Abstract
Human adolescents drink partly to facilitate their social interactions, a social facilitatory effect of ethanol also seen in adolescent rats tested under familiar test circumstances. To explore the role of hedonic affect in ethanol-induced social facilitation, this study assessed 50 kHz ultrasonic vocalizations (USVs) in pair-housed adolescent (P29-37) and adult (P71-79) Sprague-Dawley male rats during social interactions. On each of 8 test days, animals were socially isolated for 3 hr and then injected intraperitoneally with 0 (saline), 0.25 or 0.5 g/kg ethanol. Twenty five min later they were placed alone in a familiar test chamber for a 5-min period, followed by a 10-min encounter with a similarly injected peer. USVs were recorded during this 10-min period, while social interactions were videotaped for later scoring. BECs were measured immediately post-test on day 8. Although the 0.25 g/kg dose of ethanol facilitated play fighting in adolescents but not adults, ethanol had little to no effect on 50 kHz USV production under these test circumstances. USV production was higher in adults than adolescents, despite adolescents consistently engaging in more social behavior. To the extent that 50 kHz USVs index the hedonic value of social interactions, these findings support the conclusion that elevations in social behavior normally evident in adolescents may not be related to increases in hedonic sensitivity for social stimuli.
Keywords: Adolescence, 50 kHz USV, ethanol, social behavior
Introduction
Human adolescents are at an increased risk of alcohol use. According to the 2006 Monitoring the Future National Survey, 6% of 8th graders, 19% of 10th graders and 30% of 12th graders reported being drunk within the past 30 days [21]. Similarly high levels of ethanol intake are seen in adolescents of other species, with adolescent rats, for instance, showing 2–3 times higher g/kg ethanol intake relative to their more mature counterparts [8, 14, 27, 55].
Interactions with peers are thought to be of principal importance for human adolescents, with individuals spending more time with peers during adolescence than at any other developmental period [19, 26]. Similarly, adolescent rats show high levels of social behavior, with these social interactions being essential for developing the ability to express and understand intraspecific communication signals [47]. Interactions with peers during adolescence provide a substantial source of positive experiences for humans [26, 28], and, in the same way, an opportunity to interact with peers is more rewarding for adolescent rats than for their more mature counterparts [15].
An important property of alcohol that contributes to adolescent drinking is its ability to facilitate certain forms of social behavior, particularly interactions with peers [2, 39]. For instance, adolescents at risk for extensive alcohol use strongly attribute to alcohol the property of enhancing their effectiveness in social situations [1]. This ethanol-induced social facilitation is not restricted to human adolescents. Acute exposure to relatively low experimental doses of ethanol (0.5 – 0.75 g/kg) administered intraperioneally (i.p.) has been shown to facilitate social interactions in group-housed adolescent rats tested under familiar, non-anxiogenic circumstances [40, 50, 51], with the effective dose even lower in socially-deprived animals [53]. These doses produces blood ethanol concentrations (BECs) commensurate with the moderate (20–80 mg/dl) consumption range in humans [16].
Alcohol expectancy research indicates that heavy drinkers and/or problem drinkers strongly expect alcohol to make them more sociable and relaxed [6]. In human adolescents, expectancy for ethanol-induced social facilitation and their drinking experience might influence each other in a reciprocal, positive feed-back fashion, with expectancies from drinking such as increased social confidence and comfort helping to produce those very consequences, and drinking experiences further reinforcing initial expectancies [39]. Therefore, adolescents may be particularly sensitive to ethanol-induced social facilitation due to ethanol-induced enhancement of the salience of social stimuli. Given that this important issue in alcohol research has not been addressed experimentally, the present study was designed to examine whether ethanol might increase hedonic value of social interactions more strongly in adolescent than in adult rats when indexed via assessment of 50 kHz ultrasonic vocalizations (USVs).
Under certain circumstances, USVs produced by rats have been used as a measure of affective states [see 25 for review]. Relatively long (300–3000ms), low frequency (around 22 kHz) USVs are associated with negative affective states in rats [7, 37] since they are emitted by threatening or painful stimuli such as foot shock [44], predator odor [4], or lithium chloride administration [9], and are generated during defensive and submissive displays associated with inter-male fighting [42]. In contrast, shorter (20–80 ms) high frequency (around 50 kHz) USVs [5, 18] are seen in contexts involving potential reward, including play fighting [23], social exploration [5], male agonistic behaviors [36], and by males during sexual approach, copulation and ejaculation [32]. Increases in 50 kHz USVs are also seen in experimental situations, including tickling by the experimenter [11, 30, 35], electrical stimulation of the reward pathway and cues predicting such stimulation [10], amphetamine administration [22, 43], and cues associated with morphine administration [10]. These 50 kHz USVs are postulated to be an index of positive affective states in rats, given that they are increased in contexts involving appetitive stimuli [23, 24, 35]. The main objective of the present study was to determine the impact of ethanol administration on social affect in adolescent and adult male Sprague-Dawley rats using USV production as an index of the hedonic value of social interactions. The approach used was to examine production of 50 kHz USVs during social interactions following exposure to either saline or ethanol under dose and administration conditions previously shown to facilitate social interactions in adolescent rats [50, 51, 53].
Ethanol-induced changes in different components of social behavior and motivation for social contacts were also scored and analyzed. Thus a major goal of this study was to determine if 50 kHz USVs co-vary with the facilitation of social behavior by ethanol during adolescence [50, 51]. Although it has been shown that 50 kHz USVs correlate with dorsal contacts in pairs of isolate-housed adolescent rats [23], the relationship of USVs and social behaviors has yet to be studied in adult rats or among socially housed adolescents exhibiting pharmacologically-produced elevations in social interactions. Using a CPP paradigm, it has been previously shown that social stimuli are more rewarding for adolescent rats than adults, especially when the animals are housed in pairs [15]. A second goal of the present study was to further examine age differences in the rewarding value of social stimuli in both ethanol-treated and control animals.
