GraphPad Software, San Diego, CA).
RESULTS
Patients and Specimens
Pretreatment blood specimens were available from 60 patients with KS (46 were paired with PBMCs and plasma, 14 were with plasma alone) and from 43 patients with AIDS-NHL (41 were paired with PBMCs and plasma, two were with PBMCs alone). None of the patients with AIDS-NHL enrolled on the study had a history of KS, and none developed KS during the course of the study. Similarly, none of the patients had a diagnosis of primary effusion lymphoma or other KSHV-associated lymphoproliferative disease. Specimens from 20 healthy blood product donors were also studied.
KSHV in PBMCs and Plasma
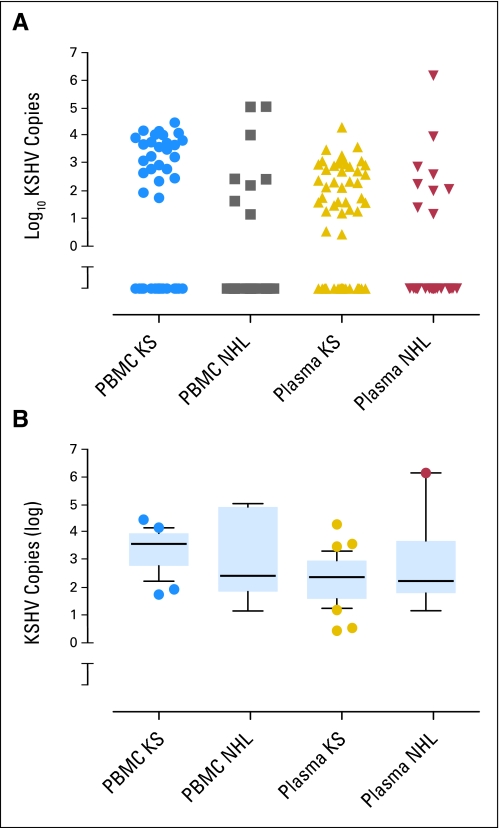
KSHV DNA was not detected in any specimens from healthy donors. KSHV DNA was detected in PBMCs and in plasma from more patients with AIDS-KS than patients with AIDS-NHL (Table 2; Fig 1A). Patients with KS also showed significantly higher median viral copy numbers in PBMCs and in plasma than patients with NHL. KSHV copy numbers in PBMCs and in plasma did not correlate with HIV RNA levels, absolute CD4 counts, or CD19 counts (Appendix Table A1, online only). Of the patients with NHL who were studied for KSHV copy number, 62% were on antiretroviral regimens at the time of diagnosis. The frequency of detection of KSHV DNA in PBMCs or in plasma did not differ between patients on antiretroviral therapy and those naïve to antiretroviral therapy (P = .69, two-tailed Fisher's exact test).
Table 2.
Pretreatment KSHV Copy Number in PBMC and Plasma From Patients With AIDS-KS or AIDS-NHL
| Variable | Tumor |
P | |
|---|---|---|---|
| KS (n = 60) | Lymphoma (n = 43) | ||
| PBMC specimen | 46 | 43 | |
| Positive KSHV | .0008* | ||
| No. | 25 | 8 | |
| % | 54 | 19 | |
| Median copy number/106 cells | 170 | 0 | .0004† |
| Plasma specimen | 60 | 41 | |
| Positive KSHV | .0041* | ||
| No. | 37 | 9 | |
| % | 62 | 22 | |
| Median copy number/106 cells | 37 | 0 | .0002† |
Abbreviations: KSHV, Kaposi's sarcoma herpesvirus; PBMC, peripheral-blood mononuclear cell; NHL, non-Hodgkin's lymphoma; KS, Kaposi's sarcoma.
Fisher's exact two-tailed test.
Mann-Whitney two-tailed test.
Fig 1.
