Abstract
Background
It is important to determine the efficacy of intraportal islet transplantation in comparison with other transplant sites. In this study, we tried to determine the optimal number of islets to achieve normoglycemia following transplantation into the liver versus kidney using a mouse model.
Methods
Streptozotocin induced diabetic mice (Balb/C) were transplanted with syngeneic islets via the intraportal (IP) and renal subcapsular (SC) routes. The transplanted islet numbers were zero to 800 (n=3 to 5). We calculated the parameters indicated below, assessed the correlation between these parameters and islet numbers and compared the parameters in the IP versus SC groups. The parameters were: (1) Percentage of normoglycemia; (2) Postoperative days to normoglycemia; (3) Mean blood glucose level at various points from pre-transplantation to end of the study (postoperative day 28); (4) Mean serum insulin; and (5) Area under the curve (AUC) of blood glucose levels after injection of glucose.
Results
Two hundred islets yielded normoglycemia in renal subcapsular grafts, while 800 islets were the minimum required in intraportal transplantation. The transplant efficacy in subcapsular transplantation was 2 to 5 times that of intraportal transplantation. The days to normoglycemia were significantly different between intraportal and renal subcapsular islets (13.25 ± 4.38 days vs. 4.50 ± 0.81 days, p = 0.007).
Conclusion
Efficacy of islet transplantation in murine diabetic models is significantly higher under the kidney capsule. Clinical islet transplantation could benefit from trials of alternative transplantation sites.
INTRODUCTION
Islet transplantation is considered one of the useful therapeutic options for type 1 diabetes mellitus (T1DM). Intraportal transplantation is the most effective method at present (1, 2). However, transplanted islets are very unstable partly due to nonspecific inflammation, hypoxia, and the metabolic condition of the recipient (3). Approximately 60% of transplanted islets are lost in the first couple days (3). Immune and nonimmune phenomenon, including the instant blood-mediated inflammatory reaction (IBMIR) are current obstacles for success of intraportal islet transplantation (4). In clinical practice, multiple transplantations are often required for one recipient to maintain normoglycemia (5).
Therefore, it is important to determine the efficacy of intraportal transplantation in comparison with other transplant sites. In this study, we tried to determine the optimal number of islets to achieve normoglycemia following their transplantation into the liver, as done currently in clinical islet transplantation, and in the kidney, the site used most frequently in animal studies.
MATERIALS AND METHODS
Animals
Balb/C female mice weighing 22–27g (Charles River Laboratories. Inc., Boston, MA, USA) were used as donor and recipient. The Institutional Animal Care Use Committee approved the subsequent experimental protocol. Recipient mice were submitted 200mg/kg body weight of streptozotocin (STZ, Sigma-Aldrich, St. Lois, MO, USA) for induction of Diabetes. We used these mice when the blood glucose levels before transplantation were over 250mg/dL
Islet isolation and transplantation
Mice islets were isolated by digestion with collagenase (collagenase V, Sigma-Aldrich), separation with Ficoll (Sigma-Aldrich) discontinuous garadients and purification (6, 7). After overnight culture in Medium 199 (HyClone, Logan, UT, USA), recipient diabetic mice were transplanted with syngeneic islets via the intraportal (IP) or left renal subcapsular (SC) routes. The transplanted islet number was zero (n=3, as a control labeled as “DM (0)”), 100 (n=4 for IP and SC), 200 (n=5 for IP and SC), 300 (n=4 for IP and SC), 500 (n=5 for IP and n=4 for SC) and 800 (n=4 for IP only) islet equivalent (IEQ). We performed static incubation and measured insulin content of the islets used for IP and SC transplantation, and detected no functional difference in vitro between the two series (data not shown).
Monitoring blood glucose levels
Blood glucose levels of the transplanted mice were measured in the morning at postoperative day (POD) 0 (i.e. preoperatively), 1, 2, 3, 5, 7, 10, 14, 17, 21, 24 and 28. Normoglycemia was defined as blood glucose under 150 mg/dL on two consecutive days. 100μL of blood were collected per animal at POD 0, 3, 7, 14, and 28 for serum insulin with enzyme linked immunosorbend assay (ELISA).
Glucose tolerance test
Intraperitoneal glucose tolerance test (IPGTT) was performed in transplanted mice at one month after transplantation. After 12 hours of over night fasting, mice were injected with 2.0g/kg body weight of 20% glucose solution. Blood glucose levels were measured from tail veins at 0, 15, 30, 60, 90 and 120 minutes after glucose injection.
Assessment of correlation between islet number and blood glucose
We calculated the parameters indicated below, assessed the correlation between these parameters and islet numbers and compared the parameters in the intraportal (IP) versus subcapsular (SC) groups. The parameters were: (1) Percentage achieving normoglycemia in each group; (2) Postoperative days to normoglycemia; (3) Mean blood glucose level at various points from pre-transplantation to the end of the study (POD28). We also tested the correlation between mean blood glucose levels and islet number; (4) Mean serum insulin at various points; and (5) Area under the curve (AUC) of blood glucose levels in the intraperitoneal GTT.
