Abstract
Purpose
Irinotecan plus cisplatin (IP) improved survival over etoposide plus cisplatin (EP) in Japanese patients with extensive-stage small-cell lung cancer (E-SCLC). To confirm those results and discern the potential role of population-related pharmacogenomics (PG) in outcomes, we conducted a large randomized trial of identical design to the Japanese trial in North American patients with E-SCLC.
Patients and Methods
Patients were randomly assigned to IP (irinotecan 60 mg/m2 on days 1, 8, and 15; cisplatin 60 mg/m2 day 1, every 4 weeks) or EP (etoposide 100 mg/m2 on days 1 through 3; cisplatin 80 mg/m2 day 1, every 3 weeks). Blood specimens for genomic DNA analysis were collected before random assignment in 169 patients.
Results
Of 671 patients, 651 were eligible (324 and 327 patients in the IP and EP arms, respectively). Response rates with IP and EP were 60% and 57%, respectively (P = .56). Median progression-free survival for IP and EP was 5.8 and 5.2 months, respectively (P = .07). Median overall survival for IP and EP was 9.9 and 9.1 months, respectively (P = .71). Severe diarrhea was more common with IP (19% v 3%); severe neutropenia and thrombocytopenia were higher with EP versus IP (68% v 33% and 15% v 4%, respectively). PG analysis showed that ABCB1 (C3435T)T/T (membrane transport) was associated with IP-related diarrhea; UGT1A1 (G-3156A)A/A (drug metabolism) was associated with IP-related neutropenia.
Conclusion
This large North American trial failed to confirm the previously reported survival benefit observed with IP in Japanese patients. Both regimens produced comparable efficacy, with less hematologic and greater gastrointestinal toxicity with IP. These results emphasize the potential importance of PG in interpreting trials of cancer therapy.
INTRODUCTION
Lung cancer represents the most common cause of cancer-related mortality in the United States, accounting for more than 150,000 deaths annually.1 Small-cell lung cancer (SCLC) is a distinct clinicopathologic entity that accounts for up to 20% of all new cases of lung cancer cases and deaths.2 The vast majority of deaths occur in patients with metastatic (or extensive-stage) disease.3,4 Although SCLC is initially considered chemotherapy-sensitive, rapid emergence of clinical drug resistance inevitably results in the death of more than 90% of affected patients.5,6 New systemic treatment options are needed for this highly lethal malignancy.
Over the past two decades, the cornerstone of therapy for extensive-stage SCLC has been a platinum compound (either carboplatin or cisplatin) in combination with the topoisomerase-II inhibitor etoposide.7 In 2002, a Japanese phase III study (Japan Clinical Oncology Group [JCOG] 9511) comparing etoposide plus cisplatin (EP) with cisplatin and the topoisomerase I inhibitor irinotecan (IP) was reported. In that trial, tumor response and patient survival time were found to be significantly higher in the IP group.8 Although those results were considered encouraging, the trial included only 174 patients. It was stopped early at interim analysis by the Data Safety Monitoring Board when prospectively prespecified efficacy parameters were met. The trial was also solely conducted in Japanese patients and may not have been directly applicable to non-Japanese populations as a result of pharmacogenomic factors. It is well known that potential differences exist in drug disposition related to racial variability in the distribution of relevant single-nucleotide polymorphisms in genes involved in drug metabolism or transport.9 For example, genes involved in chemotherapy transport (eg, ABCB1, OATP-C), metabolism (UGT-1A1), detoxification (GSTP1), and DNA repair (ERCC1, XPD) are reported to be involved in platinum and topoisomerase-inhibitor drug pathways and that DNA polymorphisms (including single-nucleotide polymorphisms [SNPs]) in these genes may be associated with treatment outcome and toxicity.10–12
Moreover, a separate phase III trial conducted in North America using modified dose schedules of EP and IP (both delivered over 21-day cycles) showed no benefit for the latter.13 Therefore, the Southwest Oncology Group (SWOG) designed and conducted an adequately powered phase III trial (S0124) using virtually the same eligibility criteria and the identical treatment regimens as the Japanese trial to definitively confirm these results in North American patients before a change in the standard of care. Additionally, we sought to evaluate the status of select genomic DNA polymorphisms and to correlate genotypic profiles with patient outcomes after chemotherapy.