Copy numbers of Kaposi's sarcoma herpesvirus (KSHV) genomes in peripheral-blood mononuclear cells (PBMCs) and in plasma of untreated patients with AIDS-KS and AIDS–non-Hodgkin's lymphoma. (A) Scatter plot that shows KSHV copy numbers in individual patients. Copy numbers are shown as the log10 of copies per million PBMCs or per 100 μL plasma. Specimens in which KSHV was not detected are plotted below the break in the Y axis. Median values are listed in Table 2. (B) Box-and-whiskers plot of the same data that excludes specimens in which KSHV was not detected. The top, middle, and bottom bars of each box represent the 75th, 50th, and 25th percentiles of copy numbers, respectively. Whiskers show the 10th to 90th percentiles. ● outliers.
DNAse Protection Assay
To determine whether the KSHV DNA detected in plasma was present as virion or unprotected DNA, we used real-time PCR to monitor the enzymatic digestion of viral and cellular DNA sequences. The rates of digestion of cellular sequences are more rapid than the rates of digestion of viral sequences in reconstruction experiments, in which plasma is spiked with virion DNA and cell DNA (Fig 2A); when plasma is spiked with DNA extracted from a cell line that harbors KSHV genomes but does not produce virions, the rates of digestion are indistinguishable (Fig 2B). In clinical specimens from patients with AIDS-NHL and AIDS-KS (Figs 2C and 2D), rates of digestion of viral DNA were less than the rates of digestion of cellular DNA from the same specimens (Wilcoxon matched pairs test, P 0.037, one-tailed Gaussian approximation).
Fig 2.
DNase digestion. Real-time polymerase chain reaction was used to determine copy numbers of —— Kaposi's sarcoma herpesvirus (KSHV) and – – – – β-actin sequences before and after DNase 1 digestion. Y axis, percentage of DNA that remained. (A) Cell DNA spiked with virion DNA. β-actin DNA is digested more rapidly than viral DNA. (B) Cell DNA from a cell line that harbored KSHV episomes. β-actin DNA and viral DNA sequences are digested at parallel rates. Representative digestions of plasma DNA from patients with (C) AIDS-NHL and (D) AIDS-KS. β-actin DNA is digested more rapidly than viral DNA in both instances, which is consistent with partial protection from DNase.
Viral DNA Fragment Size
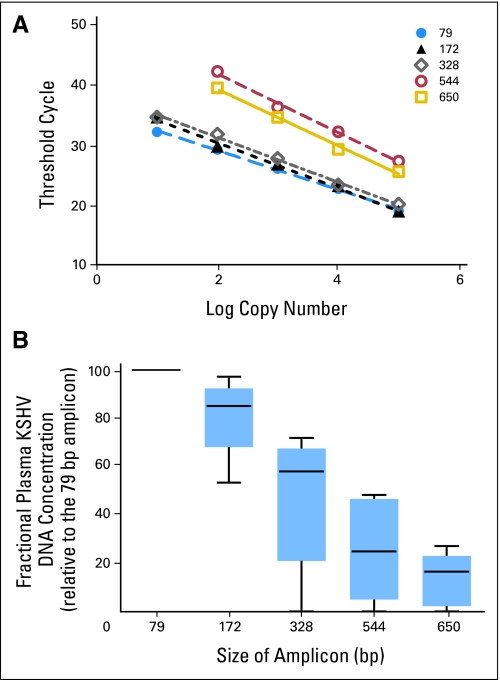
In murine models, the length ofDNA released from cells undergoing apoptosis is almost uniformly less than 180 bp (the internucleosomal length).14 To characterize the fractional concentration of viral DNA of varying lengths in plasma, we adapted an approach previously used to characterize Epstein-Barr virus (EBV) DNA in cell-free blood in patients with nasopharyngeal carcinoma and lymphoma.15 Tissue apoptosis and necrosis both have been reportedly associated with the presence of plasma DNA.14 We designed real-time PCR primer sets to amplify overlapping KSHV sequences of various sizes (Table 1). The standard curves for each amplicon are shown in Figure 3A. The absolute concentration of each amplicon size was estimated by real-time PCR. The fractional concentration was determined by dividing the absolute concentration of viral DNA of a particular amplicon size by the absolute concentration of viral DNA of the smallest amplicon size (ie, 79 bp). As illustrated in the box plot in Figure 3B, amplicons larger than 180 bps were well represented, and the median fractional concentration of the 328 bp amplicon was 58%.
