Abstract
The aim of the current study is to determine whether butein (3,4,2′,4′-tetrahydroxychalcone) exhibits antiproliferative effects against tumor cells through suppression of the signal transducer and activator of transcription 3 (STAT3) activation pathway. We investigated the effects of butein on constitutive and inducible STAT3 activation, role of tyrosine kinases and phosphatases in STAT3 activation, STAT3-regulated gene products, and growth modulation of tumor cells. We found that this chalcone inhibited both constitutive and interleukin-6-inducible STAT3 activation in multiple myeloma (MM) cells. The suppression was mediated through the inhibition of activation of the upstream kinases c-Src, Janus-like kinase (JAK) 1, and JAK2. Vanadate treatment reversed the butein-induced down-regulation of STAT3 activation, suggesting the involvement of a tyrosine phosphatase. Indeed, we found that butein induced the expression of the tyrosine phosphatase SHP-1 and deletion of SHP-1 gene by small interfering RNA abolished the ability of butein to inhibit STAT3 activation, suggesting the critical role of SHP-1 in the action of this chalcone. Butein down-regulated the expression of STAT3-regulated gene products such as Bcl-xL, Bcl-2, cyclin D1, and Mcl-1, and this led to the suppression of proliferation and induction of apoptosis. Consistent with these results, overexpression of constitutive active STAT3 significantly reduced the butein-induced apoptosis. Moreover, we found that butein significantly potentiated the apoptotic effects of thalidomide and Velcade in MM cells. Overall, these results suggest that butein is a novel blocker of STAT3 activation and thus may have potential in suppression of tumor cell proliferation and reversal of chemoresistance in MM cells.
Signal transducer and activator of transcription (STAT) is a family of six different transcription factors, first discovered in 1993 by James Darnell, that play major roles in cytokine signaling (Shuai et al., 1993). A typical STAT protein consists of a coiled-coil domain, a DNA binding domain, a linker, an SH2 domain, and a transactivation domain. The transactivation domain contains tyrosine and serine phosphorylation sites that are needed for the activation of STAT. Engagement of cell surface cytokine receptors activates the Janus-like kinase (JAK) family of protein kinases, which in turn phosphorylates and activates latent cytoplasmic STAT3 protein to an active dimer capable of translocating to the nucleus and inducing the transcription of specific target genes. Several other kinases have been implicated in the phosphorylation of STAT3, including members of the Src family (hck, src), Erb B1, Erb B2, anaplastic lymphoma kinase, protein kinase C-δ, c-fes, Gp130, and epithelial growth factor (EGF) receptor (Nelson et al., 1998; Jain et al., 1999; Zhang et al., 2000).
The role of STAT3 in cancer is indicated by numerous avenues of evidence, including the following: 1) STAT3 is often constitutively active in many human cancer cells, including multiple myeloma, lymphomas, leukemia, breast cancer, prostate cancer, and head and neck squamous cell carcinoma (Aggarwal et al., 2006); 2) STAT3 is activated by growth factors (e.g., EGF, transforming growth factor-α, IL-6, hepatocyte growth factor) and oncogenic kinases (e.g., Src); 3) this transcription factor has been shown to regulate the expression of genes that mediate proliferation (e.g., c-myc and cyclin D1), suppress apoptosis (e.g., Bcl-xL and survivin), and promote angiogenesis (e.g., vascular endothelial growth factor); and 4) STAT3 activation has been linked with chemoresistance and radioresistance (Otero et al., 2006; Bhardwaj et al., 2007). Thus, inhibitors of STAT3 activation have the potential for both prevention and treatment of cancer.
Numerous cell culture, animal, and epidemiological studies in humans suggest that fruits and vegetables can prevent cancer (Willett, 1994), but it is often unclear what constituents in fruit and vegetable prevent cancer and how they do it. One of the potential sources of STAT3 inhibitors is an agent derived from natural sources. Butein (3,4,2′,4′-tetrahydroxychalcone) is one such agent that has been identified from numerous plants, including the stem bark of cashews (Semecarpus anacardium), the heartwood of Dalbergia odorifera, and the traditional Chinese and Tibetan medicinal herbs Caragana jubata and Rhus verniciflua Stokes. It is established that butein can suppress the proliferation of a wide variety of human tumor cells, including breast carcinoma (Wang et al., 2005), colon carcinoma (Yit and Das, 1994), osteosarcoma (Jang et al., 2005), lymphoma (Lee et al., 2004), acute myelogenous leukemia (Kim et al., 2001), melanoma (Iwashita et al., 2000), and hepatic satellite cells (Lee et al., 2006); however, the mechanism by which it mediates its effects is not fully understood. Because of the critical role of STAT3 in tumor cell survival, proliferation, and angiogenesis, we hypothesized that butein mediates its effects in part through the suppression of STAT3 pathway. We present evidence to support this hypothesis.
Materials and Methods
Reagents. Butein, with the chemical structure shown in Fig. 1A, was obtained from Alexis Laboratories (San Diego, CA). A 50 mM solution of butein was prepared in dimethyl sulfoxide, stored as small aliquots at -20°C, and then diluted as needed in cell culture medium. Hoechst 33342, MTT, Tris, glycine, NaCl, SDS, and bovine serum albumin were purchased from Sigma-Aldrich (St. Louis, MO). RPMI 1640, fetal bovine serum (FBS), 0.4% trypan blue vital stain, and antibiotic-antimycotic mixture were obtained from Invitrogen (Carlsbad, CA). Rabbit polyclonal antibodies to STAT3, phosphospecific Akt (Ser473), Akt, and mouse monoclonal antibodies against phospho-STAT3 (Tyr705), Bcl-2, Bcl-xL, Mcl-1, SHP-1, cyclin D1, procaspase-3, and poly(ADP-ribose) polymerase were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to phosphospecific Src (Tyr416), Src, phosphospecific JAK1 (Tyr1022/1023), JAK1, and JAK2 were purchased from Cell Signaling Technology (Danvers, MA). Goat anti-mouse horseradish peroxidase was purchased from Transduction Laboratories (Lexington, KY), and goat anti-rabbit Alexa Fluor 594 was purchased from Invitrogen. AG490 was obtained from Calbiochem (San Diego, CA). Bacteria-derived recombinant human IL-6 was kindly provided by Novartis Pharmaceuticals (East Hanover, NJ). The siRNA for SHP-1, STAT3, and the scrambled control were obtained from Ambion (Austin, TX). Velcade (PS-341) was obtained from Millennium Pharmaceuticals (Cambridge, MA). Thalidomide was obtained from Tocris Cookson (St. Louis, MO). GST-JAK2 substrate was kindly provided by Dr. Zhizhuang Joe Zhao (Department of Pathology, University of Oklahoma Health Sciences Center, Oklahoma City, OK). The constitutively active STAT3 construct was kindly provided by Dr. John DiGiovanni from The University of Texas M.D. Anderson Cancer Center (Smithville, TX).
