Abstract
Purpose
The Ewing sarcoma family of tumors (ESFT) is a group of malignant tumors of soft tissue and bone sharing a chromosomal translocation affecting the EWS locus. The Intergroup INT-0091 demonstrated the superiority of a regimen of vincristine, cyclophosphamide, doxorubicin (VDC), and dactinomycin alternating with ifosfamide and etoposide (IE) over VDC for patients with nonmetastatic ESFT of bone. The goal of this study was to determine whether a dose-intensified regimen of VDC alternating with IE would further improve the outcome for patients with nonmetastatic ESFT of bone or soft tissue.
Methods
Patients with previously untreated, nonmetastatic ESFT of bone or soft tissue were eligible. They were randomly assigned to receive standard doses of VDC/IE over 48 weeks or a dose-intensified regimen of VDC/IE over 30 weeks.
Results
Four hundred seventy-eight patients met eligibility requirements: 231 patients received the standard regimen; 247 patients received the intensified regimen. The 5-year event-free survival (EFS) and overall survival rates for all eligible patients were 71.1% (95% CI, 67.7% to 75.0%) and 78.6% (95% CI, 74.6% to 82.1%), respectively. There was no significant difference (P = .57) in EFS between patients treated with the standard (5-year EFS, 72.1%; 95% CI, 65.8% to 77.5%) or intensified regimen (5-year EFS, 70.1%; 63.9% to 75%). Patients with soft tissue tumors accounted for 20% of the study population; there was no difference in outcome between patients with soft tissue and bone primary sites.
Conclusion
Dose escalation of alkylating agents as tested in this trial did not improve the outcome for patients with nonmetastatic ESFT of bone or soft tissue.
INTRODUCTION
The Ewing sarcoma family of tumors (ESFT) consists of Ewing sarcoma and primitive neuroectodermal tumors (PNET) of bone and soft tissue. These tumors share the presence of a translocation t(11;22) or one of its variants.1 The use of multimodality therapy dramatically improved the outcome for these patients from 15% to 20% with surgery and/or radiation2,3 to 60% to 70%.4–6
Intergroup study INT-0091 demonstrated that the addition of ifosfamide and etoposide (IE) to vincristine, doxorubicin, and cyclophosphamide (VDC) resulted in a significant improvement in event-free survival (EFS) and overall survival (OS) for patients with nonmetastatic ESFT of bone.7 Patients treated with the experimental arm had a 5-year OS rate of 72%, as compared with 61% for patients treated with the standard regimen (P = .01).
The earlier Intergroup Study (IESS-II) demonstrated improved survival for patients with nonpelvic disease treated with a high-dose intermittent regimen (5-year disease-free survival rate, 68%) compared with the less intense regimen (5-year disease-free survival rate, 48%.)8 Although Smith et al9,10 attributed this difference to the dose-intensity of doxorubicin, these regimens also differed in the total doses of alkylating agents. The goal of this study was to determine whether a dose-intensified regimen of VDC and IE would further improve the outcome for these patients.
Dose intensification is defined as the amount of drug over unit time.11 One may dose intensify therapy by keeping the interval stable while escalating the dose(s) of the chemotherapeutic agents, or one can shorten the interval between cycles. Because ESFT are exquisitely sensitive to alkylating agents,12,13 and hematopoietic growth factors allow for dose intensification, the investigational regimen of this study used dose-intensified alkylating agents yet kept the cumulative doses of the drugs similar between the two arms.
Previous clinical trials of ESFT limited eligibility to patients with bone primaries. There is a subset of soft tissue tumors histologically identical to ESFT that share the same chromosomal translocation.14 A secondary objective of this study was to determine the incidence of primary bone versus soft tissue tumors and to determine whether the prognosis differed by soft tissue or bone site. Additional goals were to determine the impact of histologic subtype on prognosis, the incidence of serious toxicities by regimen, and to estimate the occurrence of second malignant neoplasms (SMNs). This article reports the results of this study.
METHODS
Patients
National Cancer Institute protocol INT-0154 (Children's Cancer Group 7942/Pediatric Oncology Group 9354) was opened to all member institutions of the Children's Cancer Group and the Pediatric Oncology Group between May 1995 and September 1998. Eligible patients were younger than 30 years at enrollment and had ESFT of bone or soft tissue excluding the brain and no metastases identified before enrollment. ESFTs were eligible on the basis of a light microscopic appearance of a small round-cell neoplasm consistent with Ewing sarcoma or PNET. The following immunohistochemical studies were required: desmin or muscle-specific actin, leukocyte common antigen, and MIC2 antibody (12E7 or HBA 71). Patients without immunohistochemical or ultrastructural evidence excluding Ewing sarcoma or PNET were eligible. Patients with immunohistochemical evidence supporting a diagnosis of rhabdomyosarcoma were ineligible. All pathology specimens underwent central review. Eligibility requirements included normal liver, renal, and cardiac function. Patients must have started protocol therapy within 30 days of diagnostic biopsy and not have had any systemic anticancer therapy before study entry. All patients/guardians gave written informed consent according to institutional and National Cancer Institute guidelines, and the protocol was approved by the institutional review boards at all participating centers.