Previous studies have shown that doses of ethanol in the social facilitatory range do not increase general locomotor activity in the social interaction test [50, 52], however it is unclear if these doses of ethanol may have motor activating properties in the absence of a social stimulus. To explore this issue, experimental subjects in this study were placed alone in the testing chamber daily for 5 min to analyze ethanol’s effects on general locomotor activity.
Methods
Subjects
Sprague-Dawley male rats bred and reared in our colony at Binghamton University were used in this study. Female rats have been shown to emit differing levels of USVs dependent upon their estrous cycle [31], and therefore were not included in this experiment. A total of 96 rats derived from 24 litters were used. The day after birth, all litters were culled to 8–10 pups (6 males and 4 females when possible) and housed with their mother and father in standard breeding cages until weaning on postnatal day (P) 21 at which point they were pair-housed with a same-sex littermate. Housing pups with both parents is a standard procedure in our breeding colony, since use of established breeding pairs minimizes stress of individually housing pre-parturient dams, and may potentially enrich the experience of the offspring. On either P26 or P68-70 (two days prior to the onset of testing), subjects were re-housed with a same-sex, same-age, non-littermate assigned to the same ethanol dose condition. All animals were housed in a temperature-controlled (20–22°C) vivarium on a 14-/10-hr light/dark cycle (lights on at 0700) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and tap water. All animals were treated in accordance with guidelines established by the National Institutes of Health using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
Experimental Design
The design of the study was a 2 (age: adolescent, adult) × 3 (ethanol dose: 0, 0.25, 0.5 g/kg) factorial. Eight experimental animals and 8 partner animals (each from a different litter than the experimental animals with which they were paired) were assigned to each of the 6 (i.e., 2 age × 3 dose) groups. Animals were assigned to these groups randomly, with the constraint that no more than one animal from a given litter was placed in any particular test group to avoid confounding litter with treatment effects [20, 57].
Experimental Protocol
All testing was conducted between 1300 and 1700 hr under dim light. Testing was initiated on P28 (adolescents) or on P70-72 (adults). On the day prior to testing, all animals were placed individually in the test chamber for 30 min to habituate them to the testing situation, given that placement in a novel context notably suppresses social behavior [17, 45]. On test days, subjects were individually isolated in holding cages in the colony room for 3 hr prior to ethanol administration in order to increase social motivation [46] while avoiding the stress of isolate-housing. All experimental animals were then injected with a 0, 0.25, or 0.5 g/kg dose of ethanol and returned to their holding cages for 25 min prior to placement in the test apparatus. Partners received the same dose of ethanol as the experimental animal with which they were treated similarly, except that they were retained in the holding cage for 30 min post-injection prior to placing them in the testing apparatus. We have shown previously that adolescent animals tested at P28 or P35 responded to 0.5 g/kg of ethanol by facilitation of play fighting [50, 51]. However, pre-test social deprivation increases sensitivity of adolescent rats to ethanol’s facilitatory effects, with a dose of 0.25 g/kg becoming effective under these circumstances [53]. Therefore, three doses of ethanol (0, 0.25, and 0.5 g/kg) were tested in the present study.
Each experimental animal was placed alone into the social interaction chamber for a 5-min period (animal placed first) during which its behavior was video recorded on test days 1, 3, 5 and 7 and later scored for activity (see section on behavioral measures). Its partner was then placed into the chamber (animal placed second) with the experimental animal, and the dyad was allowed to interact for 10 min. Testing continued for a total of eight days, with the ethanol dose and partner for each animal remaining constant throughout. USVs were recorded during the test period on all 8 test days for later analysis. It should be noted that USV production is the sum of that produced by the experimental animal and its partner. The social interaction periods were videotaped on test days 1, 3, 5, and 7, with the behavior of each animal later scored using procedures described below.
Ethanol Administration
Ethanol was administered i.p. as a 12.6% (v/v) solution in physiological saline, with dose of ethanol varied by altering volume rather than concentration to avoid concentration-induced differences in ethanol absorption rate [29]. Control subjects were injected with physiological saline isovolumetric to the highest dose of ethanol administered (0.5% body weight). All solutions were administered at room temperature. The i.p. route of ethanol administration was chosen for use in the present experiment in part for comparability with the vast majority of other research examining ethanol sensitivity during ontogeny, including our studies characterizing ontogenetic differences in responsiveness to the social consequences of ethanol [50, 51, 52]. Moreover, this route produces very little variability in BECs in either adolescents or adults and is unaffected by recency of food ingestion.
Social Interaction Test
The social interaction testing chambers were located in a testing room adjacent to the colony room. Subjects were not exposed to the testing room except for the time they were tested. Each social interaction test apparatus consisted of a Plexiglas (Binghamton Plate Glass, Binghamton, NY) chamber (30 × 20 × 20 cm for adolescents and 45 × 30 × 20 cm for adults) containing clean pine shavings. Each chamber was divided into two equally sized compartments by a Plexiglas partition containing an aperture (7 × 5 cm for adolescents and 9 × 7 cm for adults) to allow movement of the animals between compartments [48, 49]. During testing sessions, behaviors were recorded by a video camera for later behavioral scoring. After each testing session the social interaction chambers were wiped clean with a 3% hydrogen peroxide solution and refilled with clean shavings.
Behavioral Measures
Behaviors of experimental subjects and their partners were scored separately from videotapes of the sessions recorded on test days 1, 3, 5, and 7. During the 5-min period prior to the social interaction test, crosses through the center aperture were scored as a measure of overall locomotor activity. Behaviors scored during the interaction period included social investigation (sniffing of any part of the partner’s body), contact behavior (crawling over or under, grooming of the partner), and play fighting (pouncing or playful nape attack, chasing, following, and pinning) [47, 50, 51]. Serious fighting defined by either threats (a sideways or upright stance with head movements toward the partner with attempts to bite) or “serious” attacks (a fierce lunging at the partner’s rump often accompanied by biting) [3] did not occur in the present experiment. Crosses of the center aperture either toward or away from the social partner were recorded. A social preference coefficient was calculated for each animal by subtracting the crosses away from the partner from the crosses towards the partner and dividing by the total number of crosses. Positive values of the coefficient indicated social preference, whereas negative values of this coefficient reflected social avoidance [48, 49]. Behaviors were scored from videotapes in real-time by an observer without knowledge of test group assignment.
