Abstract
We have employed whole-cell and single-channel electrophysiology to examine the kinetic and pharmacological properties of GABA-A receptors consisting of γ2L-β2-α1 and β2-α1 subunit concatemeric constructs expressed in human embryonic kidney cells. Concatemeric receptors activated by GABA exhibited the same single-channel conductance, channel opening rate constant, and basic open- and closed-time properties as receptors containing free subunits. However, the whole-cell GABA dose-response and the single-channel effective opening rate curves were shifted to higher GABA concentrations, suggesting that the concatemeric receptors have a lower affinity to GABA. Pharmacological tests demonstrated that the concatemeric receptors were potentiated by pentobarbital, diazepam, and the neurosteroid (3α,5α)-3-hydroxypregnan-20-one (3α5αP), and were insensitive to Zn2+. Selective introduction of the α1Q241L mutation, previously shown to abolish α1β2γ2L channel potentiation by neurosteroids, into one of the two concatemeric constructs had a relatively small effect on receptor activation by GABA or macroscopic potentiation by the neurosteroid 3α5αP. Single-channel measurements showed that the kinetic mechanism of action of the steroid is unchanged when the mutation is introduced to the γ2L-β2-α1 concatemer. We infer that a single wild-type α subunit is capable of mediating the full set of kinetic effects in the presence of steroids. Introduction of the α1Q241W mutation, previously shown to mimic the effect of the steroid on α1β2γ2L channels, selectively into either concatemeric construct altered the mode of activity elicited by P4S, but the presence of mutations in both α subunits was required to affect open-time distributions. The data indicate that the α1Q241W mutation acts as a partial steroid modulator.
The GABA-A receptor is a pentameric protein consisting of five homologous subunits. Functional receptors containing one or two types of subunits can be formed under certain conditions, but the majority of mammalian GABA-A receptors probably contain three kinds of subunits. The most common type of GABA-A receptors in the mammalian brain is one consisting of two α subunits, two β subunits, and one γ subunit (McKernan and Whiting, 1996).
Studies of GABA-A receptors expressed in heterologous expression systems allow the manipulation of subunit composition as well as easy introduction of mutations to the receptor and are therefore widely used. However, in many instances, heterologous expression results in multiple subunit populations, needlessly complicating the interpretation of results. For example, cells nominally expressing αβγ receptors may contain a population of receptors that lack the γ subunit. These receptors are functional, and, as shown by heterologous expression of α and β subunits, possess distinctive biophysical and pharmacological characteristics. Likewise, the analysis of the data from conventional mutational studies is hindered when the target subunit is present in two (or more) copies per receptor because of multiple changes being introduced to receptor structure. This raises issues of additivity and specificity of the effects of structural changes. In the case of neurosteroids that have two potentiating sites per receptor, mutagenesis of the α subunit results in structural changes in both binding sites, preventing study of a single modified binding site to determine whether the individual binding sites are functionally equivalent.
Previous work has shown that functional GABA-A receptors can be formed from concatemeric or tandem subunits where the carboxyl terminus of one subunit is joined with a short linker region to the amino terminus of the other subunit. The simplest tandem subunit consists of linked α and β subunits, which, when coexpressed with a single monomeric subunit, produces receptors that respond to GABA (Im et al., 1995; Baumann et al., 2001; Boileau et al., 2005). Further work has shown that combination of a trimeric concatemer (e.g., γβα) with a dimer (βα) produces receptors that are activated by GABA and potentiated by the benzodiazepine diazepam (Baumann et al., 2002). The presence of the γ subunit in the concatemeric construct (in theory, at least) ensures that the subunit gets included in the receptor complex. The use of nonidentical concatemeric constructs, neither of which alone can produce functional receptors, allows an approach in which mutations are selectively introduced to one of the two α or β subunits of the receptor.
In this study, we have examined the kinetic and pharmacological properties of GABA-A receptors formed of γ2L-β2-α1 and β2-α1 concatemeric constructs by using single-channel and whole-cell macroscopic recordings. We show that the linkage of subunits does not affect the single-channel conductance, the channel opening rate constant, or the basic pharmacological properties, including the mechanism of action of the neurosteroid 3α5αP. However, compared with receptors containing free subunits, the concatemeric receptors have lower affinity to GABA. Introduction of a mutation that confers insensitivity to the potentiating action of the neurosteroid 3α5αP to a single α subunit did not affect the mechanism of action of the steroid, indicating that steroid interaction with a single α subunit is sufficient to produce the range of kinetic effects observed in the presence of steroids.
Materials and Methods
The experiments were conducted on human embryonic kidney (HEK) cell lines (293 and tsA 201) transiently expressing rat GABA-A receptors. The cells were grown in Dulbecco's modified Eagle's medium/Ham's F-12 medium with 10% (v/v) fetal bovine serum (Atlanta Biologicals, Atlanta, GA), penicillin (100 U/ml) and streptomycin (100 μg/ml) in a humidified atmosphere with 5% CO2 at 37°C, and passaged twice a week at 80 to 90% confluence.
Concatemeric subunits were created using the rat α1, β2, and γ2L subunits as described previously (Bracamontes and Steinbach, 2009). In brief, we first generated the β2-α1 concatemer (referred to as the βα construct), which had a linker consisting of 23 amino acid residues: Q3(Q2A3PA)2AQ5. The subunit contained a FLAG tag on the N terminus of the β2 subunit between residues 4 and 5 of the mature peptide. The subunits were joined together with the linker through site-directed mutagenesis by overlap extension (Ho et al., 1989) and subcloned into pcDNA3. The γ2L-β2-α1 concatemer (referred to as the γβα construct) was generated by joining the γ2L and β2 subunits using the 26-amino acid residue linker: Q5A3PAQ2(QA)2A2PA2Q5. The resulting PCR product was subcloned into the βα construct. Mutated concatemers containing Q241L and Q241W were made by DNA subcloning, digesting mutated single subunits, and ligating them into fragments of digested concatemers.
The GABA-A receptor free subunit or concatemeric construct cDNAs were subcloned into the pcDNA3 expression vector (Invitrogen, Carlsbad, CA) and transiently transfected into HEK 293 or tsA 201 cells using a calcium phosphate precipitation-based technique. A total of 3 μg of cDNA in the ratio of 1:1 (γβα/βα) or 1:1:1 (α/β/γ) was mixed with 12.5 μl of 2.5 M CaCl2, and deionized H2O to a final volume of 125 μl. The solution was added slowly, without mixing, to an equal volume of 2× BES-buffered solution. The combined mixture was incubated at room temperature for 10 min followed by mixing the contents and incubating for an additional 15 min. The precipitate was added to the cells in a 35-mm dish for overnight incubation at 37°C, followed by replacement of medium in the dish. The experiments were conducted during the next 2 days after changing the medium.
Cells expressing GABA-A receptors were identified using a bead-binding technique. The amino termini of the βα concatemeric construct and the free α1 subunit were tagged with the FLAG epitope (Ueno et al., 1996). Surface expression of the FLAG peptide was determined using a mouse monoclonal antibody to the FLAG epitope (M2; Sigma-Aldrich, St. Louis, MO), which had been adsorbed to immunobeads with a covalently attached goat anti-mouse IgG antibody (Invitrogen).
The experiments were carried out using whole-cell voltage-clamp and single-channel patch-clamp methods. The bath solution contained 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM d-glucose, and 10 mM HEPES, pH 7.4. In whole-cell recordings, the pipette solution contained 140 mM CsCl, 4 mM NaCl, 4 mM MgCl2, 0.5 mM CaCl2, 5 mM EGTA, and 10 mM HEPES, pH 7.4. In single-channel recordings, the pipette solution contained 120 mM NaCl, 5 mM KCl, 10 mM MgCl2, 0.1 mM CaCl2, 20 mM tetraethylammonium, 5 mM 4-aminopyridine, 10 mM d-glucose, and 10 mM HEPES, pH 7.4.