Study Design
Patients were randomly assigned to standard or intensified therapy (Fig 1). Chemotherapy courses were VDC alternating with IE delivered every 3 weeks (Fig 1). The standard regimen prescribed 17 cycles of chemotherapy administered over 48 weeks; the intensified regimen prescribed 11 cycles given over 30 weeks. Granulocyte colony-stimulating factor support for both regimens (5 μg/kg/d) was prescribed. The total doses of all agents were similar (Fig 1). The intent was to deliver similar cumulative doses of the agents to determine the effect of early dose intensification without a change in total chemotherapeutic drug exposure.
Fig 1.
Treatment schema with cumulative doses of agents by regimen.
Local control was performed after week 12 and consisted of resection or radiotherapy. Patients who had close or positive margins received postresection radiotherapy. Patients were eligible for study if a resection had been done before study entry; if these patients did not have a prechemotherapy complete resection, it was the treating physician's option to give radiation or perform a repeat resection after week 12 of therapy. Patients who were treated with radiation therapy only after week 12 could receive delayed surgical resection after week 30. Patients with bone primaries who received radiation therapy as primary therapy, and those with gross residual disease after surgery, received a dose of 45 Gy to the initial tumor volume (plus 2-cm margin) with a boost of 10.8 Gy, for a total dose of 55.8 Gy to the post–neoadjuvant therapy tumor volume. For patients with extraosseous tumor who had a complete response to induction chemotherapy, the initial tumor volume plus a 2-cm margin received 45 Gy followed by a boost of 5.4 Gy with a 1-cm margin, to a total dose of 50.4 Gy. Patients with postoperative microscopic residual or close margins, defined as less than 1 cm for bone, less than 5 mm for fat and muscle, and less than 2 mm for facial planes, received 45 Gy to the primary initial volume plus 2-cm margin followed by a boost to the site of close or positive margins, to a total dose of 50.4 Gy. No radiation was recommended for patients with complete tumor resection before or at the local control time point, regardless of the extent of necrosis or size of the original tumor.
Statistical Analysis
The study was designed to accrue 423 eligible patients. The primary end point for the estimation of relative efficacy was EFS. Risk of adverse event was compared between regimens using a log-rank test. The sample size allowed detection of a 1.5-fold decrease in failure rate, with probability of 0.80 when using a two-sided test of size 5%. The expected accrual duration was 4.7 years with 3 years of follow-up after the last patient was entered. Data and follow-up received through December 2006 were included in the analysis data set. EFS was defined as the time from study entry until the occurrence of an analytic event or date of last patient contact, whichever came first. An analytic event was defined as disease progression, diagnosis of SMN, or death before the development of disease progression or SMN. OS was defined as the time from study entry until the death or date of last patient contact, whichever came first. Death, regardless of cause, was considered an event.
EFS and OS were estimated by the method of Kaplan and Meier.15 Risk of adverse event was compared across groups defined by treatment or prognostic factors using the log-rank test. Comparisons involving the chemotherapy randomization were conducted with patients' outcomes assigned to the treatment arm to which they were randomly assigned at enrollment. The prognostic significance and associated relative risk for various patient characteristics measured at study entry were assessed by a proportional hazards regression model with the characteristic of interest as the only component.16 CIs for relative risks were derived from the proportional hazards regression model. Interim monitoring was conducted 30 and 42 months after the study was opened. A P value of less than .001 was used as the monitoring boundary for all interim analyses.
EFS events were further classified into six modes: (1) disease progression or recurrence at the initial site of disease (local recurrence), (2) disease progression or recurrence at a site not initially involved by disease (distant recurrence), (3) disease progression or recurrence at the initial site of disease as well as at least one site not initially involved by disease (local plus distant recurrence), (4) disease progression or recurrence, but not enough details were supplied to determine the exact sites of progression (disease recurrence– undetermined site), (5) diagnosis of an SMN, and (6) death without an intervening disease- or SMN-related event. The cumulative incidence of each failure mode was calculated by the method as described by Gray.17 For each event type, the equality of risk for each such event type was tested using the relative risk regression model, with the other events that defined the outcome considered as censoring events.16 Possible heterogeneity in risk for EFS event was examined using a relative risk regression model that included terms for site of bone primary, randomized treatment, and interaction terms for site and treatment. The hypothesis of no interaction terms was tested using the difference in partial likelihoods for the models including and excluding the interaction terms.
Toxicity was graded according to the Pediatric Oncology Group/ Children's Cancer Group toxicity criteria (schedule of toxicities available on request from Children's Oncology Group). Each patient was classified according to the maximum grade of toxicity experienced at any time during protocol therapy for each of the 37 categories evaluated.