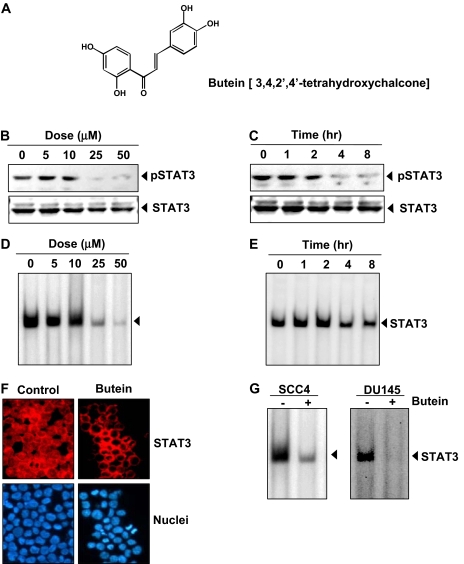
Fig. 1.
Butein inhibits constitutively active STAT3 in U266 cells. A, the chemical structure of butein (2′,4′,3,4-tetrahydroxychalcone). B, butein suppresses phospho-STAT3 levels in a dose-dependent manner. U266 cells (2 × 106/ml) were treated with the indicated concentrations of butein for 4 h, after which whole-cell extracts were prepared, and 30 μg of protein was resolved on 7.5% SDS-PAGE gel, electrotransferred onto nitrocellulose membranes, and probed for phospho-STAT3. C, butein suppresses phospho-STAT3 levels in a time-dependent manner. U266 cells (2 × 106/ml) were treated with 50 μM butein for the indicated durations and analyzed for phospho-STAT3 levels. The results shown are representative of three independent experiments. D, U266 cells (2 × 106/ml) were treated with the indicated concentrations of butein for 4 h and analyzed for nuclear STAT3 levels by EMSA. E, U266 cells (2 × 106/ml) were treated with 50 μM butein for the indicated durations and analyzed for nuclear STAT3 levels by EMSA. F, butein causes inhibition of translocation of STAT3 to the nucleus. U266 cells (1 × 105/ml) were incubated with or without 50 μM butein for 4 h and then analyzed for the intracellular distribution of STAT3 by immunocytochemistry. The same slides were counterstained for nuclei with Hoechst (50 ng/ml) for 5 min. G, SCC4 (2 × 106/ml) and DU145 (2 × 106/ml) cells were incubated with 50 μM butein for 4 h, and nuclear extracts were prepared and analyzed for STAT3 activation by EMSA. The results shown are representative of three independent experiments.
Cell Lines. Human multiple myeloma (MM) U266, MM.1S (dexamethasone-sensitive), human prostate cancer DU145, human head and neck cancer SCC4, and human embryonic kidney A293 cells were obtained from the American Type Culture Collection (Manassas, VA). Cell line U266 (American Type Culture Collection TIB-196) is a plasmacytoma of B-cell origin and is known to produce monoclonal antibodies and IL-6 (Nilsson et al., 1970; Kawano et al., 1988). The MM.1S cell line, established from the peripheral blood cells of a patient with IgA myeloma, secretes L chain, is negative for the presence of the EBV genome, and expresses leukocyte antigen DR, plasma cell Ag-1, and T9 and T10 antigens. U266, MM.1S, and DU145 cells were cultured in RPMI 1640 medium containing 1× penicillin-streptomycin solution (Invitrogen) with 10% FBS; A293 cells were cultured in DMEM containing 1× penicillin-streptomycin solution (Invitrogen) with 10% FBS. SCC4 cells were cultured in DMEM containing 1× penicillin-streptomycin solution (Invitrogen), minimum essential amino acid, vitamin solution, and glutamine with 10% FBS.
Electrophoretic Mobility Shift Assay for STAT3-DNA Binding. STAT3-DNA binding was analyzed by EMSA using a 32P-labeled high-affinity sis-inducible element probe (5′-CTTCATTTCCCGT AAATCCCT AAA GCT-3′ and 5′-AGCTTTAGGGATTTACGGGAAATGA-3′). In brief, nuclear extracts were prepared from butein-treated cells and incubated with high-affinity sis-inducible element probe. The DNA-protein complex formed was separated from free oligonucleotide on 5% native polyacrylamide gels. The dried gels were visualized, and the radioactive bands were quantitated with a Storm 820 and Imagequant software (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK).
Western Blotting. To detect various proteins, we treated either U266 or MM.1S cells (2 × 106/ml) with butein. The cells were then washed and extracted by incubation for 30 min on ice in 0.05 ml of buffer containing 20 mM HEPES, pH 7.4, 2 mM EDTA, 250 mM NaCl, 0.1% Nonidet P-40, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, 0.5 μg/ml benzamidine, 1 mM dithiothreitol, and 1 mM sodium vanadate. The lysate was centrifuged, and the supernatant was collected. Whole-cell protein (30 μg) was resolved on 7.5 to 12% SDS-PAGE, electrotransferred onto a nitrocellulose membrane, blotted with antibodies, and then detected by enhanced chemiluminescence (GE Healthcare). Blots were analyzed by densitometry using a Kodak Image Station (Carestream Health, Rochester, NY).
