Abstract
The quaternary structure of G protein-coupled receptors (GPCRs) can influence their trafficking and ability to transduce signals. GPCR oligomers are generally portrayed as long-lived entities, although the stability of these complexes has not been studied. Here we show that D2 dopamine receptor protomers interact transiently at a specific oligomer interface. Selective immobilization of cyan fluorescent protein-D2 receptors (C-D2Rs) in the plasma membrane failed to completely immobilize coexpressed D2-venus receptors (D2R-Vs), suggesting that the two did not form stable oligomers with each other. Oxidative cross-linking stabilized C-D2R-D2R-V oligomers such that immobilization of C-D2R also immobilized D2R-V. This stabilization required the presence in both C-D2R and D2R-V of a cysteine residue in transmembrane domain 4 (TM4), a region identified as a putative oligomer interface in these and other class A GPCRs. These results suggest that the interaction of D2 receptor protomers at TM4 is transient unless stabilized and that the quaternary structure of these receptors may thus be subject to physiological or pharmacological regulation.
GPCRs can exist and function in cells as dimers or higher-order oligomers (Bouvier, 2001; Milligan, 2007). However, the structural arrangement of GPCR protomers within oligomers and the dynamics of oligomer assembly and disassembly are not well understood (Gurevich and Gurevich, 2008). The most is known about class C (metabotropic glutamate-like) oligomers; the interfaces between class C protomers involve either extracellular disulfide bonds or intracellular coiled-coils and are believed to be relatively stable (Bouvier, 2001). By comparison, the interfaces between class A (rhodopsin-like) protomers are poorly defined, although it is likely that transmembrane regions are involved. Transmembrane class A interfaces identified by molecular models and crystallography occupy relatively small protein surfaces (Liang et al., 2003; Lodowski et al., 2007), suggesting that oligomers formed at these interfaces might not be stable. However, it is difficult to predict class A GPCR oligomer stability because these interfaces are buried in a hydrophobic environment and because the proteins are concentrated in a two-dimensional membrane.
To directly assess the stability of a class A GPCR oligomer interface, we selectively immobilized a subset of protomers on the surface of cells using specific antibodies. The lateral mobility of non-cross-linked protomers was then measured using fluorescence recovery after photobleaching (FRAP). The mobility of non-cross-linked protomers should have been decreased if these protomers formed stable oligomers with antibody-cross-linked protomers. We chose to study D2 dopamine receptors because oxidative cross-linking studies have identified the fourth transmembrane helix (TM4) of this receptor as part of a conformationally sensitive homo-oligomer interface (Guo et al., 2003, 2005). TM4 (together with TM5) has also been identified as an interface between protomers of rhodopsin and other class A GPCRs (Kota et al., 2006; González-Maeso et al., 2008); thus, this helix may be a general class A oligomer interface.
Materials and Methods
Molecular Biology. D2R-V was generously provided by Dr. Jonathan Javitch (Columbia University College of Physicians and Surgeons, New York, NY). This construct lacks three native cysteine residues (C118S/C371S/C373S), none of which is located at the putative TM4 interface. C-D2R was constructed by amplifying D2R from D2R-V and inserting this fragment behind a signal sequence from human growth hormone and enhanced cyan fluorescent protein (ECFP) using the polymerase chain reaction. β2-Adrenoreceptorvenus fusion (β2AR-V) was constructed by fusing venus to the C terminus of the human β2AR. The transmembrane domain in C-TM-V and C-TM was the first transmembrane domain from the human μ-opioid receptor. All constructs were verified by automated sequencing.
Cell Culture and Transfection. Human embryonic kidney 293 cells (American Type Culture Collection, Manassas, VA) were plated on poly(l-lysine)-coated coverslips in six-well tissue culture plates and cultured in minimal essential medium supplemented with 10% fetal bovine serum for 24 to 48 h before transfection. Cells that were 50 to 70% confluent were transfected with a 5:1 ratio of plasmid DNA encoding C-D2R and D2R-V using polyethylenimine. Cells were used for experiments 12 to 24 h after transfection.
