Abstract
We have studied the molecular mechanism by which the nuclear xenobiotic receptors pregnane X receptor (PXR) and constitutive active/androstane receptor (CAR) regulate transcription of the vitamin D3 24-hydroxylase (CYP24A1) gene. In the absence of vitamin D3, PXR activates the CYP24A1 gene by directly binding to and transactivating vitamin D-response elements (VDREs) within its promoter. Vitamin D3 activates the CYP24A1 promoter by dissociating the corepressor silencing mediator for retinoid and thyroid hormone receptors (SMRT) from the vitamin D receptor (VDR) on those VDREs. PXR strongly represses vitamin D3 activation of the CYP24A1 gene, in which PXR indirectly binds to and prevents vitamin D3-dependent dissociation of SMRT from the CYP24A1 promoter. The degree of the PXR-mediated locking of SMRT depends on the relative concentration of vitamin D3 to the human PXR activator rifampicin; SMRT increased its dissociation as this ratio increased. CAR is also found to prevent dissociation of SMRT from the CYP24A1 promoter. Thus, our present study defines the novel molecular mechanism by which PXR and CAR mediate drug interactions with vitamin D3 to regulate the CYP24A1 gene. Pxr(+/+) and Pxr(-/-) mice were continuously treated with mouse PXR activator PCN to evaluate the hypothesis that induction of the Cyp24a1 gene is responsible for the loss of bone mineral density often observed in patients treated continuously with PXR-activating drugs. PCN-dependent loss of mineral density is observed in the metaphyseal bones of only the Pxr(+/+) mice. This loss, however, does not correlate with the expression levels of the Cyp24a1 gene in these mice.
Vitamin D3 is essential for the maintenance of calcium homeostasis and for the development and maintenance of bones by activating the vitamin D receptor (VDR) (Jones et al., 1998; Christakos et al., 2003). Vitamin D3 24-hydroxylase CYP24A1, the gene that is activated by vitamin D3 via VDR, converts the active vitamin D3 into an inactive metabolite, thus becomes the feedback regulatory factor regulating calcium homeostasis (Sutton and MacDonald, 2003). Long-term treatments with antimicrobial (e.g., rifampicin) and antiepileptic (e.g., phenobarbital and phenytoin) drugs sometime decrease serum vitamin D levels and lead to the development of metabolic bone diseases in patients; the side effects of which are similar to vitamin D deficiency (Shah et al., 1981; Brodie et al., 1982; Pack and Morrell, 2004). Given the fact that these drugs activate the nuclear xenobiotic receptors pregnane X receptor (PXR) and/or constitutive active/androstane receptor (CAR), these receptors have been implicated as being the cause of the side effects of these drugs (Pascussi et al., 2005; Zhou et al., 2006; Moreau et al., 2007). Pascussi and his group showed that PXR directly binds to and activates the CYP24A1 promoter, thus claiming the CYP24A1 gene as the target gene responsible for this drug side effect. Another study by Zhou et al. (2006), however, rejected this claim by reporting that PXR neither binds nor activates the CYP24A1 gene and even showed that PXR represses the CYP24A1 gene. The role of PXR and CAR in regulation of the CYP24A1 gene remains controversial and, more critically, the molecular mechanism by which the nuclear xenobiotic receptors might regulate the CYP24A1 gene remains virtually unknown at the present time.
Here, we investigated the molecular mechanisms by which PXR and CAR regulate the CYP24A1 gene in different manners, for which the human CYP24A1 promoter was analyzed by using cell-based transfection, gel shift, and ChIP assays. PXR activated and repressed the CYP24A1 promoter in the absence and presence of the vitamin D3-activated VDR, respectively. PXR mediated the drug action of rifampicin to activate CYP24A1 promoter by directly binding to the VDRE region in the absence of vitamin D3. On the other hand, PXR indirectly bound to the VDRE region and locked the corepressor silencing mediator for retinoid and thyroid hormone receptors (SMRT) onto VDR to repress the CYP24A1 promoter in the presence of vitamin D3. We present experimental results that highlight the unique mechanism of drug interactions with vitamin D3 via nuclear receptors and a coregulator.
Materials and Methods
Materials. DMSO, rifampicin, and PK11195 were purchased from Sigma-Aldrich (St. Louis, MO); 1α,25-dihydroxyvitamin D3 was from Calbiochem (San Diego, CA), CITCO was from BIOMOL Research Laboratories (Plymouth Meeting, PA); restriction endonucleases and DNA-modifying enzymes were from New England Biolabs (Ipswich, MA); and [32P]dATP was from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK).
Animals. Six Pxr(+/+) and Pxr(-/-) male mice (originally provided by Dr. Jeff Staudinger, University of Kansas) were implanted with either slow-release capsules of PCN (total, 36 mg) or of control matrix (Innovative Research of America, Sarasota, FL) and were maintained on a 12-h light/dark cycle for 6 weeks: the released dose of PCN was 20 mg per 30 g of body weight per day.
