Abstract
The mammalian copper transporter 1 (CTR1) is responsible for the uptake of copper from the extracellular space. In this study, we used an isogenic pair of CTR1(+/+) and CTR1(-/-) mouse embryo fibroblasts to examine the contribution of CTR1 to the influx of cisplatin (DDP), carboplatin (CBDCA), oxaliplatin (L-OHP), and transplatin. Exposure to DDP triggered the rapid degradation of CTR1, suggesting that its contribution to influx was likely to be on the initial phase of drug entry. Loss of CTR1 decreased the initial binding of DDP to cells and reduced influx measured over the first 5 min of drug exposure by 81%. Loss of CTR1 almost completely eliminated the initial influx of CBDCA and reduced the initial uptake of L-OHP by 68% but had no effect on the influx of transplatin. Loss of CTR1 rendered cells resistant to even high concentrations of DDP when measured in vitro, and re-expression of CTR1 in the CTR1(-/-) cells restored both DDP uptake and cytotoxicity. The growth of CTR1(-/-) tumor xenografts in which CTR1 levels were restored by infection with a lentivirus expressing wild-type CTR1 was reduced by a single maximum tolerated dose of DDP in vivo, whereas the CTR1(-/-) xenografts failed to respond at all. We conclude that CTR1 mediates the initial influx of DDP, CBDCA, and L-OHP and is a major determinant of responsiveness to DDP both in vitro and in vivo.
CTR1 has been recognized as an essential copper transporter that is present on the plasma membrane and variably distributed on a variety of intracellular membranes (Lee et al., 2000; Aller et al., 2004). CTR1 is one of several transports that mediate the movement of copper across the plasma membrane; it is essential for early embryonic development because knockout of both CTR1 alleles causes in utero lethality (Kuo et al., 2001; Lee et al., 2002). The details of the mechanism by which CTR1 transports copper have not been elucidated fully (Dancis et al., 1994b; Moller et al., 2000; De Feo et al., 2007). Copper is required for the function of many enzymes (Madsen and Gitlin, 2007) but also has substantial potential for toxicity because of its ability to readily undergo redox cycling in the interior of the cell. To serve the needs of these enzymes for copper, a complex system of transporters and chaperones has evolved that continuously bind and shield Cu+ such that essentially none of it is free in the cell (Bertinato and L'Abbe, 2004; Balamurugan and Schaffner, 2006; Kim et al., 2008). Once CTR1 has transported copper across the plasma membrane, the copper is transferred to one of several chaperones that function to both protect the copper from oxidation and move it to various locations in the cell. At least three chaperones are involved in the distribution of copper, but the mechanism by which CTR1 hands off copper to these proteins has not yet been elucidated fully (Hamza et al., 2001, 2003).
Platinum serves as the basis for three clinically important and widely used chemotherapeutic agents, including cisplatin [cis-dichlorodiammine platinum(II)] (DDP), carboplatin [cis-diammine [1,1-cyclobutane-dicarboxylato (2-)-O,O′]-,(SP-4-2) platinum(II)] (CBDCA), and oxaliplatin [cis-[(1R,2R)-1,2-cyclohexanediamine-N,N′] [oxalato(2-)-O,O′] platinum(IV)] (L-OHP). All three of these drugs are used in the treatment of multiple forms of cancer, including bladder, breast, ovarian, prostate, testicular, non-small-cell lung, and colorectal carcinomas. DDP, CBDCA, and L-OHP are all highly polar molecules that do not readily diffuse across lipid membranes (Hall et al., 2008). Once inside the cell, they become aquated and form adducts in DNA that react with nucleophilic sites in a variety of targets (Kelland, 2007). However, the mechanism by which these drugs enter tumor cells has remained largely undefined until recently. Ishida et al. (2002) showed that yeast lacking yCTR1 had reduced accumulation of DDP; this was confirmed in yeast by Lin et al. (2002) and in more detailed studies in mammalian cells by Holzer and Howell (2006). High concentrations of copper have been shown to trigger the degradation of CTR1 in both yeast (Dancis et al., 1994a; Ooi et al., 1996) and mammalian cells (Petris et al., 2003). More recent studies have shown that not only is DDP uptake regulated by CTR1, but DDP also triggers the rapid degradation of CTR1 and does so at much lower concentrations and more rapidly than copper (Holzer et al., 2006b). Blockade of proteosome function prevents the DDP-induced degradation of CTR1 but it is not known whether or how this alters DDP accumulation (Holzer and Howell, 2006).
In the current study, we examined the role of CTR1 as a determinant of the cellular accumulation of DDP, CBDCA, L-OHP and transplatin in more detail using cell lines in which both alleles of CTR1 were knocked out and a subline in which expression of wild-type CTR1 was restored. We have documented that the loss of CTR1 impairs the initial influx of DDP, CBDCA, and L-OHP but has no effect on the influx of transplatin. We report here that the loss of CTR1 is associated with resistance to DDP both in vitro and in vivo.