Fig 3.
Plot of fractional concentrations of plasma Kaposi's sarcoma herpesvirus (KSHV) DNA as measured with amplicons of different length (79 bp to 650 bp). Both large fragments and small fragments were present in plasma, which is consistent with the notion that KSHV is in virion particles. The median fractional KSHV DNA concentration is listed below the box plot of each amplicon size. The top, middle, and bottom bars of each box represent the 75th, 50th, and 25th percentiles of the fractional concentrations, respectively. ● outliers; X-axis, size of amplicons (in bp); Y-axis, fractional concentration of plasma KSHV DNA.
Effect of chemotherapy.
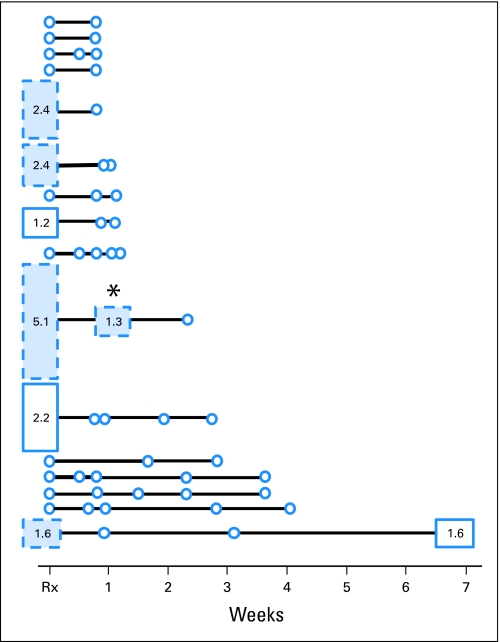
Patients with AIDS-NHL received a standard lymphoma chemotherapy regimen (ie, cyclophosphamide, doxorubicin, vincristine and prednisone [CHOP]) with (CHOP-R) or without rituximab. Follow-up specimens were evaluated for all patients in whom pretreatment study showed KSHV detection as well as for 10 patients in whom KSHV was not detected in pretreatment specimens. In all six patients with KSHV detectable in PBMC at baseline, KSHV became undetectable by the first or second post-treatment measurement (Fig 4). In one patient, KSHV again became detectable more than a year after the conclusion of chemotherapy. KSHV DNA copy number in PBMC remained undetectable in 10 patients in whom KSHV had been undetectable at baseline.
Fig 4.
Time line. Peripheral-blood mononuclear cell (PBMC) specimens from patients with AIDS–non-Hodgkin's lymphoma (NHL) before the initiation of combination chemotherapy and before follow-up specimens were assayed for Kaposi's sarcoma herpesvirus (KSHV). ○ failure to detect KSHV; □ detection of KSHV in PBMCs; numbers within and the height of the box, the log10 of the viral copy number. Every patient with a PBMC specimen also had corresponding plasma specimens assayed. ■ detection of KSHV in plasma. One patient (data not shown in figure) had a pretreatment plasma specimen but had no PBMC specimen and no follow-up specimens of either sort. X axis, relationship to the initiation of chemotherapy as measured in weeks; * patient treated with CHOP alone who had residual CD19+ cells detected at the first assay point after lymphoma chemotherapy was initiated.
Clinical Outcomes
There were 14 patients with AIDS-NHL who had KSHV detected in PBMCs, in plasma, or in both at the start of therapy. Complete remission (CR) was achieved in nine patients (64%). This is similar to the remission rate in the overall study (54%).10
DISCUSSION
KSHV genomes were detected in PBMC and in plasma from most patients with AIDS-KS. KSHV genomes were also detected in PBMC and in plasma in some patients with AIDS-NHL who had no history of AIDS-KS. As expected, the differences between patients with KS and NHL were significant. However, among patients with KSHV detected in either PBMCs or in plasma, the copy number range and interquartile range did not distinguish between patients with KS and NHL (Fig 1B). This finding—that, among AIDS patients with detectable KSHV DNA in plasma, KSHV copy number did not differentiate patients with KS from those with NHL—suggests that the KSHV DNA being measured in plasma is unlikely to be tumor-derived, even in patients with KS. Campbell et al16 reached similar conclusions, and they showed that among patients in Zimbabwe coinfected with HIV and KSHV, plasma KSHV levels did not differ between patients with and without KS.