PATIENTS AND METHODS
Patients had cytologically or histologically confirmed small-cell lung cancer; extensive-stage disease (defined by distant metastasis, contralateral hilar-node metastasis, or both; those with pleural effusion alone were excluded); no prior radiotherapy, chemotherapy, or surgery; a Zubrod performance status of 0 or 1, a life expectancy of at least 3 months; and adequate hematologic, hepatic, and renal end-organ function. Because of the increased risk of fetal or infant harm from chemotherapy, pregnant or nursing women were not allowed to participate in this trial. All patients had a thoracic and upper abdominal computed tomography scan within 28 days before registration and a pretreatment computed tomography or magnetic resonance imaging scan of the brain to evaluate for intracranial metastatic disease within 42 days before registration.
Treatment Assignment and Drug Administration
Patients were randomly assigned to receive either a combination of EP or IP. Randomization was performed at the SWOG statistical center, balanced on the following stratification factors: number of metastatic sites (single v multiple); weight loss in prior 6 months (≤ 5% v > 5%); and lactate dehydrogenase levels (≤ upper limit of normal v > upper limit of normal).
The treatment regimens and dose modification criteria used in JCOG 9511 were used for this confirmatory trial. The IP regimen consisted of four cycles of irinotecan 60 mg/m2 of body-surface area on days 1, 8, and 15 and cisplatin 60 mg/m2 of body-surface area on day 1. Cycle length for this arm was 4 weeks. The EP regimen consisted of four cycles of etoposide 100 mg/m2 on days 1, 2, and 3 and cisplatin 80 mg/m2 on day 1. Cycle length for this arm was 3 weeks. Both regimens required hydration and administration of antiemetic drugs. Day 1 treatment criteria included an absolute neutrophil count ≥ 1,500/μL, platelet count ≥ 100,000/μL, serum creatinine ≤ upper limits of normal, and calculated or measured creatinine clearance ≥ 50 mL/min. Recombinant human granulocyte colony-stimulating factor use was allowed per investigator discretion to support the neutrophil count. However, granulocyte colony-stimulating factor was not given within 24 hours of chemotherapy administration.
Dose Modifications and Modifications in the Treatment Schedule
Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria (version 2). In the irinotecan arm, the development of grade 4 neutropenia, febrile neutropenia, grade 4 thrombocytopenia, and/or grade 3 to 4 diarrhea necessitated withholding of irinotecan until recovery of toxicity to ≤ grade 1. On recovery, the day 15 dose was skipped, a reduction in the irinotecan dose by 10 mg/m2 was implemented, and further cycles were given on days 1 and 8 every 3 weeks. Etoposide and cisplatin doses were reduced in 25% decrements in subsequent cycles for grade 4 neutropenia, febrile neutropenia, or grade 4 thrombocytopenia. In both arms, the dose of cisplatin was reduced by 25% of the planned dose in patients with grade 2 renal toxicity.
Evaluations
All patients underwent evaluations every cycle that included an assessment of symptoms, a physical examination, a complete blood cell count, and blood chemistry studies. Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors, assessed by computed tomographic scanning and by the same tests used initially to stage the tumor.14 A complete response was defined as the disappearance of all clinical and radiologic evidence of tumor for at least 4 weeks; a partial response was defined as a decrease of 30% or more in the sum of longest diameters of all target measurable lesions; and progressive disease was defined as an increase of more than 20% of the sum of longest diameters of all target measurable lesions or the appearance of new lesions. All other circumstances were considered to indicate no change (or stable disease).
Patients were removed from protocol treatment when at least one of the following was observed: (1) completion of four cycles of protocol treatment, (2) unacceptable toxicity, (3) progression of disease, (4) ≥ 3-week delay in protocol treatment for any reason, or (5) more than three dose reductions in any one drug. Patients were allowed to withdraw their consent from the study at any time for any reason.
Study Design and Statistical Analysis
The primary objective of this study was to compare the survival in patients with extensive stage small-cell lung cancer treated with EP (standard arm) with that in comparable patients treated with the IP (experimental). The primary analyses were done on an intent-to-treat basis. IP would be judged superior to the standard if the true increase in median survival was 33%. A median survival of 10 months was anticipated on the EP arm. Assuming exponential survival, 4 years of patient accrual, and an additional 1 year of follow-up, it was estimated that 310 patients per arm would result in 90% power to detect a 33% increase in median survival in the experimental arm, using a one-sided stratified log-rank test at level .025.