The agonist and modulator were applied through the bath using a fast perfusion stepper system (SF-77B; Warner Instruments, Hamden, CT) in whole-cell experiments, or added to the pipette solution in single-channel recordings. The recording and analysis of whole-cell currents were carried out as described previously (Li et al., 2006). In most experiments, the cells were clamped at -60 mV. The currents were recorded using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA), low-pass-filtered at 2 kHz, and digitized with a Digidata 1320 series interface (Molecular Devices) at 10 kHz. The analysis of whole-cell currents was carried out using the pClamp 9.0 software package.
The recording and analysis of single-channel currents have been described in detail previously (Akk et al., 2001, 2004). Most experiments were conducted in the cell-attached configuration. The pipette potential was held at +60 to +80 mV, which translates to a potential difference of approximately -120 to -100 mV across the patch membrane. Channel activity was recorded using an Axopatch 200B amplifier, low-pass-filtered at 10 kHz, and acquired with a Digidata 1320 series interface at 50 kHz using pClamp software.
All experiments were conducted at room temperature. The statistical analysis was done using the Systat 7.0 (Systat Software, Inc., San Jose, CA) software package. The statistical tests used and the significance levels are given in the text. Curve fitting was carried out using the program NFIT (The University of Texas Medical Branch at Galveston).
Results
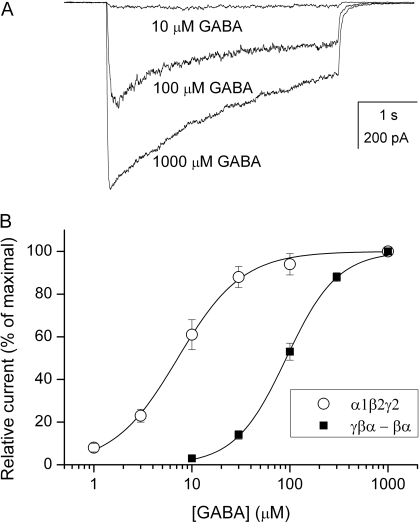
Macroscopic Activation Properties of Receptors Formed of Wild-Type γβα and βα Concatemeric Constructs. Cells transfected with cDNA for the wild-type γ2L-β2-α1 and β2-α1 constructs (γβα-βα receptors) responded to applications of GABA in a concentration-dependent manner. Sample macroscopic currents and the GABA dose-response relationship are shown in Fig. 1. Compared with receptors formed of free α1, β2, and γ2L subunits (α1β2γ2L receptors), the GABA dose-response curve for the concatemeric receptors was shifted by ∼10-fold to higher agonist concentrations. The EC50 for the concatemeric receptor was 92 ± 2 μM, and the Hill coefficient was 1.7 ± 0.1 (best-fit ± S.D. of the parameter estimate).
Fig. 1.
Receptors formed of wild-type concatemeric γβα-βα subunits are activated by GABA. A, sample responses from a HEK cell transfected with γβα and βα constructs exposed to 10, 100 or 1000 μM GABA. B, GABA dose-response properties for the γβα-βα concatemeric receptors and receptors consisting of free α1β2γ2L subunits. The data show mean ± S.E.M. from 15 (concatemeric receptors) or 6 cells (α1β2γ2L receptors). The curves were fitted to the Hill equation. For concatemeric receptors, the best-fit parameters are as follows: EC50 = 92 ± 2 μM, nH = 1.7 ± 0.1. For α1β2γ2L receptors, the best-fit parameters are as follows: EC50 = 7.2 ± 0.3 μM, nH = 1.3 ± 0.1.
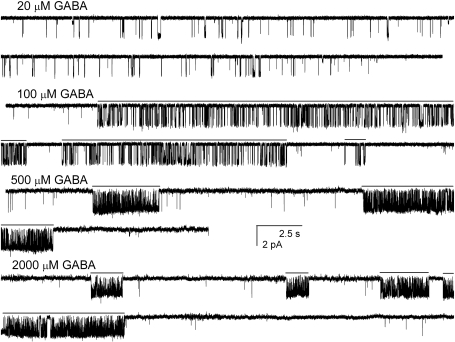
Single-Channel Properties of Wild-Type Concatemeric Receptors Activated by GABA. We examined the properties of single-channel currents arising from concatemeric receptors. Sample currents elicited by 20, 100, 500, or 2000 μM GABA are shown in Fig. 2. The increase in transmitter concentration led to specific changes in receptor kinetics. At 20 μM GABA, the activity consisted of single openings and isolated bursts, but at higher concentrations, channel activity was condensed into easily distinguishable clusters of activity.
Fig. 2.
Sample single-channel currents from cells expressing wild-type concatemeric γβα-βα GABA-A receptors. The cells were exposed to 20, 100, 500, or 2000 μM GABA. The increase in agonist concentration results in an increase in cluster open probability. At 20 μM GABA, only isolated openings and short bursts were observed. Channel openings are shown as downward deflections. The putative clusters at 100 to 2000 μM GABA are shown with lines above the current traces.
At all GABA concentrations studied (20-5000 μM), the channel open-time histograms were best-fitted to sums of three exponentials. The mean durations of the three components were 250 to 500 μs (OT1), 3 to 5 ms (OT2), and 10 to 13 ms (OT3). The OT2 component was the most prevalent, with slightly more than half of all open events falling to this class of openings. The open-time findings are summarized in Table 1. Compared with receptors containing free α1β2γ2L subunits, the concatemeric receptors demonstrate significant increases in the mean durations of OT2 (p < 0.01; t test) and OT3 (p < 0.01) (Fig. 3).
TABLE 1.
Open times for wild-type concatemeric γβα-βα receptors
Receptors were activated by 20 to 5000 μM GABA. The table gives the mean durations (OT1-3) and prevalence (fraction OT1-3) for the three open time components and the number of patches for each condition. The data are presented as mean ± S.D.
| [GABA] | OT1 | Fraction OT1 | OT2 | Fraction OT2 | OT3 | Fraction OT3 | n |
|---|---|---|---|---|---|---|---|
| ms | ms | ms | |||||
| 20 μM | 0.45 ± 0.09 | 0.30 ± 0.03 | 4.9 ± 0.5 | 0.57 ± 0.09 | 11.8 ± 1.6 | 0.14 ± 0.12 | 3 |
| 50 μM | 0.27 ± 0.13 | 0.24 ± 0.11 | 3.9 ± 0.6 | 0.58 ± 0.15 | 12.6 ± 4.0 | 0.18 ± 0.11 | 4 |
| 100 μM | 0.34 ± 0.20 | 0.20 ± 0.08 | 3.9 ± 1.0 | 0.62 ± 0.24 | 11.0 ± 2.2 | 0.17 ± 0.16 | 4 |
| 200 μM | 0.23 ± 0.16 | 0.16 ± 0.13 | 4.1 ± 1.4 | 0.53 ± 0.29 | 9.6 ± 3.7 | 0.30 ± 0.16 | 3 |
| 500 μM | 0.27 ± 0.10 | 0.10 ± 0.01 | 4.6 ± 0.8 | 0.64 ± 0.16 | 11.1 ± 4.2 | 0.26 ± 0.15 | 3 |
| 1000 μM | 0.40 ± 0.14 | 0.16 ± 0.06 | 3.8 ± 0.6 | 0.73 ± 0.10 | 10.2 ± 2.4 | 0.11 ± 0.10 | 5 |
| 2000 μM | 0.38 ± 0.07 | 0.17 ± 0.03 | 3.5 ± 0.4 | 0.67 ± 0.17 | 10.4 ± 2.4 | 0.16 ± 0.15 | 3 |
| 5000 μM | 0.42 ± 0.17 | 0.16 ± 0.13 | 2.8 ± 0.7 | 0.67 ± 0.18 | 6.5 ± 1.9 | 0.17 ± 0.11 | 5 |
Fig. 3.