Insofar as DNA tumor markers in cell-free blood are attracting increasing attention in breast, lung, gastrointestinal, head and neck, and other cancers,17–19 it is worth considering how the situation with AIDS-KS and AIDS-NHL patients differs from viral DNA markers in some other settings. EBV DNA markers are among the best studied. In patients with nasopharyngeal carcinoma, a series of studies has shown that EBV DNA in cell-free blood indicates the presence of tumor.15,20 In rare instances when tumor can be surgically excised, viral DNA is cleared within hours. With more standard treatments (ie, external-beam radiotherapy, chemotherapy), viral DNA clears with successful treatment.21 Quantification of viral DNA at diagnosis augments TNM tumor stage in predicting clinical outcome.22 The inability to clear viral DNA, or the reappearance of viral DNA after it is cleared, signals refractory or relapsing disease, respectively.21
The molecular character of the KSHV DNA in plasma in patients with AIDS malignancy differs from that of EBV DNA in nasopharyngeal cancer and some other EBV-associated malignancies. DNase protection indicative of the presence of virion DNA in AIDS-KS and AIDS-NHL, as shown here, has not been reported in nasopharyngeal cancer.15 The different assay results are not likely to simply reflect technical differences between laboratories, as our own studies show that there is no protection from DNase in plasma from EBV-associated Hodgkin's lymphoma (data not shown). DNase resistance data in patients in Zimbabwe who have HIV with and without KS also led others to conclude that virion DNA was present in plasma.16 Intracellular nuclease activity in the presence of nucleosomes, such as accompanies apoptotic cell death, yields DNA fragments less than 180 bp. Our finding of a high fractional concentration of KSHV fragment sizes in plasma larger than 180 bp also suggests an origin other than release of free DNA from cells undergoing apoptotic cell death. Again, the contrast with EBV DNA in plasma from patients with nasopharyngeal carcinoma, as reported by others,15 and with EBV-associated Hodgkin's lymphoma in our laboratory (unpublished data) is striking.
Thus, the clinical observation that the presence of plasma KSHV DNA does not correspond to the presence or absence of tumor and the molecular characterization that suggests that the KSHV DNA in plasma is virion DNA rather than tumor-derived free DNA are congruent. These results are consistent with our previous observation that the clinical outcome of interferon therapy for AIDS-KS was not correlated with changes in KSHV copy number.23 This finding does not preclude the possibility suggested by others that, among patients with AIDS KS, there may be a relationship between copy number and KS disease activity.24,25
Our findings did not suggest a relationship between CD4 cell count or HIV RNA and KSHV DNA. Some investigators have reported such a relationship,24,26 whereas others have not.27 Clearly, the determinants of KSHV copy number in PBMCs and in plasma requires additional investigation.
Several investigators have reported that KSHV copy number corresponds with KS tumor burden or activity.24 Our investigations yielded similar results (Table 2). Thus, our results confirm the observations already reported by others. However, when the analysis is restricted to patients in whom virus is detected, patients with AIDS-KS and patients with AIDS-NHL can no longer be distinguished (Fig 1B).
Why did KSHV DNA copy number decrease after the initiation of chemotherapy in patients with lymphoma? The impact of various pharmacologic therapies on KSHV copy number has been investigated in other settings. Trials with antiherpesvirus agents (ie, ganciclovir, foscarnet, cidofovir) have shown no impact on KSHV copy number in blood, although a dramatic effect on oral shedding of virus has been reported.27–29 In contrast, antiretroviral therapy lowers KSHV copy number in PBMCs and in plasma, but the decrease is much less rapid than that documented here and generally begins after a year of therapy.30 Of the six patients in our study with detectable KSHV in PBMCs before the initiation of lymphoma therapy, all but one were already on highly active antiretroviral therapy.