Immunocytochemistry for STAT3 Localization. Butein-treated cells were plated on a glass slide by centrifugation using a Cytospin 4 (Thermoshendon, Pittsburg, PA), air-dried for 1 h at room temperature, and fixed in 4% formaldehyde. After a brief washing in phosphate-buffered saline, slides were blocked with 5% normal goat serum for 1 h and then incubated with rabbit polyclonal anti-human STAT3 antibody (dilution, 1:100). After overnight incubation, the slides were washed and then incubated with goat anti-rabbit IgG-Alexa 594 (1:100) for 1 h and counterstained for nuclei with Hoechst (50 ng/ml) for 5 min. Stained slides were mounted with mounting medium and analyzed under an epifluorescence microscope (Labo-phot-2; Nikon, Tokyo, Japan). Pictures were captured using a Photometrics Coolsnap CF color camera (Nikon) and MetaMorph version 4.6.5 software (Molecular Devices, Sunnyvale, CA).
MTT Assay. The cytotoxic effect of butein was determined by the MTT dye uptake method.
Live/Dead Assay. Viability of cells was also determined by Live/Dead assay (Invitrogen) that measures intracellular esterase activity and plasma membrane integrity. To see the effect of butein in cells transfected with STAT3 siRNA, SCC4 cells were plated in each chamber of four-chamber slides and allowed to adhere for 24 h. On the day of transfection, 6 μl of HiPerFect transfection reagents (QIAGEN, Valencia, CA) were added to 50 nM STAT3 siRNA in a final volume of 100 μl of culture medium. After 48 h of transfection, cells were treated with butein for 24 h, and cell viability was determined by Live/Dead assay.
RNA Analysis and RT-PCR. U266 cells were left untreated or treated with butein, washed, and suspended in TRIzol reagent. Total RNA was extracted according to the manufacturer's instructions (Invitrogen). One microgram of total RNA was converted to cDNA by Superscript reverse transcriptase and then amplified by Platinum Taq polymerase using Superscript One Step RT-PCR kit (Invitrogen). The relative expression of Bcl-2 and Bcl-xL and SHP-1 was analyzed using quantitative RT-PCR with GAPDH as an internal control. The RT-PCR reaction mixture contained 12.5 μl of 2 × reaction buffer, 1 μg of total RNA, 0.5 μl each of forward and reverse primers, and 1 μl of RT-Platinum Taq in a final volume of 50 μl. The reaction was performed at 50°C for 30 min, 94°C for 2 min, 94°C for 30 cycles of 15 s each, 60°C for 30 s, and 72°C for 1 min with extension at 72°C for 10 min. PCR products were run on 2% agarose gel and then stained with ethidium bromide. Stained bands were visualized under UV light and photographed.
Kinase Assay. To determine the effect of butein on JAK2 activation, we performed an immunocomplex kinase assay using GST-JAK2 as the substrate as described previously (Li et al., 2007). In brief, the JAKs complex from whole-cell extracts was precipitated with antibody against JAK2 and treated with protein A/G-agarose beads (Pierce, Rockford, IL). After 2 h, the beads were washed with whole-cell extract buffer and then resuspended in a kinase assay mixture containing 50 mM HEPES, pH 7.4, 20 mM MgCl2, 2 mM dithiothreitol, 20 μCi [γ-32P]ATP, 10 μM unlabeled ATP, and 2 μg of substrate GST-JAK2. After incubation at 30°C for 30 min, the reaction was terminated by boiling with SDS sample buffer for 5 min. Finally, the protein was resolved on 10% SDS-PAGE, the gel was dried, and the radioactive bands were visualized with the Storm 820 imaging system. To determine the total amounts of JAK2 in each sample, 30 μg of whole-cell proteins was resolved on 10% SDS-PAGE, electro-transferred to a nitrocellulose membrane, and then blotted with anti-JAK2 antibody.
Transfection with SHP-1 siRNA. SCC4 cells were plated in each well of six-well plates and allowed to adhere for 24 h. On the day of transfection, 12 μl of HiPerFect transfection reagent (QIAGEN) was added to 50 nM SHP-1 siRNA in a final volume of 100 μl of culture medium. After 48 h of transfection, cells were treated with butein for 4 h, and whole-cell extracts were prepared for SHP-1, STAT3, and phospho-STAT3 analysis by Western blot.
Transfections with Constitutive STAT3 Construct. A293 cells were plated in six-well plates or in chamber slides in DMEM containing 10% FBS. After 24 h, the cells were transfected with constitutive STAT3-plasmid by FuGene 6 according to manufacturer's protocol (Roche, Indianapolis, IN). Twenty-four hours after the transfection, cells were harvested to confirm transfection by Western blot. Cells transfected in the chamber slides were treated with butein for 24 h, and viability of the cells was determined by Live/Dead assay.
Results
The present study was undertaken to determine the effect of butein on STAT3 signaling pathway. We investigated the effect of butein on both constitutive and IL-6-inducible STAT3 activation in MM, human prostate cancer (DU145), and human head and neck cancer (SCC4) cells. We also evaluated the effect of butein on various mediators of cellular proliferation, cell survival, and apoptosis. The structure of butein is shown in Fig. 1A.
Butein Inhibits Constitutive STAT3 Phosphorylation in MM Cells. We investigated whether butein can modulate constitutive STAT3 activation in MM cells. U266 cells were incubated with different concentrations of butein for 4 h, and whole-cell extracts were prepared and examined for phosphorylated STAT3 by Western blot analysis using an antibody that recognizes STAT3 phosphorylated at tyrosine 705. As shown in Fig. 1B, butein inhibited the constitutive activation of STAT3 in U266 cells, with maximum inhibition occurring at 25 μM. Under these conditions, butein had no effect on the expression of STAT3 protein (Fig. 1B, bottom) or on cell viability (data not shown). We also determined the incubation time with butein required for suppression of STAT3 activation in U266 cells. As shown in Fig. 1C, the inhibition was time-dependent, with maximum inhibition occurring at 4 h, again with no effect on the expression of STAT3 protein (Fig. 1C, bottom). This time of exposure had no effect on cell viability (data not shown).