Comparison of open-time properties for receptors containing concatemeric versus free subunits. A, mean open durations for the three open-time components at 20 to 5000 μM GABA. Each symbol corresponds to data from one patch. The lines correspond to mean ± S.D. for the open-time durations from α1β2γ2L receptors (Steinbach and Akk, 2001). The data indicate that the mean durations of OT2 and OT3 are increased in concatemeric receptors. B, the prevalence (fraction) of the three open-time components at 20-5000 μM GABA. Each symbol corresponds to data from one patch. The lines correspond to mean ± S.D. for the relative frequencies of OT1-3 from α1β2γ2L receptors (Steinbach and Akk, 2001).
The intracluster closed-time distributions were best-fitted to sums of three (at 50-200 μM GABA) or four (at 500-5000 μM GABA) exponentials. The summary of the closed-time analysis is given in Table 2. The briefest closed-time component, CT1, was observed at all agonist concentrations. It had a mean duration of 0.14 ± 0.02 ms (mean ± S.D.; averaged from data from 30 patches at 20-5000 μM GABA), and it formed 52 ± 7% of all intracluster closed events. A closed-time component with a mean duration of 1.9 ± 1.8 ms and a prevalence of 12 ± 8% was designated CT2. In the presence of high (500-5000 μM) concentrations of GABA, we observed a long-lived closed state (CT4). This closed state had a mean duration of 23 ± 10 ms and a relative frequency of 1 ± 1%. This closed interval is likely to originate from sojourns in a short-lived desensitized state (Steinbach and Akk, 2001). In addition, we observed a closed state the duration of which is dependent on GABA concentration (CTβ). The CTβ closed-time component arises from receptor occupation of mono- and unliganded closed states. As the agonist concentration is increased, the time spent in the unliganded and monoliganded closed states is reduced, and the mean duration of CTβ decreases.
TABLE 2.
Closed times for wild-type concatemeric γβα-βα receptors
Receptors were activated by 20-5000 μM GABA. The intracluster closed-time histograms were fitted to sums of three (50-200 μM GABA) or four exponentials. At 20 μM GABA, no clusters were evident. For analysis sections of data containing no overlapping currents were selected. The table gives the mean durations (CT1-4) and prevalence (fraction CT1-4) for the closed-time components. CTβ signifies the GABA concentration-dependent closed time component that arises from dwells in the di-, mono-, and unliganded closed states. The data are presented as mean ± S.D. The number of patches for each condition is given in Table 1.
| [GABA] | CT1 | Fraction CT1 | CT2 | Fraction CT2 | CTβ | Fraction CTβ | CT4 | Fraction CT4 |
|---|---|---|---|---|---|---|---|---|
| ms | ms | ms | ms | |||||
| 20 μM | 0.13 ± 0.02 | 0.57 ± 0.12 | 1.0 ± 0.2 | 0.12 ± 0.08 | 94 ± 52 | 0.31 ± 0.05 | ||
| 50 μM | 0.13 ± 0.01 | 0.55 ± 0.05 | 3.6 ± 4.3 | 0.14 ± 0.09 | 34 ± 8 | 0.31 ± 0.08 | ||
| 100 μM | 0.15 ± 0.02 | 0.53 ± 0.05 | 0.9 ± 0.2 | 0.14 ± 0.03 | 15 ± 8 | 0.33 ± 0.05 | ||
| 200 μM | 0.14 ± 0.01 | 0.48 ± 0.11 | 1.6 ± 0.6 | 0.22 ± 0.06 | 9.4 ± 1.5 | 0.30 ± 0.10 | ||
| 500 μM | 0.12 ± 0.00 | 0.52 ± 0.03 | 0.7 ± 0.1 | 0.20 ± 0.05 | 2.4 ± 0.6 | 0.27 ± 0.05 | 9.1 ± 1.1 | 0.02 ± 0.01 |
| 1000 μM | 0.14 ± 0.02 | 0.49 ± 0.03 | 2.9 ± 1.6 | 0.06 ± 0.04 | 0.6 ± 0.1 | 0.44 ± 0.03 | 28 ± 9 | 0.003 ± 0.001 |
| 2000 μM | 0.14 ± 0.03 | 0.53 ± 0.10 | 2.3 ± 0.4 | 0.09 ± 0.10 | 0.5 ± 0.1 | 0.37 ± 0.03 | 19 ± 8 | 0.01 ± 0.01 |
| 5000 μM | 0.16 ± 0.01 | 0.50 ± 0.10 | 1.8 ± 0.3 | 0.09 ± 0.10 | 0.5 ± 0.1 | 0.43 ± 0.09 | 27 ± 6 | 0.003 ± 0.001 |
The relationship between the inverse of CTβ, defined as the effective opening rate, and GABA concentration is shown in Fig. 4A. The curve was fitted with the Hill equation, yielding the maximal effective opening rate (i.e., the channel opening rate constant) of 2950 ± 686 s-1 and an EC50 of 1452 ± 488 μM. The data indicate that in concatemeric receptors, the effective opening rate curve is shifted to higher GABA concentrations. The rightward shift probably results from reduced affinity to agonist. In addition, the channel opening rate constant, estimated from fitting the effective opening rate curve, is somewhat higher for concatemeric receptors compared with α1β2γ2L receptors. We note, however, that the estimates for the channel opening rate constant were poorly defined as a result of large error limits.
Fig. 4.
Single-channel activation properties of the wild-type concatemeric γβα-βα receptor. A, relationship between the effective opening rate and GABA concentration. The effective opening rate is the inverse duration of the GABA concentration-dependent closed-time component. Each symbol corresponds to data from one patch. The curve was fitted to the Hill equation. The best-fit parameters are as follows: β (maximal effective opening rate) = 2950 ± 686 s-1, EC50 = 1452 ± 488 μM, nH = 1.4 ± 0.1. The dashed line is from previous data on α1β2γ2L receptors (Steinbach and Akk, 2001), with the following best-fit parameters: β = 1883 ± 686 s-1, EC50 = 359 ± 162 μM, nH = 1.7 ± 0.2. B, relationship between the intracluster open probability (Po) and GABA concentration. Each symbol corresponds to data from one patch. The curve was fitted to the Hill equation. The best-fit parameters are as follows: Pomax = 0.88 ± 0.03, EC50 = 83 ± 10 μM, nH = 1.4 ± 0.2. The dashed line is from previous data on α1β2γ2L receptors (Steinbach and Akk, 2001), with the following best-fit parameters: Pomax = 0.82 ± 0.04, EC50 = 70 ± 9 μM, nH = 1.2 ± 0.2.
To more fully assess the gating properties of concatemeric receptors, we compared the intracluster closed-time properties at 5 mM GABA for receptors formed of free versus concatemeric subunits. The mean duration of the closed-time component associated with the channel opening rate constant was 0.45 ± 0.05 ms (n = 5 patches) in receptors containing free subunits, and 0.47 ± 0.07 ms (n = 5 patches) in concatemeric receptors. The difference was not statistically significant (p > 0.75; t test). We conclude that the channel opening rate constant is unchanged in concatemeric receptors.
We have defined the rate of entry into the CTβ component as the channel closing rate constant. The averaged channel closing rate constant for the concatemeric receptors is 104 ± 35 s-1 (n = 30 patches at 20-5000 μM GABA). For comparison, the closing rate constant for receptors containing free subunits is 135 ± 65 s-1 (Steinbach and Akk, 2001).
Figure 4B shows the relationship between cluster open probability (Po) and GABA concentration. The curve was fitted to the Hill equation yielding a maximal Po of 0.88 ± 0.03, an EC50 of 70 ± 9 μM, and a Hill coefficient of 1.4 ± 0.2.
Single-Channel Conductance of Concatemeric Receptors. The single-channel conductance of γβα-βα concatemeric receptors was estimated in inside-out patches, where the membrane is exposed to similar chloride concentrations on both sides. The receptors were activated by 20 μM GABA, and the membrane voltage was held at -100 mV. In three patches, the conductance was 25.6 ± 1.1 pS. This is statistically indistinguishable from the single-channel conductance of receptors formed of free α1β2γ2L subunits, estimated in an identical experimental setting (27.3 ± 1.2 pS, n = 4 patches).