It has been suggested that lymphoid tissue sustains KSHV viremia and that B cells are the lymphoid reservoir.31 We have previously reported the rapid decline (within 24 hours) of EBV viral copy number in PBMCs of patients with post-transplant lymphoma who were treated with rituximab alone.32 This corresponds to a disappearance of B cells from blood that accompanies rituximab treatment. Thus, we entertained the possibility that, in this study, the decrease in viral copy number reflected the disappearance of B cells from the blood. In a recent report that detailed treatment and re-treatment of Castleman disease in HIV-infected patients, the clinical response to rituximab treatment was accompanied by a marked decrease in KSHV copy number in blood.33 Disease recurrence was accompanied by an increase in KSHV copy number, and rituximab monotherapy again led to a clinical response and a decrease in KSHV copy number in blood.
In this study, depletion of B cells coincided with a decrease in KSHV in PBMCs and in plasma. In two patients treated with CHOP-R chemotherapy, flow cytometry confirmed depletion of CD19+ B cells to less than 0.1% of PBMCs just before cycle 4 (data not shown). Flow cytometry also showed a relative depletion of B cells in four patients treated with CHOP alone. CD19+ cells constituted less than 1% of mononuclear cells in three of these patients. In the remaining patient, CD19+ cells accounted for 4% of PBMCs. Of note, this latter patient is the patient with residual virus DNA detectable in PBMCs and in plasma (Fig 4). Similarly, the reappearance of KSHV approximately a year after the conclusion of treatment with CHOP-R may correspond to a time when B-cell levels return to pretreatment levels. Thus, KSHV copy number in PBMCs and in plasma may reflect the size of the B lymphocyte pool. It should be noted, however, that in a study of infusional chemotherapy (without rituximab) for AIDS-NHL combined with antiretroviral therapy, in which 68 patients survived more than 3 months, two of the surviving patients developed KS.34 Thus, the decrease in KSHV identified in this study notwithstanding, it should not be presumed that lymphoma chemotherapy without rituximab confers protection against the development of KS. Indeed, there are a series of reports of KS exacerbation or de novo occurrence in HIV-infected patients with Castleman disease who were treated with rituximab.35–38 In addition, exacerbation of classic Castleman disease has been reported in a patient with autoimmune hemolytic anemia who was treated with rituximab.39
How the KSHV genome accesses the endothelial cells that lead to KS tumors is not understood. Viral DNA episomes might be transported in latently infected lymphocytes, and a direct cell-cell interaction might lead to infection of the precursor cells. Alternatively, virions produced elsewhere might be carried in cell-free blood to precursor cells. The suggestion has even been made that sustained tumor cell proliferation might require continuous reinfection.32 In any of these scenarios, lymphoma therapy might limit the delivery of virus (as infected PBMCs or virions) to premalignant or malignant cells and might prevent or delay the development of KS.
In summary, KSHV DNA is commonly detected in PBMCs and in plasma of patients with AIDS-NHL. Among patients in whom viral DNA is detected, viral copy number does not serve to discriminate between patients with AIDS -KS and AIDS -NHL. The characteristics of the viral DNA detected in plasma are consistent with the presence of virion DNA. Standard lymphoma chemotherapy with CHOP or CHOP-R led to rapid declines in viral DNA in PBMCs and in plasma. The changes in viral copy number may reflect changes in the size of the B-cell pool.
Appendix
Table A1.
Spearman Rank Correlation Coefficients
| Variable* | Coefficient (r) | P | No. of XY Pairs |
|---|---|---|---|
| KSHV PBMC | |||
| KSHV plasma | 0.984 | < .0001 | 33 |
| HIV RNA | |||
| KSHV PBMC | 0.099 | .598 | 31 |
| KSHV plasma | 0.112 | .481 | 42 |
| CD4 | |||
| KSHV PBMC | 0.055 | .737 | 40 |
| KSHV Plasma | 0.006 | .966 | 54 |
| CD19 | |||
| KSHV PBMC | 0.046 | .876 | 14 |
| KSHV Plasma | 0.003 | .993 | 14 |
Abbreviations: KSHV, Kaposi's sarcoma herpesvirus; PBMC, peripheral-blood mononuclear cell.