Butein Inhibits STAT3 DNA Binding Activity in MM Cells. Because tyrosine phosphorylation causes dimerization of STATs and their translocation to the nucleus, where they bind to DNA and regulate gene transcription (Yu et al., 1995), we determined whether butein suppresses DNA binding activity of STAT3. EMSA analysis of nuclear extracts prepared from U266 cells showed that butein decreased STAT3 DNA binding activity in a dose-(Fig. 1D) and time-dependent manner (Fig. 1E). These results show that butein abrogates the DNA-binding ability of STAT3.
Butein Depletes Nuclear Pool of STAT3 in MM Cells. Because nuclear translocation is central to the function of transcription factors and because it is not certain whether phosphorylation is mandatory for nuclear transport of STAT3 and its oncogenic functions (Bowman et al., 2000), we determined whether butein suppresses nuclear translocation of STAT3. Figure 1F clearly demonstrates that butein inhibited the translocation of STAT3 to the nucleus in U266 cells.
Inhibition of STAT3 Activation by Butein Is Not Cell Type-Specific. We examined the effect of butein on constitutive STAT3 activation in head and neck carcinoma SCC4 and prostate carcinoma DU145 cells. EMSA showed that butein inhibited STAT3-DNA binding in both cell types (Fig. 1G), suggesting that inhibition of STAT3 by butein is not only limited to multiple myeloma.
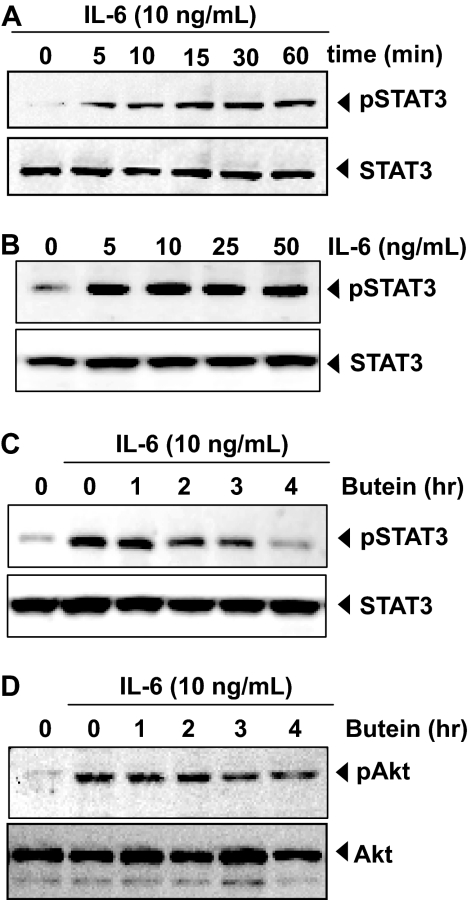
Butein Inhibits Inducible STAT3 Phosphorylation in Human MM Cells. Because IL-6 is a growth factor for MM and induces STAT3 phosphorylation (Kawano et al., 1988), we determined whether butein could inhibit IL-6-induced STAT3 phosphorylation. MM.1S cells, which lack constitutively active STAT3, were treated with IL-6 at different times and concentrations and examined for phosphorylated STAT3. As shown in Fig. 2A, IL-6 activated STAT3 as early as 5 min, and that continued to increase at 60 min. IL-6 induced phosphorylation of STAT3 at 5 ng/ml (Fig. 2B). In MM.1S cells incubated with butein for different times, IL-6-induced STAT3 phosphorylation was suppressed. Exposure of cells to butein for 4 h was sufficient to completely suppress IL-6-induced STAT3 phosphorylation (Fig. 2C).
Fig. 2.
Butein down-regulates IL-6-induced phospho-STAT3 and Akt. A, MM.1S cells (2 × 106/ml) were treated with IL-6 (10 ng/ml) for the indicated time, whole-cell extracts were prepared, and phospho-STAT3 was detected by Western blot. The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. B, MM.1S cells (2 × 106/ml) were treated with IL-6 with the indicated concentrations for 15 min, whole-cell extracts were prepared, and phospho-STAT3 was detected by Western blot. The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. C, MM.1S cells (2 × 106/ml) were treated with 50 μM butein for the indicated times and then stimulated with IL-6 (10 ng/ml) for 15 min. Whole-cell extracts were then prepared and analyzed for phospho-STAT3 by Western blotting. The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. The results shown are representative of three independent experiments. D, MM.1S cells (2 × 106/ml) were treated with 50 μM butein for the indicated times and then stimulated with IL-6 (10 ng/ml) for 15 min. Whole-cell extracts were then prepared and analyzed for phospho-Akt by Western blotting. The same blots were stripped and reprobed with Akt antibody to verify equal protein loading.
Butein Inhibits IL-6-Inducible Akt Phosphorylation in Human MM Cells. Activated Akt has been shown to play a critical role in the mechanism of action of IL-6. Moreover, activation of Akt has also been linked with STAT3 activation. We also examined whether butein could modulate IL-6-induced Akt activation. Treatment of MM.1S cells with IL-6 induced phosphorylation of Akt and treatment of cells with butein partially suppressed the activation (Fig. 2D). Under these conditions, butein had no effect on the expression of Akt protein.
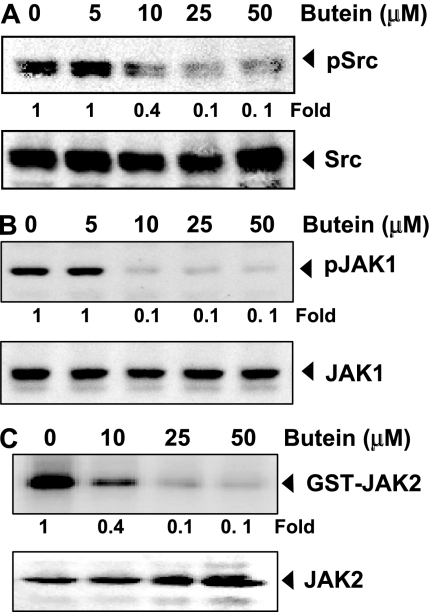
Butein Suppresses Constitutive Activation of c-Src. STAT3 has also been reported to be activated by soluble tyrosine kinases of the Src kinase families. Hence, we determined the effect of butein on constitutive activation of Src kinase in U266 cells. We found that butein suppressed the constitutive phosphorylation of c-Src kinase (Fig. 3A). The levels of nonphosphorylated c-Src kinase remained unchanged under the same conditions.