RESULTS
Patient Characteristics
Of the 492 patients enrolled, 478 patients with nonmetastatic ESFT were eligible. Thirteen patients were ineligible (13 patients had incorrect diagnosis and one patient had lung metastases). The incorrect diagnosis included rhabdomyosarcoma (n = 3), undifferentiated sarcoma (n = 1), neuroblastoma (n = 2), and other tumors (n = 7). Characteristics at time of diagnosis for patients enrolled onto the study are listed in Table 1. Two hundred sixty-three patients (55%) were male; 401 patients (84%) were white and 18 patients (4%) were African American, reflecting the rarity of ESFT among people of African ethnicity. Ninety-four patients (20%) had soft tissue tumors, 377 patients (80%) had bone primaries, and seven patients had insufficient data to classify site. Among patients with bone tumors, 70 patients (19%) had pelvic, 175 patients (46%) had appendicular, and 75 patients (20%) had thoracic tumors.
Table 1.
Patient Characteristics
| Characteristic | Standard (n = 231) |
Intensified (n = 247) |
Overall (N = 478) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Sex | ||||||
| Male | 127 | 55 | 136 | 55 | 263 | 55 |
| Female | 104 | 45 | 111 | 45 | 215 | 45 |
| Age at diagnosis, years | ||||||
| 1-9 | 71 | 31 | 77 | 31 | 148 | 31 |
| 10-17 | 121 | 52 | 144 | 58 | 265 | 55 |
| 18+ | 39 | 17 | 26 | 11 | 65 | 14 |
| Race/ethnicity | ||||||
| White | 190 | 82 | 211 | 85 | 401 | 84 |
| African American | 11 | 5 | 7 | 3 | 18 | 4 |
| Asian | 20 | 9 | 24 | 10 | 44 | 9 |
| Other | 10 | 4 | 5 | 2 | 15 | 3 |
| Primary tumor site | ||||||
| Bone | 177 | 78 | 200 | 82 | 377 | 80 |
| Soft tissue | 50 | 22 | 44 | 18 | 94 | 20 |
| Not reported | 4 | 3 | 7 | |||
| Primary site, bone lesions | ||||||
| Pelvis | 30 | 17 | 40 | 20 | 70 | 19 |
| Thoracic | 30 | 23 | 35 | 18 | 75 | 20 |
| Other axial | 27 | 15 | 30 | 15 | 57 | 15 |
| Appendicular | 80 | 45 | 95 | 48 | 175 | 46 |
| Not reported | 4 | 3 | 7 | |||
Outcome
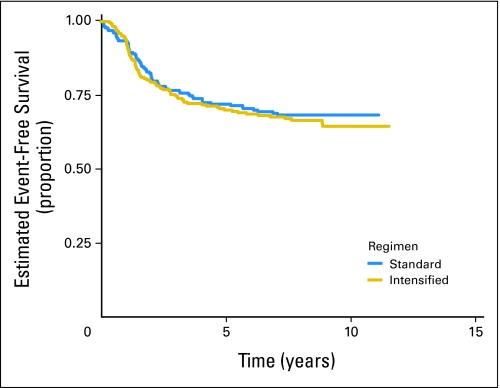
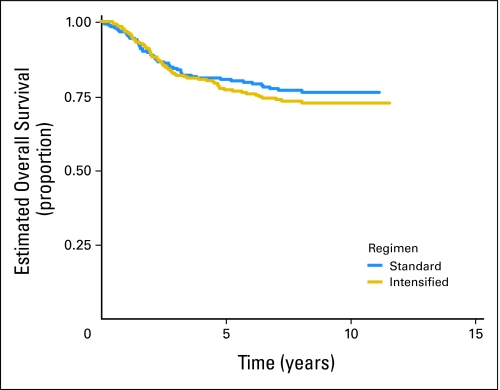
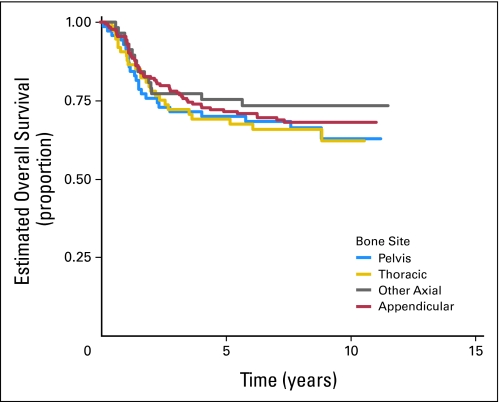
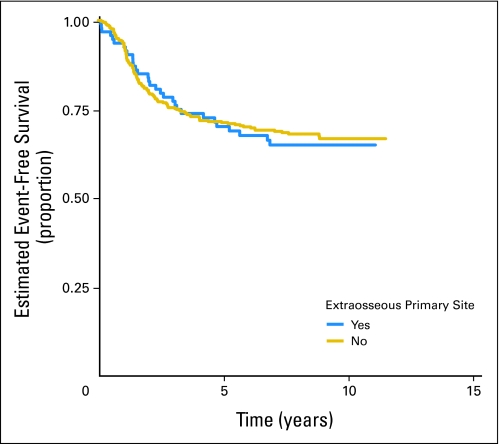
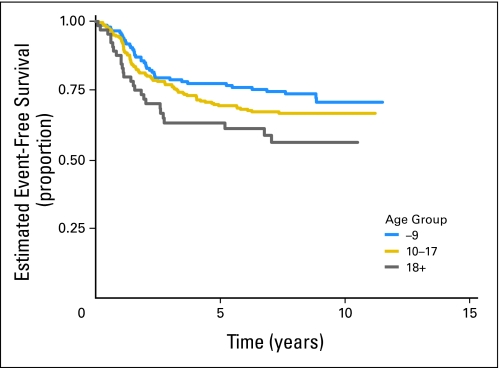
Of 478 eligible patients, 119 patients experienced disease progression, 18 patients developed SMNs, and 14 patients died as their first event. Patients who did not experience an EFS event were followed for a median of 8.3 years. The EFS rate for all patients was 71.1% (95% CI, 66.7% to 75.0%) at 5 years. The OS rate was 78.6% (95% CI, 74.6% to 82.1%) at 5 years. There was no significant difference in risk for EFS event or death when the standard and intensified regimens are compared (Fig 2; Appendix Fig A1, online only). The 5-year EFS rate for the standard regimen is 72.1% (95% CI, 65.8% to 77.5%), and the 5-year EFS rate for the intensified regimen is 70.1% (95% CI, 63.9% to 75.5%). Similarly, there was no difference in OS between the two regimens (5-year OS for the standard regimen, 80.5% [95% CI, 74.7% to 85.1%], and for