Variable of Y for each X.
Footnotes
Supported in part by Grants No. U01CA083118, U01CA070079, U01CA70058, U01CA070047, U01CA070054, U01CA070072, U01CA070080, U01CA083035, U01CA071375, U01CA083038, U01CA070062, U01CA70081, U01CA070019, U01 CA121947, and P50 CA96888 from the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Lan Lin, Lawrence D. Kaplan, Richard F. Ambinder
Financial support: Richard F. Ambinder
Administrative support: Richard F. Ambinder
Provision of study materials or patients: Lawrence D. Kaplan, Bruce J. Dezube, Ariela Noy, Susan E. Krown, Yanxing Yu, Gary S. Hayward, Richard F. Ambinder
Collection and assembly of data: Lan Lin, Richard F. Ambinder
Data analysis and interpretation: Lan Lin, Jeannette Y. Lee, Bruce J. Dezube, Ariela Noy, Susan E. Krown, Alexandra M. Levine, Gary S. Hayward, Richard F. Ambinder
Manuscript writing: Lan Lin, Jeannette Y. Lee, Bruce J. Dezube, Ariela Noy, Susan E. Krown, Alexandra M. Levine, Richard F. Ambinder
Final approval of manuscript: Lan Lin, Jeannette Y. Lee, Lawrence D. Kaplan, Bruce J. Dezube, Ariela Noy, Susan E. Krown, Alexandra M. Levine, Yanxing Yu, Gary S. Hayward, Richard F. Ambinder
REFERENCES
- 1.Moore PS. The emergence of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) N Engl J Med. 2000;343:1411–1413. doi: 10.1056/NEJM200011093431912. [DOI] [PubMed] [Google Scholar]
- 2.Boshoff C, Schulz TF, Kennedy MM, et al. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1:1274–1278. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- 3.Li JJ, Huang YQ, Cockerell CJ, et al. Localization of human herpes-like virus type 8 in vascular endothelial cells and perivascular spindle-shaped cells of Kaposi's sarcoma lesions by in situ hybridization. Am J Pathol. 1996;148:1741–1748. [PMC free article] [PubMed] [Google Scholar]
- 4.Orenstein JM, Alkan S, Blauvelt A, et al. Visualization of human herpesvirus type 8 in Kaposi's sarcoma by light and transmission electron microscopy. AIDS. 1997;11:F35–F45. doi: 10.1097/00002030-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Blasig C, Zietz C, Haar B, et al. Monocytes in Kaposi's sarcoma lesions are productively infected by human herpesvirus 8. J Virol. 1997;71:7963–7968. doi: 10.1128/jvi.71.10.7963-7968.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon JS, Nicholas J, Orenstein JM, et al. Heterogeneity of viral IL-6 expression in HHV-8-associated diseases. J Infect Dis. 1999;180:824–828. doi: 10.1086/314956. [DOI] [PubMed] [Google Scholar]
- 7.Chiou CJ, Poole LJ, Kim PS, et al. Patterns of gene expression and a transactivation function exhibited by the vGCR (ORF74) chemokine receptor protein of Kaposi's sarcoma-associated herpesvirus. J Virol. 2002;76:3421–3439. doi: 10.1128/JVI.76.7.3421-3439.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington WJ, Jr, Bagasra O, Sosa CE, et al. Human herpesvirus type 8 DNA sequences in cell-free plasma and mononuclear cells of Kaposi's sarcoma patients. J Infect Dis. 1996;174:1101–1105. doi: 10.1093/infdis/174.5.1101. [DOI] [PubMed] [Google Scholar]