Materials and Methods
Drugs and Reagents. DDP was a gift from Bristol-Myers Squibb (Princeton, NJ); it contains DDP at a concentration of 3.33 mM in 0.9% NaCl. L-OHP was a gift from Sanofi-Aventis (Malvern, PA); the powder was dissolved in ddH2O at a concentration of 10 mM. CBDCA was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in ddH2O at a concentration of 10 mM. Transplatin was obtained from Sigma-Aldrich (St. Louis, MO) and was resuspended in 0.9% NaCl at a concentration of 3.33 mM. The drugs were diluted into OptiMEM Reduced Serum Media (Invitrogen, Carlsbad, CA) to produce final concentrations of 10, 30, and 100 μM. Bradford reagent was purchased from Bio-Rad Laboratories, Inc. (Hercules, CA), and sulforhodamine B was obtained from Sigma-Aldrich; 0.4% sulforhodamine B (w/v) was solubilized in 1% (v/v) acetic acid solution.
Cell Types, Culture, and Engineering. Parental mouse embryonic fibroblasts containing wild-type alleles of CTR1 [CTR1(+/+)] and a subline in which both copies of CTR1 had been somatically knocked out [CTR1(-/-)] were a gift from Dr. Dennis Thiele (Lee et al., 2002). The CTR1(-/-/R) subline was constructed by infecting the CTR1(-/-) cells with a lentivirus expressing a wild-type human CTR1 cDNA using the ViraPower Lentiviral Induction kit (Invitrogen). Cell survival after exposure to increasing concentrations of drugs was assayed using the sulforhodamine B assay system (Monks et al., 1991). Five thousand cells were seeded into each well of a 96-well tissue culture plate. Cells were incubated overnight at 37°C, 5% CO2, and then exposed for 5 min by the addition of 100 μl of platinum drug-containing OptiMEM medium. After 5 min, the drug-containing media were aspirated off, cells were washed once with 37°C PBS, PBS was aspirated off, and cells were covered in 200 μl of complete medium. Cells were allowed to grow for 5 days, after which the media were removed, and the protein was precipitated with 50% trichloroacetic acid and stained using 100 μl of 0.4% sulforhodamine B in 1% acetic acid at room temperature for 15 min. After washing, the absorbance of each well at 515 nm was recorded using a Versamax Tunable Microplate Reader (Molecular Devices, Sunnyvale, CA). All experiments were repeated at least three times using three cultures for each drug concentration.
Measurement of Cellular Drug Accumulation. CTR1(+/+), CTR1(-/-/R), and CTR(-/-) cells were grown to 90% confluence in T-150 tissue culture flasks. Cells were then harvested using trypsin, and 7.5 × 105 cells were placed into each well of six-well tissue culture plates and allowed to grow overnight in 2.5 ml of media at 37°C in 5% CO2. All data presented are the means of at least three independent experiments each performed with six wells per concentration tested. The next day, medium was removed by aspiration, and the cells were exposed to 500 μl of drug-containing OptiMEM medium (Invitrogen) at 37°C for either 0 or 5 min, after which the drug-containing medium was removed, and the plates were washed three times with ice-cold PBS and then placed on ice. In the case of the time 0 samples, the drug-containing medium was aspirated within 15 s of the start of drug exposure. Concentrated (50-70%) nitric acid (215 μl) was added to each well, and the plate was incubated overnight at room temperature. The following day, the acid was moved into Omni-vials (Wheaton, Millville, NJ) and incubated at 65°C overnight to thoroughly dissolve all cellular debris. The following day, the nitric acid was diluted with 3 ml of buffer (0.1% Triton X-100, 1.4% nitric acid, 1 ppb in ddH2O) and incubated again at 65°C overnight. Platinum concentration was measured using a PerkinElmer Element 2 inductively coupled plasma mass spectrometry (PerkinElmer Life and Analytical Sciences, Waltham, MA), located at the Analytical Facility at Scripps Institute of Oceanography at the University of California, San Diego. Values were normalized to either protein concentration as determined using the Bradford reagent or total sulfur content, as measured by inductively coupled plasma optical emission spectroscopy, on the same samples.
64Cu Influx. Copper uptake was determined as described previously (Holzer et al., 2006a) with the following changes. Plates were incubated for 1 h rather than 30 min in the presence of copper. Plates that were pretreated with DDP were exposed to 30 μM DDP for 15 min at 37°C followed by three washings with room temperature PBS, followed by exposure to 64Cu.
qRT-PCR. CTR1 mRNA levels were measured by qRT-PCR. cDNA was generated from mRNA isolated using TRIzol (Invitrogen). Purified mRNA was converted to cDNA using Oligo(dT)20 priming and the SuperScript III First-Strand Kit (Invitrogen). qRT-PCR was performed on a Bio-Rad MyIQ qPCR machine. Primers were as follows: hCTR1: forward, gatgatgatgcctatgacct and tcttgagtccttcatagaac; reverse, actgttgggcaacagatgct and ctgctgctactgcaatgcag. mCTR1: forward, gatgatgatgcctatgacct and ctctcgggctatcttgagtc; reverse, tccaggtagtcatcagct and tggcagtgctctgtgatgtc. Mouse β-actin: forward, aggtgacagcattgcttctg; reverse, gctgcctcaacacctcaac. Reactions were prepared using iQ SYBR Green Supermix (Bio-Rad) according to the manufacturer's recommendations. Samples were prepared in quadruplicate, and three independent RNA isolates were used in three independent experiments. Analysis was done using the Bio-Rad iQ5 system software.