Two interim analyses were planned, the first after accrual of 310 patients, and the second after two thirds of expected deaths had occurred, which was estimated to take place after accrual of approximately 550 patients. For each interim analysis, evidence suggesting early termination of the trial would have been present if the null hypothesis of no difference were rejected at the .0025 level or if the alternative hypothesis of a 33% improvement in survival in favor of the experimental arm were rejected at the.0025 level. Using the modified Haybittle-Peto approach to α spending, the final analysis would conclude in favor of the experimental arm if the null hypothesis of no difference between arms were rejected at the .02 level. Secondary analyses was done regarding progression-free survival (using a stratified log-rank test), and response and toxicity (using stratified χ2 tests). With 310 patients per arm, response and toxicity rates were projected to be estimated within at worst ± 6% (95% CI).
Pharmacogenomic Studies
In an exploratory hypothesis-generating substudy, genomic DNA was obtained from 169 consenting patients to assess the status of genes—specifically, SNPs—involved in chemotherapy metabolism (uridine diphosphate-glucuronosyltransferase [UGT]), detoxification (glutathione S-transferase), or transport (organic anion transporting polypeptide; adenosine triphosphate binding cassette [ABC]), as well as DNA repair (xeroderma pigmentosum group D; excision repair cross-complementing). Of these 169 patients, 142 had completed all planned protocol therapy and had complete toxicity data at the time of the PG analysis. Eighty-seven patients from the IP arm and 82 patients on the EP arm were included. A subset of the hematologic and nonhematologic toxicities presented in Table 1 were selected a priori for these analyses. Exploratory associations between these toxicity toxicities and genotype within each arm and overall were assessed with logistic regression. As this was a hypothesis-generating substudy, no adjustment for multiple comparisons was made. Significance was set at P < .05.
Table 1.
Selected Toxicities
| Toxicity Type | Cisplatin/Irinotecan (n = 317) |
Cisplatin/Etoposide (n = 324) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 3 |
Grade 4 |
Grade 5 |
Grade 3 |
Grade 4 |
Grade 5 |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Anemia | 17 | 5 | 1 | < 1 | 0 | 35 | 11 | 4 | 1 | 0 | ||
| Leukopenia | 34 | 11 | 23 | 7 | 0 | 76 | 24 | 31 | 10 | 2 | < 1 | |
| Neutropenia | 60 | 19 | 46 | 15 | 1 | < 1 | 65 | 20 | 154 | 48 | 1 | < 1 |
| Thrombocytopenia | 11 | 3.5 | 1 | < 1 | 0 | 39 | 12 | 8 | 3 | 1 | < 1 | |
| Packed RBC transfusion | 35 | 11 | 0 | 0 | 61 | 19 | 0 | 0 | ||||
| Platelet transfusion | 1 | < 1 | 0 | 0 | 12 | 4 | 0 | 0 | ||||
| Thrombosis/embolism | 5 | 2 | 3 | 1 | 0 | 8 | 3 | 2 | 7 | 0 | ||
| Infection with neutropenia | 15 | 5 | 1 | < 1 | 4 | 1 | 15 | 5 | 4 | 1 | 3 | 1 |