the intensified regimen, 77.0% [95% CI, 71.1% to 81.8%]). Patients with pelvic primary tumors had an EFS and OS similar to that of patients with other primary tumors of bone (Fig 3; P = .57). Outcome did not differ significantly between bone and soft tissue primary tumors: 5-year EFS, 71.3% (95% CI, 66.4% to 75.6%) versus 70.2% (95% CI, 59.6% to 78.5%; P = .78), respectively (Appendix Fig A2, online only). Overall 5-year EFS by age group at enrollment (1 to 9 years, 77.5% [95% CI, 69.8% to 83.4%], v 10 to 17 years, 69.4% [95% CI, 63.4% to 74.7%] v 18+ years, 63.2% [95% CI, 49.9% to 73.8%]) yielded a P value of .018 (trend), with the younger patients having better outcome (Appendix Fig A3, online only).
Fig 2.
Event-free survival for all eligible patients by regimen.
Fig 3.
Overall survival for all eligible patients by primary bone site.
Local Control
Of the 478 eligible patients, 77 patients were excluded from the analysis of local control. Three patients experienced an analytic event before the completion of induction chemotherapy. Data was insufficient to determine the local control for the remaining excluded patients. One patient with a femur tumor was reported not to have any local control; this individual is excluded from the subsequent analysis. Among the remaining 400 patients, the local control method used did not significantly differ between the two regimens (Appendix Table A1, online only).
Patterns of Failure
The cumulative incidences of the various types of events were not statistically different when the two regimens were compared (Table 2). Of the 55 disease recurrences in patients enrolled onto the standard regimen, 15 were local, 32 were distant, and six had both local plus distant components; the site of recurrence was not reported in two cases. Of the 64 disease recurrences in patients enrolled onto the intensified regimen, 13 were local, 34 were distant, and 15 had both local plus distant components; the site of recurrence was not reported in two cases. Eighteen SMNs were reported; seven among patients receiving the standard regimen and 11 among patients receiving the intensified regimen. Eight patients on the standard and six on the intensified regimen died before any EFS event.
Table 2.
Cumulative Incidences According to Randomized Treatment Assignment
| Event Type | 5-Year Cumulative Incidence (%) |
P | |
|---|---|---|---|
| Standard | Intensified | ||
| Local recurrence | 6.2 | 5.4 | .60 |
| Distant recurrence | 12.9 | 13.3 | .97 |
| Local plus distant recurrence | 2.2 | 6.2 | .072 |
| Indeterminant site of recurrence | 0.9 | 0.8 | .95 |
| Second malignant neoplasm | 3.1 | 2.1 | .41 |
| Death without other EFS event | 2.6 | 2.1 | .52 |
Abbreviation: EFS, event-free survival.
Local control with radiation therapy only was associated with a significantly increased risk for failure at local and distant sites (Table 3). No other significant differences in pattern of failure as it related to local control modality were noted.
Table 3.