Plasmids. pCMX/hRXR, pCR3/hPXR, pCR3/hCAR, pcDNA3.1/mVDR, pcDNA3.1/mouse SRC1, and pcDNA3.1/human GRIP were constructed previously. pCMX/hSMRT and pCMX/hNcoR were kindly provided by Dr. Ronald Evans (The Salk Institute for Biological Studies, La Jolla, CA); pcDNA3.1/mouse CBP was provided by Dr. Yasutomi Kamei (Tokyo Medical and Dental University, Tokyo, Japan); pCR3/hPGC1α was provided by Dr. Anastasia Kralli (Scripps Research Institute, La Jolla, CA); and pcDNA3.1/VDR-interacting protein was provided by Dr. Joyce Goldstein (National Institute of Environmental Health Sciences, Research Triangle Park, NC). The prefixes h and m denote the origins of human and mouse, respectively. To construct the reporter plasmids pGL3/CYP24A1-5 kb (-5003/+40) and pGL3/CYP24A1-3 kb (-2870/+40), amplified sequences from human genomic DNA was cloned into the KpnI and XhoI sites of pGL3-basic from Promega (Madison, WI). Primers used for amplifications were the following: 5′-CGGGGTACCCTCATCTCCTTTGCAATAAAGCAGCTC-3′ and 5′-CCGCTCGAGAGGAGGGTAGATGAGATGCTGCT-3′ for the -5-kb promoter and 5′-CGGGGTACCTGTCTCAGTAATGATGGCAGGCATT-3′ and 5′-CCGCTCGAGAGGAGGGTAGATGAGATGCTGCT-3′ for the -3-kb promoter. Underlined segments indicate either KpnI or XhoI sites. In the context of pGL3/CYP24A1-3 kb, VDRE1 and VDRE2 were singularly or simultaneously deleted by PCR (Pfu polymerase; Promega) using the following primers: 5′-CACCCCAGGCCCGGACGCTGACTCCATCCT-3′ and 5′-AGGATGGAGTCAGCGTCCGGGCCTGGGGTG-3′ for VDRE1 deletion; 5′-GAGGCGCGTTCGAAGCCGGGCTTCGCATGA-3′ and 5′-TCATGCGAAGCCCGGCTTCGAACGCGCCTC-3′ for VDRE2 deletion; and 5′-GAGGCGCGTTCGAAGCGCTGACTCCATCCT-3′ and 5′-AGGATGGAGTCAGCGCTTCGAACGCGCCTC-3′ for double deletion.
Cells and Transfection Assays. Cells stably expressing hPXR were generated by transfection of HepG2 cells and with pCR3/hPXR, and the expression was confirmed using Western blot analysis, for which more details are described elsewhere. HepG2 and hPXR stable cells were cultured in minimum essential medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin in an atmosphere of 5% CO2 at 37°C. Cells were plated on a 24-well plate at a density of 4.0 × 105 cells/well 24 h before plasmid transfection using FuGENE 6 (Roche, Indianapolis, IN) according to the manufacturer's instructions: a CYP24A1 promoter-firefly luciferase (50 ng), pRL-CMV (5 ng; Promega), and a given gene expression plasmid (10 ng), and the total of transfected plasmids was adjusted by adding empty vector pcDNA3-V5-His (Invitrogen, Carlsbad, CA). After 24 h, transfected cells were treated with drug in fetal bovine serum-free media for an additional 24 h before harvesting to prepare lysates for luciferase assays by using Dual-Luciferase Reporter Assay System (Promega).
qRT-PCR. From cells and tissues, total RNA was extracted using TRIzol reagent (Invitrogen), from which cDNA was synthesized using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed with an ABI prism 7700 sequence detection system (Applied Biosystems). Assays-on-Demand probes (Applied Biosystems) were used for PCR and included Hs00167999_m1 for the human CYP24A1 gene, Hm00487244_m1 for the mouse Cyp24a1 gene, and Hm00731567_m1 for the mouse Cyp3a11 gene. The TaqMan human β-actin and rodent glyceraldehyde-3-phosphate dehydrogenase (Applied Biosystems) were used as internal control.
Gel-Shift Assays. A given protein was produced using the in vitro transcription/translation system (TNT T7 quick-coupled system; Promega) and were incubated with 32P-labeled VDRE1 double-strand DNA (5′-GATCCCGGACGCCCTCGCTCACCTCGCTGAGATC-3′, 100,000 cpm) probe in 10 μl of binding buffer containing 10 mM Tris, pH 8.0, containing 1 mM dithiothreitol, 6% glycerol, and 1.5 μg of poly(dI-dC). The proteins were separated on a 5% acrylamide gel in 45 mM Tris-boric acid buffer containing 1 mM EDTA at 150 V for 2 h, and the gel was dried under vacuum and subjected to autoradiography at -70°C.