Determination of Drug Sensitivity in Vivo. To grow the various types of cells as xenografts, 3 × 106 cells in 100 μl were inoculated at four subcutaneous sites into 20 g female nu/nu mice. Cell types were randomized between shoulder and hip, left and right, ensuring that there were always tumors of the same type on left and right. Tumors were allowed to grow until they were ∼2 mm in diameter, at which point each mouse received a single dose of DDP 10 mg/kg by intraperitoneal injection. Tumor size was monitored three times per week for 5 weeks. Tumor volume was estimated using the equation (length × width2)/2.
Immunohistochemistry and Chemiluminescent Immunoblotting. Immunohistochemical analysis was performed as described previously (Holzer et al., 2006b). Western blots were performed as described previously (Holzer et al., 2004), with the following changes: polyvinylidene difluoride membrane (Millipore, Billerica, MA) was used in place of nitrocellulose, and Pierce Super-Signal chemiluminescence detection was used (Thermo Fisher Scientific, Waltham, MA).
Statistical Analysis. All two-group comparisons were done using Student's t test, where two-tailed p ≤ 0.05 was determined to be statistically significant.
Results
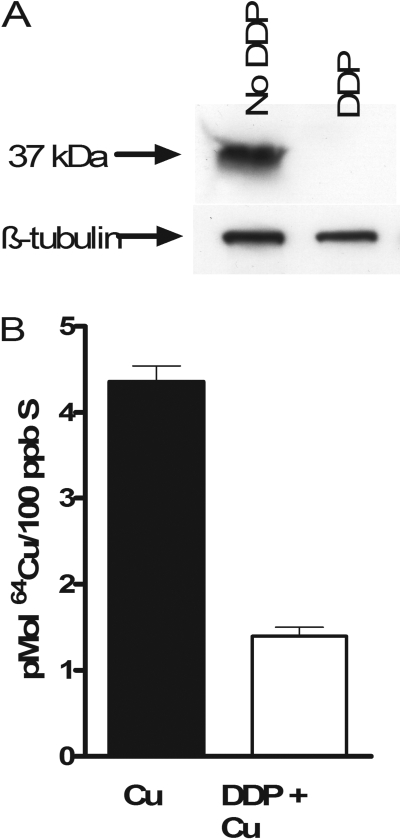
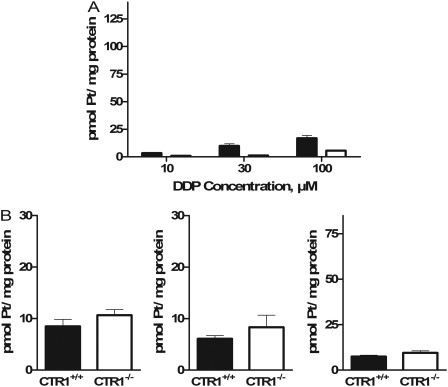
Effect of CTR1 on Initial Influx of Platinum-Containing Drugs. The effect of CTR1 on the cellular accumulation of DDP was examined using a pair of mouse embryonic fibroblast lines, one containing wild-type alleles of CTR1 [CTR1(+/+)], and another in which both copies of the CTR1 gene had been somatically knocked out [CTR1(-/-)]. Our prior studies with this pair of cell lines had indicated that a loss of CTR1 reduced the accumulation of DDP to 36% of control when uptake was measured after a 1-h exposure to either 2 or 10 μM DDP. However, as shown in Fig. 1A, even brief exposure of the CTR1(+/+) cells to 30 μM DDP triggered rapid degradation and disappearance of CTR1 staining when examined by Western blot. The reduction in CTR1 was functionally significant. As shown in Fig. 1B, when cells were exposed to DDP for 15 min before being exposed to copper for 1 h, the accumulation of copper was reduced by 68% (p < 0.0006). The rapid degradation of CTR1 suggests that the major contribution of this transporter to DDP uptake may occur during the initial phase of DDP influx. To examine this in more detail, CTR1(+/+) and CTR1(-/-) cells were exposed to 10, 30, or 100 μM DDP, and the drug was either removed immediately or after a 5-min exposure. The cells were then thoroughly washed, and the amount of cell-associated platinum was measured by inductively coupled plasma mass spectrometry. The range of concentrations was selected to encompass the range of concentrations anticipated in the peritoneal cavity after intraperitoneal instillation of DDP during treatment for ovarian cancer. Figure 2A shows that even when the drug was removed as quickly as possible and cells washed immediately with ice-cold saline, there was a clear difference in the amount of DDP that became associated with the CTR1(+/+) versus CTR1(-/-) cells at all three concentrations tested. The platinum that became immediately bound to the CTR1(-/-) cells was only 33 ± 10% (S.E.M.) of that associated with the CTR1(+/+) cells when exposed to 10 μM DDP. When exposed to 30 or 100 μM DDP, the CTR1(-/-) cells bound 48 ± 20 and 29 ± 5% (S.E.M.) of the amount bound by the CTR1(+/+) cells. Because the period of exposure was <15 s, this suggests that most of the difference in cell-associated platinum was the result of rapid binding of DDP to the extracellular domain of CTR1 rather than a difference in transport across the plasma membrane. It is interesting that, as shown in Fig. 2B, there was no significant difference in the immediate binding of either CBDCA or L-OHP between the CTR1(+/+) and CTR1(-/-) cells, a finding consistent with the generally slower rate of association of these drugs with nucleophilic targets. There was also no significant difference between the 4 drugs with respect to the amount of platinum that became immediately bound to the CTR1(+/+) cells.