Antibody and Oxidative Cross-Linking. Medium was removed from cells and washed three times with buffer containing 150 mM NaCl, 10 mM sodium-HEPES, 12.8 mM d-glucose, 2.5 mM KCl, 0.5 mM MgCl2, and 0.5 mM CaCl2, pH 8.0, at room temperature. For antibody cross-linking, cells were incubated sequentially for 5 min each (separated by three buffer washes) in 1:200 anti-GFP rabbit IgG (Invitrogen, Carlsbad, CA) and biotin-XX goat anti-rabbit (Invitrogen). For oxidative cross-linking, cells were first incubated for 10 min in a solution containing 1 mM CuSO4 and 2 mM 1,10-phenanthroline (CuP), washed in buffer three times, and then cross-linked with antibodies as described above. Control cells in these experiments were incubated in CuP-free buffer for the equivalent time before antibody cross-linking.
Imaging and Fluorescence Recovery after Photobleaching. FRAP experiments were performed at room temperature on the stage of an inverted Leica SP2 laser scanning confocal microscope (Leica, Wetzlar, Germany). ECFP and venus excitation intensities were standardized by setting an acousto-optic tunable filter to allow transmission of 6% of the 458-nm laser line and 1% of the 514-nm laser line (or an equivalent ratio of these two lines) to reach the specimen; ECFP and venus emissions were sampled at 460 to 500 nm and 520 to 700 nm, respectively. After scanning at low power for 10 s, a 5-μm circular region of interest (ROI) was photobleached with a single scan at high power (75-100% transmission), after which scanning was resumed at low power for 180 s. Background-subtracted average intensity in a sub-ROI containing the bleached plasma membrane was divided by a control ROI to correct for bleaching during low-power scanning. Recovery was defined as the fractional recovery after 180 s.
Immunoblotting. Transfected cells plated on poly(l-lysine)-coated 100-mm cell culture dishes were washed twice with PBS and incubated for 10 min in either PBS or CuP (see above). After washing, cells were then incubated for 20 min at room temperature in a solution containing PBS supplemented with 1 mM CaCl2 and 0.1 mM MgCl2, 20 mM N-ethylmaleimide, and protease inhibitor cocktail (Sigma, St. Louis, MO). Cells were scraped on ice, centrifuged at 1500 rpm at 4°C for 5 min, and lysed in 1% dodecylmaltopyranoside containing 20 mM N-ethylmaleimide. Extracts were isolated by centrifugation at 20,000 rpm at 4°C for 30 min, and total protein concentration was measured using BCA Protein Assay (Pierce, Rockford, IL). Equal amounts of protein were mixed with an equal volume of nonreducing 2× sample buffer and warmed to 37°C for 15 min. Proteins were resolved on 4 to 20% gradient SDS-polyacrylamide gel electrophoresis precast gels (Pierce) and transferred onto polyvinylidene difluoride membrane. Membranes were probed with anti-GFP rabbit IgG (1:1000) and horseradish peroxidase-conjugated anti-rabbit (Pierce; 1:10,000). Proteins were detected using enhanced chemiluminescence (SuperSignal West Pico; Pierce).
Electrophysiology and Data Analysis. Whole-cell voltage-clamp recordings were made using standard procedures from transfected cells on the stage of an inverted fluorescence microscope. Cells were held at a membrane potential of -60 mV and were stepped to -100 mV for 0.2 s and then ramped from -100 to 0 mV at a rate of 0.18 mV/ms. Electrodes (4-5 MΩ) were filled with a solution containing 140 mM potassium gluconate, 5 mM KCl, 0.2 mM EGTA, 10 mM HEPES, 3 mM MgATP, and 0.3 mM Na2GTP, pH 7.2, ∼295 mOsm/kg H2O. Cells were perfused with a solution containing 122.5 or 150 mM NaCl, 30 mM or 5 mM KCl, 10 mM HEPES, 10 mM glucose, 1.5 mM CaCl2, and 2.5 mM MgCl2, pH 7.2, ∼320 mOsm/kg H2O. Solution changes were made using a multiport attachment, and perfusion capillary was positioned directly in front of the cell under study. Statistical comparisons were made using the unpaired t test; P < 0.001 was considered statistically significant.