Fig. 3.
A, Butein suppresses phospho-Src levels. U266 cells (2 × 106/ml) were treated with the indicated concentrations of butein, after which whole-cell extracts were prepared, and 30-μg aliquots of those extracts were resolved on 10% SDS-PAGE, electrotransferred onto nitrocellulose membranes, and probed for phospho-Src antibody. The same blots were stripped and reprobed with Src antibody to verify equal protein loading. The blots were quantitated, and relative induction is mentioned as a -fold. B, butein suppresses phospho-JAK1 expression. U266 cells (2 × 106/ml) were treated with the indicated concentrations of butein, after which whole-cell extracts were prepared and 30-μg aliquots of those extracts were resolved on 10% SDS-PAGE, electrotransferred onto nitrocellulose membranes, and probed for phospho-JAK1 antibody. The same blots were stripped and reprobed with JAK1 antibody to verify equal protein loading. The blots were quantitated, and relative induction is mentioned as a -fold. C, butein suppresses JAK2 activity. U266 cells (2 × 106/ml) were treated with the indicated concentrations of butein, after which whole-cell extracts were prepared, and kinase assay was performed. The blots were quantitated, and relative induction is mentioned as a -fold. The results shown are representative of three independent experiments.
Butein Suppresses Constitutive Activation of JAK1. STAT3 has been reported to be activated by soluble tyrosine kinases of the Janus family (JAK), we determined whether butein affects constitutive activation of JAK1 in U266 cells. We found that butein suppressed the constitutive phosphorylation of JAK1 (Fig. 3B). The levels of nonphosphorylated JAK1 remained unchanged under the same conditions (Fig. 3B, bottom).
Butein Suppresses Constitutive JAK2 Activity. We investigated whether butein affects JAK2 activity in U266 cells. We found that butein suppressed the constitutive activation of JAK2 without affecting the expression of the kinase protein (Fig. 3C).
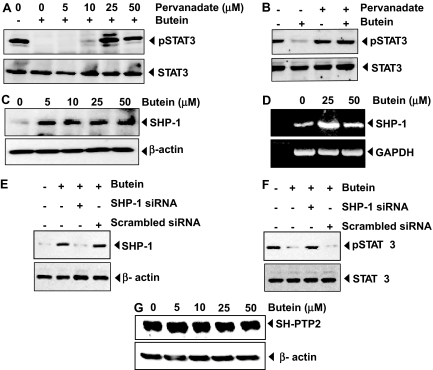
Tyrosine Phosphatases Are Involved in Butein-Induced Inhibition of STAT3 Activation. Because protein tyrosine phosphatases have been implicated in STAT3 activation, we determined whether butein-induced inhibition of STAT3 tyrosine phosphorylation could be due to activation of a protein tyrosine phosphatase (PTPase). Treatment of U266 cells with the broad-acting tyrosine phosphatase inhibitor sodium pervanadate prevented the butein-induced inhibition of STAT3 activation (Fig. 4A). This suggests that tyrosine phosphatases are involved in butein-induced inhibition of STAT3 activation. Whether constitutive phosphorylation of STAT3 is modulated by pervanadate was also examined. We found that sodium pervanadate did not affect the basal level of STAT3 phosphorylation but reversed the butein-induced inhibition of STAT3 phosphorylation (Fig. 4B).
Fig. 4.
A and B, pervanadate reverses the phospho-STAT3 inhibitory effect of butein. A, U266 cells (2 × 106/ml) were treated with the indicated concentration of pervanadate and 50 μM butein for 4 h, after which whole-cell extracts were prepared, and 30-μg portions of those extracts were resolved on 7.5% SDS-PAGE gel, electrotransferred onto nitrocellulose membranes, and probed for phospho-STAT3 and STAT3. B, pervanadate affects the phosphorylation level of STAT3 in the presence or absence of butein. C and D, butein induces the levels SHP-1 and mRNA expression in U266 cells. Cells (2 × 106/ml) were treated with the indicated concentrations of butein for 4 h; after completion of incubation, either whole-cell extract or RNA was isolated. Portions (30 μg) of those extracts were resolved on 10% SDS-PAGE, electrotransferred onto nitrocellulose membranes, and probed for SHP-1 antibody. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. RNA samples were subjected to RT-PCR with SHP-1 specific primers. E and F, effect of SHP-1 knockdown on butein-induced expression of SHP-1. SCC4 cells (1 × 105/ml) were transfected with either SHP-1-specific or scrambled siRNA (50 nM). After 48 h, cells were treated with 50 μM butein for 4 h, and whole-cell extracts were subjected to Western blot analysis for SHP-1. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading (E), and transfection with SHP-1 siRNA reverses butein-induced suppression of STAT3 activation. The same whole-cell extracts were subjected to phospho-STAT3 and STAT3 (F). G, the effect of butein on SH-PTP2. U266 cells (2 × 106/ml) were treated with the indicated concentrations of butein, after which whole-cell extracts were prepared, and Western blot was performed against SH-PTP2 antibody. The same blot was reprobed with β-actin antibody to verify equal protein loading. The results shown are representative of three independent experiments.
Butein Induces the Expression of SHP-1 in MM Cells. SHP-1 is a nontransmembrane protein tyrosine phosphatase expressed most abundantly in hematopoietic cells (Wu et al., 2003). We therefore examined whether butein can modulate the expression of SHP-1 in U266 cells. Cells were incubated with different concentrations of butein for 4 h, and whole-cell extracts were prepared and examined for SHP-1 protein by Western blot analysis. As shown in Fig. 4C, butein induced the expression of SHP-1 protein in U266 cells.