Modulation of Wild-Type Concatemeric Receptors by Diazepam, Pentobarbital, Zn2+, and 3α5αP. We next examined the basic pharmacological properties of wild-type concatemeric receptors by probing receptor modulation by the benzodiazepine diazepam, pentobarbital, Zn2+, and the neurosteroid 3α5αP.
Coapplication of 10 μM diazepam with 30 μM GABA (approximately EC20) potentiated the peak whole-cell current to 320 ± 125% of control (n = 5 cells). When 100 μM pentobarbital was coapplied with 30 μM GABA, the peak response was increased to 644 ± 331% of control (n = 5 cells). Exposure to 100 μM ZnCl2 was without effect (97 ± 3% of control; n = 4 cells) on the peak response elicited by 1 mM GABA. These findings are consistent with previously published data on α1β2γ2L receptors (Draguhn et al., 1990; Pistis et al., 1997; Walters et al., 2000; Li et al., 2006). We note that the extent of potentiation is sensitive to the fractional activation of the control response. Accordingly, the findings merely qualitatively confirm the concatemeric receptor modulation by diazepam and pentobarbital. The lack of effect of Zn2+ on the peak response elicited by a saturating concentration of GABA is indistinguishable from the findings on α1β2γ2L receptors (Li et al., 2006).
Coapplication of the neurosteroid 3α5αP with GABA resulted in potentiation of whole-cell responses. Sample currents and the potentiation dose-response curve are shown in Fig. 5, A and B. The effect of the steroid was concentration-dependent, with the EC50 at 63 ± 9 nM.
Fig. 5.
The neurosteroid 3α5αP potentiates wild-type concatemeric γβα-βα GABA-A receptors. A, sample whole-cell responses to 40 μM GABA in the absence and presence of 1 μM 3α5αP. B, potentiation dose-response properties. The data show mean ± S.E.M. from seven cells. The test applications lasted 4 s and were separated from flanking control (GABA alone) applications by 30 s washouts. The curve was fitted to the Hill equation with an offset (fixed at 100%). The best-fit parameters are as follows: maximal potentiation = 386 ± 11%, EC50 = 63 ± 9 nM, and nH = 1.5 ± 0.2. The dashed line applies to α1β2γ2L receptors (Akk et al., 2008), with best-fit parameters as follows: maximal potentiation = 351 ± 4%, EC50 = 41 ± 2 nM, and nH = 1.2 ± 0.1. C, sample single-channel clusters elicited by 50 μM GABA in the absence and presence of 1 μM 3α5αP. The clusters are shown with lines above the traces. The intracluster open- and closed-time histograms from the respective patches are shown next to data traces. For GABA, the open times were 0.17 ms (24%), 4.3 ms (58%) and 17 ms (18%), and the closed times were 0.13 ms (54%), 1.5 ms (10%) and 42 ms (36%). For GABA + 3α5αP, the open times were 0.24 ms (47%), 1.4 ms (14%) and 26 ms (39%), and the closed times were 0.19 ms (72%), 1.5 ms (22%) and 39 ms (6%).
We examined the kinetic mechanism of steroid potentiation of concatemeric receptors using single-channel patch clamp. The receptors were activated by 50 μM GABA in the absence and presence of 1 μM 3α5αP. Sample currents under both conditions are shown in Fig. 5C, and the summary of kinetic parameters is given in Table 3. The presence of steroid affected both open- and closed-time distributions. In accordance with data from receptors consisting of free subunits (Akk et al., 2005), concatemeric receptors exposed to 3α5αP demonstrated an increase in the mean duration of OT3, and a reduction in the prevalence of CTβ. However, in contrast to our previous findings from α1β2γ2L receptors, the increase in the prevalence of OT3 in γβα-βα receptors was not statistically significant.
TABLE 3.
Neurosteroid 3α5αP potentiates currents from the wild-type concatemeric γβα-βα receptor
The effect of 1 μM 3α5αP on currents elicited by 50 μM GABA. Mean durations and prevalence for the three open- and closed-time components are given. The data are presented as mean ± S.D. from four (GABA) or five patches (GABA + 3α5αP). The significance levels apply to comparison with the data in the absence of steroid.
| [GABA] | [3α5αP] | OT1 | Fraction OT1 | OT2 | Fraction OT2 | OT3 | Fraction OT3 | CT1 | Fraction CT1 | CT2 | Fraction CT2 | CTβ | Fraction CTβ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ms | ms | ms | ms | ms | ms | ||||||||
| 50 μM | 0.27 ± 0.13 | 0.24 ± 0.11 | 3.9 ± 0.6 | 0.58 ± 0.15 | 12.6 ± 4.0 | 0.18 ± 0.11 | 0.13 ± 0.01 | 0.55 ± 0.05 | 3.6 ± 4.3 | 0.14 ± 0.09 | 34 ± 8 | 0.31 ± 0.08 | |
| 50 μM | 1 μM | 0.27 ± 0.05† | 0.45 ± 0.10* | 2.1 ± 1.8† | 0.24 ± 0.06** | 22.7 ± 5.5* | 0.31 ± 0.09† | 0.16 ± 0.05† | 0.68 ± 0.07* | 1.2 ± 0.3† | 0.27 ± 0.05* | 23.4 ± 9.7† | 0.05 ± 0.03*** |
P < 0.05.
P < 0.01.
P < 0.001.
Not significant.
The α1Q241L Mutation in a Single α Subunit Does Not Disrupt Channel Potentiation by the Neurosteroid 3α5αP. Introduction of the Q241L mutation to the α subunit in the receptor containing free α1β2γ2L subunits affects channel activation by GABA. The macroscopic dose-response curve is shifted to higher agonist concentrations. In single-channel records, the major effect of the mutation is to reduce the mean open time (Akk et al., 2008). In addition, the α1Q241L mutation disrupts channel potentiation by the neurosteroid 3α5αP, possibly by preventing steroid binding to the steroid binding site (Hosie et al., 2006; Akk et al., 2008). In this study, we examined activation by GABA, and potentiation by 3α5αP, of concatemeric receptors containing the α1Q241L mutation in the γβα, the βα, or both concatemeric constructs.
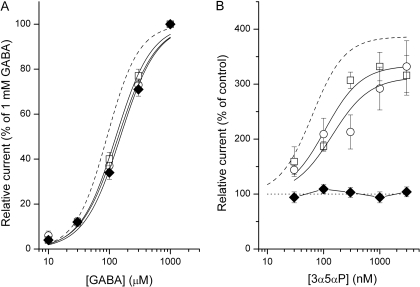
In whole-cell recordings, the GABA concentration-response relationships for receptors containing the mutation were shifted to slightly higher agonist concentrations (Fig. 6A). The midpoint of the GABA activation curve was 140 or 128 μM when the α1Q241L mutation was in the γβα or βα constructs, respectively. When both α subunits contained the mutation, the GABA EC50 was 152 μM. For comparison, the EC50 of the wild-type γβα-βα receptor is 92 μM GABA (Fig. 1B).
Fig. 6.