Cumulative Incidences of Specific Event Types After Local Control According to Local Control Modality Used
| Event Type | 5-Year Cumulative Incidence (%) |
P | ||
|---|---|---|---|---|
| Surgery Only | Radiation Therapy Only | Surgery Plus Radiation Therapy | ||
| Local recurrence | 5.1 | 9.2 | 2.0 | .16 |
| Distant recurrence | 11.8 | 13.9 | 15.8 | .62 |
| Local plus distant recurrence | 2.7 | 8.0 | 5.9 | .038 |
| Indeterminant site of recurrence | 0 | 0 | 2 | —* |
| Second malignant neoplasm | 2.8 | 3.5 | 2.0 | .30 |
| Death without other EFS event | 2.4 | 2.3 | 0 | .99 |
Abbreviation: EFS, event-free survival.
Only one event occurred. The P value could not be calculated.
SMNs
There were 12 patients with secondary leukemia and seven patients with secondary solid tumors (Appendix Tables A2 and A3, online only). Among the patients with leukemia, nine patients had cytogenetic data available; all had cytogenetics consistent with secondary leukemia: seven 11q23 abnormalities, one del7. Seven patients with secondary leukemia were treated with standard and five with intensified therapy. Six of the seven patients with secondary solid tumors were treated on the intensified regimen. Of these, three patients had radiation to the site of the second malignancy. One patient with secondary osteosarcoma never received radiation. The one patient randomly assigned to the standard regimen who developed a solid tumor never received radiation therapy; this patient developed renal cell carcinoma 3 years after disease progression of Ewing sarcoma.
Toxicity
The following grade 3 and 4 toxicities were reported more frequently among patients treated on the investigational regimen: neutropenia, thrombocytopenia, anemia, diarrhea, stomatitis, Fanconi syndrome (primarily grade 3), fungal stomatitis, hematuria, sepsis, and infection. Appendix Table A4 (online only) gives the comparison of selected grade 3 or worse toxicities by treatment regimen.
DISCUSSION
Dose intensification as studied in this clinical trial did not result in an improved outcome for patients with nonmetastatic ESFT. The dose of cyclophosphamide during the 12-week induction on the investigational arm was 350% greater than the cyclophosphamide dose on the standard arm; during weeks 13 to 30 on the investigational arm and weeks 13 to 48 on the standard arm, the dose-intensity for cyclophosphamide was the same. The ifosfamide dose was 133% greater throughout the 30 weeks of the investigational regimen compared with the standard regimen. Thus despite significant intensification of both early cyclophosphamide and ifosfamide dose, we could not demonstrate improved survival for patients on the investigational therapy. This is similar to the failure of chemotherapy dose intensification to improve survival in patients for metastatic ESFT. Unexpectedly, patients with pelvic tumors did not have an inferior survival as compared with that of patients with primary tumors at other sites. This is a new finding and did not differ between the regimens.18 The percentage (19%) of patients with bone primary with pelvic tumors is slightly lower than that of other published series (23% to 25%)6,7 but did not differ between regimens and thus would not impact comparison between the regimens.
The majority of patients on this study were treated with surgery alone for local therapy. European groups have advocated the use of surgery followed by radiation in patients with large tumors and those with a poor pathologic response to chemotherapy as ascertained at the time of surgical local control. In contrast, we used radiation therapy after surgery only in patients with close or positive margins after resection. There is no evidence that our patients with large tumors would have derived a benefit from adding radiation therapy, as local control rates are comparable to those reported in European studies.6 An analysis of the chest wall tumors treated on this study and INT-0091 demonstrated that there was no benefit to the addition of radiation to the treatment of patients with chest wall tumors with negative margins after resection.19
There was no difference between the two regimens in regard to the incidence of secondary leukemia. The incidence of secondary leukemia and solid tumors among patients on this study is similar to that reported for patients treated on INT-0091 and other contemporary series. One study did report a higher incidence of secondary leukemia; the highly intensified Regimen C in INT-009120,21 for patients with metastatic Ewing sarcoma of bone found a cumulative incidence of secondary leukemia or myelodysplasia of 11% at 5 years. That protocol differs from the experimental arm of this study in that patients were exposed to twice the cumulative dose of alkylating agents and had a higher dose rate and cumulative dose of doxorubicin. Thus the excess incidence of leukemia and myelodysplasia on INT-0091 Regimen C for patients with metastatic disease was probably related to the cumulative agent exposure and/or dose rate of doxorubicin rather than dose intensification of alkalating agents alone.
The incidence of solid tumors among the INT-0154 patients reported here is small. The incidence of secondary solid tumors increases over time, thus it may be too soon to obtain an accurate estimate of the incidence of secondary solid tumors.22 Most reports of secondary solid tumors report a strong association with radiation and alkylating agent exposure.22,23 We note that half of the patients who developed a secondary solid tumor did not receive radiation to the site of the secondary malignancy.