The activation of STAT3 has been shown to be mediated by negative regulation of protein tyrosine phosphatase activity through methylation-mediated gene-silencing, as noted in leukemia and lymphoma (Oka et al., 2002). Whether modulation of SHP-1 by butein is regulated at the transcriptional level was examined. We found that treatment of butein induces the expression of the mRNA for SHP-1 (Fig. 4D). Therefore, these results suggest that stimulation of SHP-1 expression by butein may mediate the down-regulation of constitutive STAT3 activation in U266 cells.
SHP-1 siRNA Down-Regulates the Expression of SHP-1 and Reverses the Inhibition of STAT3 Activation by Butein. We determined whether the suppression of SHP-1 expression by siRNA would abrogate the inhibitory effect of butein on STAT3 activation. Western blotting showed that butein-induced SHP-1 expression was effectively abolished in the cells treated with SHP-1 siRNA; treatment with scrambled siRNA had no effect (Fig. 4E). We also found that butein failed to suppress STAT3 activation in cells treated with SHP-1 siRNA (Fig. 4F). These results suggest the critical role of SHP-1 in the suppression of STAT3 phosphorylation by butein.
Effect of Butein on SH-PTP2. Whether the effect of butein on SHP-1 activation is specific or whether it also induces other PTPases was investigated. We found that butein had no effect on SH-PTP2 activation (Fig. 4G). These results indicate that the effect of butein on SHP-1 activation is specific.
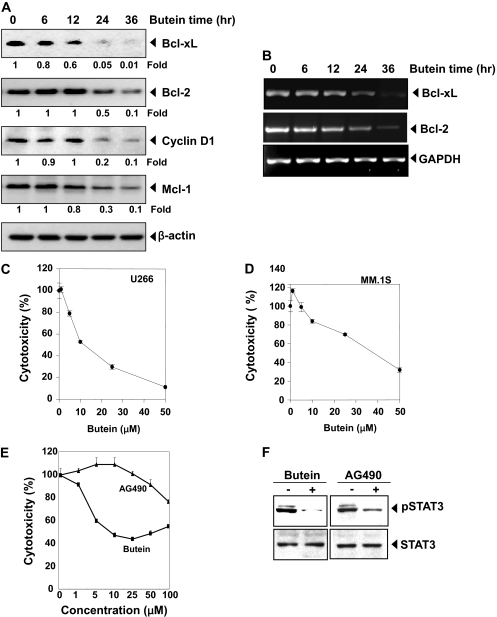
Butein Down-Regulates the Expression of Bcl-xL, Bcl-2, cyclin D1, and Mcl-1. STAT3 activation has been shown to regulate the expression of various gene products involved in cell survival and proliferation. The expression of the antiapoptotic proteins Bcl-xL, Bcl-2, and Mcl-1 and the cell cycle regulator protein cyclin D1 have all been shown to be regulated by STAT3 (Bowman et al., 2000). We found that expression of all of these gene products was down-regulated by butein in a time-dependent manner (Fig. 5A).
Fig. 5.
A, butein down-regulates the expression of antiapoptotic proteins. U266 cells (2 × 106/ml) were treated with 25 μM butein for the indicated time intervals, cells were harvested after incubation, and whole-cell extracts were prepared; 30-μg portions of those extracts were resolved on 10% SDS-PAGE and probed against Bcl-xL, Bcl-2, cyclin D1, and Mcl-1 antibodies. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. The blots were quantitated, and relative induction is mentioned as a -fold. B, butein inhibits gene expression. U266 cells (106/ml) were treated with butein (25 μM) for the indicated times, and total RNA was extracted and examined for the expression of Bcl-2 and Bcl-xL by RT-PCR. GAPDH was used as an internal control to show equal RNA loading. C and D, cytotoxic effects of butein on U266 (C) and MM.1S (D) cells. Cells were plated in triplicate, treated with indicated concentrations of butein for 72 h, and then subjected to MTT assay to analyze the viability of cells. E and F, butein is more effective than AG490. U266 cells were plated in triplicate, treated with the indicated concentrations of butein and AG490 for 24 h, and then subjected to MTT assay (E). U266 cells were treated with 50 μM concentration of butein and 100 μM AG490 for 4 h, whole-cell extracts were prepared, and 30-μg portions of those extracts were resolved on 10% SDS-PAGE and probed against p-STAT3 and STAT3 (F). The results shown are representative of three independent experiments.
Butein Modulates mRNA Expression of Antiapoptotic Genes. To determine whether butein affects the transcription antiapoptotic genes, we examined the mRNA expression of Bcl-2 and Bcl-xL. The mRNA of both these genes was constitutively expressed, and butein treatment suppressed the expression in a time-dependent manner (Fig. 5B). These results suggest that butein modulates the expression of genes at the transcriptional level.
Butein Inhibits Survival of MM Cells. Because butein suppressed the activation of STAT3 and STAT3-regulated gene products linked to cell survival, we next determined whether butein modulates survival of MM cells. Results in Fig. 5 show that butein suppressed the survival of U266 (Fig. 5C) and MM.1S cells (Fig. 5D).
We examined whether butein exhibits cytotoxicity against normal cells by using normal human peripheral blood mononuclear cells. The results show that butein had a minimal effect on normal peripheral blood mononuclear cells (Supplementary Fig. 1).
Butein Is More Effective than AG490 in Inhibiting the Survival of MM Cells. AG490 is the best-known rationally designed inhibitor of JAK2 kinase linked to STAT3 activation (Meydan et al., 1996). We examined how the activity of butein compares with AG490 for the suppression of cell survival. As shown in Fig. 5E, butein was more potent than AG490 against MM cells. The 50% cell growth inhibitory concentration was 25 μM for butein and 100 μM for AG490. These results coincide with their relative effects on STAT3 phosphorylation (Fig. 5F).