Fig. 1.
Degradation of CTR1 and inhibition of copper uptake induced by DDP. A, Western blot analysis of lysates from CTR1(+/+) cells treated with or without 30 μM DDP for 15 min. B, 64Cu accumulation during a 1-h exposure to 2 μM copper in CTR1(+/+) cells treated previously without (▪) or with (□) 30 μM DDP for 15 min. Each bar represents the mean of at least three independent experiments each performed with six separate cultures. Vertical bars, S.E.M.
Fig. 2.
Pt associated with CTR1(+/+) and CTR1(-/-) cells after exposure to and immediate removal of DDP, CBDCA, L-OHP and transplatin. A, cell-associated platinum after the addition and immediate removal of 10, 30, or 100 μM DDP (exposure, <15 s); B, cell-associated platinum after the addition and immediate removal of 30 μM CBDCA, L-OHP and transplatin. ▪, CTR1(+/+) cells; □, CTR1(-/-) cells. Each bar represents the mean of at least three independent experiments each performed with six separate cultures. Vertical bars, S.E.M.
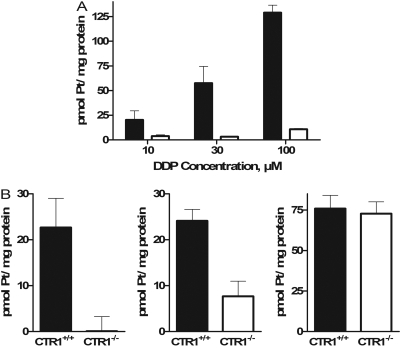
Figure 3A shows the net uptake of platinum when the CTR1(+/+) and CTR1(-/-) cells were exposed to 10, 30, or 100 μM DDP for 5 min; the values shown are the total platinum associated with the cells at 5 min less that bound to the cells at time 0. At all three concentrations tested, the net accumulation of DDP was reduced in the CTR1(-/-) cells; this reduction was significant for the 30 (p < 0.05) and 100 μM (p < 0.0005) concentrations. There was a trend toward a greater effect at higher DDP concentrations: as the concentration was increased from 10 to 100 μM, the uptake in the CTR1(-/-) cells diminished from 19 to 8% of that in the CTR1(+/+) cells. Thus, loss of CTR1 had a large effect on the initial uptake of DDP, and by comparison to prior studies (Holzer et al., 2006a), the effect was substantially larger with a 5-min relative to a 1-h period of drug exposure.
Fig. 3.
Net platinum accumulation in CTR1(+/+) (▪) and CTR1(-/-) (□) cells after a 5-min exposure. A, exposure to 10, 30, or 100 μM DDP. B, exposure to 30 μM CBCDA, L-OHP, or transplatin. Each bar represents the mean of at least three independent experiments each performed with six separate cultures. Vertical bars, S.E.M.
To determine whether the loss of CTR1 affected uptake of the other platinum-containing drugs, the CTR1(+/+) and CTR1(-/-) cells were exposed to CBDCA, L-OHP, or transplatin at a concentration of 30 μM for 5 min. As shown in Fig. 3B, the magnitude of the effect for CBDCA was even greater than for DDP; the CTR1(-/-) cells accumulated only 0.4% as much CBDCA as the CTR1(+/+) cells (p < 0.03). In contrast, although the CTR1(-/-) cells also took up less L-OHP, the uptake was fully 32% of that in the CTR1(+/+) cells (p < 0.05), and there was no effect of the loss of CTR1 on the uptake of transplatin at all. Thus, CTR1 regulates the initial influx of the three clinically effective drugs, although the magnitude of the effect was less for l-OHP than for DDP and CBDCA as had been reported previously for 1-h drug exposures (Holzer et al., 2006a). However, it has no effect on the accumulation of transplatin, a molecule having the same composition as DDP, but with the chlorides in a trans-rather than cis-configuration around the central platinum atom, and that is very much less cytotoxic than DDP.
A comparison of data presented in Fig. 3, A and B, indicates that the initial influx of transplatin in the CTR1(+/+) cells was 1.3-fold higher than that of DDP, whereas the influx of CBDCA and l-OHP was 39 and 42% of that for DDP, respectively. The fact that the accumulation of transplatin was even greater than that of DDP and that the loss of CTR1 had no effect on the accumulation of transplatin suggests that transplatin enters cells by quite a different mechanism than DDP.