ChIP Assays. Huh7 cells were cross-linked and processed using a ChIP assay kit (Millipore, Billerica, MA) according to the manufacturer's instructions. Precleared chromatin solution was incubated with 5 μg of anti-VDR antibody (Santa Cruz Biotechnology, Santa Cruz, CA), anti-hPXR antibody (Perseus Proteomics, Tokyo, Japan), anti-hCAR antibody (Perseus Proteomics), anti-SMRT antibody (Affinity BioReagents, Golden, CO) or normal rabbit IgG (Santa Cruz Biotechnology) at 4°C overnight, from which immunoprecipitates were collected to purify DNA using QIAQuick PCR purification kit (QIAGEN, Valencia, CA). The -296/-55 region of the CYP24A1 promoter was amplified using the purified DNA as template and the primers 5′-CGAAGCACACCCGGTGAACT-3′ and 5′-CCAATGAGCACGCAGAGGAG-3′.
Bone Mineral Density. The left femur of each mouse was used for analysis of bone mineral density. The bone mineral density (measured in milligrams per centimeter squared) was measured by dual-energy X-ray absorptiometry using the Lunar PIXImus2 densitometer (GE Healthcare).
Results
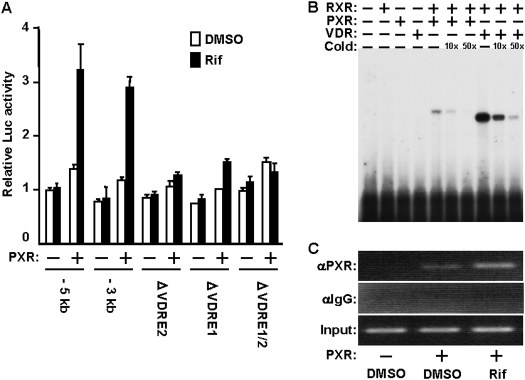
PXR Activation of the CYP24A1 Promoter. Both -5kb and -3 kb promoters of the CYP24A1 gene were activated by hPXR activator rifampicin in the presence of hPXR 3-fold in Huh7 cells (Fig. 1A). This activation was greatly reduced with the internal deletions of either VDRE1 (-170/-156) or VDRE2 (-291/-277) and was essentially abolished with the simultaneous deletion of both VDREs in the context of the -3-kb promoter (Fig. 1A). Gel-shift assays showed specific binding of PXR to VDRE1, although this binding was extremely weak compared with that of VDR (Fig. 1B). The similar binding patterns were also observed with VDRE2 (data not shown). Despite this weak in vitro binding, PXR was enabled to bind to a VDRE region of the CYP24A1 promoter in Huh7 cells, and the binding was increased after rifampicin treatment as demonstrated by ChIP assays (Fig. 1C). These results indicated the PXR activates the CYP24A1 promoter through the direct binding to VDRE in the absence of vitamin D3. Consistent with this PXR activation, the endogenous CYP24A1 gene was induced by rifampicin in both transiently transfected Huh7 cells with hPXR expression plasmid and the HepG2 cells stably expressing hPXR, but not in control HepG2 cells (Supplement Fig. 1).
Fig. 1.
PXR activation of VDRE. A, transient transfection assays were performed by transfecting pCR3/hPXR with various pGL3 constructs of the CYP24A1 promoter-luciferase reporter gene in Huh7 cells. After 24 h, cells were treated with DMSO or rifampicin (Rif, 10 μM) for an additional 24 h before being harvested for luciferase assays. Relative luciferase activities were expressed relative to the activity of the DMSO-treated cells transfected with the empty plasmid and pGL3/CYP24A1-5 kb reporter plasmid. Bars, mean ± S.D. B, PXR binding to VDRE. Gel-shift assays were performed by incubating radiolabeled VDRE1 probe with in vitro transcribed and translated proteins: PXR, RXR, PXR/RXR, VDR, or VDR/RXR as described under Materials and Methods. C, chromatin binding of PXR. ChIP assays were performed using nuclear extracts prepared from pCR3/hPXR-transfected Huh7 cells. Cells were transfected for 24 h and subsequently treated with DMSO or rifampicin (10 μM) as indicated and incubated for an additional 2 h. By scanning three independent assays, the relative intensities of the bands were calculated based on the inputs: 0.2 ± 0.2, 1.6 ± 0.4, and 5.0 ± 0.9 for DMSO, PXR plus DMSO, and PXR plus Rif, respectively.
