Abstract
Loss of von Hippel-Lindau tumor suppressor gene function occurs in familial and most sporadic clear cell renal cell carcinoma, resulting in the aberrant expression of genes that control cell proliferation, metabolism, invasion and angiogenesis. The molecular mechanisms by which loss of function leads to tumorigenesis are not yet fully defined. The von Hippel-Lindau gene product is part of an ubiquitin ligase complex that targets hypoxia inducible factors for polyubiquitination and proteasomal degradation, linking hypoxia response genes to renal cell carcinoma oncogenesis. Loss von Hippel-Lindau gene function also promotes cell invasiveness in response to hepatocyte growth factor, an important regulator of kidney development and renal homeostasis. Increased cell invasiveness is mediated by another ubiquitin ligase target with relevance to the molecular pathogenesis of renal cell carcinoma: β-catenin. This discovery and other recent insights into kidney cancer oncogenesis implicate convergent developmental and homeostatic signaling pathways in tumorigenesis, tumor invasiveness and metastasis.
Keywords: VHL, HIF, oncogenesis, HGF, β-catenin, renal cell carcinoma
Signalling Network Facts
Loss of VHL gene function occurs in hereditary and sporadic forms of ccRCC
The VHL gene product, pVHL, is part of an oxygen sensor that targets HIFs for degradation
pVHL also targets β-catenin for degradation in adults, thereby attenuating developmental responses to HGF and Wnts
Dysregulated HGF and Wnt signaling also occurs in ccRCC, partly due to pVHL loss
Further insight into signaling can be found at: http://www.genome.ad.jp/kegg/pathway/hsa/hsa05211.html
Introduction
von Hippel-Lindau (VHL) syndrome is an autosomal dominant hereditary neoplastic disorder characterized by the development of tumors in the cerebellum, spine, retina, inner ear, pancreas, adrenal glands, and kidneys (reviewed in Linehan et al., 2007). VHL-associated bilateral multifocal renal tumors are malignant and metastatic; up to 40% of untreated patients with VHL syndrome have died of advanced clear cell renal cell carcinoma (ccRCC). Affected individuals inherit an altered copy of the VHL tumor suppressor gene, located on chromosome 3 (3p25-26), and the remaining wild type copy is later inactivated in somatic cells, most often by loss of chromosome 3p or VHL gene hypermethylation. Alterations or deletions of 3p also occur in the majority of sporadic ccRCC cases, which claims more than 13,000 lives each year in the U.S. alone (Linehan et al., 2007). Reconstitution of wild type VHL expression in ccRCC-derived cell lines has been shown to regulate tumorigenesis in athymic nude mice, confirming a fundamental role for VHL in ccRCC oncogenesis (reviewed in Kaelin, 2002, Linehan et al., 2007).
Functions
Biochemical studies of the protein encoded by VHL (pVHL) have revealed that it forms a stable complex with other proteins possessing E3 ubiquitin ligase activity. This complex is best known for targeting hypoxia inducible factors (HIFs) for polyubiquitination and subsequent proteasomal degradation (Kaelin, 2002). HIF family transcription factors control the expression of genes involved in the cellular response to hypoxia. HIFs are constitutively expressed and under normoxic conditions, protein levels, and thus activity, are continuously suppressed by pVHL. Under hypoxic conditions or when the VHL gene is mutated or lost, HIFs accumulate, leading to the increased expression of genes encoding vascular endothelial growth factor, platelet derived growth factor, transforming growth factor-α, erythropoietin, the hepatocyte growth factor (HGF) receptor, Met, and others, all of which are potentially important in RCC oncogenesis (Kaelin, 2002, Linehan et al., 2007). Cultured VHL-negative ccRCC cells contain elevated levels of HIFs and, unlike normal, fully differentiated renal epithelial cells, respond to HGF treatment with increased motility, matrix invasion and morphogenesis (Peruzzi et al., 2006). These HGF responses are abolished when wild type VHL expression is reconstituted in ccRCC cells, directly linking loss of VHL function to an invasive tumor phenotype (Peruzzi et al., 2006).
The mechanism by which pVHL represses HGF-driven ccRCC cell invasiveness was elucidated by Peruzzi et al. (2006), who hypothesized that pVHL negatively regulates Met-driven β-catenin signaling in mature renal tubule epithelial cells. β-catenin normally maintains adherens junctions and, in response to HGF or Wnt stimulation, accumulates in the cytoplasm and translocates to the nucleus where it associates with T-cell factor/lymphoid enhancer factor (TCF/LEF) family members to function as a transcriptional co-activator (Clevers, 2006). Consistent with a shift in function from adhesion to signaling, Peruzzi et al. (2006) found that HGF stimulated the redistribution of β-catenin from peripheral to cytoplasmic and nuclear pools in VHL-negative ccRCC cells and that restoration of pVHL production repressed HGF-stimulated β-catenin target gene activation. Moreover, ectopic expression of a dominant-negative form of TCF blocked the invasive HGF response of pVHL-negative ccRCC cells (Peruzzi et al., 2006). In addition to revealing an important novel target for pVHL, these findings identified β-catenin as a potential target for biomarker and therapeutic development in ccRCC.