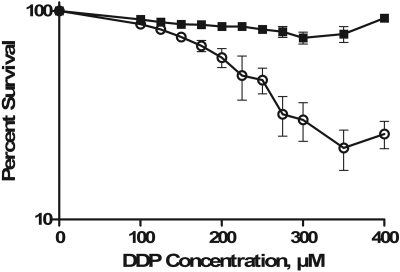
Effect of CTR1 on the Cytotoxicity of DDP. CTR1 is not the only route by which DDP can enter cells, as shown by the fact that loss of CTR1 did not block uptake completely. If the DDP entering the cell via CTR1 is an important component of the pool of intracellular drug that contributes to cell killing, then the reduction in DDP uptake that accompanies the loss of CTR1 function should be matched by a reduction in cytotoxicity. CTR1(+/+) and CTR1(-/-) cells were treated for 5 min with increasing concentrations of DDP, ranging from 0 to 400 μM, after which they were allowed to grow for 5 days before being stained with sulforhodamine B. Figure 4 shows that for CTR1(+/+) cells, there was a concentration-dependent inhibition of growth. However, even a concentration of 400 μM DDP produced no inhibition of the growth of CTR1(-/-) cells. Thus, the DDP that enters the cell via a CTR1-mediated process contributes directly to the cytotoxic pool of intracellular drug rather than being effectively detoxified by sequestration into endosomes or other subcellular compartments from which it has no access to critical cytotoxic targets, as might be expected if DDP were simply chelated to CTR1, endocytosed into the cell, and retained in a subcellular compartment.
Fig. 4.
Inhibition of CTR1(+/+) (○) and CTR1(-/-) (▪) growth rate as a function of concentration after a 5-min exposure to DDP. Each data point presents the mean of three independent experiments each performed with triplicate cultures for each drug concentration. Vertical bars, S.E.M.
Effect of Re-Expression of CTR1. To further demonstrate the importance of CTR1 to initial DDP influx and to demonstrate that hCTR1 and mCTR1 could mediate DDP transport, the CTR1(-/-) cells were infected with a lentiviral vector expressing an hCTR1 cDNA, and a clone of cells in which CTR1 expression had been restored was isolated and designated as CTR1(-/-/R). Using primers specific for mCTR1 and hCTR1 mRNA, the CTR1(-/-/R) cells were found to express hCTR1 mRNA at a level ∼50% of the mCTR1 mRNA expression in the CTR1(+/+) cells, as normalized to β-actin expression. As shown in Fig. 5A, re-expression of hCTR1 restored the initial influx of DDP over the first 5 min to a level similar to that observed in the CTR1(+/+) cells. Re-expression of hCTR1 also rendered the CTR1(-/-) cells sensitive to the growth-inhibitory effects of DDP (Fig. 5B). Thus, the reduced DDP influx and cytotoxicity in the CTR1(-/-) cells was due specifically to the loss of CTR1, and hCTR1 was as effective as mCTR1 in mediating the initial influx and cytotoxicity of DDP.
Fig. 5.
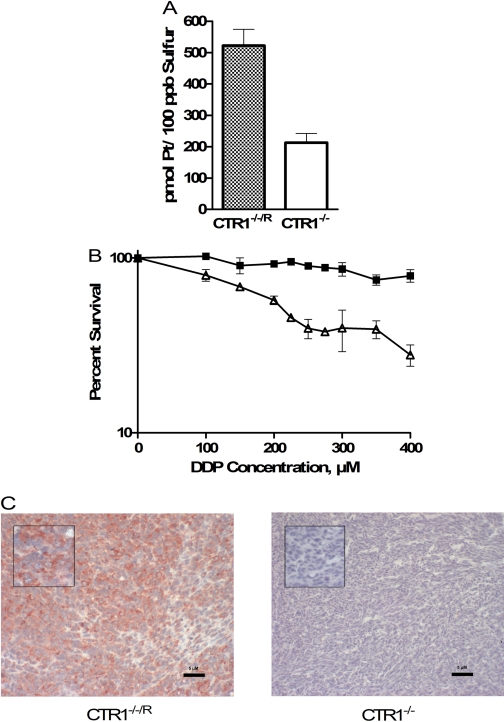
Effect of re-expression of CTR1 in CTR1(-/-) cells on drug uptake and in vitro cytotoxicity. A, platinum accumulation after a 5-min exposure to 30 μM DDP. B, concentration-survival curves for CTR1(-/-) (▪) and CTR1(-/-/R) (▵) cells after a 5-min exposure to DDP. C, CTR1 expression in CTR1(-/-/R) and CTR1(-/-) xenografts as determined by immunohistochemical analysis. Magnification: main image, 200×; inset, 400×. Vertical bars, S.E.M.