Results
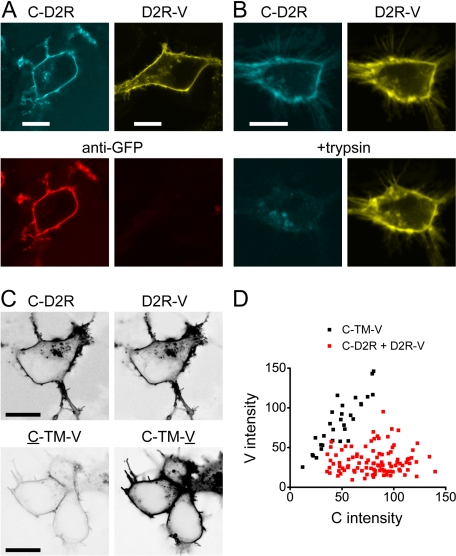
ECFP (C) and the yellow fluorescent protein venus (V) were fused to the extracellular N terminus and intracellular C terminus, respectively, of a cysteine-depleted D2 receptor used in previous studies of the TM4 interface (Guo et al., 2003, 2005). When expressed in human embryonic kidney 293 cells, these fusion proteins (C-D2R and D2R-V) trafficked to the plasma membrane and adopted the expected orientation (Fig. 1, A and B). The C moiety of C-D2R was extracellular, and the V moiety of D2R-V was intracellular, as shown by immunostaining of intact cells with an anti-GFP antibody (Fig. 1A) and susceptibility to trypsin digestion (Fig. 1B). C-D2R and D2R-V were both functional, as shown by activation of inwardly rectifying potassium channels (C-D2R, 167 ± 32 pA, n = 7; D2R-V, 352 ± 62 pA, n = 6; no-receptor control, 17 ± 9 pA, n = 5).
Fig. 1.
Orientation and stoichiometry of coexpressed C-D2R and D2R-V. A, confocal images of live cells expressing either D2R-V or C-D2R stained with Alexa-633-conjugated anti-GFP. Only C-D2R is exposed to the antibody in intact cells; scale bar, 10 μm. B, confocal images of a single cell expressing both D2R-V and C-D2R before (top) and after (bottom) treatment with trypsin (1 mg/ml) for 1 min; scale bar, 10 μm. C, confocal images of cells expressing C-D2R and D2R-V (top) or C-TM-V (bottom) acquired with identical settings; C excitation intensity was attenuated to normalize C-D2R and D2R-V emission intensity. The lookup table is inverted for clarity; scale bar, 10 μm. D, C and V intensities measured at the plasma membrane; each point represents a single cell. The C/V ratio is higher for cells expressing C-D2R and D2R-V than for cells expressing C-TM-V.
C-D2R and D2R-V were coexpressed under conditions such that C-D2R were more abundant than D2R-V in most cells, with the expectation that this mismatch would favor assembly of D2R-V-containing oligomers that also contained a C-D2R protomer. Cells were selected for imaging based on the relative intensities of C-D2R and D2R-V. The ratio of C-D2R and D2R-V expression in these same cells was measured by calibrating C and V intensity at the cell surface with a standard, fixed-stoichiometry transmembrane protein (C-TM-V; Fig. 1, C and D). With standardized imaging conditions, the C/V intensity ratio at the plasma membrane was 0.60 ± 0.02 (n = 30) for C-TM-V and 3.05 ± 0.16 (n = 103) for coexpressed C-D2R and D2R-V; thus, the average C-D2R: D2R-V expression ratio in the cells studied was ∼5:1.