The effect of the α1Q241L mutation on the activation and modulation of concatemeric receptors. A, GABA dose-response properties for the γβαQ241L-βα (hollow circles), γβα-βαQ241L (hollow squares), and γβαQ241L-βαQ241L receptors (filled diamonds). The data show mean ± S.E.M. from 8-19 cells. The curves were fitted to the Hill equation. The best-fit parameters are: EC50 = 140 ± 13 μM, nH = 1.4 ± 0.2 (γβαQ241L-βα); EC50 = 128 ± 8 μM, nH = 1.5 ± 0.1 (γβα-βαQ241L); EC50 = 152 ± 14 μM, nH = 1.5 ± 0.2 (γβαQ241L-βαQ241L). The dashed curve applies to data from the wild-type γβα-βα receptor (reproduced from Fig. 1B). B, potentiation dose-response properties for the γβαQ241L-βα (hollow circles), γβα-βαQ241L (hollow squares), and γβαQ241L-βαQ241L receptors (filled diamonds). The data show mean ± S.E.M. from 4-6 cells. The test applications lasted 4 s and were separated from flanking control (30 μM GABA alone; ∼EC20) applications by 30 s washouts. The curve was fitted to the Hill equation with an offset (fixed at 100%). The best-fit parameters for the γβαQ241L-βα are: maximal potentiation = 313 ± 27%, EC50 = 142 ± 65 nM. The best-fit parameters for the γβα-βαQ241L are: maximal potentiation = 334 ± 28%, EC50 = 106 ± 41 nM. No fitting was attempted for the data from the γβαQ241L-βαQ241L receptor. The dashed curve applies to data from the wild-type γβα-βα receptor (reproduced from Fig. 5B).
The presence of a single α1Q241L mutation per receptor had a relatively small effect on channel potentiation by the neurosteroid 3α5αP. When the γβα concatemer contained the α1Q241L mutation, the midpoint of the steroid potentiation curve was at 140 nM. When the mutation was in the βα concatemer, the steroid EC50 was at 106 nM (Fig. 6B). For comparison, the steroid EC50 of the wild-type γβα-βα receptor is at 63 nM (Fig. 5B). The maximal potentiation was slightly reduced when one α1 subunit contains the Q241L mutation, but the effect is not statistically significant (Fig. 6B). A concatemeric receptor with the Q241L mutation in both α subunits was not potentiated by the neurosteroid 3α5αP (Fig. 6B).
Previous studies employing single-channel patch clamp have suggested that the open- and closed-time effects that collectively underlie channel potentiation by neuroactive steroids are mediated by steroid interactions with distinct sites (Akk et al., 2004; Li et al., 2007). This raises a possibility that the two α subunits mediate a different set of kinetic effects; e.g., steroid interactions with one α subunit may underlie the changes in open times, whereas steroid binding to the other α subunit leads to the closed-time effect. To test this hypothesis, we examined the kinetic mechanism of potentiation of the concatemeric mutant receptors by 3α5αP.
The single-channel currents from the γβαQ241L-βα receptor activated by 100 μM GABA demonstrated the presence of three intracluster open-time and three closed-time components. The kinetic parameters (mean durations and prevalence) of the open and closed times (Table 4) were indistinguishable from those observed for the wild-type concatemeric receptor (Tables 1 and 2). Coapplication of 1 μM 3α5αP with GABA enhanced the channel open probability by prolonging the mean open duration and reducing the mean intracluster closed-time duration (Fig. 7 and Table 4). The effect in the mean open duration was mediated by an increase in the mean duration (from 8.0 to 21.4 ms) and the prevalence (from 19 to 30%) of the longest-lived open-time component. The effect on the mean closed-time duration was predominantly mediated by a reduction in the prevalence (from 24 to 11%) of the longest intracluster closed-time component. We conclude that steroid interactions with a single wild-type α1 subunit (in the βα concatemer) can cause the full set of kinetic changes observed in the presence of 3α5αP.
TABLE 4.
The neurosteroid 3α5αP potentiates single-channel currents from the concatemeric mutant γβαQ241L-βα receptor
Effect of 1 μM 3α5αP on currents elicited by 100 μM GABA. Mean durations and prevalence for the three open- and closed-time components are given. The data are presented as mean ± S.D. from five (GABA) or four patches (GABA + 3α5αP). The significance levels apply to comparison with the data in the absence of steroid.
| [GABA] | [3α5αP] | OT1 | Fraction OT1 | OT2 | Fraction OT2 | OT3 | Fraction OT3 | CT1 | Fraction CT1 | CT2 | Fraction CT2 | CTβ | Fraction CTβ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ms | ms | ms | ms | ms | ms | ||||||||
| 100 μM | 0.27 ± 0.17 | 0.16 ± 0.03 | 3.1 ± 0.6 | 0.65 ± 0.10 | 8.0 ± 2.1 | 0.19 ± 0.10 | 0.16 ± 0.02 | 0.64 ± 0.05 | 1.6 ± 0.7 | 0.12 ± 0.03 | 13 ± 2 | 0.24 ± 0.05 | |
| 100 μM | 1 μM | 0.32 ± 0.08† | 0.36 ± 0.08*** | 2.5 ± 1.1† | 0.39 ± 0.08*** | 21.4 ± 5.9** | 0.39 ± 0.08 | 0.16 ± 0.04† | 0.64 ± 0.06† | 1.3 ± 0.3* | 0.25 ± 0.06** | 12 ± 5† | 0.11 ± 0.04** |
P < 0.05.
P < 0.01.
P < 0.001.
Not significant.
Fig. 7.
The potentiation of single-channel currents from the concatemeric γβαQ241L-βα receptor by the neurosteroid 3α5αP. A, a sample single-channel cluster from the γβαQ241L-βα receptor activated by 10 μM GABA, and the open- and closed-time histograms. The open times were 0.13 ms (15%), 2.5 ms (73%), and 7.1 ms (12%), and the closed times were 0.15 ms (57%), 1.0 ms (11%), and 13.0 ms (31%). B, a sample single-channel cluster from the γβαQ241L-βα receptor activated by 10 μM GABA in the presence of 1 μM 3α5αP, and the open- and closed-time histograms. The open times were 0.33 ms (44%), 1.6 ms (22%), and 16.9 ms (34%), and the closed times were 0.16 ms (72%), 1.0 ms (22%), and 15.6 ms (6%). Averaged data are shown in Table 5.
As a result of low expression levels, we were unable to record single-channel currents from the γβα-βαQ241L or γβαQ241L-βαQ241L receptors. Based on the macroscopic measurements (Fig. 6B) as well as previous work with receptors formed from free subunits (Akk et al., 2008), we believe that 3α5αP is ineffective at modulating the single-channel currents from the γβαQ241L-βαQ241L receptor. The similarities in the macroscopic potentiation curves for the γβαQ241L-βα and γβα-βαQ241L receptors suggest that the single-channel currents may be modified in a similar fashion. If so, this indicates that 3α5αP interactions with either α subunit is functionally equivalent.
The α1Q241W Mutation in a Single α Subunit Affects Closed but Not Open Times. The α1Q241W is a gain-of-function mutation that affects the α1β2γ2L channel activation by the low-efficacy agonist piperidine-4-sulfonic acid (P4S) by increasing the mean open-time and decreasing the mean closed-time durations (Akk et al., 2008). This leads to the emergence of high open probability single-channel clusters in contrast to the monotonous, low-frequency channel openings seen in the absence of the mutation.
We have investigated the effect of the Q241W mutation in a single α subunit on concatemeric channel activation by P4S. Exposure of wild-type concatemeric channels to 1 mM P4S resulted in low Po (∼0.05) channel activity with no clear-cut clusters present (Fig. 8A). Segments of channel activity containing no overlapping currents were selected for open- and closed-time analysis. The open-time analysis of channel activity revealed two components with the mean durations of 1.2 ms (OT1) and 2.4 ms (OT2). The OT2 component was less frequent, constituting 28% of all open events (Table 5). We observed two closed-time components within the records. The briefer closed event (CT1) had a mean duration of 0.42 ms and a prevalence of 10% (Table 6). The longer-lived closed event had a mean duration of 42 ms. We caution, however, that in records without clear-cut clusters, even in the absence of overlapping currents, the neighboring openings may originate from the activation of different receptor-complexes. So, little mechanistic meaning can be assigned to the durations of long closed events.
Fig. 8.