PXR Repression of the VDR-Activated CYP24A1 Promoter. When Huh7 cells transfected with VDR expression plasmid were treated with vitamin D3, the endogenous CYP24A1 gene was greatly induced more than 3000-fold (Fig. 2A). Cotransfection of PXR expression plasmid and treatment with rifampicin clearly repressed vitamin D3 induction of the CYP24A1 gene (Fig. 2A). This repression is consistent with findings by Zhou et al. (2006), in which short-term treatment with mouse PXR activator PCN repressed the vitamin D3 induction of the Cyp24a1 gene in mouse kidney. In direct reflection of PXR repression of the CYP24A1 gene, PXR also down-regulated the vitamin D3 activation of the CYP24A1 promoter (Fig. 2B). Although the internal deletion of one VDRE (either VDRE1 or VDRE2) resulted in an approximately 75% decrease of CYP24A1 promoter activity, PXR still repressed the activities of both deletion promoters equally and as effectively as it did the CYP24A1 promoter (Fig. 2C). As expected, simultaneous deletion of both VDREs nearly abolished the activity of the CYP24A1 promoter and the repression by PXR. These results suggested that PXR represses the vitamin D3 activation of the CYP24A1 promoter through the VDR-VDRE. Moreover, the underlying mechanism of this repression can take place at either of the two VDREs and does not require the presence of both VDREs.
Fig. 2.
PXR repression of vitamin D3-activated CYP24A1 promoter. pCR3/hPXR was transfected with or without pcDNA3.1/mVDR into Huh7 cells. After 24 h, cells were treated with DMSO, VD3 (10 nM), and Rif (10 μM) for an additional 24 h before being harvested for qRT-PCR. Relative CYP24A1 mRNA levels were expressed by taking the level of the DMSO-treated cells transfected without VDR and PXR as one. Bars, mean ± S.D. B, transient transfection assays were performed by transfecting pGL3/CYP24A1-3 kb with or without pcDNA3.1/mVDR and pCR3/hPXR into Huh7 cells. After 24 h, cells were treated with DMSO, VD3 (10 nM), and Rif (10 μM) for an additional 24 h before being harvested for luciferase assays. C, various pGL3 constructs of the CYP24A1 promoter-luciferase reporter gene were transfected with or without pcDNA3.1/mVDR and pCR3/hPXR into Huh7 cells. After 24 h, cells were treated with DMSO, VD3 (10 nM), and rifampicin (10 μM) for an additional 24 h before being harvested for transient transfection assays. Relative luciferase activities in both B and C are expressed relative to the activity of the DMSO-treated cells transfected with the empty plasmid. Bars, mean ± S.D.
Locking SMRT onto the CYP24A1 Promoter. In an effort to decipher the underlying molecular mechanism by which PXR represses the VDR-activated CYP24A1 promoter, various coregulators were examined as to whether they up- or down-regulate the repression by PXR: SRC1, DRIP, NcoR, CBP, GRIP, PGC1α, and SMRT. Among these seven coregulators examined, only the corepressor SMRT was found to affect the degree to which PXR repressed the vitamin D3-activated CYP24A1 promoter and, moreover, effectively potentiated the PXR-dependent repression (Fig. 3A and Supplement Fig. 2, A-C). In the presence of ectopic SMRT, rifampicin enhanced the PXR repression of the CYP24A1 promoter as its concentration increased in the presence but not the absence of PXR (Supplement Fig. 2, B and C). Given the finding that SMRT was involved in the PXR-mediated repression of the CYP24A1 promoter, ChIP assays were used to examine the interactions of VDR, PXR, and SMRT with a VDRE region of the promoter (Fig. 3B). To this end, VDR and PXR were expressed either by themselves or together in Huh7 cells, which were then treated with DMSO, vitamin D3, and vitamin D3 plus rifampicin. First, as expected, ChIP assays showed that VDR binding to the VDRE region was increased in response to vitamin D3 treatment with the concomitant dissociation of SMRT from the VDRE region (Fig. 3B). Although VDR binding remained in the presence of PXR, PXR was also found to bind to the VDRE region, suggesting that both VDR and PXR coexist on the VDRE region during the repression by PXR of the CYP24A1 promoter. This chromatin binding of PXR occurred in conjunction with the retained binding of SMRT to the VDRE region (Fig. 3B). Thus, these results indicate that PXR prevents the dissociation of SMRT from the CYP24A1 promoter to repress the VDR-mediated activation, locking this corepressor onto the promoter.
Fig. 3.

PXR repression via SMRT. A, transient transfection assays were performed by transfecting pGL3/CYP24A1-3 kb with various combinations of pcDNA3.1/mVDR, pCMX/hSMRT, and pCR3/hPXR into Huh7 cells. After 24 h, cells were treated with DMSO, VD3 (10 nM), and/or Rif (10 μM) for an additional 24 h before being harvested for luciferase assays. Relative luciferase activities were expressed relative to the activity of the DMSO-treated cells transfected with the empty -3-kb reporter plasmid. Bars, mean ± S.D. B, Huh7 cells were transfected with or without pcDNA3.1/mVDR and pCR3/hPXR. After 24 h, transfected cells were treated with DMSO, VD3 (10 nM), and/or rifampicin (10 μM) for an additional 2 h before being harvested for ChIP assays. Cross-linked chromatin-protein complexes were immunoprecipitated with anti-VDR (αVDR), anti-PXR (αPXR), anti-SMRT (αSMRT), or normal mouse IgG (αIgG). By scanning three independent assays, the relative intensities of the bands were calculated based on the inputs: in the VDR binding, 0.8 ± 0.1, 3.3 ± 0.7, 3.7 ± 1.1, and 3.8 ± 0.9% for VDR plus DMSO, VDR plus VD3, VDR plus PXR and VD3, and VDR plus PXR, VD3, and Rif, respectively; in the PXR binding, 0.2 ± 0.2, 0.3 ± 0.3, 2.7 ± 0.8, and 5.2 ± 1.2% for VDR plus DMSO, VDR plus VD3, VDR plus PXR and VD3, and VDR plus PXR, VD3, and Rif, respectively; in the SMRT binding, 4.7 ± 1.0, 0.0 ± 0.1, 1.3 ± 0.6, and 4.0 ± 0.4% for VDR plus DMSO, VDR plus VD3, VDR plus PXR and VD3, and VDR plus PXR, VD3, and Rif, respectively.
