Abstract
Objective
Impairment of white matter connecting frontal and temporal cortices has been reported in schizophrenia. Yet, not much is known about the effects of age on fibers connecting these brain regions. Using diffusion tensor imaging tractography, we investigated the relationship between age and fiber integrity in patients with schizophrenia vs. healthy adults.
Methods
DTI tractography was used to create 3D reconstructions of the cingulum, uncinate and inferior occipito-frontal fasciculi in 27 patients with schizophrenia and 34 healthy volunteers (23–56 years of age, group-matched on age). Fractional anisotropy (FA), describing fiber integrity, was then calculated along the entire length of these tracts, and correlated with subjects’ age.
Results
Patients revealed a significant decline in FA with age in both the cingulum and uncinate, but not in the inferior occipito-frontal fasciculi. No statistically significant correlations were found in these fiber bundles in controls.
Conclusions
These results suggest an age-associated reduction of frontal–temporal connectivity in schizophrenia, but not in healthy controls.
Keywords: White matter, DTI, Tractography, Fronto-temporal connections, Age effects
1. Introduction
Fronto-temporal circuitry disruption is likely involved in the pathophysiology of schizophrenia (Akbarian et al., 1996). Speculations concerning the disconnectivity between fronto-temporal brain regions in schizophrenia, in fact, date back to the beginning of the last century (Wernicke, 1906). These speculations have been refuelled by recent histological as well as genetic findings pointing to abnormalities in glial cells, especially oligodendrocytes, further suggesting that white matter regions that connect fronto-temporal brain regions may be impaired in this disorder (Hakak et al., 2001; Uranova et al., 2004).
Over the past two decades, structural MRI studies have been successful in delineating fronto-temporal gray matter abnormalities in schizophrenia (Shenton et al., 2001), further suggesting an impairment in connectivity between these brain regions. Moreover, connections between gray matter regions provided by white matter tracts are an important new area to investigate. Structural imaging studies have also focused on delineating white matter abnormalities, but have been less successful (Suddath et al., 1990; Wible et al., 1995), likely due to the fact that white matter volume measurements are not sufficiently sensitive for detecting white matter abnormalities involving fiber tract integrity.
Diffusion tensor imaging (DTI) offers the possibility of in vivo detection of white matter integrity disruption. This method is an improvement over conventional magnetic resonance imaging (MRI) in that information is provided concerning the directionality of water diffusion in brain tissue, making it a sensitive tool to detect axonal and myelin disruptions. There is also considerable evidence that less myelination results in lower diffusion fractional anisotropy (FA) (Beaulieu, 2002). We note, however, that at current levels of resolution, we cannot determine whether loss in integrity as evinced from FA measures is due to smaller diameters of axons, fewer axons, or disruptions in myelin sheaths.
Of further note, studies in normal aging have uniformly shown decreased FA in late adulthood, predominantly in prefrontal, temporal and parietal areas of the brain (Salat et al., 2005). These brain regions evince the last myelination in the course of brain development, as well as being most vulnerable to myelin breakdown as a consequence of normal aging (Bartzokis et al., 2004; Marner et al., 2003). Sullivan and Pfefferbaum (2006) have also observed age-associated white matter integrity changes in healthy volunteers across the lifespan.
At the current level of research, however, we do not know the timing of brain abnormalities in schizophrenia or at which stage of brain maturation such abnormalities take place. Many brain abnormalities are observed when a patient first becomes symptomatic (Shenton et al., 2001), but it is still not known whether brain abnormalities observed early in the course of illness are more “static”, and seemingly more trait like, or if they are more “dynamic”, where progressive changes may dominate. Studies in first episode schizophrenia (Price et al., 2005; Szeszko et al., 2005) have shown less pronounced FA abnormalities than chronic schizophrenia studies. This might indicate progressive white matter changes that are additional to neurodevelopmental abnormalities observed in first episode schizophrenia. Thus it would seem important to closely evaluate the relationship between age and white matter integrity in patients with schizophrenia to determine whether or not there are different patterns of aging in controls and in schizophrenia subjects. Unfortunately, not much research has been conducted in this area. To date, there are only two cross-sectional DTI studies investigating age-related FA changes in schizophrenic patients, one showing FA increases with age (Jones et al., 2006), and the other showing the opposite age effect (Mori et al., 2007).