There was no difference in EFS and OS between ESFT patients with extraosseous and those with bone primary tumors. This is consistent with a recent report from France24 describing the treatment of EOE and bone primary ESFT patients on the same protocol, as compared with previous protocols, during which EOE patients received treatment on malignant mesenchymal tumor trials.
In conclusion, dose intensification of alkylating agents as tested here did not improve OS and EFS for patients with ESFT. Twenty percent of patients had soft tissue tumors and outcome for those patients was the same as those with ESFT of bone. The incidence of secondary leukemia did not differ between the regimens. Secondary solid tumors were more frequent among patients on the investigational regimen, and half of these tumors were not associated with radiation therapy.
Appendix
Fig A1.
Survival for all eligible patients by regimen.
Fig A2.
Comparison of event-free survival for all eligible patients by primary tumor site (bone v soft tissue).
Fig A3.
Event-free survival for all eligible patients by age at diagnosis.
Table A1.
Local Control Modality According to Randomized Treatment Assignment
| Local Control Method | Randomized Treatment Assignment |
|||
|---|---|---|---|---|
| Standard |
Intensified |
|||
| No. | % | No. | % | |
| Surgery | 122 | 66 | 139 | 65 |
| Radiation therapy | 37 | 20 | 50 | 23 |
| Surgery and radiation therapy | 27 | 15 | 25 | 12 |
NOTE. P value for the hypothesis of no difference in choice of local control modality across randomized regimen = .57.
Table A2.
Patients With a Diagnosis of Secondary Leukemia After Enrollment Onto Study
| Secondary Leukemia | Regimen | Cytogenetics | Radiation Therapy Received During Study Therapy |
|---|---|---|---|
| Acute monoblastic | A | Not done | Yes |
| Acute myeloid | A | del(7) | No |
| Acute lymphoblastic | A | del(11)(q23) | No |
| Acute myeloid | A | t(9:11) | Yes |
| Acute myelomonocytic | A | Not done | No |
| Acute myeloid | A | Not done | Yes |
| Acute myeloid | A | del(9)(q21q32) | Yes |
| Acute monoblastic | B | t(9:11) | No |
| Acute myeloid | B | del(11)(q23) | No |
| Acute lymphoblastic | B | del(11)(q23) | Yes |
| Acute myeloid | B | t(9:11) | Yes |
| Acute lymphoblastic | B | der(8)t(1:8)(q21;p21) | No |
Table A3.
Patients With a Diagnosis of Secondary Solid Tumor After Enrollment on Study
| Secondary Solid Tumor | Regimen | Radiation Therapy Received During Study Therapy | Did Tumor Occur in Radiation Therapy Field? |
|---|---|---|---|
| Poorly differentiated sarcoma | B | Yes | Yes |
| Papillary carcinoma of the thyroid | B | Yes | No |
| Infiltrating duct carcinoma | B | No | — |
| Osteosarcoma | B | No | — |
| Osteosarcoma | B | Yes | Yes |
| Papillary and follicular adenocarcinoma | B | Yes | Yes; edge of the margin of the field |
| Renal cell sarcoma* | A | No | — |
Tumor was diagnosed after the patient experienced progression of Ewing sarcoma.
Table A4.
All Grade 3 or 4 Toxicities Reported in ≥ 5% of Eligible Patients in at Least One Treatment Group of INT-0154
| Toxicity | Regimen A (standard) |
Regimen B (intensified) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ever |
Never |
Total No. | Ever |
Never |
Total No. | |||||
| No. | % | No. | % | No. | % | No. | % | |||
| ANC | 203 | 87.9 | 28 | 12.1 | 231 | 238 | 96.4 | 9 | 3.6 | 247 |
| Allergic reaction | 9 | 3.9 | 222 | 96.1 | 231 | 13 | 5.3 | 234 | 94.7 | 247 |
| Bilirubin | 6 | 2.6 | 225 | 97.4 | 231 | 19 | 7.7 | 228 | 92.3 | 247 |
| Constipation | 10 | 4.3 | 221 | 95.7 | 231 | 23 | 9.3 | 224 | 90.7 | 247 |
| Diarrhea | 14 | 6.1 | 217 | 93.9 | 231 | 49 | 19.8 | 198 | 80.2 | 247 |
| Echo, FS | 16 | 6.9 | 215 | 93.1 | 231 | 9 | 3.6 | 238 | 96.4 | 247 |
| Fanconi syndrome | 8 | 3.5 | 223 | 96.5 | 231 | 27 | 10.9 | 220 | 89.1 | 247 |
| Fungal esophagitis | 4 | 1.7 | 227 | 98.3 | 231 | 15 | 6.1 | 232 | 93.9 | 247 |