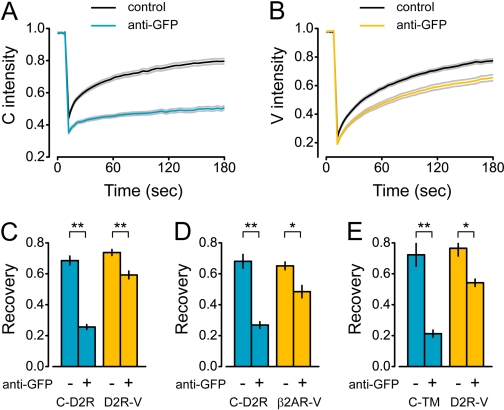
The lateral mobilities of coexpressed C-D2R and D2R-V were then measured with FRAP. The rates of fluorescence recovery were indistinguishable for these two receptors (Fig. 2A and B), suggesting that the dimensions of the transmembrane elements of both proteins (or oligomers containing them) were similar. C-D2R at the cell surface were cross-linked by sequential incubation of live cells with a polyclonal anti-GFP antibody and a secondary antibody. Antibody cross-linking virtually immobilized C-D2R, as indicated by a dramatic decrease in fluorescence recovery after photobleaching (Fig. 2, A and C). The antibodies used for cross-linking had no access to the V moiety of D2R-V, yet cross-linking would be expected to also immobilize these protomers if they formed stable oligomers with C-D2R. In contrast, antibody cross-linking had a relatively modest effect on D2R-V mobility (Fig. 2, B and C). Activation of C-D2R and D2R-V with the agonist quinpirole had no effect on D2R-V mobility after antibody cross-linking (P = 0.82; n = 7). To determine whether the effect of antibody cross-linking on D2R-V mobility reflected a specific protein interaction, we performed the analogous experiment with C-D2R and a β2AR-V, a protein that is not known to interact with D2Rs. Antibody cross-linking of C-D2R had a similar effect on the mobility of β2AR-V (Fig. 2D) when the two were expressed at relative levels similar to C-D2R and D2R-V (C/V intensity ratio = 3.94 ± 0.42; n = 31). This suggested that the slowing of D2R-V mobility by immobile C-D2R was not entirely specific. In fact, we found that antibody cross-linking of even a single TM control protein (C-TM) decreased D2R-V mobility (Fig. 2E; C/V intensity ratio = 4.98 ± 1.06; n = 18). This suggests that immobile TM proteins may nonspecifically decrease the lateral mobility of other TM proteins, which is consistent with previous reports that membrane protein crowding decreases the lateral mobility of transmembrane proteins (Frick et al., 2007). We cannot rule out an alternative possibility, namely that these control proteins interact with D2Rs in a manner similar to the interaction of C-D2R and D2R-V with each other.
Fig. 2.
Antibody cross-linking of C-D2R does not immobilize D2R-V. A, recovery of C-D2R fluorescence after photobleaching (at time = 10 s) in cells expressing C-D2R and D2R-V is nearly abolished by anti-GFP antibody cross-linking; lines represent the mean of all experiments, gray lines represent the mean ± S.E.M. B, recovery of D2R-V fluorescence in the same cells as A. C to E, recovery of normalized fluorescence (measured at time = 180 s) for cells expressing C-D2R and D2R-V (C; control, n = 59; anti-GFP, n = 61), C-D2R and β2AR-V (D; control, n = 15; anti-GFP, n = 16), and C-TM and D2R-V (E; control, n = 9; anti-GFP, n = 9); *, P < 0.002; **, P < 0.001; bars represent mean ± S.E.M.