In previous investigations (Kubicki et al., 2002b; Kubicki et al., 2003) we showed reduced FA in the region of interest studies of cingulum bundle (CB) and uncinate fasciculus (UF) in schizophrenia. Most recently our group has begun to investigate fronto-temporal abnormalities in schizophrenia using fiber tractography, a method that makes possible the computation of diffusion tensor values along the whole course of the fiber bundles, as opposed to a region of interest approach, where only a small portion of the fiber bundle can be measured. In the current study we used a DTI tractography method, based on a principle diffusion direction, to investigate the impact of aging on fronto-temporal and fronto-occipital connections in schizophrenia, more specifically the CB, UF, and the inferior occipito-frontal fasciculus (IOFF). We hypothesized that we would find a negative correlation between age and FA decrease within the fronto-temporal (e.g., the CB and the UF), but not fronto-occipital white matter connections (e.g., the IOFF). We further hypothesized this relationship to be much more pronounced (greater FA decreases earlier in the lifespan) in schizophrenia subjects, compared with healthy controls.
2. Methods
Twenty-seven patients diagnosed with schizophrenia were recruited from out-patient, in-patient, day treatment, and foster care programs at the VA Boston Healthcare System, Brockton, MA. Thirty-four healthy comparison subjects were recruited from advertisements, and were group-matched to patients on age, gender, handedness, and parental socioeconomic status (PSES). Mean ages were 39.8 years (SD=9) for patients, and 41.7 (SD=9.3) for controls (F=0.38, df=58, P=0.15). Detailed diagnostic information and inclusion and exclusion criteria are described elsewhere (Kubicki et al., 2003). The local IRB committee at the VA and BWH approved the study and written informed consent was obtained from all subjects. Table 1 describes the demographic and clinical information.
Table 1.
Demographic data for schizophrenic (SZ) and normal control (NC) subjects
| SZ subjects (n=27) |
NC subjects (n=32) |
Student t-test (two-tailed) |
|||
|---|---|---|---|---|---|
| t | df | P | |||
| Sex ratio (% male) | 100 | 100 | |||
| Age (y) | 39.1±9.1 | 42.4±8.4 | 1.47 | 58 | 0.15 |
| Socioeconomic status (SES)a | 3.9±1.1 | 2.4±1.2 | −4.92 | 57 | 0.01 |
| Parental SES | 3.1±1 | 2.6±1.3 | −1.61 | 53 | 0.11 |
| Handedness | 0.77±0.21 | 0.77±0.21 | 0.012 | 57 | 0.99 |
| Chlorpromazine equivalent (CPZ) | 453.9±343.1 | ||||
The MRI Protocol, as well as details of the DTI processing is described elsewhere (Kubicki et al., 2003). Additionally, a more complete description of the fiber tracking procedures can be found in Park et al. (Park et al., (2004). Briefly, after obtaining Line Scan Diffusion Tensor Images of the entire brain (1.7×1.7×4 mm coronal slices, 6 gradient directions with B=1000, 2 B0 scans) and eddy current correction, all of the following fiber tractography steps were performed using dodti software (Park et al., 2004, http://neuroimage.yonsei.ac.kr/~dodti).
Whole brain fiber tractography was performed by seeding points in each voxel inside the white matter segmentation of B0 images. For the tractography stopping criteria, similarly to Jones et al. (2006), we used FA <0.15 and an angle of curvature above 20° per 1 mm.