Vocalization Measures
Ultrasonic vocalizations were measured using Avisoft UltraSoundGate CM16 microphones, recorded by an UltraSoundGate 416-200 recording device, digitized by Avisoft-Recorder USG, and stored as .wav-files for later analysis (Avisoft Bioacoustics, Berlin, Germany). The microphones were sensitive to frequency ranges between 10 and 125 kHz with a high sensitivity at 50 kHz and moderate directional properties. The microphones were mounted on the outside edge of the social interaction chambers straddling the center divider in order to record USVs from both sides of the chamber. Sound analysis was performed using Avisoft-SASLab Pro software (Avisoft Bioacoustics, Berlin, Germany). Sound files were converted to spectrograms for manual visual counts of calls. A sound was considered to be a 50 kHz USV if it fell between 35 and 70 kHz with a duration of 30–80 ms [see [25] for discussion]. Calls were considered in the 22 kHz category if they fell between 18 and 32 kHz with duration of 300–3000 ms. Twenty two kHz calls were seen in very few animals, and thus were not included in the statistical analyses. All sound files were scored by trained experimenters without knowledge of test group assignment. Inter-rater reliability exceeded 90%.
Blood Ethanol Determination
Immediately following testing on day 8, tail blood samples were collected in heparinized tubes and frozen at −80°C until analysis of blood ethanol concentration (BEC). Samples were assessed for BECs via headspace gas chromatography using a Hewlett Packard (HP) 5890 series II Gas Chromatograph (Wilmington, DE). At the time of assay, blood samples were thawed and 25-μl aliquots were placed in airtight vials. Vials were placed in a HP 7694E Auto-Sampler, which heated each individual vial for 8 min, and then extracted and injected a 1.0 ml sample of the gas headspace into the HP 5890 series Gas Chromatograph. Ethanol concentrations in each sample were determined using HP Chemstation software which compares the peak area under the curve in each sample with those of standard curves derived from reference standard solutions.
Data Analyses
Repeated measures analyses of variance (ANOVAs) were used to analyze crosses during the 5-min period prior to social interactions and 50 kHz USV production during the interaction period, with test day serving as the within-subjects factor, and age and ethanol dose serving as between-subjects measures. Nested-repeated measures ANOVAs were used for all social behavioral measures, with test day and partner animal serving as within-subjects factors, and age, ethanol dose, and placement (placement into apparatus first or second) serving as the between-subjects measures. Significant effects and interactions were further investigated using Fisher’s LSD post-hoc tests. Spearman’s R tests were used to correlate USV production with the behavioral measures at each age.
Results
Locomotor Activity
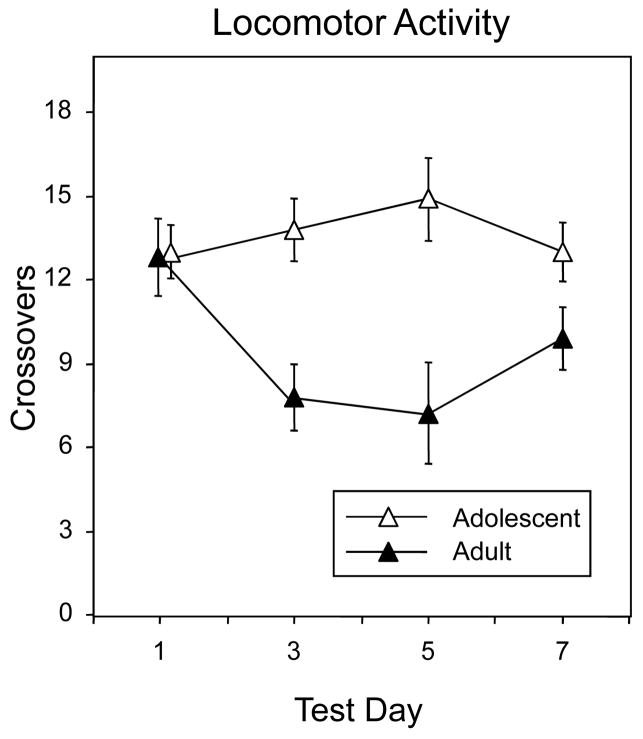
Ethanol had no impact on locomotor activity of experimental animals during the 5-min period prior to the onset of the social interaction test. As seen in Fig. 1 (data are collapsed across ethanol dose), in general, the number of crosses through the center aperture was significantly higher in adolescents than in adults [main effect of age, F(1, 120) = 6.66, p < 0.005]. This age effect was tempered by test day [age × day interaction, F(3, 120) = 8.00, p < 0.0001]. Overall locomotor activity of adolescent animals remained similar across test days, whereas in adults the number of crossovers on test day 1 was significantly higher than on all subsequent test days. Adolescents and adults exhibited a similar number of crosses on test days 1 and 7; however locomotor activity of adolescents was significantly higher than that of adults on test days 3 and 5.
Figure 1.
Frequency of crossing through the center aperture between the two sides of the test chamber during the 5-min period prior to the social interaction test. Data collapsed across ethanol dose. Vertical lines in these and subsequent figures reflect SEM values.