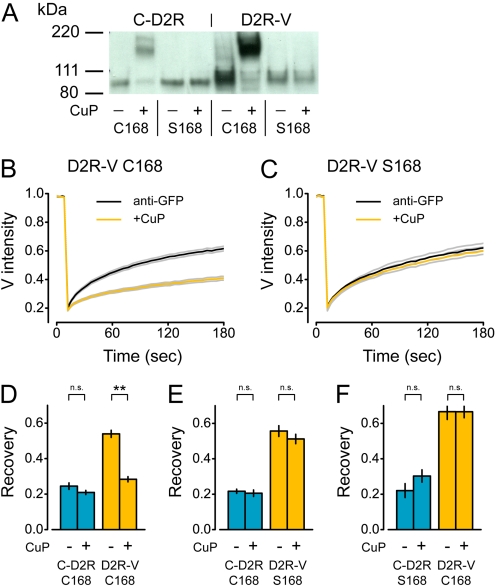
The mobility of D2R-V after selective cross-linking of C-D2R implied that the majority of these protomers did not reside in stable oligomers that also contained a C-D2R protomer. Thus, either C-D2R and D2R-V did not form hetero-oligomers at all, or else C-D2R-D2R-V hetero-oligomers were not stable. The TM4 interface between D2R protomers can be stabilized by oxidative cross-linking of either native or introduced cysteine residues (Guo et al., 2003, 2005). We reasoned, therefore, that oxidative cross-linking would stabilize this interaction if C-D2R and D2R-V protomers could associate at the TM4 interface. We verified that both receptors (expressed at a 1:1 ratio) migrated as monomers on nonreducing SDS-polyacrylamide gel electrophoresis and that treatment of intact cells with the oxidizing reagent CuP induced the formation of SDS-resistant dimers (Fig. 3A). CuP did not induce C-D2R or D2R-V dimers when cysteine 168 in TM4 was mutated to serine, as shown previously for D2R (Fig. 3A). We then measured C-D2R and D2R-V mobility in cells that were cross-linked with antibodies alone or with antibodies and CuP. Immobilization of C-D2R was not changed by oxidative cross-linking (Figs. 2C and 3D). However, immobile C-D2R produced a significantly greater slowing of D2R-V mobility in CuP-treated cells than in untreated controls (P < 0.001; Fig. 3D). Fluorescence recovery of D2R-V in CuP-treated cells was similar to that of C-D2R, suggesting that these protomers formed highly stable hetero-oligomers after oxidative cross-linking. In contrast, CuP did not enhance immobilization of D2R-V when either C-D2R or D2R-V incorporated a serine residue at position 168 (Ser168; Fig. 2, E and F). This result rules out a nonspecific effect of CuP on D2R-V mobility mediated by factors intrinsic to the cells and specifically indicates the involvement of TM4 in the formation of stable C-D2R-D2R-V hetero-oligomers after oxidative cross-linking. These results suggest that C-D2R and D2R-V could interact at the TM4 interface in these experiments but that this interaction was transient unless it was stabilized by oxidative cross-linking.
Fig. 3.
Oxidative cross-linking stabilizes C-D2R-D2RV hetero-oligomers via Cys168. A, immunoblot probed with anti-GFP primary antibody of lysates from cells transfected with equal amounts of C-D2R or D2R-V (both Cys168 or Ser168) plasmid DNA, with or without CuP treatment; the predicted molecular mass of both protomers is ∼80 kDa. B and C, recovery of D2R-V fluorescence after antibody cross-linking alone (anti-GFP) or antibody and oxidative cross-linking (+CuP); lines represent the mean ± S.E.M. of all cells expressing C-D2R Cys168 and D2R-V Cys168 (B) or C-D2R Cys168 and D2R-V Ser168 (C). D to F, recovery of normalized fluorescence for cells expressing (measured at time = 180 s) for C-D2R Cys168 and D2R-V Cys168 (D; control, n = 46; CuP, n = 48), C-D2R Cys168 and D2R-V Ser168 (E; control, n = 25; CuP, n = 31), and C-D2R Ser168 and D2R-V Cys168 (F; control, n = 11; CuP, n = 17); **, P < 0.001; n.s., P > 0.1; bars represent mean ± S.E.M.
Discussion
The quaternary structure of GPCRs has been studied extensively. Oligomerization has been shown to alter GPCR trafficking and function, and in some cases, oligomers possess unique pharmacological or signaling properties (Park and Palczewski, 2005). However, the structural elements involved in oligomerization and the stability of oligomers are largely unknown (Gurevich and Gurevich, 2008). The possibility that GPCR oligomers might be short-lived entities in the plasma membrane has not been explored, perhaps because few experimental methods can measure the stability of multiprotein complexes in the plasma membrane of live cells. Here, we used a method whereby selective immobilization of one component of such complexes provides a simple means to assess the association of other components. Using this method, we have shown that at least one GPCR oligomer interface is relatively unstable, to the point where immobile protomers (C-D2R) have no detectable specific effect on the mobility of other protomers (D2R-V). Thus, the lifetime of D2R oligomers joined at the TM4 interface must be short relative to the time scale of macroscopic diffusion. This does not imply that D2R protomers interact randomly at this interface, because biologically significant protein-protein interactions can be transient. Higher resolution methods are required to determine the absolute stability of D2R interactions at TM4. We also emphasize that our results do not rule out the possibility that C-D2R and D2R-V form more stable homo-oligomers at another interface (Guo et al., 2008). For example, we cannot rule out the possibility that mobile D2R-V exists partly or entirely as stable D2R-V-D2RV homooligomers. However, if this is the case, these oligomers do not interface at TM4. It is important to consider the possibility that GPCR oligomers need not be highly stable to function as oligomers. For example, transient oligomerization might facilitate the exchange of protomers during receptor activation (Guo et al., 2005). If class A GPCR oligomers in general are not highly stable, then the dynamics of oligomer association and dissociation may be subject to physiological regulation or pharmacological intervention. After this report was submitted, a similar study using this approach appeared and showed that some GPCR protomers (but not others) apparently interact transiently (Dorsch et al., 2009).