Two regions of interest (ROI) per tract were used to extract each of the three fiber tracts (IOFF, UF and CB). All ROIs were drawn on the FA map blind to the diagnosis, but were overinclusive in order to avoid investigator’s bias (Kubicki et al., 2002a; Nakamura et al., 2005). In short, for the CB, one ROI was placed above the genu, and one above the splenium of the corpus callosum; for the UF, one ROI was placed within the temporal stem and the second one included the entire temporal pole white matter. For the IOFF, the first ROI was identical to that used for UF, the second included the posterior temporal lobe white matter at the level of the splenium of the corpus callosum.
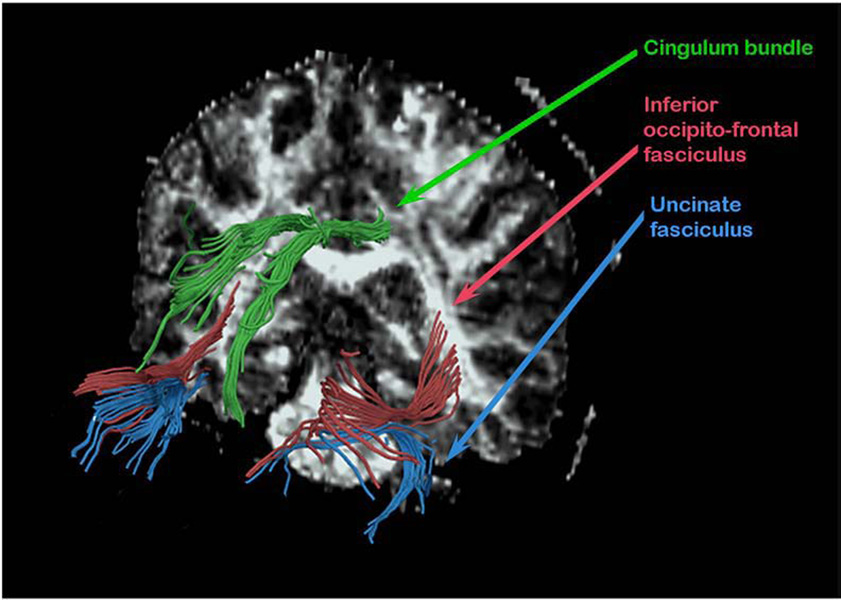
Using an algorithm that automatically excludes fibers not passing through the selected ROIs, the fiber tracts belonging to UF, CB and IOFF for the left and right sides, respectively, were extracted from whole brain white matter (Fig. 1). The final output of tractography analysis, provided by the dodti program, is the averaged value of FA, averaged length of the tract, as well as total number of fiber tracts, and these numbers were obtained for each fiber bundle, respectively.
After reconstructing the fiber bundles in three dimensions, we checked for consistency with respect to neuroanatomy by checking atlases (e.g., Crosby et al., 1962; Dejerine, 1895) and by qualitative visual assessment of 3D reconstruction for each rater (performed by MK).
Two independent raters completed measurements as described above on nine randomly selected cases, blind to diagnosis. The Intraclass Correlation Coefficient was greater than 0.88 for each of the tracts.
Fig. 1.
3D reconstruction of all three fiber bundles.
In order to minimize the Type 1 error, and avoid the need for multiple comparisons correction, as well as due to the lack of specific hypothesis regarding lateralization of white matter abnormalities and aging, both sides were averaged, and treated as a single tract.
We used the statistical software package SPSS 15 (SPSS Inc., Chicago) for data analysis. Demographic data were analyzed using t-tests, as comparisons were between two groups only. Age and DTI were first computed separately for each group using Pearson’s correlation. Next, the effects of group and age on FA were tested using a hierarchical regression model.
3. Results
Groups did not differ in age (P=0.15), PSES (P= 0.11), or handedness (P=0.99). No correlations were observed between diffusion measures and IQ, PSES, or handedness for either group. In the patient group, there were also no statistically significant correlations between the diffusion measures and age of onset or duration of illness for the three fiber tracts (CB P=0.290; UF P=0.810; IOFF P=0.709) or for the dosage of medication, the latter assessed using mean chlorpromazine equivalence, where we used Stoll’s (2001) conversions (CB P=0.118; UF P=0.155; IOFF P=0.830). No statistically significant correlations were found between age and total SANS (r=0.099; P=0.631) in the patient group, although total SAPS (r=−0.379; P=0.062) showed a trend toward statistical significance, where olfactory hallucinations (r=−0.445,P=0.026), grandiose delusions (r=−0.421; P=0.032), and delusions of guilt and sin (r=−0.427; P=0.030) resulted in significant correlations with age.