Ultrasonic Vocalizations
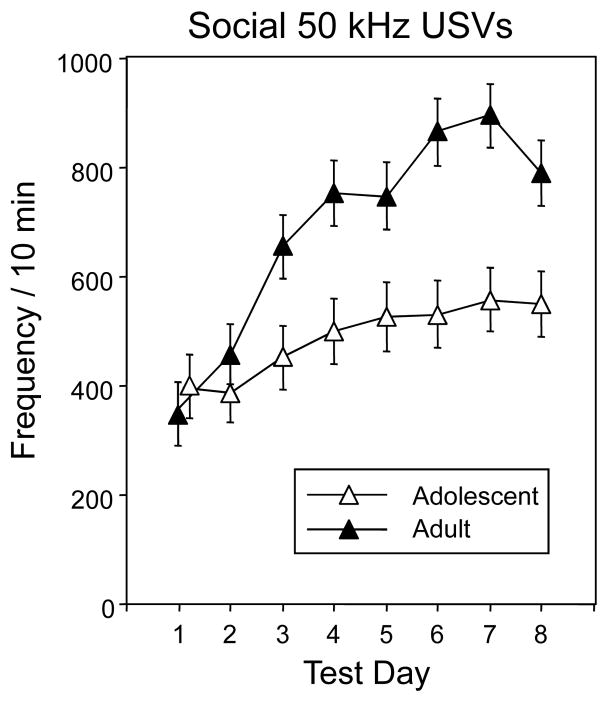
The average number of 50 kHz USVs emitted by the social pairs during the social interaction test are shown in Fig. 2. The ANOVA of these data revealed significant effects of age, F(1,42) = 8.35, p < 0.01, with adults overall showing higher levels of 50 kHz USV production during social interactions than adolescents, and test day, F(7,147) = 21.41, p < 0.001, tempered by a significant interaction of test day with age, F(7,294) = 6.44, p < 0.001. Although adolescents and adults produced comparable amounts of 50 kHz USVs in the social context on test days 1 and 2, the adults notably increased their 50 kHz USV production across days whereas adolescents did not, resulting in significant differences between the ages on test days 3 through 8 (see Fig. 2, with data collapsed across ethanol dose). Test day also interacted significantly with dose, F(14,294) = 2.00, p < 0.05. Post-hoc analysis of the data collapsed across age to explore this interaction revealed that it was only on test day 8 that USV production was significantly higher in animals injected with 0.25 g/kg ethanol (808.75±81.01) than in saline-injected controls (586.88±73.65) (data not shown).
Figure 2.
Total number of 50 kHz USVs emitted by each pair of animals during the social interaction test across days. Data are collapsed across ethanol dose.
Social Behavior
Adolescents demonstrated more play fighting and contact behavior than their adult counterparts. Animals that were placed into the test apparatus first exhibited higher levels of all social measures, an effect that often was more marked among adolescents than adults. The social preference/avoidance coefficient was also higher in animals placed in the test apparatus first, whereas the animals placed second tended to exhibit negative values, indicating social avoidance. Adolescents showed ethanol-induced facilitation of play fighting at a dose of 0.25 g/kg ethanol on earlier test days. In contrast, no stimulatory effects of ethanol on any measure of social behavior were seen in adults.
Play Fighting
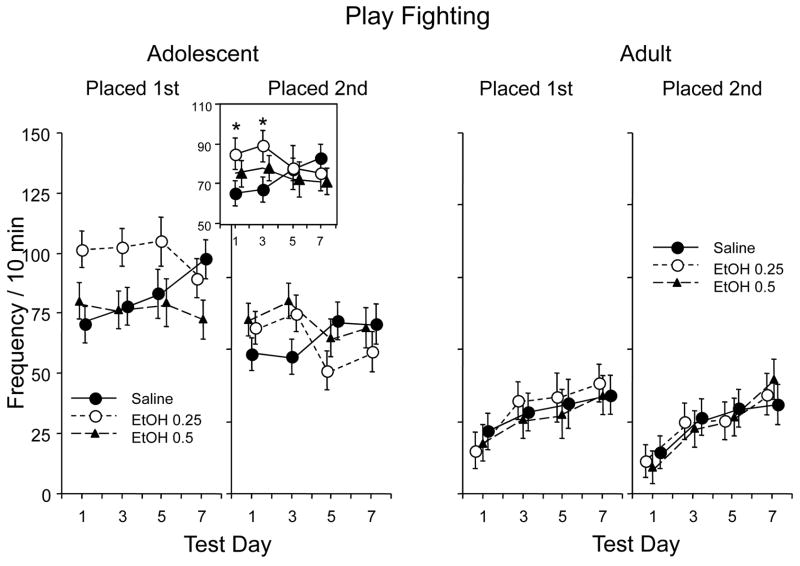
As was expected, play fighting – an adolescent characteristic form of social interactions (Fig. 3), differed as a function of age, F(1,42) = 122.29, p < 0.001, with adolescents exhibiting notably more play fighting than adults. Significant effects of placement, F(1,42) = 16.91, p < 0.001, and placement by age, F(1,42) = 6.30, p < 0.05, revealed that adolescents placed first exhibited more play behavior than their partners, a placement order effect not seen among adults. A main effect of test day, F(3,126) = 8.28, p < 0.001, along with interactions of test day and age, F(3,126) = 6.89, p < 0.001, and test day by age by ethanol dose, F(6,126) = 2.17, p < 0.05, were also seen, with post-hoc comparisons reflecting a significant low dose increase in play fighting among adolescents but not adults on test days 1 and 3. As can be seen in Fig. 3, this ethanol effect was more notable in adolescents placed first, although placement order did not interact significantly with these variables.
Figure 3.
Frequency of play fighting in adolescent and adult animals across test days. Figure insert shows frequency of play fighting in adolescents collapsed across placement order. Asterisk (*) indicates significant difference from saline-injected group in post-hoc analyses on data collapsed across placement order.