- 9.Whitby D, Howard MR, Tenant-Flowers M, et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet. 1995;346:799–802. doi: 10.1016/s0140-6736(95)91619-9. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan LD, Lee JY, Ambinder RF, et al. Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lymphoma: AIDS-Malignancies Consortium Trial 010. Blood. 2005;106:1538–1543. doi: 10.1182/blood-2005-04-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noy A, Scadden DT, Lee J, et al. Angiogenesis inhibitor IM862 is ineffective against AIDS-Kaposi's sarcoma in a phase III trial, but demonstrates sustained, potent effect of highly active antiretroviral therapy: From the AIDS Malignancy Consortium and IM862 Study Team. J Clin Oncol. 2005;23:990–998. doi: 10.1200/JCO.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 12.Cianfrocca M, Cooley TP, Lee JY, et al. Matrix metalloproteinase inhibitor COL-3 in the treatment of AIDS-related Kaposi's sarcoma: A phase I AIDS malignancy consortium study. J Clin Oncol. 2002;20:153–159. doi: 10.1200/JCO.2002.20.1.153. [DOI] [PubMed] [Google Scholar]
- 13.Cannon JS, Ciufo D, Hawkins AL, et al. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi's sarcoma herpesvirus-containing supernatant. J Virol. 2000;74:10187–10193. doi: 10.1128/jvi.74.21.10187-10193.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahr S, Hentze H, Englisch S, et al. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- 15.Chan KC, Zhang J, Chan AT, et al. Molecular characterization of circulating EBV DNA in the plasma of nasopharyngeal carcinoma and lymphoma patients. Cancer Res. 2003;63:2028–2032. [PubMed] [Google Scholar]
- 16.Campbell TB, Borok M, White IE, et al. Relationship of Kaposi sarcoma (KS)-associated herpesvirus viremia and KS disease in Zimbabwe. Clin Infect Dis. 2003;36:1144–1151. doi: 10.1086/374599. [DOI] [PubMed] [Google Scholar]
- 17.Johnson PJ, Lo YM. Plasma nucleic acids in the diagnosis and management of malignant disease. Clin Chem. 2002;48:1186–1193. [PubMed] [Google Scholar]
- 18.Jiang WW, Zahurak M, Goldenberg D, et al. Increased plasma DNA integrity index in head and neck cancer patients. Int J Cancer. 2006;119:2673–2676. doi: 10.1002/ijc.22250. [DOI] [PubMed] [Google Scholar]
- 19.Dulaimi E, Hillinck J, Ibanez de Caceres I, et al. Tumor suppressor gene promoter hypermethylation in serum of breast cancer patients. Clin Cancer Res. 2004;10:6189–6193. doi: 10.1158/1078-0432.CCR-04-0597. [DOI] [PubMed] [Google Scholar]
- 20.Ryan JL, Fan H, Swinnen LJ, et al. Epstein-Barr Virus (EBV) DNA in plasma is not encapsidated in patients with EBV-related malignancies. Diagn Mol Pathol. 2004;13:61–68. doi: 10.1097/00019606-200406000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Chan AT, Lo YM, Zee B, et al. Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst. 2002;94:1614–1619. doi: 10.1093/jnci/94.21.1614. [DOI] [PubMed] [Google Scholar]
- 22.Leung SF, Zee B, Ma BB, et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol. 2006;24:5414–5418. doi: 10.1200/JCO.2006.07.7982. [DOI] [PubMed] [Google Scholar]
- 23.Krown SE, Lee JY, Lin L, et al. Interferon-alpha2b with protease inhibitor-based antiretroviral therapy in patients with AIDS-associated Kaposi sarcoma: An AIDS malignancy consortium phase I trial. J Acquir Immune Defic Syndr. 2006;41:149–153. doi: 10.1097/01.qai.0000194237.15831.23. [DOI] [PubMed] [Google Scholar]
- 24.Laney AS, Cannon MJ, Jaffe HW, et al. Human herpesvirus 8 presence and viral load are associated with the progression of AIDS-associated Kaposi's sarcoma. AIDS. 2007;21:1541–1545. doi: 10.1097/QAD.0b013e3282202b7d. [DOI] [PubMed] [Google Scholar]
- 25.Marcelin AG, Motol J, Guihot A, et al. Relationship between the quantity of Kaposi sarcoma-associated herpesvirus (KSHV) in peripheral blood and effusion fluid samples and KSHV-associated disease. J Infect Dis. 2007;196:1163–1166. doi: 10.1086/521625. [DOI] [PubMed] [Google Scholar]