Results of direct FA comparison between groups indicated that the patients had significantly lower FA in two of the three bundles examined (CB t=2.266, P=0.027, IOFF t=2.334, P=0.023; but not U t=0.396, P=0.693). Here, our main focus was on the interaction between FA and age (Table 2). [Note: Group differences are already published for UF and CB, and presented as an abstract for IOFF (Kubicki et al., 2006). The purposes of this study were to use tractography, as opposed to region of interest analyses, and to focus more closely on age and its association with these fiber bundles.]
Table 2.
FA values averaged along the fiber bundles
| Fractional anisotropy | SZ subjects (n=27) | NC subjects (n=32) | Student t-test (two-tailed) | ||
|---|---|---|---|---|---|
| t | df | P | |||
| Cingulum bundle | 0.446±0.034 | 0.466±0.033 | 2.266 | 57 | 0.027 |
| Uncinate fasciculus | 0.360±0.020 | 0.362±0.020 | 0.396 | 58 | 0.693 |
| Inferior occipito-frontal fasciculus | 0.416±0.025 | 0.430±0.023 | 2.334 | 58 | 0.023 |
In addition, to make sure that there was no systematic bias in terms of differences in fiber number or tract length for either group, we calculated t-tests to compare the number of fibers, mean length, and mean angle for each of the three fiber tracts that we compared (i.e., UF, IOFF and CB). No statistically significant differences were observed for any of these variables for the three fiber tracts.
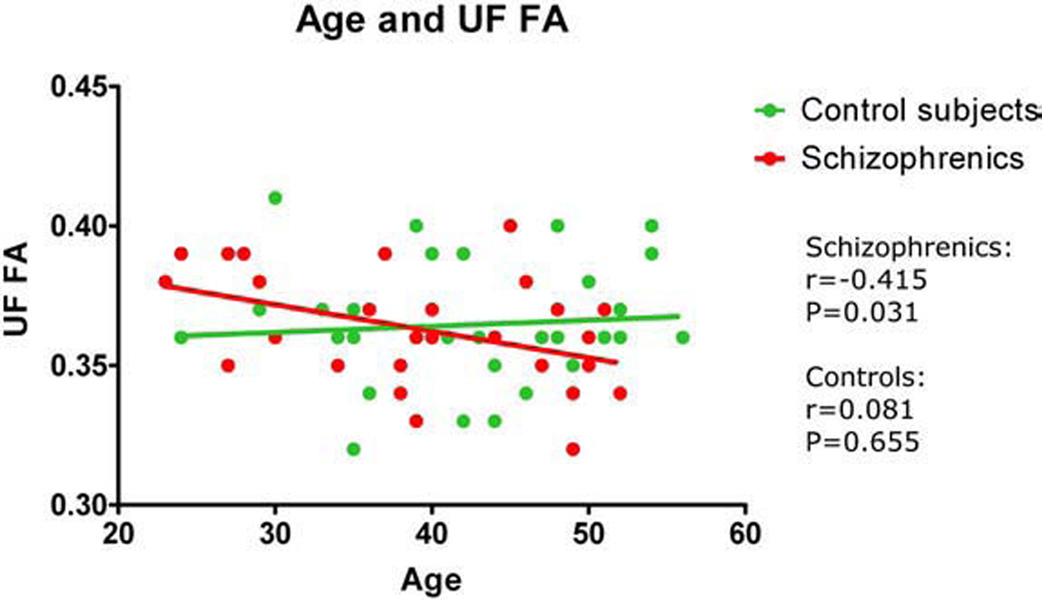
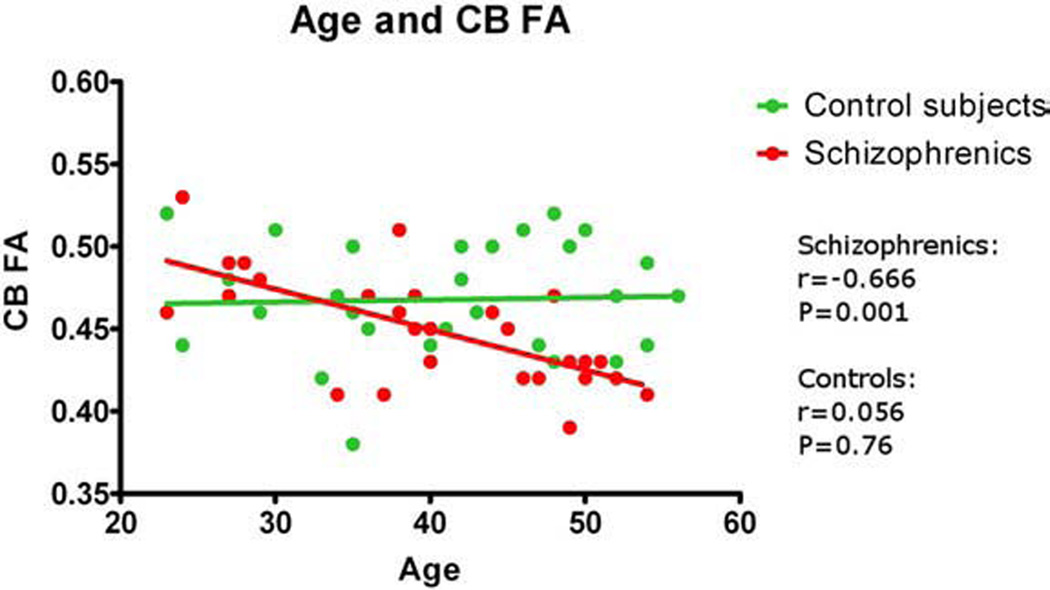