Cascades
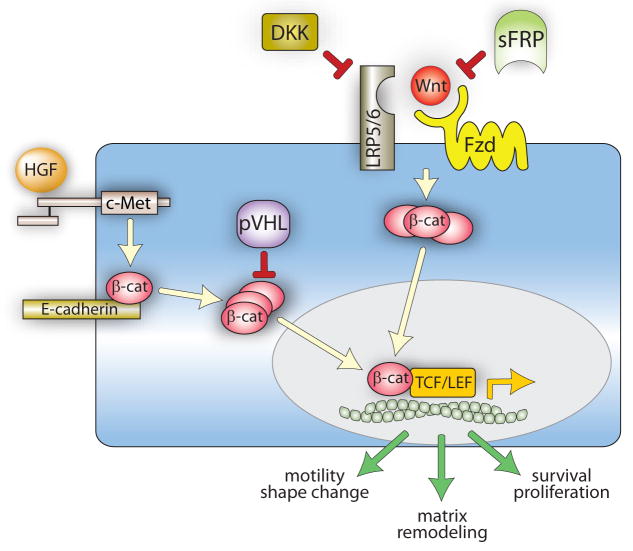
HGF signaling between mesenchymal and epithelial cells is a driving force in kidney morphogenesis and differentiation, and inappropriate Met signaling in cancer can resemble HGF-regulated developmental transitions between epithelial and mesenchymal cell types (Birchmeier et al., 2003). β-catenin is likely to mediate both HGF and Wnt signaling during nephrogenesis (Perantoni, 2003); relevant aspects of these convergent networks are schematically depicted in Figure 1.
Figure 1. Hypothetical model of HGF and Wnt signaling in kidney development and adulthood.
During development, HGF-Met binding promotes receptor kinase activation and tyrosyl phosphorylation of junctional β-catenin, resulting in its dissociation from E-cadherin and cytoplasmic accumulation. Wnt binding to Fzd and LRP5/6 induces Fzd activation, which stabilizes cytoplasmic β-catenin by protecting it from constitutive degradation. Cytoplasmic β-catenin from either pathway translocates to the nucleus, where it associates with members of TCF/LEF transcription factor family and promotes the expression of genes controlling cell motility, proliferation and matrix remodeling. In adult, fully differentiated renal epithelial cells, pVHL targets cytosolic β-catenin for degradation and balanced expression of Wnts and the negative regulators DKKs and sFRPs keeps Fzd activation, and thus cytosolic β-catenin, to a minimum.
Like HGF, Wnts act broadly in embryogenesis and adulthood, including kidney development and homeostasis (Dressler, 2006). Aberrant Wnt/β-catenin signaling occurs in many cancers, particularly colorectal cancer (Clevers, 2006). Although activation of the pathway in cancer is usually attributed to deregulation of downstream effectors (e.g. β-catenin) or suppressors (adenomatous polyposis coli protein or Axin), autocrine mechanisms may also be involved (Rubin et al., 2006). All of these signaling events are mediated by Wnt receptors in the Frizzled (Fzd) family and the low density lipoprotein (LDL) receptor-related proteins 5 and 6 (LRP5/6) co-receptors. Wnt-stimulated Fzd/LRP activation inhibits the otherwise constitutive degradation of cytosolic β-catenin, permitting nuclear translocation, TCF/LEF binding and the activation of genes controlling cell proliferation, migration and morphogenesis (Clevers, 2006). Unlike HGF signaling, which is primarily paracrine, normal Wnt signaling is frequently autocrine. Wnt signaling is also regulated at the cell surface by secreted Frizzled-related proteins (sFRPs), which bind Wnts and typically antagonize Wnt signaling, and Dickkopfs (DKKs), which bind LRP5/6 and block Wnt-dependent β-catenin transcriptional activity (Rubin et al., 2006). It is widely thought that negative regulation of autocrine Wnt signaling by sFRPs and DKKs is likely to be important in both developmental and homeostatic contexts.