The presence of the α1Q241W mutation affects channel activation by P4S. A, sample single-channel currents from the γβα-βα receptor exposed to 1 mM P4S. No clusters were evident. A portion of the current trace is shown at higher resolution in the bottom trace. B, sample single-channel currents from the γβαQ241W-βα receptor exposed to 1 mM P4S. One cluster is shown. A portion of the cluster is shown at higher resolution in the bottom trace. C, sample single-channel currents from the γβα-βαQ241W receptor exposed to 1 mM P4S. One cluster is shown. A portion of the cluster is shown at higher resolution in the bottom trace. D, sample single-channel currents from the γβαQ241W-βαQ241W receptor exposed to 1 mM P4S. One cluster is shown. A portion of the cluster is shown at higher resolution in the bottom trace.
TABLE 5.
The effect of the αQ241W mutation on channel activation by P4S in the absence and presence of 3α5αP: open times
The open-time histograms were fitted to sums of two exponentials. The table gives the mean durations (OT1-2) and relative contributions (fraction OT1-2) for the open-time components. For data obtained in the absence of 3α5αP, the significance levels (ANOVA with Bonferroni correction) apply to comparison with the remaining three receptor types. For data obtained in the presence of P4S + 3α5αP, the significance levels apply to comparison with the same type of receptor activated by P4S in the absence of 3α5αP.
| Receptor | P4S | 3α5αP | OT1 | Fraction OT1 | OT2 | Fraction OT2 | n |
|---|---|---|---|---|---|---|---|
| ms | ms | ||||||
| γβα-βα | 1 mM | 1.2 ± 0.2†,†,† | 0.72 ± 0.16†,†,** | 2.4 ± 0.6†,†,† | 0.28 ± 0.16†,†,** | 4 | |
| γβα-βα | 1 mM | 1 μM | 0.6 ± 0.2** | 0.48 ± 0.03* | 12.5 ± 0.5*** | 0.52 ± 0.03* | 4 |
| γβαQ241W-βα | 1 mM | 1.0 ± 0.2†,†,† | 0.58 ± 0.20†,†,* | 3.2 ± 1.1†,†,† | 0.42 ± 0.20†,†,* | 8 | |
| γβαQ241W-βα | 1 mM | 1 μM | 0.8 ± 0.4† | 0.37 ± 0.06* | 6.9 ± 1.9*** | 0.63 ± 0.06* | 5 |
| γβα-βαQ241W | 1 mM | 1.4 ± 0.7†,†,† | 0.61 ± 0.28†,†,* | 4.0 ± 1.2†,†,† | 0.39 ± 0.28†,†,* | 6 | |
| γβα-βαQ241W | 1 mM | 1 μM | 1.1 ± 0.6† | 0.33 ± 0.08* | 11.8 ± 4.8** | 0.67 ± 0.08* | 7 |
| γβαQ241W-βαQ241W | 1 mM | 0.8 ± 0.2†,†,† | 0.27 ± 0.03**,*,* | 3.4 ± 0.8†,†,† | 0.73 ± 0.03**,*,* | 6 | |
| γβαQ241W-βαQ241W | 1 mM | 1 μM | 0.8 ± 0.4† | 0.27 ± 0.13† | 3.7 ± 1.4† | 0.73 ± 0.13† | 6 |
P < 0.05.
P < 0.01.
P < 0.001.
Not significant.
TABLE 6.
The effect of the αQ241W mutation on channel activation by P4S in the absence and presence of 3α5αP: closed times
The closed-time histograms were fitted to sums of two (γβα-βα with P4S) or three exponentials. The table gives the mean durations (CT1-3) and relative contributions (fraction CT1-3) for the closed-time components. For data obtained in the presence of P4S + 3α5αP, the significance levels apply to comparison with the same type of receptor activated by P4S (t test) and to the γ2Lβ2α1Q241W-β2α1Q241W receptor activated by P4S + 3α5αP (ANOVA with Dunnett's correction). Due to lack of clusters, statistical comparison for the effect of steroid was not carried out for the wild-type concatemeric receptor.
| Receptor | P4S | 3α5αP | CT1 | Fraction CT1 | CT2 | Fraction CT2 | CT3 | Fraction CT3 |
|---|---|---|---|---|---|---|---|---|
| ms | ms | ms | ||||||
| γβα-βα | 1 mM | 0.42 ± 0.34 | 0.10 ± 0.04 | 42 ± 33 | 0.90 ± 0.04 | |||
| γβα-βα | 1 mM | 1 μM | 0.21 ± 0.08‡,† | 0.40 ± 0.10‡,* | 1.2 ± 0.3‡,* | 0.39 ± 0.07‡,† | 9 ± 3‡,† | 0.21 ± 0.06‡,* |
| γβαQ241W-βα | 1 mM | 0.14 ± 0.04 | 0.17 ± 0.06 | 3.6 ± 3.1 | 0.33 ± 0.23 | 15 ± 6 | 0.50 ± 0.24 | |
| γβαQ241W-βα | 1 mM | 1 μM | 0.21 ± 0.09†,† | 0.39 ± 0.05***,* | 1.6 ± 0.7†,† | 0.35 ± 0.08†,† | 13 ± 8†,† | 0.26 ± 0.07†,† |
| γβα-βαQ241W | 1 mM | 0.27 ± 0.12 | 0.19 ± 0.10 | 2.9 ± 2.4 | 0.20 ± 0.19 | 29 ± 12 | 0.61 ± 0.19 | |
| γβα-βαQ241W | 1 mM | 1 μM | 0.18 ± 0.03†,† | 0.35 ± 0.04**,† | 1.4 ± 0.4†,* | 0.29 ± 0.07†,† | 18 ± 7†,* | 0.36 ± 0.07**,† |
| γβαQ241W-βαQ241W | 1 mM | 0.23 ± 0.10 | 0.24 ± 0.05 | 2.5 ± 0.3 | 0.42 ± 0.11 | 9 ± 3 | 0.34 ± 0.15 | |
| γβαQ241W-βαQ241W | 1 mM | 1 μM | 0.27 ± 0.03†,‡ | 0.28 ± 0.06†,‡ | 2.6 ± 1.0†,‡ | 0.37 ± 0.07†,‡ | 8 ± 3†,‡ | 0.35 ± 0.09†,‡ |
P < 0.05.
P < 0.01.
P < 0.001.
Not significant.
Not applicable.
In receptors in which the α1Q241W mutation was introduced to both the γβα and βα constructs, the prevalence of OT2 was increased to 73% (Table 5). The mean durations of the open-time components were not significantly altered. It is noteworthy that the presence of the mutation in both α subunits did not result in the emergence of the third, long-lived open state (Akk et al., 2008). In addition, the mutations altered the modal behavior of channel activity. Concatemeric receptors containing the α1Q241W mutation in both α subunits demonstrated clear-cut clusters when activated by 1 mM P4S (Fig. 8D). The cluster Po was 0.40 ± 0.08 (n = 6 patches).
The presence of a single Gln-to-Trp mutation per receptor was without effect on the open-time distributions (Table 5). In particular, a single mutation was incapable of producing a statistically significant increase in the prevalence of OT2. The data indicate that the major effect of the α1Q241W mutation on channel open distributions is an increase in the prevalence of OT2, but the mutation must be present in both α subunits to produce the effect.
Both receptor types containing a single α1Q241W mutation exhibited single-channel clusters when exposed to 1 mM P4S (Fig. 8, B and C). Similar to the γβαQ241W-βαQ241W receptor, the intracluster closed-time histograms contained three components. The mean durations and prevalence of the three closed states are given in Table 6.
The comparison of closed-time distributions for the four types of receptors is complicated by the absence of clusters in the data from wild-type receptors. Among the receptors containing the Q241W mutation, the major difference in the intracluster closed-time distributions lies in the duration of the longest-lived closed-time component (CT3). The mean duration of CT3 was 9 ms for receptors containing the Q241W mutation in both α subunits, and 15 or 29 ms for receptors containing the mutation in the γβα or βα constructs, respectively (Table 6).