To test the hypothesis of an age-related reduction in FA for patients, we first performed Pearson correlations, which showed that FA in CB (r=−0.666; P=0.001) and UF (r=−0.415; P=0.031), but not IOFF (r=0.264; P=0.184) was significantly correlated with age in schizophrenia (Fig. 2 and Fig 3). In contrast, age did not correlate with FA for controls. Using hierarchical regression, we next tested the effects of group and age on FA. For the CB, the age by group interaction accounted for the greatest amount of reduction in bilateral FA (F=9.085, df=1, 55, P=0.004). As indicated by the semi-partial correlation value of 0.377, approximately 14% of the variance in the reduction of FA for bilateral CB could be specifically and uniquely accounted for by the interaction of age by group. For the UF, the age by group interaction approached significance (F=3.4, df=1, 56, P=0.065), accounting for approximately 8% (r square) of the variance in reduction in bilateral FA.
Fig. 2.
FA of uncinate fasciculus (UF) correlated with age.
Fig. 3.
Fractional anisotropy (FA) of cingulum bundle (CB) correlated with age.
4. Discussion
Results of our study reveal a significant age-related decline of fiber tract integrity within CB and UF, but not IOFF in schizophrenia. Of note, such a decline was not observed in age-matched controls. In addition, the decline in fiber tract integrity observed in schizophrenia was not associated with either illness duration, medication effects, or total positive or negative symptoms (though a trend was noted toward a statistically significant negative correlation between positive symptoms and FA in schizophrenia).