| Respiratory infection with neutropenia | 1 | < 1 | 0 | 2 | < 1 | 0 | 2 | < 1 | 0 | |||
| Febrile neutropenia | 10 | 3 | 0 | 1 | < 1 | 25 | 8 | 5 | 1.5 | 2 | < 1 | |
| Flu-like symptoms | 0 | 1 | < 1 | 1 | < 1 | 0 | 0 | 2 | < 1 | |||
| Anorexia | 33 | 10 | 3 | 1 | 0 | 15 | 5 | 2 | < 1 | 0 | ||
| Fatigue/malaise/lethargy | 33 | 10 | 11 | 3.5 | 0 | 32 | 10 | 3 | 1 | 0 | ||
| Dehydration | 48 | 15 | 4 | 1 | 0 | 27 | 8 | 0 | 0 | |||
| Hypotension | 11 | 3.5 | 2 | < 1 | 0 | 6 | 2 | 1 | < 1 | 1 | < 1 | |
| Nausea | 45 | 14 | 0 | 0 | 35 | 11 | 0 | 0 | ||||
| Vomiting | 33 | 10 | 0 | 0 | 29 | 9 | 1 | < 1 | 0 | |||
| Diarrhea | 56 | 18 | 4 | 1 | 0 | 9 | 3 | 0 | 0 | |||
| Ileus | 0 | 1 | < 1 | 1 | < 1 | 0 | 0 | 0 | ||||
| Hypoxia | 6 | 2 | 1 | < 1 | 0 | 4 | 1 | 1 | < 1 | 1 | < 1 | |
| Dyspnea | 23 | 7 | 4 | 1 | 0 | 13 | 4 | 6 | 2 | 2 | < 1 | |
| Cerebrovascular ischemia | 0 | 0 | 1 | < 1 | 5 | 1.5 | 2 | < 1 | 0 | |||
| Cardiovascular ischemia/infarction | 0 | 1 | < 1 | 0 | 0 | 2 | < 1 | 0 | ||||
| Cardiovascular (other) | 0 | 0 | 1 | < 1 | 0 | 1 | < 1 | 0 | ||||
| Death within 30 days of treatment; treatment not ruled out as factor | 0 | 0 | 1 | < 1 | 0 | 0 | 1 | < 1 | ||||
| Maximum grade, any toxicity | 134 | 42 | 68 | 22 | 11 | 3.5 | 93 | 29 | 173 | 53 | 8 | 2.5 |
RESULTS
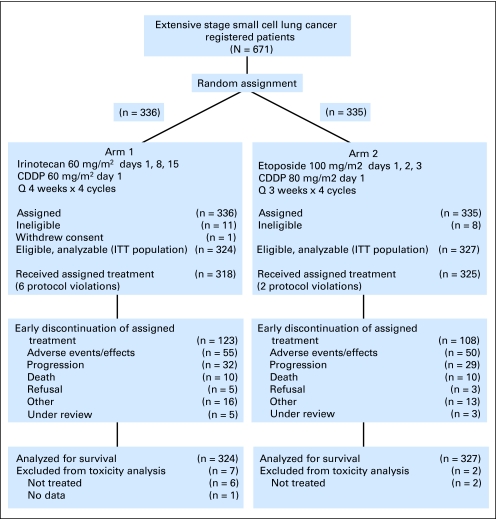
From November 2002 through March 2007, 671 patients were accrued, with 652 patients deemed eligible. One patient withdrew consent, leaving 324 patients randomly assigned to IP and 327 patients randomly assigned to EP. Patient disposition as per the CONSORT criteria is illustrated in Figure 1. Patient characteristics, which were well distributed between the arms, are listed in Table 2. Median age was 62 years (range, 22 to 85 years) in the IP arm and 63 years (range, 35 to 86 years) in the EP arm. There were 188 men (58%) in the IP arm and 182 (55%) in the EP arm. Weight loss ≤ 5% was reported in 62% of patients in both arms.
Fig 1.
Patient disposition (as per CONSORT). CDDP, cisplatin; ITT, intent to treat.
Table 2.
Patient Characteristics
| Characteristic | Cisplatin/Irinotecan (n = 324) |
Cisplatin/Etoposide (n = 327) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 62 | 63 | ||
| Minimum | 22 | 35 | ||
| Maximum | 85 | 86 | ||
| Sex | ||||
| Male | 188 | 58 | 182 | 56 |
| Female | 136 | 42 | 145 | 44 |
| Hispanic ethnicity | ||||
| Yes | 7 | 2 | 7 | 2 |
| No | 289 | 89 | 288 | 88 |
| Unknown | 28 | 9 | 32 | 10 |
| Race | ||||
| White | 300 | 93 | 304 | 93 |
| Black | 14 | 4 | 18 | 6 |
| Asian | 4 | 1 | 1 | 0 |
| Native American | 2 | 1 | 0 | 0 |
| Multiracial | 2 | 1 | 0 | 0 |
| Unknown | 2 | 1 | 4 | 1 |
| LDH | ||||
| ≤ ULN | 115 | 35 | 109 | 33 |
| > ULN | 209 | 65 | 218 | 67 |
| Metastatic sites | ||||
| Single | 77 | 24 | 81 | 25 |
| Multiple | 247 | 76 | 246 | 75 |
| Weight loss | ||||
| ≤ 5% | 202 | 62 | 204 | 62 |
| > 5% | 122 | 38 | 123 | 38 |
Abbreviations: LDH, lactate dehydrogenase; ULN, upper limit of normal.