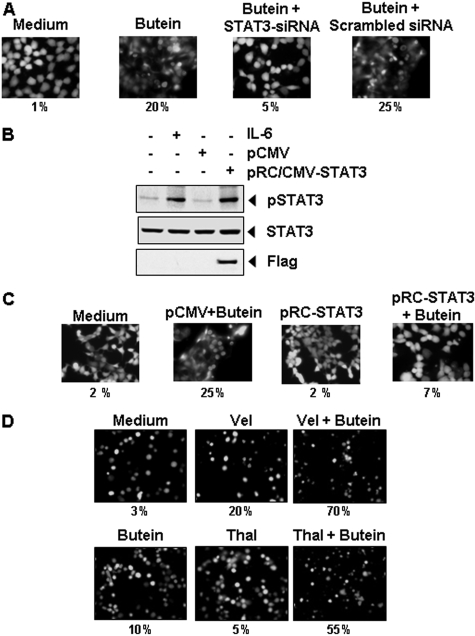
STAT3 siRNA Reduces Butein-Induced Apoptosis. We determined whether the suppression of STAT3 expression by siRNA would abrogate the inductive effects of butein on apoptosis. Apoptotic effects of butein were measured through esterase staining (live and dead) assay. Results shown in Fig. 6A reveal that butein-induced apoptosis was effectively abolished in the cells transfected with STAT3 siRNA, whereas treatment with scrambled siRNA had no effect (Fig. 6A). These results suggest that induction of apoptosis is mediated through the suppression of STAT3 by butein.
Fig. 6.
A, knockdown of STAT3 siRNA inhibited the apoptotic effect of butein. SCC4 cells were transfected with either STAT3-specific or scrambled siRNA (50 nM). After 48 h, cells were treated with 25 μM butein for 24 h and analyzed for the percentage of apoptosis by Live/Dead assay, and 20 random fields were counted. B and C, overexpression of constitutive STAT3 rescues A293 cells from butein-induced cytotoxicity. First, A293 cells were transfected with constitutive STAT3 plasmid. The cells were harvested 24 h after transfection, and the transfection was confirmed by Western blot analysis (B). A293 cells were transfected with constitutive STAT3 plasmid. After 24 h of transfection, the cells were treated with 25 μM butein for 24 h, and then the cytotoxicity was determined by Live/Dead assay, and 20 random fields were counted (C). D, butein potentiates the apoptotic effect of thalidomide and Velcade. U266 cells (1 × 106/ml) were treated with 25 μM butein and 10 ng/ml thalidomide or 20 nM Velcade alone or in combination for 24 h at 37 °C. Cells were stained with a Live/Dead assay reagent for 30 min and then analyzed under a fluorescence microscope, and 20 random fields were counted. The results shown are representative of three independent experiments.
Overexpression of Constitutively Active STAT3 Rescues Butein-Induced Apoptosis. We assessed whether the overexpression of constitutive active STAT3 can rescue butein-induced apoptosis. A293 cells were transfected with constitutively active STAT3 plasmid for 24 h, and cells were incubated with butein for the next 24 h and examined for apoptosis by esterase staining assay. The transfection of constitutively active STAT3 plasmid led to the expression of STAT3 protein as indicated by Western blotting (Fig. 6B). The results show that the forced expression of STAT3 significantly reduces the butein-induced apoptosis (Fig. 6C).
Butein Potentiates the Apoptotic Effect of Velcade and Thalidomide in MM Cells. Velcade, an inhibitor of proteasome, and thalidomide, an inhibitor of TNF expression, have been approved for the treatment of MM in patients (Cavo, 2006). We examined whether butein can potentiate the effect of these drugs. For this, U266 cells were treated with butein together with either thalidomide or Velcade, and then apoptosis was measured by the live/dead assay, which determines plasma membrane stability, using esterase staining. As shown in Fig. 6D, butein significantly enhanced the apoptotic effects of Velcade from 20 to 70% and of thalidomide from 5 to 55%.
Discussion
The goal of this study was to determine whether butein exerts its antiproliferative effects through the abrogation of the STAT3 signaling pathway in MM cells. We found that this chalcone suppressed both constitutive and IL-6-inducible STAT3 activation in parallel with the inhibition of c-Src and JAK1 and JAK2 activation. Butein specifically stimulated the expression of nontransmembrane protein tyrosine phosphatase SHP-1, and it down-regulated the expression of STAT3-regulated gene products, including, Bcl-xL, Bcl-2, cyclin D1, and Mcl-1. It induced the inhibition of proliferation, increased apoptosis, and significantly potentiated the apoptotic effects of Velcade and thalidomide in MM cells. Furthermore, knockdown of the expressions of SHP-1 and STAT3 using siRNA abolished the effects of butein.
The effects of butein on STAT3 phosphorylation correlated with the suppression of upstream protein tyrosine kinases JAK1, JAK2, and c-Src. Although we also observed that butein suppressed nuclear translocation and DNA binding activity of STAT3, the phosphorylation of STAT3 plays a critical role in the transformation and proliferation of tumor cells (Aggarwal et al., 2006). All Src-transformed cell lines have persistently activated STAT3, and dominant-negative STAT3 blocks transformation (Bowman et al., 2000). Dominant-negative STAT3 has also been shown to induce apoptosis in cells with constitutively active STAT3 (Catlett-Falcone et al., 1999). Constitutive activation of STAT3 has been reported in a large number of tumors, including breast cancer, prostate cancer, head and neck squamous cell carcinoma, lymphomas and leukemias, brain tumor, colon cancer, Ewing sarcoma, gastric cancer, esophageal cancer, ovarian cancer, nasopharyngeal cancer, and pancreatic cancer (Aggarwal et al., 2006). Hence, the suppression of constitutively active STAT3 in MM cells raises the possibility that this novel STAT3 inhibitor might also exhibit cytotoxicity against other types of cancer cells that display constitutively active STAT3. We found that butein inhibited the constitutive active STAT3 in both head and neck squamous cell carcinoma SCC4 and in human prostate carcinoma DU145 cells.