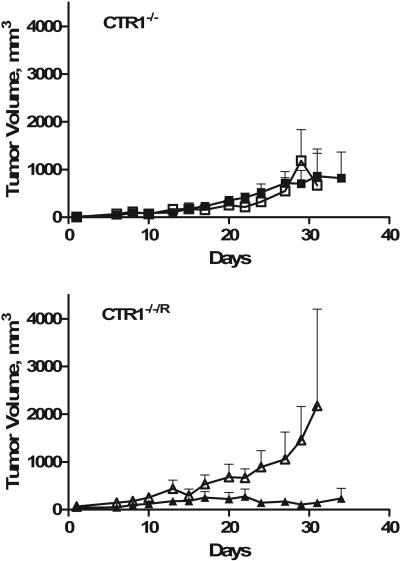
Effect of CTR1 on Sensitivity to DDP in Vivo. CTR1(-/-) and CTR1(-/-/R) cells were inoculated subcutaneously into nu/nu mice, and both types of cells formed tumors with equal frequency. Immunohistochemical analysis of sections from these tumors demonstrated robust expression of CTR1 in the CTR1(-/-/R) cells but none in the CTR1(-/-) cells (Fig. 5C). As shown in Fig. 6, the CTR1(-/-/R) tumors grew more rapidly than the CTR1(-/-) tumors. After a single intraperitoneal injection of a maximum tolerated dose of DDP (10 mg/kg), there was a clear reduction in the growth of the CTR1(-/-/R) tumors but no effect on the growth of the CTR1(-/-) tumors. Thus, consistent with the impairment of influx, loss of CTR1 rendered the tumor cells completely resistant to a maximum tolerated dose of DDP in vivo.
Fig. 6.
Sensitivity of CTR1(-/-) and CTR1(-/-/R) xenografts to treatment with DDP. Open symbols, treatment with vehicle only; closed symbols, mice treated with a single dose of DDP 10 mg/kg. Vertical bars, S.E.M.
Discussion
CTR1 plays a critical role in the regulation of copper uptake into the cell during normal development, and previous studies from this and other laboratories documented that it is also involved in the uptake of all three of the clinically used platinum-containing drugs. Loss of CTR1 was found to reduce the uptake of DDP, CBDCA, and L-OHP when measured at 1 h (Holzer et al., 2006a). Enhanced expression of CTR1 was also shown to increase DDP uptake in several different cell systems (Holzer et al., 2004; Song et al., 2004). The results of the current study provide further detail about the role and substrate-specificity of CTR1 as a platinum drug transporter.
As was observed previously in human ovarian cancer cells expressing hCTR1 (Holzer and Howell, 2006), exposure to DDP triggered rapid degradation of mCTR1 in the murine CTR1(+/+) cells, suggesting that the major contribution of CTR1 to DDP influx was likely to be during the first few minutes of drug exposure. The initial interaction of DDP with CTR1 seems to be very rapid, as demonstrated by the fact that measurable levels of platinum became associated with the cells in a CTR1-dependent manner within 15 s. The extracellular domain of CTR1 is rich in both methionine and histidine motifs potentially capable of chelating DDP, but attempts to produce the extracellular domain in Escherichia coli as a recombinant protein with which to further analyze binding have been unsuccessful because of its insolubility. It is noteworthy that the amount of platinum that became associated with the CTR1(+/+) cells when they were exposed briefly to CBDCA and L-OHP was less than that after exposure to DDP, and that the initial binding of these two drugs did not differ significantly between the CTR1(+/+) and CTR1(-/-) cells, indicating that the initial binding of these two drugs was not as dependent on CTR1 expression.
The availability of an isogenic pair of CTR1(+/+) and CTR1(-/-) cells allowed unequivocal demonstration that CTR1 plays a major role in the initial influx of DDP. Over a wide concentration range, loss of CTR1 markedly reduced the initial influx of DDP; when exposed to 10 μM DDP for 5 min, initial influx was reduced by 81%. There was a trend toward a greater magnitude of the effect at higher DDP concentrations, suggesting that other mechanisms which contribute to initial influx become saturated such that initial influx is even more dependent on CTR1. The dependence of initial DDP influx on CTR1 was further demonstrated by restoring CTR1 expression in the CTR1(-/-) cells. The re-expression of human rather than mouse CTR1 served to document both that restoration of CTR1 increased DDP uptake and that human and mouse CTR1 were capable of mediating this effect.
Loss of CTR1 had an even greater effect on reducing the initial influx of CBDCA than that of DDP, but, as observed when uptake was measured at 1 h in a previous study (Holzer et al., 2006a), there was a smaller effect on the initial influx of L-OHP whose uptake was reduced to only 32% of control. In addition, loss of CTR1 had no effect on the initial influx of transplatin. The fact that loss of CTR1 had a large effect on the initial influx of DDP but no effect on that of transplatin might suggest that the cis configuration is essential to the participation of CTR1; however, Kabolizadeh et al. (2007) reported recently that the trinuclear trans-platinum compound BBR3464 is also dependent on CTR1 for influx, making the structural significance of the cis versus trans configuration with respect to CTR1 transport uncertain at this time. In the case of cisplatin versus transplatin, we surmise that the cis configuration is required to allow the drug to bind to the methionine- and histidine-rich motifs of the extracellular domain of CTR1. Whether the differences in the magnitude of the effect of the loss of CTR1 on the initial influx of DDP versus CBDCA and l-OHP are related to their ability to interact with the extracellular domain of CTR1 remains to be determined. The fact that loss of CTR1 did not alter the initial binding of CBDCA and L-OHP detectable with exposures of <15 s is consistent with their generally slower rate of reaction with nucleophilic targets but is inconsistent with the observation that loss of CTR1 had as large an affect on the initial uptake of CBDCA as it did for DDP.