- 26.Nsubuga MM, Biggar RJ, Combs S, et al. Human herpesvirus 8 load and progression of AIDS-related Kaposi sarcoma lesions. Cancer Lett. 2008;263:182–188. doi: 10.1016/j.canlet.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphrey RW, O'Brien TR, Newcomb FM, et al. Kaposi's sarcoma (KS)-associated herpesvirus-like DNA sequences in peripheral blood mononuclear cells: Association with KS and persistence in patients receiving anti-herpesvirus drugs. Blood. 1996;88:297–301. [PubMed] [Google Scholar]
- 28.Little RF, Merced-Galindez F, Staskus K, et al. A pilot study of cidofovir in patients with kaposi sarcoma. J Infect Dis. 2003;187:149–153. doi: 10.1086/346159. [DOI] [PubMed] [Google Scholar]
- 29.Casper C, Krantz EM, Corey L, et al. Valganciclovir for suppression of human herpesvirus-8 replication: A randomized, double-blind, placebo-controlled, crossover trial. J Infect Dis. 2008;198:23–30. doi: 10.1086/588820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourboulia D, Aldam D, Lagos D, et al. Short- and long-term effects of highly active antiretroviral therapy on Kaposi sarcoma-associated herpesvirus immune responses and viraemia. AIDS. 2004;18:485–493. doi: 10.1097/00002030-200402200-00015. [DOI] [PubMed] [Google Scholar]
- 31.Campbell TB, Staskus KA, Folkvord J, et al. Persistence of Kaposi sarcoma-associated herpesvirus (KSHV)-infected cells in KSHV/HIV-1-coinfected subjects without KSHV-associated diseases. J Infect Dis. 2005;191:367–371. doi: 10.1086/427194. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Tao Q, Flinn IW, et al. Characterization of Epstein-Barr virus-infected B cells in patients with posttransplantation lymphoproliferative disease: Disappearance after rituximab therapy does not predict clinical response. Blood. 2000;96:4055–4063. [PubMed] [Google Scholar]
- 33.Powles T, Stebbing J, Montoto S, et al. Rituximab as retreatment for rituximab pretreated HIV-associated multicentric Castleman disease. Blood. 2007;110:4132–4133. doi: 10.1182/blood-2007-08-106187. [DOI] [PubMed] [Google Scholar]
- 34.Bower M, Stebbing J, Tuthill M, et al. Immunologic recovery in survivors following chemotherapy for AIDS-related non-Hodgkin lymphoma. Blood. 2008;111:3986–3990. doi: 10.1182/blood-2007-10-115659. [DOI] [PubMed] [Google Scholar]
- 35.Bower M, Powles T, Williams S, et al. Brief communication: Rituximab in HIV-associated multicentric Castleman disease. Ann Intern Med. 2007;147:836–839. doi: 10.7326/0003-4819-147-12-200712180-00003. [DOI] [PubMed] [Google Scholar]
- 36.Casquero A, Barroso A, Fernandez Guerrero ML, et al. Use of rituximab as a salvage therapy for HIV-associated multicentric Castleman disease. Ann Hematol. 2006;85:185–187. doi: 10.1007/s00277-005-0038-4. [DOI] [PubMed] [Google Scholar]
- 37.Gerard L, Berezne A, Galicier L, et al. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus associated multicentric Castleman's disease: ANRS 117 CastlemaB Trial. J Clin Oncol. 2007;25:3350–3356. doi: 10.1200/JCO.2007.10.6732. [DOI] [PubMed] [Google Scholar]
- 38.Pantanowitz L, Fruh K, Marconi S, et al. pathology of rituximab-induced kaposi sarcoma flare. BMC Clin Pathol. 2008;8:7. doi: 10.1186/1472-6890-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clifford KS, Demierre MF. Progression of classic Kaposi's sarcoma with rituximab. J Am Acad Dermatol. 2005;53:155–157. doi: 10.1016/j.jaad.2004.12.048. [DOI] [PubMed] [Google Scholar]