| Fungal stomatitis | 10 | 4.3 | 221 | 95.7 | 231 | 20 | 8.1 | 227 | 91.9 | 247 |
| Hematuria | 30 | 13.0 | 201 | 87.0 | 231 | 38 | 15.4 | 209 | 84.6 | 247 |
| Hemoglobin | 170 | 73.6 | 61 | 26.4 | 231 | 227 | 91.9 | 20 | 8.1 | 247 |
| Infection NOS/UNK | 111 | 48.1 | 120 | 51.9 | 231 | 203 | 82.2 | 44 | 17.8 | 247 |
| Potassium | 91 | 39.4 | 140 | 60.6 | 231 | 123 | 49.8 | 124 | 50.2 | 247 |
| Local | 29 | 12.6 | 202 | 87.4 | 231 | 32 | 13.0 | 215 | 87.0 | 247 |
| Nausea | 7 | 3.0 | 224 | 97.0 | 231 | 15 | 6.1 | 232 | 93.9 | 247 |
| Other bacterial | 56 | 24.2 | 175 | 75.8 | 231 | 64 | 25.9 | 183 | 74.1 | 247 |
| Path fracture | 8 | 3.5 | 223 | 96.5 | 231 | 19 | 7.7 | 228 | 92.3 | 247 |
| Platelets | 151 | 65.4 | 80 | 34.6 | 231 | 232 | 93.9 | 15 | 6.1 | 247 |
| AST, ALT | 17 | 7.4 | 214 | 92.6 | 231 | 31 | 12.6 | 216 | 87.4 | 247 |
| Sensory | 30 | 13.0 | 201 | 87.0 | 231 | 47 | 19.0 | 200 | 81.0 | 247 |
| Sepsis, bacteria | 64 | 27.7 | 167 | 72.3 | 231 | 97 | 39.3 | 150 | 60.7 | 247 |
| Stomatitis | 67 | 29.0 | 164 | 71.0 | 231 | 158 | 64.0 | 89 | 36.0 | 247 |
| Thrombosis | 12 | 5.2 | 219 | 94.8 | 231 | 10 | 4.0 | 237 | 96.0 | 247 |
| Vomiting | 9 | 3.9 | 222 | 96.1 | 231 | 29 | 11.7 | 218 | 88.3 | 247 |
| WBC | 10 | 4.3 | 221 | 95.7 | 231 | 13 | 5.3 | 234 | 94.7 | 247 |
| Zoster | 9 | 3.9 | 222 | 96.1 | 231 | 24 | 9.7 | 223 | 90.3 | 247 |
Abbreviations: ANC, absolute neutrophil count; FS, shortening fraction; NOS, not otherwise specified; UNK, unknown.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Linda Granowetter, Richard Womer, Mark Bernstein, Mark Gebhardt, John Healey, Robert Cooper Shamberger, Allen Goorin, James Miser, James Meyer, Carola A.S. Arndt, Scott Sailer, Elizabeth Perlman, Paul Dickman, Holcombe E. Grier
Provision of study materials or patients: Linda Granowetter, Richard Womer, Mark Bernstein, John Healey, Allen Goorin, Carola A.S. Arndt, Scott Sailer, Elizabeth Perlman, Paul Dickman, Holcombe E. Grier
Collection and assembly of data: Linda Granowetter, Meenakshi Devidas, Mark Krailo, Patrick Leavey, Scott Sailer, Karen Marcus, Paul Dickman, Holcombe E. Grier
Data analysis and interpretation: Linda Granowetter, Meenakshi Devidas, Mark Krailo, Chenguang Wang, Mark Bernstein, Neyssa Marina, Patrick Leavey, Mark Gebhardt, John Healey, Robert Cooper Shamberger, Scott Sailer, Karen Marcus, Holcombe E. Grier
Manuscript writing: Linda Granowetter, Richard Womer, Meenakshi Devidas, Mark Krailo, Mark Bernstein, Neyssa Marina, Patrick Leavey, Mark Gebhardt, John Healey, Robert Cooper Shamberger, Allen Goorin, Scott Sailer, Holcombe E. Grier
Final approval of manuscript: Linda Granowetter, Richard Womer, Mark Krailo, Mark Bernstein, Neyssa Marina, Patrick Leavey, Mark Gebhardt, John Healey, Robert Cooper Shamberger, Allen Goorin, James Miser, Carola A.S. Arndt, Scott Sailer, Karen Marcus, Elizabeth Perlman, Paul Dickman, Holcombe E. Grier
REFERENCES
- 1.Delattre O, Zucman J, Melot T, et al. The Ewing family of tumors: A subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med. 1994;331:294–299. doi: 10.1056/NEJM199408043310503. [DOI] [PubMed] [Google Scholar]
- 2.Dahlin DC, Coventry MB, Scanlon PW. Ewing's sarcoma. A critical analysis of 165 cases. J Bone Joint Surg Am. 1961;43-A:185–192. [PubMed] [Google Scholar]
- 3.Falk S, Alpert M. Five-year survival of patients with Ewing's sarcoma. Surg Gynecol Obstet. 1967;124:319–324. [PubMed] [Google Scholar]
- 4.Bacci G, Dallari D, McDonald D, et al. Neoadjuvant chemotherapy for localized Ewing's sarcoma of the extremities: Preliminary results of a protocol which uses surgery (alone or followed by radiotherapy) for local control. Tumori. 1989;75:456–462. doi: 10.1177/030089168907500511. [DOI] [PubMed] [Google Scholar]
- 5.Barbieri E, Emiliani E, Zini G, et al. Combined therapy of localized Ewing's sarcoma of bone: Analysis of results in 100 patients. Int J Radiat Oncol Biol Phys. 1990;19:1165–1170. doi: 10.1016/0360-3016(90)90223-7. [DOI] [PubMed] [Google Scholar]