Contact Behavior
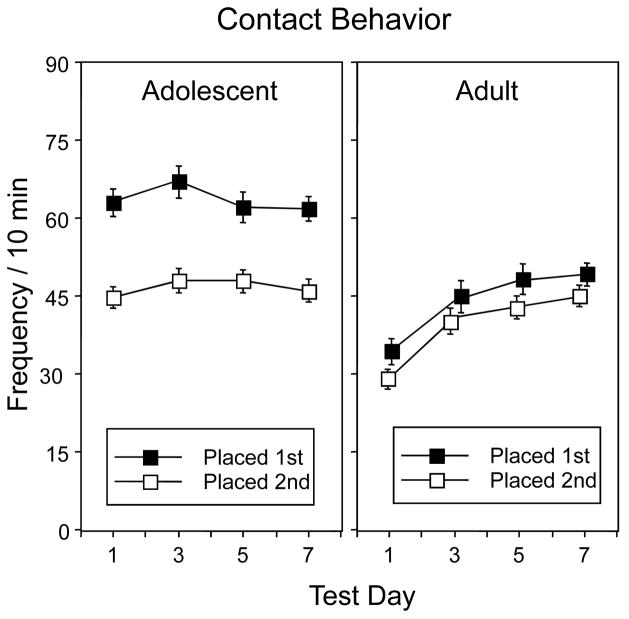
Contact behavior (Fig. 4) was also generally more pronounced in adolescents than adults [main effect of age, F(1,42) = 33.24, p < 0.001], with placement order also influential [main effect of placement order, F(1,42) = 44.63, p < 0.001], and both of these effects tempered by a significant interaction of these two variables, F(1,42) = 16.33, p < 0.001. Adolescents exhibited more contact behavior than adults regardless of placement order. However, as can be seen in Fig. 4, adolescents placed first exhibited significantly more contact behavior than those placed second, a placement order effect not seen in adults. No significant main effects or interactions involving ethanol dose were seen for contact behavior, therefore the data are collapsed across ethanol dose in this figure. There were, however, significant effects of test day, F(3,126) = 12.42, p < 0.001, and test day by age, F(3,126) = 10.22, p < 0.001, with adolescents exhibiting consistent levels of contact behavior across days, whereas adults modestly increased the incidence of this behavior across test days.
Figure 4.
Frequency of social contact behavior in adolescent and adult animals. Data are collapsed across ethanol dose.
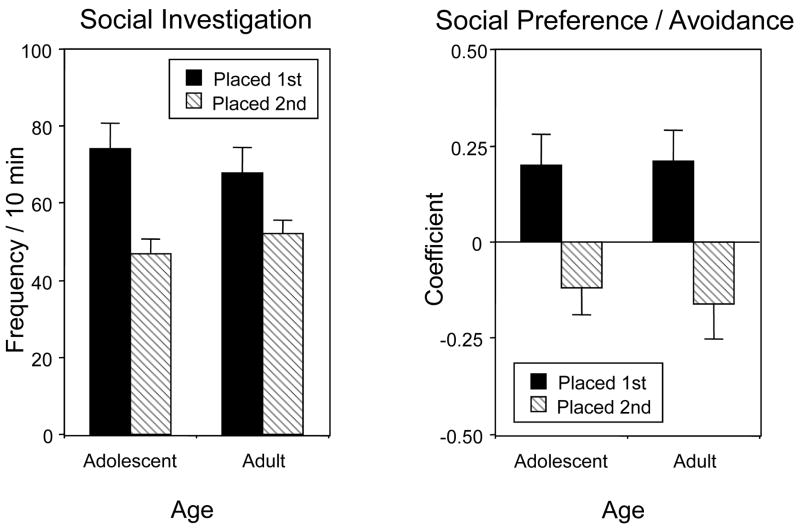
Social Investigation
Analysis of social investigation revealed significant effects of placement order, F(1,42) = 61.88, p < 0.001, as well as age by placement order, F(1,42) = 4.60, p < 0.05. These data are shown in Figure 5 (left) collapsed across non-significant variables of dose and test day. Animals placed into the test apparatus first engaged in notably more social investigation than those placed second regardless of age; a placement effect that tended to be more marked in adolescents than in adults.
Figure 5.
Frequency of social investigation (left) and social preference/avoidance (right) in adolescent and adult animals Positive values of the coefficient indicate social preference, negative values indicate social avoidance. Data are collapsed across ethanol dose and test day.
Social Preference/Avoidance
Analysis of social preference/avoidance (Fig. 5, right) revealed that the animals placed first had a higher preference coefficient than animals placed second [main effect of placement order, F(1,40) = 43.55, p < 0.001] regardless of age. In general, animals placed first tended to have positive coefficients, displaying social preference, whereas the animals placed second tended to have negative coefficients, indicating relative avoidance of their partner. No effects of dose or day were evident in this analysis.
Blood Ethanol Concentrations
BECs collected immediately after testing on day 8 (see Table 1) increased in a dose-dependent fashion [main effect of ethanol dose, F(1, 52) = 121.88, p < 0.0001] and differed as a function of age, F(1, 52) = 36.45, p < 0.0001, with adolescents having significantly lower BECs than adults. There were no effects of placement order on BECs.
Table 1.
Blood ethanol concentrations (mg/100mL) ± SEM for each age, dose, and testing condition of sample collected immediately after testing on day 8
| 0.25 g/kg ethanol | Placed first | Placed second |
|---|---|---|
| adolescent | 4.39 ± 1.62 | 2.98 ± 1.34 |
| adult | 9.05 ± 1.33 | 10.86 ± 0.42 |
| 0.5 g/kg ethanol | Placed first | Placed second |
| adolescent | 16.17 ± 3.92 | 17.77 ± 3.34 |
| adult | 28.13 ± 1.57 | 27.10 ± 1.98 |
Correlations
Production of 50 kHz USVs during social interactions was significantly correlated with play fighting in adolescents and with play fighting and contact behavior in adults (see Table 2). 50 kHz USV production during social interactions was not found to correlate with social investigation at either age.
Table 2.
Correlations of social 50 kHz USVs and behaviors*
| Play Fighting | Day 1 | Day 3 | Day 5 | Day 7 |
|---|---|---|---|---|
| Adolescent | 0.19 | 0.52 | 0.36 | 0.47 |
| Adult | 0.3 | 0.28 | 0.26 | 0.20 |
| Social Contact | Day 1 | Day 3 | Day 5 | Day 7 |
| Adolescent | 0.02 | 0.16 | 0.18 | −0.07 |
| Adult | 0.38 | 0.37 | 0.43 | 0.21 |
| Social Investigation | Day 1 | Day 3 | Day 5 | Day 7 |
| Adolescent | 0.05 | −0.17 | −0.11 | −0.14 |
| Adult | 0.26 | 0.11 | 0.16 | 0.08 |
Note: values noted in bold indicate significance at p< 0.05
Discussion
The results of the present study revealed pronounced age-related differences in 50 kHz USV production and social behavior. Adults exhibited more 50 kHz USVs while engaging in less social behavior relative to adolescents. Adolescent animals generally exhibited higher levels of social behaviors and showed ethanol-induced social facilitation of play fighting that was not evident in adults; this ethanol-induced social facilitation, however, was not accompanied by increases in 50 kHz USV production. Overall locomotor activity in the non-social context was not affected by ethanol.