Similar to PXR, whereas activating the CYP24A1 gene in the absence of vitamin D3, CAR repressed vitamin D3 activation of the CYP24A1 gene, and this CAR repression was reflected directly on its promoter activity (Fig. 4, A and B). It is interesting that the human CAR agonist (CITCO) and antagonist (PK11195) were effective in their abilities to repress the CYP24A1 promoter. PK11195, peripheral benzodiazepine receptor ligand, is a newly characterized antagonist of human CAR (Li et al., 2008). Again, similar to PXR, ChIP assays showed that CAR retained SMRT binding to the VDRE region: once vitamin D3-dissociated SMRT was restored in the presence of CAR, the degree of the restoration was increased by both CITCO and PK11195 (Fig. 4C). Thus, CAR and PXR repressed the VDR-mediated activation of the CYP24A1 promoter through the same regulatory mechanism that involves SMRT locking onto the promoter.
Fig. 4.
CAR repression of vitamin D3 activation via SMRT. A, Huh7 cells were transfected with or without pcDNA3.1/mVDR and pCR3/hCAR. After 24 h, cells were treated with DMSO, VD3 (10 nM), CITCO (250 nM), and/or PK11195 (10 μM) for an additional 24 h before being harvested for qRT-PCR. Relative CYP24A1 mRNA levels were expressed by taking the level of the DMSO-treated cells transfected with empty plasmid as 1. Bars, mean ± S.D. B, pGL3/CYP24A1-3 kb was transfected with or without pcDNA3.1/mVDR and pCR3/hCAR into Huh7 cells. After 24 h, cells were treated with DMSO, VD3 (10 nM), CITCO (25, 75, and 250 nM), and PK11195 (1, 3, and 10 μM) for an additional 24 h before being harvested for luciferase assays. Relative luciferase activities were expressed relative to the activity of the DMSO-treated cells transfected with empty plasmid as 1. Bars, mean ± S.D. C, Huh7 cells were transfected with pcDNA3.1/mVDR and pcDNA3.1/mVDR plus pCR3/hCAR. After 24 h, cells were treated with DMSO, VD3 (10 nM), CITCO (250 nM), and/or PK11195 (10 μM) for an additional 2 h before being harvested for ChIP assays. Cross-linked chromatin-protein complexes were immunoprecipitated with anti-VDR (αVDR), anti-CAR (αCAR), anti-SMRT (αSMRT), or normal mouse IgG (αIgG). By scanning three independent assays, the relative intensities of the bands were calculated based the inputs: in the VDR binding, 3.4 ± 0.8, 3.3 ± 1.0, 3.4 ± 1.3, and 3.4 ± 1.2% for VDR plus VD3, VDR plus CAR and VD3, VDR plus CAR, VD3, and CITCO, and VDR plus CAR, VD3, and PK11195, respectively; in the CAR binding, 0.1 ± 0.3, 1.0 ± 0.3, 2.9 ± 0.5, and 3.7 ± 1.2% for VDR plus VD3, VDR plus CAR and VD3, VDR plus CAR, VD3, and CITCO, and VDR plus CAR, VD3, and PK11195, respectively; in the SMRT binding, 0.0 ± 0.0, 0.8 ± 0.1, 2.0 ± 0.5, and 2.1 ± 0.3% for VDR plus VD3, VDR plus CAR and VD3, VDR plus CAR, VD3, and CITCO, and VDR plus CAR, VD3, and PK11195, respectively.
Rifampicin-Vitamin D3 Interaction. Consistent with the manner of endogenous CYP24A1 gene repressed, rifampicin repressed the CYP24A1 promoter in PXR-transfected Huh7 cells. The repression by rifampicin weakened as vitamin D3 levels increased (Fig. 5A). In addition, ChIP assays showed the binding of SMRT to the VDRE region was higher at 10 μM rather than 1 μM rifampicin, both of which were attenuated as the concentration of vitamin D3 increased from 1 to 100 nM (Fig. 5B). Thus, repression by rifampicin was dependent on the doses of vitamin D3 through the function of the nuclear receptors VDR and PXR to regulate the corepressor SMRT, providing a novel mechanism of drug interactions with vitamin D3.