Consistent with the known role of HGF in regulating developmental transitions between epithelial and mesenchymal cell types, β-catenin and E-cadherin, another adherens junction protein, are upregulated early in kidney development upon transition of the mesenchyme surrounding the branching ureteric buds into the epithelium that will form the tubules of the nephron (Huber et al., 2000). This process and the ensuing tubule formation involves several Wnt family members acting in an autocrine manner (Perantoni, 2003), as well as HGF acting in a paracrine mode (van Adelsberg et al., 2001). The upregulation of VHL late in nephrogenesis, and its persistent expression throughout adulthood, is thought to be critical in attenuating HIF-mediated proliferation and morphogenesis in mature renal epithelial cells. VHL and HIF genes display reciprocal temporal expression patterns during renal development that point to a role for hypoxia in driving early nephrogenesis and for pVHL in limiting this process (Bernhardt et al., 2006, Richards et al., 1996). For example, VHL mRNA has been found in mid-phase (mesonephric) duct epithelia and tubules, but not in the early part of final (metanephric) development (Richards et al., 1996). Later, VHL expression re-emerges throughout the tubular epithelium of the metanephric kidney (Richards et al., 1996). In contrast, marked nuclear localization of HIF-1α has been found in early medullary and cortical collecting ducts and in glomerular cells, whereas HIF-2α is reportedly produced in interstitial and peritubular cells and podocytes of the more mature glomeruli, consistent with roles in tubulogenesis and renal vasculogenesis, respectively (Bernhardt et al., 2006). Upon completion of kidney development, HIF-1α and -2α proteins are no longer detected (Bernhardt et al., 2006), consistent with proteasomal targeting by upregulated pVHL expression.
The recently described convergence of pVHL, HGF and β-catenin pathways suggests that VHL upregulation also may be important for attenuating β-catenin-mediated phenotypic changes once kidney development is completed. HGF and MET expression persist in the normal adult kidney, but the response of renal epithelial cells to HGF stimulation undergoes striking changes upon completion of normal development. Morphogenic and proliferative responses are minimized, and HGF signaling in the adult becomes adapted for renal homeostasis (Liu, 2006), protecting adult kidney tissue from toxicity and ischemic stress (Matsumoto and Nakamura, 2001) and counteracting fibrosis, a major cause of renal failure (Liu, 2006). This change in response coincides with tight control of HIF protein levels in the adult kidney by pVHL. When VHL function is lost, the resulting combined derepression of HIFs and β-catenin is likely to contribute to acquisition of the malignant ccRCC phenotype, resembling the aberrant activation of a robust developmental signaling program.
Key Molecules
As inhibitors of autocrine Wnt signaling, sFRPs have been viewed as potential tumor suppressors. Consistent with this hypothesis, the chromosomal loci of sFRPs have been associated with loss of heterozygosity in various tumor types and loss of SFRP expression in cancer due to promoter hypermethylation is well documented (Rubin et al., 2006). Loss of SFRP1 expression in breast cancer correlated with decreased survival and restoration of expression in colorectal tumor cell lines resulted in an attenuated tumor phenotype (Rubin et al., 2006). Interestingly, the attenuated phenotype was observed in cells with mutations in APC or β-catenin, providing support for the concept that Wnt stimulation is needed to drive oncogenic signaling by these mutations. Reduced expression of DKKs also has been reported for various tumors (Rubin et al., 2006). Kurose et al. (2004) reported a high incidence of DKK-3 silencing specifically in RCC, suggesting that DKKs also possess tumor suppressor activity. A recent and comprehensive review of Wnt signaling in renal cancer is available (Guillen-Ahlers, 2008).
Recently, Dalh et al. (2007) and Gumz et al. (2007) reported a remarkably high incidence of SFRP1 loss in ccRCC. In addition, Wnt responsive genes were found to be dramatically upregulated in ccRCC specimens (Gumz et al., 2007). Restoration of SFRP1 expression in ccRCC cell lines decreased the expression of these genes by two- to threefold (Gumz et al., 2007). SFRP1 expressing ccRCC cell lines also displayed dramatically reduced growth in culture and in soft agar (Gumz et al., 2007). Finally, stable transfection of three clonal ccRCC cell lines with SFRP1 cDNA expression constructs resulted in complete blockade of tumor xenograft growth in mice (Gumz et al., 2007). These results provide strong evidence that loss of SFRP1 expression is a key event in ccRCC tumorigenesis, and proof of concept that SFRP1 restoration is a potential ccRCC treatment modality.