Previous studies in which overexpression of CTR1 was shown to increase whole-cell platinum levels after exposure to DDP, but not to increase the extent of DNA adduct formation or cytotoxicity (Holzer et al., 2004), raised the question of whether the DDP transferred into the cell in a CTR1-dependent manner was really available to attack critical targets or was just being trafficked to sites where it was detoxified by sequestration into subcellular organelles. A subsequent study using the same cells demonstrated that forced overexpression of CTR1 was in fact associated with increased sensitivity to DDP when apoptosis was used as a measure of cell death (Kabolizadeh et al., 2007), and the results reported here clearly demonstrate that the diminished influx of DDP associated with the loss of CTR1 was accompanied by reduced DDP cytotoxicity and that the cytotoxicity of DDP could be restored by re-expression of CTR1 in the CTR1(-/-) cells. Loss of CTR1 rendered cells completely resistant to even very high concentrations of DDP when they were exposed for 5 min in vitro. Our previous study demonstrated a 3.2-fold loss of sensitivity when cells were exposed for 1 h (Holzer et al., 2006a), which more closely approximates exposure to non-protein-bound DDP after administration of standard doses of DDP to patients. It is noteworthy that the results of the current study showed that despite the differences between the in vitro and in vivo exposures, this impairment of influx translated to loss of sensitivity in vivo as well. The CTR1(-/-/R) tumor xenografts responded well to a single maximum tolerated dose of DDP, whereas the CTR1(-/-) tumors demonstrated no detectable response at all. This suggests that CTR1 expression in tumors is likely to be an important determinant of tumor responsiveness to DDP. A broad survey of CTR1 expression in a variety of types of cancer demonstrated marked variability in the level of expression both within and between histologically defined tumor types (Holzer et al., 2006b). It remains to be determined whether CTR1 can serve as a clinically useful biomarker of responsiveness of human cancers to DDP, CBDCA, or L-OHP, and whether up-regulation of CTR1 expression using pharmacologic strategies or a gene therapy approach might enhance in vivo responsiveness.
How CTR1 mediates the uptake of DDP, CBDCA, and L-OHP remains an enigma. The pore created by the trimeric CTR1 complex through which Cu+ is believed to enter cells is too small to accommodate the platinum-containing drugs (Aller and Unger, 2006; De Feo et al., 2007), and recent studies with yeast CTR1 indicate that copper and DDP produce different changes in the structure of CTR1 (Sinani et al., 2007). Our prior studies indicated that DDP uptake by CTR1 requires micropinocytosis (Holzer and Howell, 2006), suggesting a model in which DDP binds to the extracellular domain of CTR1 and is rapidly internalized into endosomes. A similar phenomenon has been suggested by researchers studying the yeast variant of CTR1 (Sinani et al., 2007). How DDP escapes from such endosomes and finds its way to DNA in the nucleus remains one of the key questions about the cellular pharmacology of this drug.
Acknowledgments
We thank Dr. Dennis Thiele for kindly providing the isogenic pair of CTR1(+/+) and CTR1(-/-) cells used in this study. We also thank Dr. Danielle Jandial, Gerald Manorek, and Nicole Chung for their technical expertise and invaluable discussion.
This work was supported by the National Institutes of Health National Cancer Institute [Grants CA78648 and 5T32-CA121938]. The production of 64Cu at Washington University School of Medicine was supported by the National Institutes of Health [Grant R24-CA86307].
Portions of this work were presented at the 2008 Annual Meeting of the American Association for Cancer Research: Larson C, Chung N, and Howell S (2008) Role of mammalian copper transporter (CTR1) in the cellular accumulation of cisplatin, carboplatin and oxaliplatin. Proc Am Assoc Cancer Res 49:4771.
ABBREVIATIONS: CTR1, mammalian copper transporter 1; CBDCA, carboplatin; DDP, cisplatin; L-OHP, oxaliplatin; PBS, phosphate-buffered saline; ddH2O, double-distilled H2O; qRT-PCR, qualitative reverse transcriptase-polymerase chain reaction; BBR3464, [{trans-PtCl(NH3)2}2μ-(trans-Pt(NH3)2(H2N(CH2)6NH2)2)]4+.