Fig. 5.

PXR repression depending on vitamin D3 concentration. A, pGL3/CYP24A1-3 kb was cotransfected with pcDNA3.1/mVDR and pCR3/hPXR into Huh7 cells. After 24 h, cells were treated with DMSO and rifampicin and with the increased concentrations of VD3 for an additional 24 h before being harvested for luciferase assays. Relative luciferase activities were expressed relative to the activity of the cells treated with DMSO alone as 1. Bars, mean ± S.D. B, Huh7 cells were transfected with pcDNA3.1/mVDR and pCR3/hPXR. At 24 h after the transfection, cells were treated with Rif (1 and 10 μM) and VD3 (1, 10, and 100 nM) at their various concentrations for an additional 2 h before being harvested for ChIP assays. Cross-linked chromatin-protein complexes were immunoprecipitated with anti-SMRT (αSMRT) or normal mouse IgG (αIgG). By scanning three independent assays, the relative intensities of the bands were calculated based on the inputs: 4.2 ± 1.0, 2.5 ± 0.7, 1.4 ± 0.5, 5.7 ± 1.1, 4.6 ± 1.1, and 3.8 ± 0.9% for the bars from left to right.
Bone Mineral Density of PCN-Treated Mice. The CYP24A1 gene is implicated in the decrease of bone mineral density and the related bone deceases often observed in patients continuously treated with PXR-activating drugs (Shah et al., 1981). Using a slow-release capsule form of PCN, Pxr(+/+) and Pxr(-/-) mice were continuously treated for 6 weeks; livers and kidney were collected to measure the expression levels of the CYP24A1 gene, whereas femora were kept for the determination of bone mineral density (BMD). The known PXR target gene Cyp3a11 was induced in both livers and kidneys of all of the PCN-treated Pxr(+/+) mice (data not shown). Neither the basal nor the induced expressions of CYP24A1 mRNA were detected in any of these livers. In the kidneys, CYP24A1 mRNA was induced in the PCN-treated Pxr(+/+) mice only, although the degree of this induction greatly varied. BMD of whole femoral bones featured a statistically significant decrease after PCN treatment in both Pxr(+/+) and Pxr(-/-) mice (Fig. 6A, 67 ± 3 to 64 ± 1 and 62 ± 3 to 59 ± 2 mg/cm2, respectively). Although the decrease of whole BMD was PXR-independent, the BMD in the metaphyseal femoral bones exhibited a PXR-dependent decrease: PCN treatment decreased the metaphyseal BMD in the only Pxr(+/+) mice (Fig. 6A; 67 ± 7 to 60 ± 7 mg/cm2, P < 0.05). The metaphyseal BMD was already decreased in the control matrix-treated Pxr(-/-) mice and was not further reduced by PCN treatment (59 ± 4 and 58 ± 5 mg/cm2). Loss of the metaphyseal BMD, however, was not correlated with the expression levels of CYP24A1 mRNA in the kidney (Fig. 6B). Therefore, at least in mice, the Cyp24a1 gene does not seem to play a role in decreasing the mineral density of femoral bones.
Fig. 6.
Bone mineral density in PCN-treated mice. Six male Pxr(+/+) and Pxr(-/-) mice were continuously treated with PCN pellet (36 mg/mouse) or control pellet subcutaneously for 6 weeks. A. Using legs, whole and metaphyseal femoral BMDs were measured by the dual-energy X-ray absorptiometry. Bars, mean ± S.D. *, P < 0.05; **, P < 0.01. B, renal CYP24A1 mRNA was determined by qRT-PCR and was expressed by taking those in the Pxr(+/+) mice treated with control pellet as 1. Correlations between the CYP24A1 expressions and metaphyseal femoral BMD are shown.
Discussion
The CYP24A1 gene is activated by vitamin D3, which is mediated by direct binding of VDR to the VDREs within the CYP24A1 proximal promoter (Sutton and MacDonald, 2003). Here we have defined the novel molecular mechanism by which the nuclear xenobiotic receptors PXR and CAR mediate drug interactions with vitamin D3 to regulate the VDR-mediated activation of the CYP24A1 promoter. In the absence of vitamin D3, PXR directly binds to VDRE to activate the CYP24A1 promoter. Resulting induction of the CYP24A1 gene by PXR, however, is extremely weak compared with the high VDR-dependent induction by vitamin D3. In the presence of vitamin D3, PXR represses the VDR-mediated activation of the CYP24A1 promoter and the vitamin D3 induction of the CYP24A1 gene. Vitamin D3 activates the CYP24A1 gene by dissociating the corepressor SMRT from the VDR on the VDREs within the CYP24A1 proximal promoter. Upon drug activation, PXR indirectly binds to the VDRE region of the CYP24A1 promoter, thus inhibiting this dissociation of SMRT from VDR to repress the vitamin D3-mediated promoter activation. This binding of PXR should be indirect, because its extremely weak binding ability in our gel-shift assays may not allow PXR to directly bind to VDRE in the presence of vitamin D3-activated VDR, although a possibility of direct binding competition between PXR and VDR to VDRE cannot be totally excluded. How PXR indirectly binds to the VDRE region remains unsolved; whether PXR interacts with VDR, SMRT, or an as-yet-other-unidentified transcription factors remains to be seen. Likewise, the other xenobiotic nuclear receptor CAR also represses the VDR-mediated activation of the CYP24A1 promoter by inhibiting the dissociation of SMRT. Thus, locking the corepressor onto VDR seems to be the common mechanism underlying the repression of vitamin D3 activation of the CYP24A1 promoter by the nuclear xenobiotic receptors.