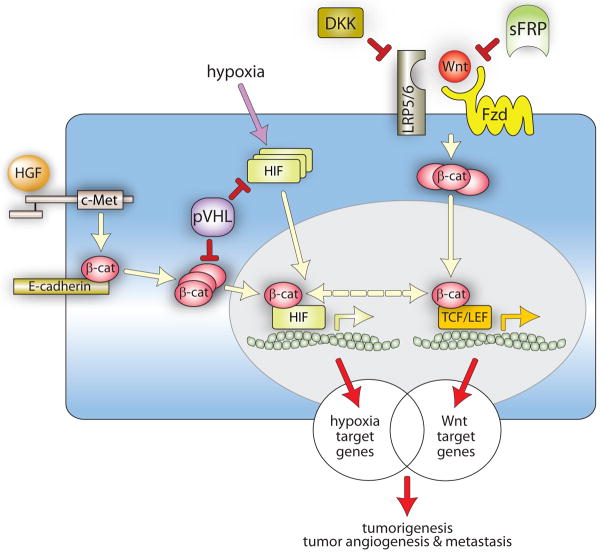
The convergence of dysregulated HIF, HGF and Wnt/β-catenin signaling networks in ccRCC may amplify their individual oncogenic effects (Figure 2). Loss of E-cadherin expression occurs in many tumor types, including ccRCC, and is associated with increased β-catenin transcriptional activity and the acquisition of an invasive cell phenotype (Russell and Ohh, 2007). Concomitant stimulation by HGF leads to Met-mediated tyrosyl phosphorylation of β-catenin, reducing its affinity for junctional E-cadherin and promoting its binding to Bcl9-2 (Brembeck et al., 2004). As a result, adherens junctions are further disrupted and cytoplasmic β-catenin is more efficiently translocated to the nucleus, enhancing cell invasiveness (Peruzzi et al., 2006). Dysregulated activation of HIF target genes, promoting cell survival, proliferation, motility, matrix remodeling and angiogenesis, compounds aberrant HGF and Wnt signaling (Kaelin, 2002, Linehan et al., 2007, Peruzzi et al., 2006). Finally, as reported by Kaidi et al. (2007), HIF-1α directly modulates β-catenin-dependent gene expression by competing with TCF/LEF proteins for binding to nuclear β-catenin. Moreover, β-catenin-HIF-1α interaction enhances HIF mediated transcription (Kaidi et al., 2007). In light of the many levels at which these pathways coincide, it is tempting to speculate that partial loss of VHL function that precedes inactivation of both alleles, combined with modest activity in the HGF and Wnt/β-catenin pathways, may, in the absence of other genetic defects, drive renal epithelial cells toward tumorigenesis.
Figure 2. Convergence of HIF and β-catenin signaling networks at multiple levels in ccRCC.
Wnt-Fzd-LRP5/6 and HGF-Met interactions promote increases in cytosolic β-catenin normally kept in check by sFRPs, DKKs and pVHL. The loss of these negative regulators in ccRCC, as well as hypoxia during tumor progression, results in the aberrant accumulation of cytoplasmic and nuclear β-catenin and HIF through multiple pathways, with compounding effects on target gene expression. Nuclear β-catenin interacts either with HIF or TCF/LEF to promote the expression of overlapping sets of genes, contributing to tumorigenesis, tumor angiogenesis and metastasis.
Associated Pathologies and Therapeutic Implications
Despite recent regulatory approval of two new drugs to treat ccRCC, there is no broadly effective, durable therapy for this disease once it becomes metastatic. While imaging techniques have improved the detection of localized ccRCC, these tumors often are asymptomatic until they have spread systemically, and patients that present with advanced disease have only an 18% two-year survival rate (Linehan et al., 2007). Consequently, early detection methods and new therapies are urgently needed. Recent progress in defining ccRCC cell invasiveness mediated by HGF, HIF and Wnt driven β-catenin signaling identifies each of these molecules, and HIF-β-catenin interaction, as potential targets for drug development. The identification of SFRP and DKK gene hypermethylation as a frequent feature of ccRCC, and the demonstration that restored SFRP1 expression can block ccRCC tumorigenesis in animals, suggest that demethylating agents or methylase inhibitors that could upregulate SFRP1 expression are also potential ccRCC treatment strategies. Finally, analysis of SFRP gene hypermethylation may have diagnostic and prognostic value in the detection and clinical management of ccRCC (Urakami et al., 2006). Continued research into the molecular pathogenesis of ccRCC will better define this collection of critical oncogenic events and facilitate the development of combination therapies that ultimately may be needed for the effective treatment of this and other human cancers.