References
- Aller SG, Eng ET, De Feo CJ, and Unger VM (2004) Eukaryotic CTR copper uptake transporters require two faces of the third transmembrane domain for helix packing, oligomerization, and function. J Biol Chem 279 53435-53441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aller SG and Unger VM (2006) Projection structure of the human copper transporter CTR1 at 6-A resolution reveals a compact trimer with a novel channel-like architecture. Proc Natl Acad Sci U S A 103 3627-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamurugan K and Schaffner W (2006) Copper homeostasis in eukaryotes: teetering on a tightrope. Biochim Biophys Acta 1763 737-746. [DOI] [PubMed] [Google Scholar]
- Bertinato J and L'Abbé MR (2004) Maintaining copper homeostasis: regulation of copper-trafficking proteins in response to copper deficiency or overload. J Nutr Biochem 15 316-322. [DOI] [PubMed] [Google Scholar]
- Dancis A, Haile D, Yuan DS, and Klausner RD (1994a) The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J Biol Chem 269 25660-25667. [PubMed] [Google Scholar]
- Dancis A, Yuan DS, Haile D, Askwith C, Eide D, Moehle C, Kaplan J, and Klausner RD (1994b) Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell 76 393-402. [DOI] [PubMed] [Google Scholar]
- De Feo CJ, Aller SG, and Unger VM (2007) A structural perspective on copper uptake in eukaryotes. Biometals 20 705-716. [DOI] [PubMed] [Google Scholar]
- Hall MD, Okabe M, Shen DW, Liang XJ, and Gottesman MM (2008) The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol Toxicol 48 495-535. [DOI] [PubMed] [Google Scholar]
- Hamza I, Faisst A, Prohaska J, Chen J, Gruss P, and Gitlin JD (2001) The metallochaperone Atox1 plays a critical role in perinatal copper homeostasis. Proc Natl Acad Sci U S A 98 6848-6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza I, Prohaska J, and Gitlin JD (2003) Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc Natl Acad Sci U S A 100 1215-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer AK and Howell SB (2006) The internalization and degradation of human copper transporter 1 following cisplatin exposure. Cancer Res 66 10944-10952. [DOI] [PubMed] [Google Scholar]
- Holzer AK, Manorek GH, and Howell SB (2006a) Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol 70 1390-1394. [DOI] [PubMed] [Google Scholar]
- Holzer AK, Samimi G, Katano K, Naerdemann W, Lin X, Safaei R, and Howell SB (2004) The copper influx transporter human copper transport protein 1 regulates the uptake of cisplatin in human ovarian carcinoma cells. Mol Pharmacol 66 817-823. [DOI] [PubMed] [Google Scholar]
- Holzer AK, Varki NM, Le QT, Gibson MA, Naredi P, and Howell SB (2006b) Expression of the human copper influx transporter 1 in normal and malignant human tissues. J Histochem Cytochem 54 1041-1049. [DOI] [PubMed] [Google Scholar]
- Ishida S, Lee J, Thiele DJ, and Herskowitz I (2002) Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci U S A 99 14298-14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabolizadeh P, Ryan J, and Farrell N (2007) Differences in the cellular response and signaling pathways of cisplatin and BBR3464 ([{trans-PtCl(NH3)2}2[mu]-(trans-Pt(NH3)2(H2N(CH2)6-NH2)2)]4+) influenced by copper homeostasis. Biochem Pharmacol 73 1270-1279. [DOI] [PubMed] [Google Scholar]
- Kelland L (2007) The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7 573-584. [DOI] [PubMed] [Google Scholar]
- Kim BE, Nevitt T, and Thiele DJ (2008) Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol 4 176-185. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Zhou B, Cosco D, and Gitschier J (2001) The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci U S A 98 6836-6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Petris MJ, and Thiele DJ (2002) Characterization of mouse embryonic cells deficient in the Ctr1 high affinity copper transporter. J Biol Chem 277 40253-40259. [DOI] [PubMed] [Google Scholar]
- Lee J, Prohaska JR, Dagenais SL, Glover TW, and Thiele DJ (2000) Isolation of a murine copper transporter gene, tissue specific expression and functional complementation of a yeast copper transport mutant. Gene 254 87-96. [DOI] [PubMed] [Google Scholar]
- Lin X, Okuda T, Holzer A, and Howell SB (2002) The copper transporter CTR1 regulates cisplatin uptake in saccharomyces cerevisiae. Mol Pharmacol 62 1154-1159. [DOI] [PubMed] [Google Scholar]
- Madsen E and Gitlin JD (2007) Copper deficiency. Curr Opin Gastroenterol 23 187-192. [DOI] [PubMed] [Google Scholar]
- Møller LB, Petersen C, Lund C, and Horn N (2000) Characterization of the hCTR1 gene: genomic organization, functional expression, and identification of a highly homologous processed gene. Gene 257 13-22. [DOI] [PubMed] [Google Scholar]
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, and Vaigro-Wolff A (1991) Feasibility of a high-flux anticancer drug screen using a diverse of cultured human tumor cell lines. J Natl Cancer Inst 83 757-766. [DOI] [PubMed] [Google Scholar]
- Ooi CE, Rabinovich E, Dancis A, Bonifacino JS, and Klausner RD (1996) Copper-dependent degradation of the Saccharomyces cerevisiae plasma membrane copper transporter Ctr1p in the apparent absence of endocytosis. EMBO J 15 3515-3523. [PMC free article] [PubMed] [Google Scholar]
- Petris MJ, Smith K, Lee J, and Thiele DJ (2003) Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem 278 9639-9646. [DOI] [PubMed] [Google Scholar]
- Sinani D, Adle DJ, Kim H, and Lee J (2007) Distinct mechanisms for CTR1-mediated copper and cisplatin transport. J Biol Chem 282 26775-26785. [DOI] [PubMed] [Google Scholar]
- Song IS SN, Siddik ZH, Liu P, Wei Y, Wu CJ, Kuo MT (2004) Roles of copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and resistant cells. Mol Cancer Ther 3 1543-1549. [PubMed] [Google Scholar]