Long-term treatment with certain antiepileptic and antimicrobial drugs that activate PXR and/or CAR often decreases serum vitamin D levels and bone mineral density (Shah et al., 1981; Brodie et al., 1982; Pack and Morrell, 2004), similar to what is observed in patients with vitamin D3 deficiency. PXR-dependent induction of the CYP24A1 gene has been implicated recently in the development of these drug side effects (Pascussi et al., 2005). Our present study does not provide support for the involvement of the CYP24A1 gene in drug-induced bone diseases: the induction of the CYP24A1 gene by rifampicin is extremely weak compared with the corresponding vitamin D3 induction, and, moreover, rifampicin even represses the CYP24A1 gene in the presence of vitamin D3. Most critically, although long-term activation of PXR resulted in the decrease of femoral bone mineral density in mice, neither PXR-independent loss of the whole femoral bone mineral density nor the PXR-dependent loss of the metaphyseal femoral bone mineral density correlated with the expression levels of the Cyp24a1 gene in those mice. Thummel and his group specified CYP3A4, not CYP24A1, as a cause of bone diseases because CYP3A4 also metabolically inactivates vitamin D3 (Xu et al., 2006; Zhou et al., 2006). Given the caveat that vitamin D3 metabolism by mouse CYP3A enzymes is not known at the present time and that there may be species differences in how vitamin D3 works, however, neither hepatic nor intestinal expressions of CYP3A11 mRNA correlated with the PCN-induced loss of the bone mineral density (Y. Konno and M. Negishi, unpublished observation). It has been reported recently that vitamin K2 activates hPXR (or steroid and xenobiotic receptor), inducing the genes that are involved in bone formation in osteoblasts, and that rifampicin, in part, could replace vitamin K2 by inducing these same genes (Tabb et al., 2003; Ichikawa et al., 2006). In this scenario, PXR activation would help osteoblasts to increase bone formation. Given the limitation of our present study, it is intriguing that the PCN-treated Pxr(+/+) mice, which showed induced levels of renal CYP24A1 mRNA, in fact, exhibited higher mineral density of the metaphyseal bones. Therefore, PXR activation may be a double-edged sword in maintaining bone homeostasis, which could be a reason for the low incidence of bone diseases in patients continuously treated with PXR-activating drugs compared with those continuously treated with CAR-activating drugs. Further investigations of drug interactions with vitamins D and K and their receptor-mediated molecular mechanism are critically important for the prevention and treatment of bone diseases.
One of the major roles of PXR and CAR is to endow cells with a defense mechanism against the toxicity of therapeutics and xenobiotics by activating genes that encode detoxification enzymes and transporters (Kodama and Negishi, 2006; Urquhart et al., 2007). However, this induction by PXR and CAR often becomes a liability, causing drug-drug interactions and side effects. Therefore, chemicals that activate these receptors, PXR in particular, are generally removed from drug development. Chemicals such as PK11195, nevertheless, can be primary drug candidates for the specific targeting of genes such as CYP24A1, because PK11195 does not activate CAR-mediated induction of drug metabolism and represses the vitamin D-dependent activation of the CYP24A1 gene. Similar to the case of VDR yet different in molecular mechanism, PXR and CAR repress the hepatic genes (i.e., CYP7A1, PEPCK 1, and G6Pase) that regulate gluconeogenesis through their protein-protein interaction with transcription factors such as FoxO1 and PGC1α (Kodama et al., 2004, 2007; Miao et al., 2006). PXR is also found to regulate fatty-acid metabolism via interacting with FoxA2 (Nakamura et al., 2007). Specifically using this direct regulation by binding to DNA or the indirect regulation via protein-protein interactions, and screening chemicals like PK11195 may provide us with an opportunity to develop drugs for treating hormone- or vitamin-related diseases such as diabetes and osteoporosis by targeting nuclear xenobiotic receptors.
Supplementary Material
Acknowledgments
We thank Dr. Toshiyuki Sakaki (Toyama Prefectural University, Japan) for his valuable discussion on this work and for the National Institute of Environmental Health Sciences sequencing core.
This work was supported by the Intramural Research Program of the National Institutes of Health National Institute of Environmental Health Sciences.