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The authors regret that not all relevant original reports could be cited due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
W. Marston Linehan, Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892 USA.
Jeffrey S. Rubin, Laboratory of Cellular and Molecular Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892 USA
Donald P. Bottaro, Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892 USA
References
- Bernhardt WM, Schmitt R, Rosenberger C, et al. Expression of hypoxia-inducible transcription factors in developing human and rat kidneys. Kidney Int. 2006;69:114–22. doi: 10.1038/sj.ki.5000062. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, Metastasis, Motility And More. Nat Rev Mol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between beta-catenin’s adhesive and transcriptional functions. Genes Dev. 2004;18:2225–30. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Dahl E, Wiesmann F, Woenckhaus M, et al. Frequent loss of SFRP1 expression in multiple human solid tumours: association with aberrant promoter methylation in renal cell carcinoma. Oncogene. 2007;26:5680–91. doi: 10.1038/sj.onc.1210345. [DOI] [PubMed] [Google Scholar]
- Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–29. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- Guillen-Ahlers H. Wnt signaling in renal cancer. Curr Drug Targets. 2008;9:591–600. doi: 10.2174/138945008784911813. [DOI] [PubMed] [Google Scholar]
- Gumz ML, Zou H, Kreinest PA, et al. Secreted frizzled-related protein 1 loss contributes to tumor phenotype of clear cell renal cell carcinoma. Clin Cancer Res. 2007;13:4740–9. doi: 10.1158/1078-0432.CCR-07-0143. [DOI] [PubMed] [Google Scholar]
- Huber SM, Braun GS, Segerer S, Veh RW, Horster MF. Metanephrogenic mesenchyme-to-epithelium transition induces profound expression changes of ion channels. Am J Physiol Renal Physiol. 2000;279:F65–F76. doi: 10.1152/ajprenal.2000.279.1.F65. [DOI] [PubMed] [Google Scholar]
- Kaelin WG., Jr Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2:673–82. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–7. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- Kurose K, Sakaguchi M, Nasu Y, et al. Decreased expression of REIC/Dkk-3 in human renal clear cell carcinoma. J Urol. 2004;171:1314–8. doi: 10.1097/01.ju.0000101047.64379.d4. [DOI] [PubMed] [Google Scholar]
- Linehan WM, Pinto PA, Srinivasan R, et al. Identification of the genes for kidney cancer: opportunity for disease-specific targeted therapeutics. Clin Cancer Res. 2007;13:671s–9s. doi: 10.1158/1078-0432.CCR-06-1870. [DOI] [PubMed] [Google Scholar]
- Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–7. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Hepatocyte growth factor: Renotropic role and potential therapeutics for renal diseases 64. Kidney International. 2001;59:2023–38. doi: 10.1046/j.1523-1755.2001.00717.x. [DOI] [PubMed] [Google Scholar]
- Perantoni AO. Renal development: perspectives on a Wnt-dependent process. Semin Cell Dev Biol. 2003;14:201–8. doi: 10.1016/s1084-9521(03)00022-3. [DOI] [PubMed] [Google Scholar]
- Peruzzi B, Athauda G, Bottaro DP. The von Hippel-Lindau tumor suppressor gene product represses oncogenic beta-catenin signaling in renal carcinoma cells. Proc Natl Acad Sci U S A. 2006;103:14531–6. doi: 10.1073/pnas.0606850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards FM, Schofield PN, Fleming S, Maher ER. Expression of the von Hippel-Lindau disease tumour suppressor gene during human embryogenesis. Hum Mol Genet. 1996;5:639–44. doi: 10.1093/hmg/5.5.639. [DOI] [PubMed] [Google Scholar]
- Rubin JS, Barshishat-Kupper M, Feroze-Merzoug F, Xi ZF. Secreted WNT antagonists as tumor suppressors: pro and con. Front Biosci. 2006;11:2093–105. doi: 10.2741/1952. [DOI] [PubMed] [Google Scholar]
- Russell RC, Ohh M. The role of VHL in the regulation of E-cadherin: a new connection in an old pathway. Cell Cycle. 2007;6:56–9. doi: 10.4161/cc.6.1.3668. [DOI] [PubMed] [Google Scholar]
- Urakami S, Shiina H, Enokida H, et al. Wnt antagonist family genes as biomarkers for diagnosis, staging, and prognosis of renal cell carcinoma using tumor and serum DNA. Clin Cancer Res. 2006;12:6989–97. doi: 10.1158/1078-0432.CCR-06-1194. [DOI] [PubMed] [Google Scholar]
- van Adelsberg J, Sehgal S, Kukes A, et al. Activation of hepatocyte growth factor (HGF) by endogenous HGF activator is required for metanephric kidney morphogenesis in vitro. J Biol Chem. 2001;276:15099–106. doi: 10.1074/jbc.M006634200. [DOI] [PubMed] [Google Scholar]