Modulation of Concatemeric α1Q241W Mutant Receptors by 3α5αP. The α1β2γ2L receptor containing the Q241W mutation is not potentiated by the neurosteroid 3α5αP (Hosie et al., 2006). In an earlier study, we proposed that the tryptophan side chain acts by mimicking the presence of steroid in the steroid binding pockets (Akk et al., 2008). Here, we examined how the Gln-to-Trp mutation modifies concatemeric channel potentiation by 3α5αP.
Single-channel currents elicited by 1 mM P4S from the γβαQ241W-βαQ241W receptor were not modulated by 3α5αP (Tables 5 and 6). In contrast, exposure of the wild-type concatemeric receptor to 1 μM 3α5αP had a strong effect on the open-time distributions. The mean duration of OT2 was prolonged from 2.4 to 12.5 ms, and the prevalence of OT2 was increased from 28 to 52% (Table 5).
Receptors with the α1Q241W mutation in one of the two concatemeric constructs showed positive modulation by 3α5αP. In receptors containing the mutated γβα construct, the average open duration was increased from 1.9 to 4.8 ms in the presence of steroid. The change was due to an increase in the mean duration and prevalence of OT2 (Table 5). When the βα tandem contained the α1Q241W mutation, the average open duration was increased from 2.5 to 8.2 ms. Likewise, the effect was due to a prolonged and more prevalent OT2 component (Table 5).
It is noteworthy that the mean duration of OT2 in wild-type concatemeric receptors or receptors containing the Q241W mutation in only one of the two α subunits, exposed to P4S + 3α5αP, is prolonged compared with that in the γβαQ241W-βαQ241W receptor activated by P4S. The mean duration of OT2 was 12.5, 6.9, or 11.8 ms for γβα-βα, γβαQ241W-βα, or γβα-βαQ241W receptors, respectively, in the presence of P4S + 3α5αP (Table 5). In contrast, the mean duration of OT2 was 3.4 ms for the γβαQ241W-βαQ241W receptor activated by P4S. In the model in which the tryptophan mutation in the α241 position acts by mimicking the presence of a steroid molecule, this finding suggests that the presence of the steroid molecule in the binding pocket is more efficacious, in terms of prolonging OT2, than the presence of the tryptophan residue.
The application of 3α5αP was largely without effect on intracluster closed times in receptors containing a single α1Q241W mutation. In γβαQ241W-βα receptors, the application of 3α5αP increased the prevalence of CT1, whereas in γβα-βαQ241W receptors the presence of steroid resulted in an increase in the prevalence of CT1 and a decrease in the prevalence of CT3.
Discussion
Concatemers of subunits can generate multimeric receptors of defined subunit composition and order. They also provide the opportunity to selectively mutate one copy of a subunit that is present in multiple copies in the assembled receptor. Concatemers of GABA-A receptor subunits have been successfully used to exploit both these advantages (e.g., Baumann et al., 2003; Boulineau et al., 2005; Baur et al., 2006). However, previous studies have almost exclusively used Xenopus laevis oocytes as expression systems (but see Boileau et al., 2005; Baur et al., 2006) and have only used macroscopic measures of receptor function. It would be extremely valuable to study properties of concatemers expressed in a somatic expression system whose properties may be more similar to other cells in the organism and that is more amenable to precise experimental analysis. The present results demonstrate that concatemers of wild-type GABA-A receptor subunits produce functional receptors when expressed in the human embryonic kidney cell line. More importantly, the receptors have functional properties essentially identical to those of receptors formed from the same subunits expressed as free subunits. Pharmacological properties of the receptors formed from concatemeric constructs are indistinguishable from those of receptors containing free subunits. Finally, we demonstrate that concatemeric constructs can be used to determine the effects of selective mutation of one copy of a subunit present in two copies in the assembled receptor, when expressed in HEK cells. Overall, these studies significantly extend the use of concatemeric subunits in studies of GABA-A receptors and validate the interpretation of results obtained in studies of receptors expressed in oocytes. To the best of our knowledge, this is the first study presenting single-channel data from concatemeric GABA-A receptors.
In the present study, we have investigated the properties of receptors consisting of concatemeric γ2L-β2-α1 and β2-α1 subunits (γβα-βα receptors). Our data indicate that the subunit linkage does not affect the single-channel conductance, the channel opening rate constant or the mechanism of action of the neurosteroid 3α5αP.
In single-channel currents, the major effect of linkage of subunits is the rightward shift in the effective opening rate curve. The midpoint of the effective opening rate curve was at ∼1.5 mM in receptors formed of concatemeric subunits, but only 0.4 mM when free subunits were used. This suggests that the concatemeric receptors have lower affinity to GABA than receptors formed of free subunits. In whole-cell recordings, the GABA dose-response curve for concatemeric receptors is shifted to higher agonist concentrations. It is noteworthy that we observed no changes in the intracluster Po properties, because the prolongation in the mean closed-time duration (due to the shift in the effective opening rate) is offset by an increase in the mean open-time duration.
Previous studies in X. laevis oocytes (Baumann et al., 2001) have observed a higher GABA EC50 for macroscopic responses from cells expressing concatemeric receptors. It has been suggested that eggs injected with cRNA for free α1, β2, and γ2 subunits express a population of high-affinity αβ receptors whose presence affects the overall GABA dose-response properties. The use of concatemeric constructs presumably prevents that by forcing the incorporation of the γ subunit in receptor-complexes, thereby revealing the true dose-response relationship for the γ-containing receptor.
We agree that the use of free subunits can result in the expression of αβ receptors (e.g., Li et al., 2006), but we believe that subunit linkage per se is the major contributor to the observed shift in the agonist dose-response relationship. First, the rightward shift in the GABA dose-response curve is also observed in the case of the tandem βα construct coexpressed with the β subunit (Baumann et al., 2001). Second, the degree of shift can be dependent on the linker length (Baumann et al., 2001), as well as the location of the linker in identically arranged receptors (Baumann et al., 2002). We also note that the α1β2 receptors have lower conductance and a lower maximal open probability than α1β2γ2L receptors, limiting their relative contribution to macroscopic responses from cells expressing mixed receptor populations. However, the strongest argument against a contribution of α1β2 receptors comes from the present single-channel data. The single-channel conductance indicates that α, β, and γ subunits are present, whereas the effective opening rate data (Fig. 4A) demonstrate that a change in affinity has occurred.
The concatemeric wild-type receptors are potentiated by the benzodiazepine diazepam, pentobarbital, and the neurosteroid 3α5αP, whereas coapplication of ZnCl2 with GABA was without effect on the macroscopic peak current. These findings are in agreement with previously published data on α1β2γ2L receptors. We caution, however, that the linkage of subunits may have minor, yet measurable effects on the kinetic mechanisms of action of these drugs. For example, whereas the application of 3α5αPon α1β2γ2L receptors leads to a decrease in the prevalence of CTβ and an increase in the mean duration and prevalence of OT3 (Akk et al., 2005), only the first two effects were consistently reproduced in concatemeric receptors (Table 3). The change in the prevalence of OT3 was not statistically significant.
Conventional mutagenesis studies of ligand-gated ion channels have shortcomings when the target subunit is present in multiple copies per receptor. Mutagenesis of the α (or β) subunit in the α1β2γ2 GABA-A receptor inevitably results in the introduction of two mutations per receptor. This complicates the interpretation of studies aimed at examining the effects of modifications to a single locus. The use of concatemeric receptors allows an approach where the mutations are selectively introduced to one of the two α (or β) subunits of the receptor. The present study suggests that concatemeric GABA-A receptors expressed in HEK cells are an acceptable model system for these purposes.