Results of our study suggest the possibility of progressive changes in white matter fiber integrity in schizophrenia over time. This association, however, while suggestive, has not been extensively studied in schizophrenia. Among the small number of studies that have addressed this issue, one structuralMRI imaging study by Mathalon et al. (2001) demonstrated fronto-temporal gray, but not white matter age-related volume decline in chronic schizophrenic patients. In a separate electrophysiological study using ERP, O’Donnell et al. (1995) observed a greater increase of P300 latency in older chronic schizophrenic patients. Another longitudinal study (van Haren et al., 2007) observed increased cortical gray matter atrophy in schizophrenic patients before the age of 45 in a 5-year-follow-up. This group also found white matter to increase excessively before the age of 32 as compared to normal healthy controls, which showed a steadier trajectory of white matter volume growth. Finally, the only study by Jones et al. (2006) utilizing DTI and fiber tractography, demonstrated, on a small sample of younger schizophrenics, an age-related increase of tract integrity within the Superior Longitudinal Fasciculus. The difference between our results and those reported by Jones et al. might be explained by different DTI acquisition methods, difference in sample sizes, as well as differences in subjects’ age distribution and illness duration (our study includes twice as many subjects as Jones’s paper, moreover our subjects were much older, and medicated/sick for much longer period of time). Nevertheless, taken together, these findings suggest that there may be a neurodegenerative process in pathological aging (Arnold and Trojanowski, 1996), which affects the white matter but does not start right after psychotic onset, but instead might be delayed because of prolonged brain maturation (Benes, 1989).
Of further note, we found no significant decline in FA with age in healthy control subjects, which differs from the findings of Sullivan and Pfefferbaum (2006), where their findings showed a continuous decline of FA starting at the age of 20. This difference between our findings and theirs might be accounted for by the fact that their study was designed to look at lifespan and not at the kind of constriction of age that characterizes the current study. Finally, a study by Raz et al. (2005) reported findings that suggested that there was a decline in white matter integrity that was accelerated in older adults. According to their findings, the mid-fifties appear to be the inflection point for an age trend in volume changes of the brain for the normal healthy population, which is consistent with our findings. It is of further interest to longitudinally follow-up our study cohort to determine whether or not we find such continued changes.
Possible limitations of the study should be noted. First, this study was not designed to longitudinally follow up patients over the course of their illness. Thus cohort effects such as severity of illness (even though overall total positive and negative symptoms were not associated with FA decline), might be a possible confound in the finding of FA and age correlations in schizophrenia. Second, we observed chronic schizophrenic patients who have been medicated for years. We note also that even though we did not demonstrate correlations between diffusion measures and age of onset, duration of illness, or medication, we can not totally rule them out as possible confounds. Further, even though there is some evidence of white matter loss after treatment with antipsychotic medications (Christensen et al., 2004), this evidence is weak, and requires further investigation. We also realize that since we study chronic schizophrenics, duration of illness is closely associated with age. However, since we observed correlations between diffusion measures and patients’ age, and did not observe such correlations with age of onset, or with duration of illness, we hypothesize that there is another factor, probably related to brain development differences in schizophrenia that plays a role in white matter pathology. Finally, additional studies examining prodrome subjects at high risk for developing schizophrenia and patients diagnosed with a first episode of schizophrenia will help to determine the impact of these factors on DTI measures in schizophrenia.
Additionally, the study cohort consisted only of male schizophrenic patients. Since theories of a possible neuroprotective potential for estrogen have been reported (Bardenstein and McGlashan, 1990), further investigations are needed to reveal possible gender differences in age effects on brain connectivity. Finally, an additional study limitation is the fact that the sequence used in this study was a relatively low resolution (especially out-of-plane) and only 6 directions of diffusion were collected. Nevertheless, we note that the findings of our study were sufficiently robust. Even at lower resolution the differences between groups and correlational changes with age were found.
Another potential limitation, often discussed in DTI reviews, is posed by the fact that tractography requires an FA threshold to be set at a certain value, which again limits the possibility of measuring voxels below a certain FA value. Kanaan et al. (2006) demonstrated using FA histograms, however, that the FA differences between schizophrenia patients and controls fall above the 0.2 FA values. Thus to eliminate possible bias, lower stopping threshold should be chosen. We note that we chose to set the threshold at 0.15 (Jones et al., 2006), which should robustly encompass any pathological white matter voxels in the schizophrenia brain.