Although adolescents generally showed a higher incidence of social behavior than adult animals, 50 kHz USVs emitted during social testing were higher in adults, indicating a developmental dissociation between social behavior and this presumptive vocal index of the hedonic value of these social interactions. One explanation of these findings could be that play behavior may somehow interfere with the ability to produce USVs; this possibility seems unlikely, however, in view of the positive correlations that were seen between 50 kHz USVs and play fighting among both the adolescents and adults. It has also been shown that rats with an increased motivation for play (i.e, isolate-housed compared to pair-housed animals) emit more 50 kHz USVs and show more play behavior while in a social situation than those less motivated to play, data that again support the conclusion that play behavior and USV production are not competing behaviors [23].
Another possible explanation of the observed age differences in USV production is related to the different pre-test experiences of the two animals in each dyad: i.e., whether they were placed in the apparatus first or second. Placement order was sufficient to dramatically alter level of social activity, differences that were particularly pronounced among adolescents. Therefore, we cannot preclude that low socially active adolescents (i.e., those placed second in the apparatus) emitted fewer USVs than highly socially active adolescents in each pair, lowering the overall amount of USVs in the adolescent pairs to levels below those of the adult dyads.
Such placement order effects may also have contributed to the lack of notable effects of ethanol on USV production. As the USV data necessarily reflect calling by both members of the dyads, it is possible that the slightly differential treatment of each of the animals in a pair may have affected overall USV production of the dyad. Given that ethanol-induced increases in play fighting tended to be driven largely by adolescent animals placed first into the test chamber, it may be the case that the minimal apparent effects of ethanol on social behavior in the adolescents placed second into the chamber may have prevented a significant increase in USVs produced by the pair.
These robust effects of placement order on social behavior (and the more modest effects seen in adults) were surprising. The 5-min period may have served as a daily habituation for the animals placed first in the apparatus, perhaps reducing their anxiety levels relative to animals placed second into the apparatus, and hence exposed simultaneously to both the experimental animal and the test apparatus. Indeed, social interactions have previously been shown to be elevated following familiarization to the test circumstances, an effect seen in both adolescent and adult animals [17, 50, 54]. At both ages, animals placed first into the chamber demonstrated social preference, as indexed by positive values of the coefficient, whereas social avoidance (i.e., negative values of the coefficient) emerged in their partners. It is unlikely that these differences are due to resident/intruder status, given that expression of dominance in a resident-intruder setting is typically expressed in terms of aggressive fighting [34], whereas animals only demonstrated play fighting in the present study. It is more likely that during this 5-min period, animals placed first had the opportunity to investigate the chamber and acclimatize to the environment, whereas the animals placed second were less familiar with the situation and, while reacquainting themselves with the testing chamber, may have made fewer attempts to engage the experimental animal in social behavior.
As expected, effects of ethanol on social behavior were age- and dose-dependent. Acute administration of 0.25 g/kg ethanol enhanced play fighting in adolescent but not adult animals, with this social facilitation seemingly driven largely by animals placed first in the apparatus. In contrast to our previous studies that have reported ethanol-induced social facilitation in adolescent animals following 0.5 and 0.75 g/kg of ethanol [40, 49–52], the dose of 0.5 g/kg did not enhance play fighting in adolescent animals. This dose difference between the present findings and those earlier studies is likely to be related to differences in duration of pre-test social deprivation. Indeed, we have shown recently that social deprivation for 90 min pre-test increases adolescent sensitivity to the socially facilitating effects of ethanol, with these socially deprived animals showing increases in play fighting in response to 0.25 g/kg, but not 0.5 g/kg ethanol [53]. In the present study, ethanol-induced facilitation of play fighting was evident not only on the first test day, but also during the second assessment of social interactions (i.e., on test day 3 of the repeated ethanol exposure period). These findings document for the first time that ethanol-induced social facilitation can still be detected when adolescents are tested repeatedly with ethanol in a social context (and with the same social partner). Several of the parameters used in this study for evaluating social behaviors varied from previous reports, arguing for the robustness of these facilitatory effects of ethanol. For instance in prior work in our laboratory [e.g., [50, 51], experimental animals were always tested with a novel, non-drug treated partner, whereas in order to examine ethanol’s effects on 50 kHz USVs during social interactions in the present study, both animals in each dyad were exposed to the same challenge dose, with their partners remaining constant across test days.
In contrast to previous studies that have reported no differences in BECs between adolescent and adult rats, in the current experiment adolescents were found to have significantly lower BECs than adults when samples were collected 40 min following administration of 0.25 g/kg or 0.5 g/kg ethanol on test day 8. Adolescents typically show similar BECs as adults at relatively low doses – e.g., at doses as low as 0.5 g/kg using tail blood samples [50], and in trunk blood samples following a dose of 1.5 g/kg ethanol [38]. However, both of these previous studies (and most other studies assessing ontogenetic differences in BECs) examined BECs following an initial challenge with ethanol. Yet, in a study of male and female adolescent and adult rats exposed daily for seven days to 1 g/kg ethanol, metabolic tolerance emerged only in adults, with lower challenge doses (similar to those used in the present study) producing similar BECs in adolescents and adults [52]. Such an age-related difference in the emergence of metabolic tolerance would seemingly foster an opposite ontogenetic pattern of BEC findings from that seen here. It is possible that repeated psychosocial stress may have played a role in the age-related differences in BECs observed in the present study, given that subjects were socially isolated for 3 hr and injected daily over the eight day test period. Indeed, repeated restraint stress has been shown to increase hepatic alcohol dehydrogenase activity in rats [33], leading to faster ethanol metabolism and therefore lower BECs. To the extent that adolescents are more sensitive to stressors [41, 56], stress-related increases in hepatic alcohol dehydrogenase activity associated with the chronic testing procedure may have precipitated more rapid ethanol metabolism among the adolescents than adults, lowering the BECs of adolescents relative to adults in the present experiment.