We used the concatemeric GABA-A receptor subunit constructs to examine the effect of α1Q241L and α1Q241W mutations on channel activation and modulation by the neurosteroid 3α5αP. Our previous work with receptors consisting of free subunits had indicated that the α1Q241L mutation prevents the interaction of 3α5αP with its binding site (Akk et al., 2008). In this study, we probed the effect of a single α1Q241L mutation per receptor on neurosteroid-mediated potentiation. The overall goal was to test the hypothesis that the two α subunits (i.e., two individual steroid binding sites) mediate different kinetic actions (e.g., only the open-time effect versus only the closed-time effect) of the steroid. When the α1Q241L mutation was included solely in the γβα concatemer, the application of 3α5αP resulted in the prolongation and increase in the prevalence of the longest open-time component and a decrease in the prevalence of the longest intracluster closed-time component. The effects were analogous to those found in the α1β2γ2L receptor (Akk et al., 2005) or the wild-type γβα-βα receptor (Table 3). We infer from the data that a single wild-type α subunit (in the βα construct) can mediate the full set of kinetic effects observed in the presence of neurosteroids. Low expression levels prevented an analogous study on the γβα-βαQ241L receptor. However, the similarities in the macroscopic potentiation curves (Fig. 6B) strongly suggest that the wild-type γβα construct is similarly capable of mediating the full range of kinetic effects of neurosteroids. In sum, the data indicate that steroid interaction with a single α subunit produces multiple kinetic changes.
When expressed in free subunits, the α1Q241W mutation prolongs the mean open-time duration and reduces the mean closed-time duration. The same kinetic effects are observed in the presence of neuroactive steroids, prompting us to propose that the presence of the bulky tryptophan residue in the α241 position mimics the presence of steroid (Akk et al., 2008).
The presence of the α1Q241W mutation in both concatemeric constructs resulted in an increase in the prevalence of long openings. In contrast, having the mutation in just one α subunit was without effect. When 3α5αP was coapplied with P4S, the prevalence of OT2 was increased, indicating that the unmutated site can interact with the steroid molecule and that this interaction produces an effect not unlike the presence of the tryptophan residue in the α241 position. It is noteworthy that the mean duration of OT2 in receptors containing a single Q241W mutation (as well as in wild-type concatemeric receptors), exposed to P4S + 3α5αP, was significantly greater than in γβαQ241W-βαQ241W receptors activated by P4S. We interpret this finding to mean that the presence of a steroid molecule in the binding pocket is more efficacious, in terms of prolonging OT2, than the presence of the tryptophan residue in the α241 position.
No changes in the kinetics of the γβαQ241W-βαQ241W receptor were observed when 3α5αP was coapplied with the agonist. The mean duration of OT2 under these conditions was significantly below the value reached when the wild-type γβα-βα receptor was exposed to P4S + 3α5αP. We interpret this finding as the Gln-to-Trp mutation being able to exclude the steroid from its binding site to prevent any additional effect, in addition to partially mimicking the effect of the steroid.
Overall, our results are in agreement with a recent study from our laboratory describing the effects of the α1Q241L and α1Q241W mutations on macroscopic currents from concatemeric receptors expressed in X. laevis oocytes (Bracamontes and Steinbach, 2009). The major conclusion of that study was that a single wild-type α subunit can support the actions of a steroid. The present study extends these findings by providing a full kinetic and pharmacologic description of the wild-type concatemeric receptor activity. We also demonstrate that neurosteroid interactions with a single α subunit produce multiple kinetic effects and show that the Q241W mutations in the individual α subunits act in concert to produce the effects seen when the mutant α subunit is expressed in the context of free subunits.
In sum, the use of concatemeric receptors is a practical approach to forcing receptor assembly, allowing the combination of different isoforms or mutated subunits within the receptor complex. Our work indicates that the wild-type concatemeric γ2Lβ2α1-β2α1 receptors expressed in human embryonic kidney cells behave qualitatively similarly to receptors consisting of free α1, β2, γ2L subunits. The major conclusion from the work with the mutant concatemers is that steroid interaction with a single wild-type α subunit is capable of producing the full set of kinetic effects seen in the wild-type receptor.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM47969] (to J.H.S.).
ABBREVIATIONS: HEK, human embryonic kidney; BES, N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid; 3α5αP, (3α,5α)-3-hydroxypregnan-20-one; P4S, piperidine-4-sulfonic acid.
References
- Akk G, Bracamontes J, and Steinbach JH (2001) Pregnenolone sulfate block of GABAA receptors: mechanism and involvement of a residue in the M2 region of the α subunit. J Physiol 532 673-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes JR, Covey DF, Evers A, Dao T, and Steinbach JH (2004) Neuroactive steroids have multiple actions to potentiate GABAA receptors. J Physiol 558 59-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, and Mennerick S (2005) Neurosteroid access to the GABAA receptor. J Neurosci 25 11605-11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Li P, Bracamontes J, Reichert DE, Covey DF, and Steinbach JH (2008) Mutations of the GABA-A receptor α1 subunit M1 domain reveal unexpected complexity for modulation by neuroactive steroids. Mol Pharmacol 74 614-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann SW, Baur R, and Sigel E (2001) Subunit arrangement of γ-aminobutyric acid type A receptors. J Biol Chem 276 36275-36280. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, and Sigel E (2002) Forced subunit assembly in α1β2γ2 GABAA receptors. J Biol Chem 277 46020-46025. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, and Sigel E (2003) Individual properties of the two functional agonist sites in the GABAA receptors. J Neurosci 23 11158-11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur R, Minier F, and Sigel E (2006) A GABAA receptor of defined subunit composition and positioning: concatenation of five subunits. FEBS Lett 580 1616-1620. [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Pearce RA, and Czajkowski C (2005) Tandem subunits effectively constrain GABAA receptor stoichiometry and recapitulate receptor kinetics but are insensitive to GABAA receptor-associated protein. J Neurosci 25 11219-11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulineau N, Baur R, Minier F, and Sigel E (2005) Consequence of the rpesence of two different β subunit isoforms in a GABAA receptor. J Neurochem 95 1724-1731. [DOI] [PubMed] [Google Scholar]
- Bracamontes JR and Steinbach JH (2009) Steroid interaction with a single potentiating site is sufficient to modulate GABA-A receptor function. Mol Pharmacol 75 972-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, and Sakmann B (1990) Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+. Neuron 5 781-788. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, and Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77 51-59. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, and Smart TG (2006) Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 444 486-489. [DOI] [PubMed] [Google Scholar]
- Im WB, Pregenzer JF, Binder JA, Dillon GH, and Alberts GL (1995) Chloride channel expression with the tandem construct of α6-β2 GABAA receptor subunit requires a monomeric subunit of α6 or γ2. J Biol Chem 270 26063-26066. [DOI] [PubMed] [Google Scholar]
- Li P, Bracamontes J, Katona BW, Covey DF, Steinbach JH, and Akk G (2007) Natural and enantiomeric etiocholanone interact with distinct sites on the rat α1β2γ2L GABAA receptor. Mol Pharmacol 71 1582-1590. [DOI] [PubMed] [Google Scholar]
- Li P, Covey DF, Steinbach JH, and Akk G (2006) Dual potentiating and inhibitory actions of a benz[e]indene neurosteroid analog on recombinant α1β2γ2 GABAA receptors. Mol Pharmacol 69 2015-2026. [DOI] [PubMed] [Google Scholar]
- McKernan RM and Whiting PJ (1996) Which GABAA-receptor subtypes really occur in the brain. Trends Neurosci 19 139-143. [DOI] [PubMed] [Google Scholar]
- Pistis M, Belelli D, Peters JA, and Lambert JJ (1997) The interaction of general anaesthetics with recombinant GABAA and glycine receptors expressed in Xenopus laevis oocytes: a comparative study. Br J Pharmacol 122 1707-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach JH and Akk G (2001) Modulation of GABAA receptor gating by pentobarbital. J Physiol 537 715-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Zorumski C, Bracamontes J, and Steinbach JH (1996) Endogenous subunits can cause ambiguities in the pharmacology of exogenous γ-aminobutyric acidA receptors expressed in human embryonic kidney 293 cells. Mol Pharmacol 50 931-938. [PubMed] [Google Scholar]
- Walters RJ, Hadley SH, Morris KD, and Amin J (2000) Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms. Nature Neurosci 3 1274-1281. [DOI] [PubMed] [Google Scholar]