Toxicity
Toxicities possibly, probably, and definitely related to protocol therapy are summarized in Table 1. There were 213 instances of grade 3 or worse adverse events reported in the IP arm of 317 assessable patients (67.2%), whereas there were 274 such instances in the EP arm of 324 assessable patients (84.6%). More grade 4 toxicities were reported with EP (173 v 68). The most common grade 3 or worse adverse events in the IP arm were gastrointestinal events (principally diarrhea) and myelosuppression. In the EP arm, the predominant grade 3 or worse toxicities were myelosuppression. Respective grade 3 and 4 toxicities in IP versus EP arms were as follows: neutropenia (33% v 68%), thrombocytopenia (4% v 15%), diarrhea (19% v 3%), infection (11% v 18%), cardiovascular (10% v 12%), renal (4% v 4%), and hepatic (3% v 5%). There were 11 treatment-related deaths on the IP arm and eight on the EP arm.
Delivery of Treatment
There were no significant differences between the two arms in treatment delivery. Six patients in the IP arm and two patients in the EP arm did not receive any protocol treatment. For the remaining 318 and 325 patients, the proportion receiving the planned four cycles of chemotherapy was 63% and 67% in the IP and EP arms, respectively. More patients in the EP arm (29%) than in the IP arm (24%) completed their assigned study treatment with no modifications in the doses or delivery schedule The mean dose-intensity (the actual dose delivered as a proportion of the planned dose, with or without delay) per arm was as follows: IP, 66% for irinotecan and 78% for cisplatin; EP, 78% for etoposide and 81% for cisplatin.
Efficacy
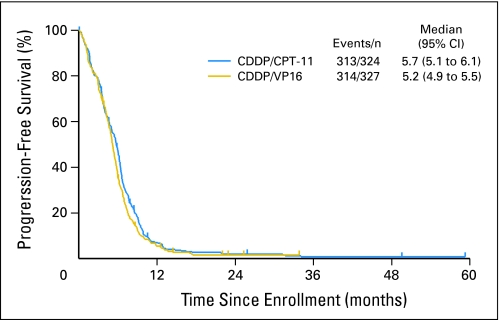
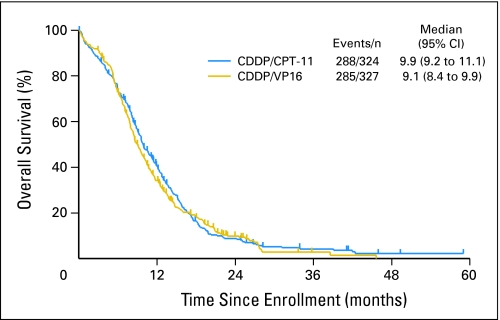
There were no statistical differences in tumor response, overall survival, or progression-free survival between the two arms. In the IP arm, the response rate was 60% (95% CI, 54% to 65%), whereas in the EP arm, it was 57% (95% CI, 53% to 63%). Median progression-free survival time for IP was 5.7 months (95% CI, 5.1 to 6.1 months). Median progression-free survival time for patients treated with EP was 5.2 months (95% CI, 4.9 to 5.5 months; Fig 2). The stratified P value for the progression-free survival comparison was .07. One-year progression-free survival rates were 7% and 6%, respectively, for IP and EP. Median overall survival time was 9.9 months (95% CI, 9.2 to 11.1 months) in the IP arm. Median overall survival time was 9.1 months (95% CI, 8.4 to 9.9 months) in the EP group. The stratified P value for the overall survival comparison was .71. Estimated 1-year survival rates were 41% and 34% for the IP and EP arms, respectively (Fig 3).
Fig 2.
Kaplan and Meier progression-free survival curves. CDDP, cisplatin; CPT-11, irinotecan; VP-16, etoposide.
Fig 3.
Kaplan and Meier overall survival curves. CDDP, cisplatin; CPT-11, irinotecan; VP-16, etoposide.
Pharmacogenomic Results
In this preliminary analysis, two genes—ABCB1 (C3435T) and UGT1A1 (G-3156A)—were found to be significantly associated with specific toxicities. ABCB1 (C3435T) T/T was associated with an increased risk of irinotecan-associated grade 3 or worse diarrhea (odds ratio [OR] = 3.9; 95% CI, 1.1 to 13.8; P = .01) as compared with C/C and C/T. UGT1A1 (G-3156A) A/A was associated with increased risk of irinotecan-associated grade 3 or worse neutropenia (OR = 24; 95% CI, 2 to 282; P = .02). Combined grade ≥ 3 neutropenia and diarrhea—previously reported as a high-risk combination of toxicities—was associated with ABCB1 (C3435) T/T (OR = 5.0; 95% CI, 1.2 to 22.9; P = .03) and UGT1A1 (G-3156A) A/A (OR = 7.6; 95% CI, 0.9 to 63; P = .06). UGT1A1*28 TA7, typically associated with increased irinotecan toxicity, was seen in only four patients (two in each arm); thus no correlation was possible. None of the genotypes seemed to be associated with efficacy outcomes. A complete and updated pharmacogenetic analysis is planned for a subsequent article.