Young drinkers have clear expectancies that alcohol will increase their effectiveness in social situations [12, 13]. However, low dose social stimulatory effects of ethanol, while perhaps contributing to the frequent initiation of ethanol use among human adolescents, are unlikely to explain adolescent-characteristic patterns of binge drinking involving relatively high consumption levels during which degradation of meaningful social interactions may occur. Rather, the high levels of consumption associated with adolescent binge drinking may be related to the relative resistance of adolescents to a number of adverse effects of ethanol that normally serve as cues to limit drinking. Indeed, there is convincing experimental evidence that adolescent rats, when compared with their adult counterparts, are less sensitive to a variety of ethanol effects, including ethanol-induced social inhibition [see [40] for references and review].
In summary, the present study demonstrates that ethanol administration sufficient to produce socially activating effects in adolescent animals does not affect USV production during social interactions under these test circumstances. Moreover, adolescent animals, though exhibiting more social activity than their adult counterparts, emit fewer USVs during interactions with their peers. Thus, although adolescents engage in increased amounts of social interactions when compared to adults, it may not be because they find social stimulation more rewarding than do adults, but rather may reflect another mechanism that has yet to be identified. Further ontogenetic investigation of indices of incentive salience and consummatory-related affect for different reinforcing stimuli may yield important clues as to the role of these reward processes in expression of adolescent-typical behaviors, and in the emergence of alcohol and substance use disorders among vulnerable adolescents.
Acknowledgments
The research presented in this article was supported by grants from the National Institute of Health (R01 DA019071 and R01 AA016887) to Linda P. Spear and (R01 AA012453) to Elena I. Varlinskaya.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beck KH, Thombs DL, Summons TG. The social context of drinking scales: Construct validation and relationship to indicants of abuse in an adolescent population. Addict Behav. 1993;18(2):159–169. doi: 10.1016/0306-4603(93)90046-c. [DOI] [PubMed] [Google Scholar]
- 2.Beck KH, Treiman KA. The relationship of social context of drinking, perceived social norms, and parental influence to various drinking patterns of adolescents. Addict Behav. 1996;21(5):633–644. doi: 10.1016/0306-4603(95)00087-9. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard RJ, Blanchard DC. Aggressive behavior in the rat. Behav Biol. 1977;21(2):197–224. doi: 10.1016/s0091-6773(77)90308-x. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two khz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol Behav. 1991;50(5):967–972. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard RJ, Yudko EB, Blanchard DC, Taukulis HK. High-frequency (35–70 khz) ultrasonic vocalizations in rats confronted with anesthetized conspecifics: Effects of gepirone, ethanol, and diazepam. Pharmacol Biochem Behav. 1993;44(2):313–319. doi: 10.1016/0091-3057(93)90467-8. [DOI] [PubMed] [Google Scholar]
- 6.Brown SA, Christiansen BA, Goldman MS. The alcohol expectancy questionnaire: An instrument for the assessment of adolescent and adult alcohol expectancies. J Stud Alcohol. 1987;48(5):483–491. doi: 10.15288/jsa.1987.48.483. [DOI] [PubMed] [Google Scholar]
- 7.Brudzynski SM, Kehoe P, Callahan M. Sonographic structure of isolation-induced ultrasonic calls of rat pups. Dev Psychobiol. 1999;34(3):195–204. doi: 10.1002/(sici)1098-2302(199904)34:3<195::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29(9):1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- 9.Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav Neurosci. 2000;114(2):320–327. [PubMed] [Google Scholar]
- 10.Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50-khz ultrasonic vocalizations in rats. Behav Neurosci. 2001;115(4):940–944. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- 11.Burgdorf J, Panksepp J. Tickling induces reward in adolescent rats. Physiol Behav. 2001;72(1–2):167–173. doi: 10.1016/s0031-9384(00)00411-x. [DOI] [PubMed] [Google Scholar]
- 12.Coleman LM, Cater SM. A qualitative study of the relationship between alcohol consumption and risky sex in adolescents. Arch Sex Behav. 2005;34(6):649–661. doi: 10.1007/s10508-005-7917-6. [DOI] [PubMed] [Google Scholar]
- 13.Darkes J, Greenbaum PE, Goldman MS. Alcohol expectancy mediation of biopsychosocial risk: Complex patterns of mediation. Exp Clin Psychopharmacol. 2004;12(1):27–38. doi: 10.1037/1064-1297.12.1.27. [DOI] [PubMed] [Google Scholar]
- 14.Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29(10):1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- 15.Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45(3):153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- 16.Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- 17.File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463(1–3):35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 18.Fu XW, Brudzynski SM. High-frequency ultrasonic vocalization induced by intracerebral glutamate in rats. Pharmacol Biochem Behav. 1994;49(4):835–841. doi: 10.1016/0091-3057(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 19.Harris JR. Where is the child’s environment? A group socialization theory of development. Psychological Review. 1995;102:458–489. [Google Scholar]
- 20.Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14(3):221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- 21.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Secondary school students. i. Bethesda, MD: National Institute on Drug Abuse; 2007. Monitoring the future national survey results on drug use, 1975–2006. [Google Scholar]
- 22.Knutson B, Panksepp J. Effects of serotonin depletion on the play of juvenile rats. Ann N Y Acad Sci. 1997;807:475–477. doi: 10.1111/j.1749-6632.1997.tb51942.x. [DOI] [PubMed] [Google Scholar]
- 23.Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol. 1998;112(1):65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- 24.Knutson B, Burgdorf J, Panksepp J. High-frequency ultrasonic vocalizations index conditioned pharmacological reward in rats. Physiol Behav. 1999;66(4):639–643. doi: 10.1016/s0031-9384(98)00337-0. [DOI] [PubMed] [Google Scholar]