Further, we note that diffusion tensor tractography is not free of its own limitations. With today’s scanning techniques, results from fiber tractography do not describe the spatial extent of individual axons but instead describe the average diffusion properties in white tissue at the scale of a millimeter. Thus the diffusion tensors at this scale should be regarded as estimators of local diffusion properties, and tractography as a visualization of features in this field. Validation of the features captured in the tensor field is thus very challenging since the definition of the tissue properties corresponding to the diffusion field is not well understood. However, similarities between DT-MRI tractography and real white matter fiber architecture in the human brain are compelling even at today’s limited resolution. We also note that this method has been used to generate white matter atlases (Mori et al., 2007). Further, in this study, and as noted previously, to make sure that there were no group differences in tract appearance, all tracts were visually inspected, and also their mean length, fiber number, and mean distribution angle were reported, and compared between groups, where no group differences were found.
One imaging parameter that could be viewed as a limiting factor in fiber tractography is the small number of diffusion directions collected (6 in our case). The literature regarding the accuracy of tensor estimation in relation to the number of directions is large, and no agreement has been reached. Some believe that since 6 directions are sufficient to generate tensors, it should be sufficient to track the main diffusion direction, and thus to perform accurate tractography. Others believe that 12 or more directions are needed to increase tensor estimation accuracy. Our 6-direction acquisition has been used before in surgical planning (Talos et al., 2007), and confirms the accuracy of tractography. However, with newer, faster MR systems, where it is not too costly to use higher directional resolution, one should certainly consider doing so. Additionally, we note that tractography stopping criteria of 20° of curvature change within 1 mm, are quite common in the literature, and have been introduced in order to deal with potential artifacts in the data that could influence the tractography output (see review in Kubicki et al., 2007).
In summary, this study underscores the need for further investigation of progressive changes in schizophrenia, as well as underscores the importance of including age as a possible confounding factor in future white matter investigations.
Acknowledgements
The authors would like to thank Nancy Maxwell for her administrative assistance. Additionally, we gratefully acknowledge the support, in part, by grants from the National Institutes of Health (R03 MH068464-01 to MK, K02 K05 MH070047 and R01 MH 50747 to MES, R01 MH 40799 to RWM), from the MIND foundation (RWM), from the Department of Veterans Affairs (a VA Merit Award toMES and RWM, a Research Enhancement Award Program to RWM and MES, and a VA Schizophrenia Research Center Grant to RWM and MES), fromthe Harvard Medical School (Milton Award to MK), from the National Alliance for Research on Schizophrenia and Depression (NARSAD: MK), from the Department of Veterans Affairs Merit Awards (MES, RWM), and from the VA Research Enhancement Award Program (REAP to RWM/MES) and the VA Schizophrenia Center Grant (RWM/MES). This work is also part of the National Alliance for Medical Image Computing (NAMIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 EB005149 (RK, MK, MES, AS).
Role of funding source
All of the study sponsors had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest
There are no conflicts of interest.