- 6.Jürgens H, Exner U, Gadner H, et al. Multidisciplinary treatment of primary Ewing's sarcoma of bone: A 6-year experience of a European Cooperative Trial. Cancer. 1988;61:23–32. doi: 10.1002/1097-0142(19880101)61:1<23::aid-cncr2820610106>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 8.Burgert EO, Jr, Nesbit ME, Garnsey LA, et al. Multimodal therapy for the management of nonpelvic, localized Ewing's sarcoma of bone: Intergroup study IESS-II. J Clin Oncol. 1990;8:1514–1524. doi: 10.1200/JCO.1990.8.9.1514. [DOI] [PubMed] [Google Scholar]
- 9.Smith MA. The impact of doxorubicin dose intensity on survival of patients with Ewing's sarcoma. J Clin Oncol. 1991;9:889–891. doi: 10.1200/JCO.1991.9.5.889. [DOI] [PubMed] [Google Scholar]
- 10.Smith MA, Ungerleider RS, Horowitz ME, et al. Influence of doxorubicin dose intensity on response and outcome for patients with osteogenic sarcoma and Ewing's sarcoma. J Natl Cancer Inst. 1991;83:1460–1470. doi: 10.1093/jnci/83.20.1460. [DOI] [PubMed] [Google Scholar]
- 11.Hryniuk WM, Goodyear M. The calculation of received dose intensity. J Clin Oncol. 1990;8:1935–1937. doi: 10.1200/JCO.1990.8.12.1935. [DOI] [PubMed] [Google Scholar]
- 12.Johnson R, Humphreys SR. Past failures and future possibilities in Ewing's sarcoma: Experimental and preliminary clinical results. Cancer. 1969;23:161–166. doi: 10.1002/1097-0142(196901)23:1<161::aid-cncr2820230121>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Samuels ML, Howe CD. Cyclophosphamide in the management of Ewing's sarcoma. Cancer. 1967;20:961–966. doi: 10.1002/1097-0142(196706)20:6<961::aid-cncr2820200605>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Mierau GW. Extraskeletal Ewing's sarcoma (peripheral neuroepithelioma) Ultrastruct Pathol. 1985;9:91–98. doi: 10.3109/01913128509055491. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. ed 2. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 17.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 18.Bernstein ML, Devidas M, Lafreniere D, et al. Intensive therapy with growth factor support for patients with Ewing tumor metastatic at diagnosis: Pediatric Oncology Group/Children's Cancer Group Phase II Study 9457—A Report From the Children's Oncology Group. J Clin Oncol. 2006;24:152–159. doi: 10.1200/JCO.2005.02.1717. [DOI] [PubMed] [Google Scholar]
- 19.Shamberger RC, LaQuaglia MP, Gebhardt MC, et al. Ewing sarcoma/primitive neuroectodermal tumor of the chest wall: Impact of initial versus delayed resection on tumor margins, survival, and use of radiation therapy. Ann Surg. 238:563–567. doi: 10.1097/01.sla.0000089857.45191.52. discussion 567-568, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacci G, Longhi A, Barbieri E, et al. Second malignancy in 597 patients with Ewing sarcoma of bone treated at a single institution with adjuvant and neoadjuvant chemotherapy between 1972 and 1999. J Pediatr Hematol Oncol. 2005;27:517–520. doi: 10.1097/01.mph.0000183270.28785.33. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia S, Krailo MD, Chen Z, et al. Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children's Oncology Group. Blood. 2007;109:46–51. doi: 10.1182/blood-2006-01-023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blatt J, Olshan A, Gula MJ, et al. Second malignancies in very-long-term survivors of childhood cancer. Am J Med. 1992;93:57–60. doi: 10.1016/0002-9343(92)90680-a. [DOI] [PubMed] [Google Scholar]
- 23.Tucker MA, D'Angio GJ, Boice JD, Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 24.Castex MP, Rubie H, Stevens MC, et al. Extraosseous localized Ewing tumors: Improved outcome with anthracyclines—the French society of pediatric oncology and international society of pediatric oncology. J Clin Oncol. 2007;25:1176–1182. doi: 10.1200/JCO.2005.05.0559. [DOI] [PubMed] [Google Scholar]