DISCUSSION
Recent trends toward “globalization” of the clinical trials process critically highlight the issue of whether the results of trials (including cancer studies) conducted outside the United States can be directly extrapolated to North American populations.15 Observed differences in toxicity and efficacy outcomes between trials testing similar or identical treatment regimens may be due to several factors, including differences in study design, eligibility criteria, patient selection, demographics, and treatment regimens. Another emerging reason for this divergence of outcomes is the presence of host-related genetic differences associated with race and/or ethnicity. This is specially relevant when comparing similarly designed trials performed in different parts of the world.
This large North American randomized trial (S0124) failed to confirm the previously reported survival benefit seen with IP in Japanese patients. Both chemotherapy regimens in S0124 produced comparable overall response rates and survival outcomes, with less hematologic and greater gastrointestinal toxicity for IP compared with EP.
Multiple potential explanations exist for the divergence of results between the two North American trials and JCOG 9511. One reason is related to early stopping of the JCOG 9511 trial. The smaller sample size of JCOG 9511 may have significantly overestimated the treatment effect, which diminishes when the number of events accrued is large, as was the case for our trial. In light of the correlation between the apparent treatment effect and the number of events, some have argued that interim analyses only be performed after a sufficient number of events have occurred to reduce the likelihood of overestimating the true treatment effect.16
In addition, JCOG 9511 also had imbalances in the distribution of patient characteristics which, although not statistically significant, may have favored the experimental arm in the context of a small trial. These characteristics included fewer patients with borderline performance status (performance status of 2), more women (who historically do better than men17), and fewer brain metastases, among others. On the other hand, the present trial benefits from a well-balanced population and its much larger sample size.
In this age of globalization of the clinical trials process, a particularly interesting reason for the difference in Japanese and North American results may be inherent genetic differences that exist between populations, resulting in divergent outcomes with the same cytotoxic agents. It is known that polymorphisms of genes involved in the metabolism or transport of chemotherapy vary among ethnic populations.18,19 The active metabolite of irinotecan, SN-38, is a target for several gene products, such as the ABCB1 family (including P-glycoprotein) which actively transports SN-38 out of the cell. SN-38 is also the target for UGT-1A1, an enzyme catalyzing glucuronidation of SN-38, making it into an inactive metabolite. The UGT and ABCB1 gene families are characterized by polymorphisms that can affect the gene product's function. SNPs may affect the function of the encoded protein in drug transport, metabolism, and receptor binding, resulting in clinically evident effects. It has been shown that polymorphisms in the promoter region of UGT1A1*28—characterized by six to seven TA repeats within each allele (ie, 6/6, 6/7, or 6/7)—influence the risk of grade 4 neutropenia after irinotecan therapy. It has been shown that the 6/6 genotype is associated with little or no neutropenia, whereas the 7/7 genotype, which corresponds to the clinical phenotype of Gilbert's disease, is associated with a 50% risk of grade 4 neutropenia. Hence pharmacogenomic variability in SNPs may help explain interindividual differences and population-related differences in toxicity and outcome after chemotherapy. In fact, when a formal comparative patient toxicity analysis of the JCOG 9511 and S0124 trials was performed, significant differences in hematologic toxicity (neutropenia, leucopenia, and anemia) were observed.20 Specifically, enhanced hematologic toxicity was seen in Japanese patients as compared with North American S0124 patients, each receiving the exact same chemotherapy regimens. To further explore this issue in S0124, we evaluated SNPs of selected genes involved in chemotherapy metabolism and transport. We found that certain SNPs seem to be associated with specific toxicities, but no clear correlation with chemotherapy efficacy was detected, likely because of the limited number of available specimens.
Interestingly, a recently published phase III European trial comparing irinotecan plus carboplatin with etoposide plus carboplatin in patients with extensive SCLC reported a survival benefit for the former.21 However, the generalizability of those results to the broader population of patients with SCLC is limited as a result of marked differences in study design, treatment regimens, and study conduct compared with other trials in this population, such as S0124. These and other issues are thoroughly discussed in an accompanying editorial coauthored by some of us (P.N.L., R.N., D.R.G.).22
In conclusion, EP remains the reference treatment standard in North America. It is possible that pharmacogenetic differences exist between Japanese and North American populations to explain these results, as is the likelihood of overestimation of treatment effect as a result of early termination of accrual in the Japanese trial.