Statistical analysis
Statistical analysis of postoperative days to normoglycemia was by Student t test. P value less than 0.05 was considered as significant difference. Other parameters were compared with islet number and we applied simple regression analysis. We considered there was a correlation among two parameters in cases where R2 value was over 0.09 (R > 0.3). We applied approximation to all variables except postoperative days to normoglycemia. The degree of efficacy between intraportal and subcapsular groups was assessed by the ratio of the coefficients using the formula Y= aX+b (a=coefficient, x=islet number, y=parameter tested).
RESULTS
Percentage of normoglycemia
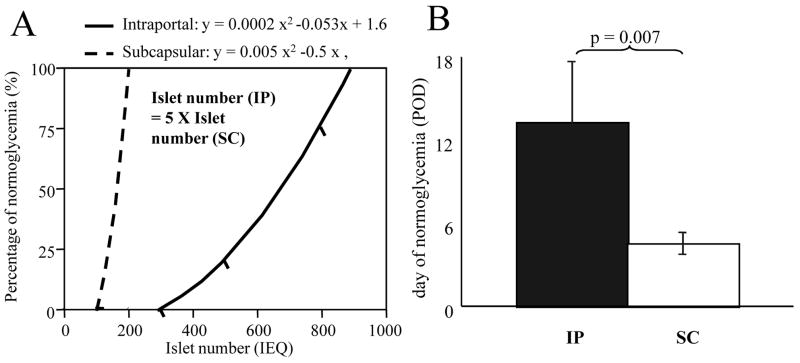
Percentage of animals achieving normoglycemia is shown in Table 1. Two hundred islets could yield normoglycemia in renal subcapsular grafts, while 800 islets were the minimum required in intraportal transplantation. Data from both group were approximated using the quadratic function. Approximations were y (=percentage of normoglycemia) = 0.0002x2 (x=islet number) in intraportal and y = 0.005x2 in subcapsular transplants. These approximations were applied when the range of percentage of normoglycemia was between zero and 100 %, excluding 0 and 100%. From the approximation assessment, it was concluded that intraportal transplants required five times the number of islets as renal subcapsular transplants for an equivalent percentage of normoglycemia (Figure 1A).
Table 1.
Summary of percentage of normoglycemia and postoperative days to normoglycemia.
| DM | 100 | 200 | 300 | 500 | 800 | |
|---|---|---|---|---|---|---|
| 1) Percentage of normoglycemia (%) | 0 (0/3) | 0 (0/4) | 0 (0/5) | 0 (0/4) | 20 (1/5) | 75 (3/4) |
| 0 (0/4) | 100 (5/5) | 100 (4/4) | 100 (4/4) | |||
|
| ||||||
| 2) POD to normoglycemia (POD) | 10 | 5, 10, 28 | ||||
| 2, 5, 5, 5, 7 | 1, 5, 7, 10 | 1, 1, 3, 7 | ||||
Top: intraportal (IP), bottom: renal subcapsular (SC)
Figure 1.
A: correlation between islet number and percentage of normoglycemia. Approximation was done by the quadratic function (rigid line for intraportal group and broken line for subcapsular group). The efficacy of the subcapsular transplantation appears as 5 times that of intraportal transplantation. B: Postoperative time to normoglycemia was significantly shorter in the subcapsular group (white area) than the intraportal group (black area). Statistical analysis was performed by Student’ t test. Significant difference = p < 0.05.
Postoperative days to normoglycemia
The days to normoglycemia did not significantly differ by the number of transplanted islets (Dunnet test, Table 1), however, they were significantly different between intraportal and renal subcapsular islets (13.25 ± 4.38 days versus 4.50 ± 0.81 days, p = 0.007; Figure 1B).