ABBREVIATIONS: VDR, vitamin D receptor; CAR, constitutive active/androstane receptor; PXR, pregnane X receptor; VDRE, vitamin D response element; ChIP, chromatin immunoprecipitation; SMRT, silencing mediator for retinoid and thyroid hormone receptors; RXR, retinoid X receptor; PGC1α, peroxisome-proliferator-activated receptor γ coactivator 1α; BMD, bone mineral density; PCN, pregnenolone 16α-carbonitrile; CITCO, 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzenyl)oxime; DMSO, dimethyl sulfoxide; PK11195, 1-(2-chlorophenyl-N-methylpropyl)-3-isoquinoline-carboxamide; Rif, rifampicin; VD3, 1α,25-dihydroxyvitamin D3; kb, kilobase; PCR, polymerase chain reaction; qRT-PCR, quantitative reverse-transcriptase polymerase chain reaction.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
References
- Brodie MJ, Boobis AR, Hillyard CJ, Abeyasekera G, Stevenson JC, MacIntyre I, and Park BK (1982) Effect of rifampicin and isoniazid on vitamin D metabolism. Clin Pharmacol Ther 32 525-530. [DOI] [PubMed] [Google Scholar]
- Christakos S, Dhawan P, Liu Y, Peng X, and Porta A (2003) New insights into the mechanisms of vitamin D action. J Cell Biochem 88 695-705. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Horie-Inoue K, Ikeda K, Blumberg B, and Inoue S (2006) Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J Biol Chem 281 16927-16934. [DOI] [PubMed] [Google Scholar]
- Jones G, Strugnell SA, and DeLuca HF (1998) Current understanding of the molecular actions of vitamin D. Physiol Rev 78 1193-1231. [DOI] [PubMed] [Google Scholar]
- Kodama S, Koike C, Negishi M, and Yamamoto Y (2004) Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol 24 7931-7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Moore R, Yamamoto Y, and Negishi M (2007) Human nuclear pregnane X receptor cross-talk with CREB to repress cAMP activation of the glucose-6-phosphatase gene. Biochem J 407 373-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S and Negishi M (2006) Phenobarbital confers its divers effects by activating the orphan nuclear receptor CAR. Drug Metab Rev 38 75-87. [DOI] [PubMed] [Google Scholar]
- Li L, Chen T, Stanton JD, Sueyoshi T, Negishi M, and Wang H (2008) The peripheral benzodiazepine receptor ligand 1-(2-chlorophenyl-methylpropyl)-3-isoquinoline-carboxamide is a novel antagonist of human constitutive androstane receptor. Mol Pharmacol 74 443-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Fang S, Bae Y, and Kemper JK (2006) Functional inhibitory cross-talk between constitutive androstane receptor and hepatic nuclear factor-4 in hepatic lipid/glucose metabolism is mediated by competition for binding to the DR1 motif and to the common coactivators, GRIP-1 and PGC-1α. J Biol Chem 281 14537-14546. [DOI] [PubMed] [Google Scholar]
- Moreau A, Maurel P, Vilarem MJ, and Pascussi JM (2007) Constitutive androstane receptor-vitamin D receptor crosstalk: consequence on CYP24 gene expression. Biochem Biophys Res Commun 360 76-82. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Moore R, Negishi M, and Sueyoshi T (2007) Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver. J Biol Chem 282 9768-9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack AM and Morrell MJ (2004) Epilepsy and bone health in adults. Epilepsy Behav 5 (Suppl 2): S24-S29. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Robert A, Nguyen M, Walrant-Debray O, Garabedian M, Martin P, Pineau T, Saric J, Navarro F, Maurel P, et al. (2005) Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J Clin Invest 115 177-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SC, Sharma RK, Hemangini, and Chitle AR (1981) Rifampicin induced osteomalacia. Tubercle 62 207-209. [DOI] [PubMed] [Google Scholar]
- Sutton AL and MacDonald PN (2003) Vitamin D: more than a “bone-a-fide” hormone. Mol Endocrinol 17 777-791. [DOI] [PubMed] [Google Scholar]
- Tabb MM, Sun A, Zhou C, Grün F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M, et al. (2003) Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem 278 43919-43927. [DOI] [PubMed] [Google Scholar]
- Urquhart BL, Tirona RG, and Kim RB (2007) Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: implications for interindividual variability in response to drugs. J Clin Pharmacol 47 566-578. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hashizume T, Shuhart MC, Davis CL, Nelson WL, Sakaki T, Kalhorn TF, Watkins PB, Schuetz EG, and Thummel KE (2006) Intestinal and hepatic CYP3A4 catalyze hydroxylation of 1α,25-dihydroxyvitamin D3: implications for drug-induced osteomalacia. Mol Pharmacol 69 56-65. [DOI] [PubMed] [Google Scholar]
- Zhou C, Assem M, Tay JC, Watkins PB, Blumberg B, Schuetz EG, and Thummel KE (2006) Steroid and xenobiotic receptor and vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia. J Clin Invest 116 1703-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.