Appendix
Dose Schedule of Cisplatin/Irinotecan and Cisplatin/Etoposide in the Hanna Trial
In the Hanna trial (Hanna N, Bunn P, Langer C, et al: J Clin Oncol 24:2038-2043, 2006) patients on the cisplatin plus irinotecan (IP) arm received cisplatin 30 mg/m2 administered intravenously (IV) plus irinotecan 65 mg/m2 IV on days 1 and 8 every 21 days, whereas those on the cisplatin plus etoposide (EP) arm received cisplatin 60 mg/m2 IV on day 1 plus etoposide 120 mg/m2 IV on days 1 through 3 every 21 days.
Reasons for Early Protocol Discontinuation
The reasons for early discontinuation were distributed similarly over both arms. The following summarizes the reasons for and rates of early discontinuation: 1 adverse events, 45%; 2 progressive disease, 26%; 3 death, 9%; and 4 patient refusal: 3%. An additional 13% discontinued for various other reasons. Eight cases or 3% were still under review at the time of database analysis.
Frequencies of ABCB1 (C3435) and UGT1A1 (G3156A)
The frequencies of the specific alleles of ABCB1 (C3435) were as follows: C/C (30%), C/T (46%), and T/T (24%). The frequencies for the specific alleles of UGT1A1 (G-3156A) were as follows: A/A (9%), A/G (47%), and G/G (45%).
Footnotes
Supported in part by the following Public Health Service Cooperative Agreement grants awarded by the National Cancer Institute, United States Department of Health and Human Services: Grants No. CA32102, CA38926, CA46441, CA58882, CA35261, CA35431, CA35119, CA22433, CA58658, CA11083, CA46441, CA37981, CA45560, CA58861, CA04919, CA67663, CA12644, CA45807, CA67575, CA35281, CA20319, CA45808, CA35178, CA58416, CA14028, CA76448, CA35090, CA52654, CA58882, CA76447, CA76429, CA35128, CA46282, CA63848, CA46113, CA58723, CA63844, CA46368, CA35192, CA68183, CA45450, CA35176, CA76132, CA13612, CA16385, CA45377, CA63850, CA74647, CA58348, CA42777, CA35279 (CALGB), CA27525, and CA21115 from the Eastern Cooperative Oncology Group; Pfizer Inc; and Grant No. CA114771 from the National Institutes of Health Strategic Partnering to Evaluate Cancer Signatures in Lung Cancer.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00045162.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Heinz Josef Lenz, Southwest Oncology Group (C) Consultant or Advisory Role: Heinz Josef Lenz, Pfizer (C); James Jett, Amgen (C), Genentech (C); David R. Gandara, Pfizer (C) Stock Ownership: None Honoraria: Primo N. Lara Jr, Pfizer; Heinz Josef Lenz, Pfizer Research Funding: Heinz Josef Lenz, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Primo N. Lara Jr, Ronald Natale, John Crowley, Heinz Josef Lenz, Mary W. Redman, David R. Gandara
Administrative support: John Crowley, Mary W. Redman, Kari Chansky
Provision of study materials or patients: Primo N. Lara Jr, Ronald Natale, Jane E. Carleton, James Jett, Corey J. Langer, J. Philip Kuebler, Shaker R. Dakhil, David R. Gandara
Collection and assembly of data: Primo N. Lara Jr, Ronald Natale, John Crowley, Mary W. Redman, Kari Chansky
Data analysis and interpretation: Primo N. Lara Jr, Ronald Natale, John Crowley, Heinz Josef Lenz, Mary W. Redman, Kari Chansky, David R. Gandara
Manuscript writing: Primo N. Lara Jr, John Crowley, Mary W. Redman, Corey J. Langer, Kari Chansky, David R. Gandara
Final approval of manuscript: Primo N. Lara Jr, Ronald Natale, John Crowley, Heinz Josef Lenz, Mary W. Redman, Jane E. Carleton, James Jett, Corey J. Langer, J. Philip Kuebler, Shaker R. Dakhil, Kari Chansky, David R. Gandara
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Govindan R, Page N, Morgensztern N, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the Surveillance, Epidemiologic, and End Results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 3.Clark R, Ihde DC. Small cell lung cancer: Treatment progress and prospects. Oncology (Williston Park) 1998;12:647–658. [PubMed] [Google Scholar]