References
- Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE, Jr, Jones EG. Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch. Gen. Psychiatry. 1996;53:425–436. doi: 10.1001/archpsyc.1996.01830050061010. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Trojanowski JQ. Cognitive impairment in elderly schizophrenia: a dementia (still) lacking distinctive histopathology. Schizophr. Bull. 1996;22:5–9. doi: 10.1093/schbul/22.1.5. [DOI] [PubMed] [Google Scholar]
- Bardenstein KK, McGlashan TH. Gender differences in affective, schizoaffective, and schizophrenic disorders. A review. Schizophr. Res. 1990;3:159–172. doi: 10.1016/0920-9964(90)90034-5. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer's disease. Neurobiol. Aging. 2004;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Benes FM. Myelination of cortical–hippocampal relays during late adolescence. Schizophr. Bull. 1989;15:585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Christensen J, Holcomb J, Garver DL. State-related changes in cerebral white matter may underlie psychosis exacerbation. Psychiatry Res.: Neuroimaging. 2004;130:71–78. doi: 10.1016/j.pscychresns.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Crosby EC, Humphrey T, Lauer EW. Correlative anatomy of the nervous system. New York: Macmillan; 1962. [Google Scholar]
- Dejerine J. Anatomie des centres nerveux. vol. 1. Paris: Rueff et Cie; 1895. [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Catani M, Pierpaoli C, Reeves SJ, Shergill SS, O'Sullivan M, et al. Age effects on diffusion tensor magnetic resonance imaging tractography measures of frontal cortex connections in schizophrenia. Hum. Brain Mapp. 2006;27:230–238. doi: 10.1002/hbm.20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan RA, Shergill SS, Barker GJ, Catani M, Ng VW, Howard R, et al. Tract-specific anisotropy measurements in diffusion tensor imaging. Psychiatry Res. 2006;146:73–82. doi: 10.1016/j.pscychresns.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am. J. Psychiatry. 2002a;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am. J. Psychiatry. 2002b;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol. Psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Bushell G, Markant D, Dreusicke M, Park HJ, Bouix S, et al. In-vivo diffusion tensor tractography suggests disruptions in connectivity between frontal and temporal lobes in schizophrenia. Biol. Psychiatry. 2006;59:57S. [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, et al. A review of diffusion tensor imaging studies in schizophrenia. J. Psychiatr. Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J. Comp. Neurol. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch. Gen. Psychiatry. 2001;58:148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- Mori T, Ohnishi T, Hashimoto R, Nemoto K, Moriguchi Y, Noguchi H, et al. Progressive changes of white matter integrity in schizophrenia revealed by diffusion tensor imaging. Psychiatry Res. 2007;154:133–145. doi: 10.1016/j.pscychresns.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Nakamura M, McCarley RW, Kubicki M, Dickey CC, Niznikiewicz MA, Voglmaier MM, et al. Fronto-temporal disconnectivity in schizotypal personality disorder: a diffusion tensor imaging study. Biol. Psychiatry. 2005;58:468–478. doi: 10.1016/j.biopsych.2005.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell BF, Faux SF, McCarley RW, Kimble MO, Salisbury DF, Nestor PG, et al. Increased rate of P300 latency prolongation with age in schizophrenia. Electrophysiological evidence for a neurodegenerative process. Arch. Gen. Psychiatry. 1995;52:544–549. doi: 10.1001/archpsyc.1995.03950190026004. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kubicki M, Westin CF, Talos IF, Brun A, Peiper S, et al. Method for combining information from white matter fiber tracking and gray matter parcellation. AJNR Am. J. Neuroradiol. 2004;25:1318–1324. [PMC free article] [PubMed] [Google Scholar]
- Price G, Bagary MS, Cercignani M, Altmann DR, Ron MA. The corpus callosum in first episode schizophrenia: a diffusion tensor imaging study. J. Neurol. Neurosurg. Psychiatry. 2005;76:585–587. doi: 10.1136/jnnp.2004.042952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb. Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol. Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr. Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll A. The Psychopharmacology Reference Card. 2001 [Google Scholar]
- Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N. Engl. J. Med. 1990;322:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci. Biobehav. Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, et al. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am. J. Psychiatry. 2005;162:602–605. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- Talos IF, Zou KH, Kikinis R, Jolesz FA. Volumetric assessment of tumor infiltration of adjacent white matter based on anatomic MRI and diffusion tensor tractography. Acad. Radiol. 2007;14:431–436. doi: 10.1016/j.acra.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr. Res. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Pol HE, Schnack HG, Cahn W, Brans R, Carati I, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol. Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Grundrisse der Psychiatrie. Leipzig: Thieme; 1906. [Google Scholar]
- Wible CG, Shenton ME, Hokama H, Kikinis R, Jolesz FA, Metcalf D, et al. Prefrontal cortex and schizophrenia. A quantitative magnetic resonance imaging study. Arch. Gen. Psychiatry. 1995;52:279–288. doi: 10.1001/archpsyc.1995.03950160029007. [DOI] [PubMed] [Google Scholar]