Comparison between IP and SC groups in blood glucose, serum insulin levels and GTT
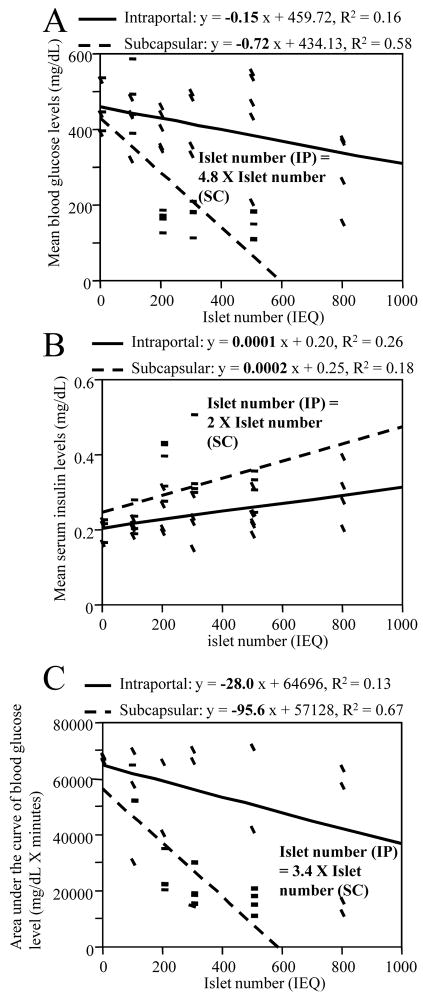
Figure 2A shows a correlation between mean blood glucose levels and islet number. There was a significant negative correlation between islet number and mean blood glucose level at various points in both intraportal (coefficient was −0.15, p = 0.046) and subcapsular (coefficient was −0.72, p < 0.0001) transplantation. The transplantation efficacy of the liver was under one fourth (21% by the ratio of the coefficient) that of the kidney in this assessment. In the assessment of serum insulin, there was a positive correlation with islet number in both groups. The insulin releasing function was twice as high in the subcapsular group compared to the intraportal group (Figure 2B). In terms of glucose response (the AUC of glucose changes in intraperitoneal GTT) there was a negative correlation between islet number and AUC in both group. The efficacy of transplantation was 3.4 times higher in the subcapsular group (Figure 2C).
Figure 2.
Correlation between islet number and mean blood glucose levels (A), mean serum insulin (B) and AUC of blood glucose in intraperitoneal GTT (C). There was a significant correlation in the three parameters and the transplant efficacies were significantly higher in subcapsular group than in the intraportal group. All the statistical assessments were performed by simple regression analysis and correlation was significant when R2 > 0.09. Transplant efficacy was calculated by ratio of the coefficients in both groups.
DISCUSSION
In this study, we compared the efficacy of intraportal and renal subcapsular transplantation in multiple analyses. We clarified the significant (approximately 2 to 5 times) efficacy of subcapsular transplantation in terms of percentage of normoglycemic recipients, their mean glucose levels, mean serum insulin and AUC of blood glucose levels after injection of glucose (Figures 1A and 2). Moreover, we determined that the postoperative time to normoglycemia was much shorter in the subcapsular group (Figure 1B). Major reasons for this variation in efficacy may be the instant blood mediated reaction IBMIR (4), and as stated by other reports (8), hypoxia of the liver due to portal vein embolization by transplanted islets. According to the latter theory, when transplanted islet emboli block the blood flow of the portal vessels, hypoxia and destruction of surrounding liver tissue occurs and causing early islet graft failure. Hypoxia and destruction within the liver were seen within a couple of days in one of our studies (data in press). Another reason for the difference in transplant efficacy may be the morphology of the islets at the transplant site. In subcapsular transplantation, islets are clustered in almost one position. In intraportal transplants on the other hand, islets are dispersed. Angiogenesis and effective blood flow might be more efficient in a mass of islets rather than in dispersed cells. Clustered islets may also have a survival advantage via paracrine and neurocrine signaling pathways.
In conclusion, efficacy of islet transplantation in murine diabetic models is significantly higher under the kidney capsule. This may reflect an advantage of maintained juxtaposition versus dispersion of islet cells. Clinical islet transplantation could benefit from trials of alternative transplantation sites. In view of the relative success of intraportal transplantation clinically, alternate transplant sites with enhanced efficacy may lead to a dramatic and universal shift in the standard of care for patients with type 1 diabetes.
Acknowledgments
This work was supported by NIH/NIDDK Grant # 1R01-DK077541 [EH].
We are very grateful to John Chrisler for his technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newsletter No. 9, Internationa Islet Transplnat Registry, Giessen, 2001.
- 2.Annual Report, Collaborative Islet Transplant Registry, Rockville, 2007.
- 3.Biarnés M, Montolio M, Nacher V, et al. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51:66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- 4.Ozmen L, Ekdahl KN, Elgue G, et al. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51:1779–1784. doi: 10.2337/diabetes.51.6.1779. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 6.Miao G, Mace J, Kirby M, et al. In vitro and in vivo improvement of islet survival following treatment with nerve growth factor. Transplantation. 2006;81:519–524. doi: 10.1097/01.tp.0000200320.16723.b3. [DOI] [PubMed] [Google Scholar]
- 7.Sakata N, Egawa S, Sumi S, et al. Optimization of glucose level to determine the stimulation index of isolated rat islets. Pancreas. 2008;36:417–423. doi: 10.1097/MPA.0b013e31815ccad2. [DOI] [PubMed] [Google Scholar]
- 8.Yin D, Ding JW, Shen J, et al. Liver ischemia contributes to early islet failure following intraportal transplantation: benefits of liver ischemic-preconditioning. Am J Transplant. 2006;6:60–68. doi: 10.1111/j.1600-6143.2005.01157.x. [DOI] [PubMed] [Google Scholar]