Acknowledgments
We thank Jonathan A. Javitch (Columbia University, New York, NY) for supplying D2R-V constructs and for guidance with oxidative cross-linking, and Pooja R. Sethi for expert technical assistance.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS045543]; the National Institutes of Health National Institute of General Medical Sciences [Grant GM078319]; and the National Science Foundation [Grant MCB-0620024].
A report of these findings was presented at the 2008 Annual Meeting of the Biophysical Society, 2008 Feb 2-6, Long Beach, CA.
ABBREVIATIONS: GPCR, G protein-coupled receptor; TM, transmembrane domain; FRAP, fluorescence recovery after photobleaching; CuP; Cu2+(phenanthroline)2; ECFP, enhanced cyan fluorescent protein; V, venus; C-D2R, cyan fluorescent protein-D2 receptor; D2R-V, D2-venus receptor; GFP, green fluorescent protein; PBS, phosphate-buffered saline; β2AR-V, β2-adrenoreceptor-venus fusion.
References
- Bouvier M (2001) Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci 2 274-286. [DOI] [PubMed] [Google Scholar]
- Dorsch S, Klotz KN, Engelhardt S, Lohse MJ, and Bünemann M (2009) Analysis of receptor oligomerization by FRAP microscopy. Nat Methods 6 225-230. [DOI] [PubMed] [Google Scholar]
- Frick M, Schmidt K, and Nichols BJ (2007) Modulation of lateral diffusion in the plasma membrane by protein density. Curr Biol 17 462-467. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, et al. (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452 93-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Shi L, Filizola M, Weinstein H, and Javitch JA (2005) Crosstalk in G protein-coupled receptors: changes at the transmembrane homodimer interface determine activation. Proc Natl Acad Sci U S A 102 17495-17500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Shi L, and Javitch JA (2003) The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J Biol Chem 278 4385-4388. [DOI] [PubMed] [Google Scholar]
- Guo W, Urizar E, Kralikova M, Mobarec JC, Shi L, Filizola M, and Javitch JA (2008) Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J 27 2293-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV and Gurevich EV (2008) How and why do GPCRs dimerize? Trends Pharmacol Sci 29 234-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota P, Reeves PJ, Rajbhandary UL, and Khorana HG (2006) Opsin is present as dimers in COS1 cells: identification of amino acids at the dimeric interface. Proc Natl Acad Sci U S A 103 3054-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Fotiadis D, Filipek S, Saperstein DA, Palczewski K, and Engel A (2003) Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J Biol Chem 278 21655-21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodowski DT, Salom D, Le Trong I, Teller DC, Ballesteros JA, Palczewski K, and Stenkamp RE (2007) Reprint of “Crystal packing analysis of Rhodopsin crystals” [J Struct Biol 158 (2007) 455-462]. J Struct Biol 159 253-260. [DOI] [PubMed] [Google Scholar]
- Milligan G (2007) G protein-coupled receptor dimerisation: molecular basis and relevance to function. Biochim Biophys Acta 1768 825-835. [DOI] [PubMed] [Google Scholar]
- Park PS and Palczewski K (2005) Diversifying the repertoire of G protein-coupled receptors through oligomerization. Proc Natl Acad Sci U S A 102 8793-8794. [DOI] [PMC free article] [PubMed] [Google Scholar]