- 25.Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128(6):961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- 26.La Greca AM, Prinstein MJ, Fetter MD. Adolescent peer crowd affiliation: Linkages with health-risk behaviors and close friendships. J Pediatr Psychol. 2001;26(3):131–143. doi: 10.1093/jpepsy/26.3.131. [DOI] [PubMed] [Google Scholar]
- 27.Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20(6):1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- 28.Larson R, Richards MH. Daily companionship in late childhood and early adolescence: Changing developmental contexts. Child Dev. 1991;62(2):284–300. doi: 10.1111/j.1467-8624.1991.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 29.Linakis JG, Cunningham CL. Effects of concentration of ethanol injected intraperitoneally on taste aversion, body temperature, and activity. Psychopharmacology (Berl) 1979;64(1):61–65. doi: 10.1007/BF00427346. [DOI] [PubMed] [Google Scholar]
- 30.Mallo T, Matrov D, Herm L, Koiv K, Eller M, Rinken A, et al. Tickling-induced 50-khz ultrasonic vocalization is individually stable and predicts behaviour in tests of anxiety and depression in rats. Behav Brain Res. 2007;184(1):57–71. doi: 10.1016/j.bbr.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Matochik JA, White NR, Barfield RJ. Variations in scent marking and ultrasonic vocalizations by long-evans rats across the estrous cycle. Physiol Behav. 1992;51(4):783–786. doi: 10.1016/0031-9384(92)90116-j. [DOI] [PubMed] [Google Scholar]
- 32.McIntosh TK, Vallano ML, Barfield RJ. Effects of morphine, beta-endorphin and naloxone on catecholamine levels and sexual behavior in the male rat. Pharmacol Biochem Behav. 1980;13(3):435–441. doi: 10.1016/0091-3057(80)90251-8. [DOI] [PubMed] [Google Scholar]
- 33.Mezey E, Potter JJ, Kvetnansky R. Effect of stress by repeated immobilization on hepatic alcohol dehydrogenase activity and ethanol metabolism. Biochem Pharmacol. 1979;28(5):657–663. doi: 10.1016/0006-2952(79)90151-5. [DOI] [PubMed] [Google Scholar]
- 34.Miczek KA. A new test for aggression in rats without aversive stimulation: Differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60(3):253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- 35.Panksepp J, Burgdorf J. 50-khz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats: Effects of social housing and genetic variables. Behav Brain Res. 2000;115(1):25–38. doi: 10.1016/s0166-4328(00)00238-2. [DOI] [PubMed] [Google Scholar]
- 36.Sales GD, Pye D. Ultrasonic communication by animals. London: Chapman & Hall; 1974. [Google Scholar]
- 37.Sales GD. Strain differences in the ultrasonic behavior of rats (rattus norvegicus) American Journal of Zoology. 1979;19:513–527. [Google Scholar]
- 38.Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20(1):45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- 39.Smith GT, Goldman MS, Greenbaum PE, Christiansen BA. Expectancy for social facilitation from drinking: The divergent paths of high-expectancy and low-expectancy adolescents. J Abnorm Psychol. 1995;104(1):32–40. doi: 10.1037//0021-843x.104.1.32. [DOI] [PubMed] [Google Scholar]
- 40.Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- 41.Stone EA, Quartermain D. Greater behavioral effects of stress in immature as compared to mature male mice. Physiol Behav. 1997;63(1):143–145. doi: 10.1016/s0031-9384(97)00366-1. [DOI] [PubMed] [Google Scholar]
- 42.Thomas DA, Takahashi LK, Barfield RJ. Analysis of ultrasonic vocalizations emitted by intruders during aggressive encounters among rats (rattus norvegicus) J Comp Psychol. 1983;97(3):201–206. [PubMed] [Google Scholar]
- 43.Thompson B, Leonard KC, Brudzynski SM. Amphetamine-induced 50 khz calls from rat nucleus accumbens: A quantitative mapping study and acoustic analysis. Behav Brain Res. 2006;168(1):64–73. doi: 10.1016/j.bbr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Tonoue T, Ashida Y, Makino H, Hata H. Inhibition of shock-elicited ultrasonic vocalization by opioid peptides in the rat: A psychotropic effect. Psychoneuroendocrinology. 1986;11(2):177–184. doi: 10.1016/0306-4530(86)90052-1. [DOI] [PubMed] [Google Scholar]
- 45.Vanderschuren LJ, Niesink RJ, Spruijt BM, Van Ree JM. Influence of environmental factors on social play behavior of juvenile rats. Physiol Behav. 1995;58(1):119–123. doi: 10.1016/0031-9384(94)00385-i. [DOI] [PubMed] [Google Scholar]
- 46.Vanderschuren LJ, Stein EA, Wiegant VM, Van Ree JM. Social play alters regional brain opioid receptor binding in juvenile rats. Brain Res. 1995;680(1–2):148–156. doi: 10.1016/0006-8993(95)00256-p. [DOI] [PubMed] [Google Scholar]
- 47.Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21(3):309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 48.Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: Role of housing conditions and partner’s activity. Physiol Behav. 1999;67(4):475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- 49.Varlinskaya EI, Spear LP, Spear NE. Acute effects of ethanol on behavior of adolescent rats: Role of social context. Alcohol Clin Exp Res. 2001;25(3):377–385. [PubMed] [Google Scholar]
- 50.Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: Role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26(10):1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- 51.Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: Social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48(2):146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- 52.Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult sprague-dawley rats. Neurotoxicol Teratol. 2007;29(1):23–30. doi: 10.1016/j.ntt.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varlinskaya EI, Spear LP. Increases in anxiety induced by acute stress are reversed by ethanol in adolescent but not adult rats. Society for Neuroscience; San Diego, CA. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varlinskaya EI, Spear LP. Social interactions in adolescent and adult sprague-dawley rats: Impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188(2):398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31(7):1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker CD, Trottier G, Rochford J, Lavallee D. Dissociation between behavioral and hormonal responses to the forced swim stress in lactating rats. J Neuroendocrinol. 1995;7(8):615–622. doi: 10.1111/j.1365-2826.1995.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 57.Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30(2):141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]