- 4.Albain KS, Crowley JJ, LeBlanc M, et al. Determinants of improved outcome in small cell lung cancer: An analysis of the 2,580 patient Southwest Oncology Group database. J Clin Oncol. 1990;8:1563–1574. doi: 10.1200/JCO.1990.8.9.1563. [DOI] [PubMed] [Google Scholar]
- 5.Schiller JH, Adak S, Cella D, et al. Topotecan versus observation after cisplatin plus etoposide in extensive stage small cell lung cancer: E7593—A phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:2114–2122. doi: 10.1200/JCO.2001.19.8.2114. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, Monnerat C, Turrisi AT, 3rd, et al. Small cell lung cancer: State of the art and future perspectives. Lung Cancer. 2004;45:105–117. doi: 10.1016/j.lungcan.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Davies AM, Lara PN, Lau DH, et al. Treatment of extensive small cell lung cancer. Hematol Oncol Clin North Am. 2004;18:373–385. doi: 10.1016/j.hoc.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly JG. Pharmacogenetics in cancer chemotherapy: Balancing toxicity and response. Ther Drug Monit. 2004;26:231–235. doi: 10.1097/00007691-200404000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Marsh S. Pharmacogenetics of colorectal cancer. Expert Opin Pharmacother. 2005;6:2607–2616. doi: 10.1517/14656566.6.15.2607. [DOI] [PubMed] [Google Scholar]
- 11.Watters JW, McLeod HL. Cancer pharmacogenomics: Current and future applications. Biochim Biochim Biophys Acta. 2003;1603:99–111. doi: 10.1016/s0304-419x(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 12.Lenz HJ. Pharmacogenomics and colorectal cancer. Adv Exp Med Biol. 2006;587:211–231. doi: 10.1007/978-1-4020-5133-3_18. [DOI] [PubMed] [Google Scholar]
- 13.Hanna N, Bunn P, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–2043. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Thiers F, Sinskey AJ, Berndt ER. From the analyst's couch: Trends in the globalization of clinical trials. Nat Rev Drug Discov. 2008;7:13–14. [Google Scholar]
- 16.Wilcox R, Djulbegovic B, Guyatt GH, et al. Randomized trials in oncology stopped early for benefit. J Clin Oncol. 2008;26:18–19. doi: 10.1200/JCO.2007.13.6259. [DOI] [PubMed] [Google Scholar]
- 17.Johnson BE, Steinberg SM, Phelps R, et al. Female patients with small cell lung cancer live longer than male patients. Am J Med. 1988;85:194–196. doi: 10.1016/s0002-9343(88)80341-3. [DOI] [PubMed] [Google Scholar]
- 18.de Jong FA, Marsh S, Mathijssen RH, et al. ABCG2 pharmacogenetics: Ethnic differences in allele frequency and assessment of influence on irinotecan disposition. Clin Cancer Res. 2004;10:5889–5894. doi: 10.1158/1078-0432.CCR-04-0144. [DOI] [PubMed] [Google Scholar]
- 19.Hoskins JM, Marcuello E, Altes A, et al. Irinotecan pharmacogenetics: Influence of pharmacodynamic genes. Clin Cancer Res. 2008;14:1788–1796. doi: 10.1158/1078-0432.CCR-07-1472. [DOI] [PubMed] [Google Scholar]
- 20.Lara PN, Redman M, Lenz HJ, et al. Cisplatin (Cis)/etoposide (VP16) compared to cis/irinotecan (CPT11) in extensive-stage small cell lung cancer (E-SCLC): Pharmacogenomic (PG) and comparative toxicity analysis of JCOG 9511 and SWOG 0124. J Clin Oncol. 2007;25(suppl):390s. abstr 7524. [Google Scholar]
- 21.Hermes A, Bergman B, Bremnes R, et al. Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: A randomized phase III trial. J Clin Oncol. 2008;26:4261–4267. doi: 10.1200/JCO.2007.15.7545. [DOI] [PubMed] [Google Scholar]
- 22.Gandara DR, Lara PN, Natale RB, et al. Progress in small-cell lung cancer: The lowest common denominator. J Clin Oncol. 2008;26:4236–4238. doi: 10.1200/JCO.2008.17.2692. [DOI] [PubMed] [Google Scholar]