We found that butein suppressed not only constitutive but also inducible STAT3 activation. The activation of STAT3 can be induced by a wide variety of growth factors including IL-6, EGF, interferon-γ, and lipopolysaccharide (Aggarwal et al., 2006). We found that activation of STAT3 induced by IL-6 was completely suppressed by butein. How butein inhibits IL-6 induced STAT3 activation is not clear. The roles of JAK1, JAK2, mitogen-activated protein kinase, and Akt have been implicated in IL-6-induced STAT3 activation. We found that butein did suppress constitutive activation of JAK1 and JAK2. In addition, we found that IL-6-induced Akt activation was also suppressed by butein, although not completely. This suggests that butein could manifest its effect on STAT3 activation through multiple mechanisms.
Numerous PTPases have been implicated in STAT3 signaling, including SHP-1, SHP-2, TC-PTPase, phosphatase and tensin homolog deleted on chromosome 10, PTPase-1D, CD45, PTPase-ε, and low molecular weight (Woetmann et al., 1999; Irie-Sasaki et al., 2001). The type of PTPase involved in the down-regulation of STAT3 phosphorylation is not clear, although loss of SHP-1 has been shown to enhance JAK/STAT3 signaling in ALK-positive anaplastic large-cell lymphoma. We also found unequivocal evidence that the butein-induced inhibition of STAT3 activation specifically involves a protein tyrosine phosphatase SHP-1. Our results clearly show that 1) SHP-1 is induced by butein, and its knocking down with siRNA abolished its STAT3 inhibitory effects; 2) the lack of effect of butein on SH-PTP2 activation indicates specificity; and 3) the up-regulation of SHP-1 by butein occurs at the transcriptional level. Hence, our studies demonstrate that butein inhibits STAT3 activation through induction of SHP-1. SHP-1 has been shown to be inactive in various human tumors, including multiple myeloma (Chim et al., 2004) and lymphoma (Han et al., 2006), and DNA methylation has been described as one of the mechanisms for inactivation of SHP-1 in different cancers.
We have shown previously that butein can also suppress NF-κB activation (Pandey et al., 2007). Whether suppression of STAT3 activation by butein is linked to inhibition of NF-κB activation is not clear. The p65 subunit of NF-κB has been shown to interact with STAT3 (Yu et al., 2002). STAT3 and NF-κB, however, are activated in response to different cytokines. For instance, although IL-6 is a major activator of STAT3, TNF is a potent activator of NF-κB. Interestingly, erythropoietin has been shown to activate NF-κB through the activation of JAK2 kinase (Digicaylioglu and Lipton, 2001). Thus, it is possible that suppression of JAK activation is the critical target for inhibition of both NF-κB and STAT3 activation by butein.
Constitutively active STAT3 has been implicated in the induction of resistance to apoptosis (Catlett-Falcone et al., 1999), which leads to chemo-resistance and radiation resistance (Otero et al., 2006). This is possibly mediated through the expression of Bcl-2 and cyclin D1 (Danial et al., 1995). Expression of Bcl-xL is regulated by STAT3 and is overexpressed in MM cells (Tu et al., 1998). Bcl-xL can also block cell death induced by a variety of chemotherapeutic agents, in parallel with an increase in chemoresistance (Simonian et al., 1997). We also report that butein suppresses the expression of several STAT3-regulated gene products, including proliferative (cyclin D1) and antiapoptotic gene products (Bcl-2, Bcl-xL, and Mcl-1). This implies that suppression of STAT3 activation by agents such as butein could facilitate apoptosis. The down-regulation of the expression of Bcl-2, and Bcl-xL is probably linked with the butein's ability to induce apoptosis in MM cells. We further observed that butein induced the down-regulation of Mcl-1 protein in U266 cells. Indeed, it has been shown that inhibition of Src or STAT3 by an Src inhibitor (PD180970) results in the down-regulation of expression of the Mcl-1 gene in melanoma cells (Niu et al., 2002). Furthermore, we observed that knocking down the expression of STAT3 by siRNA abolished the effect of butein on apoptosis, and interestingly, overexpression of STAT3 also rescues the apoptotic effects of butein, strengthening our hypothesis that antiproliferative effects of butein are mediated through the abrogation of the STAT3 signaling pathway.
MM that has relapsed after conventional-dose therapy or stem-cell transplantation is typically treated with high-dose glucocorticoids, thalidomide, or Velcade. However, significant proportions of patients do not respond to these agents. Moreover, prolonged exposure leads to the development of resistance and toxicity, and progression-free and overall survival times are short. Recently, a proteasome inhibitor (PS-341, Velcade) and a TNF inhibitor (Revlimid, an analog of thalidomide) were approved for the treatment of multiple myeloma (Cavo, 2006). We also found that butein potentiates the apoptotic effect of Velcade and thalidomide in MM cells.
We argue that the pharmacological safety of butein and its ability to down-regulate the expression of several genes involved in cell survival and chemoresistance and potentiate the effect of Velcade and thalidomide provide a sufficient rationale to further carry out preclinical studies preceding human trials. Further animal studies are required with butein alone and in combination with existing therapy to demonstrate the potential applications for butein as a small-molecule inhibitor of STAT3 signaling.
Supplementary Material
Acknowledgments
We thank Walter Pagel for carefully editing the manuscript and providing valuable comments.
This work was supported by the National Institutes of Health National Cancer Institute [Grants CA16672, 1P01-CA1248701] and the Clayton Foundation for Research.
ABBREVIATIONS: STAT3, signal transducer and activator of transcription 3; MM, multiple myeloma; FBS, fetal bovine serum; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; PTPase, protein tyrosine phosphatase; JAK, Janus-like kinase; EGF, epithelial growth factor; IL, interleukin; siRNA, small interfering RNA; DMEM, Dulbecco's modified Eagle's medium; EMSA, electrophoretic mobility shift assay; PAGE, polyacrylamide gel electrophoresis; RT-PCR, reverse transcriptase-polymerase chain reaction; NF-κB, nuclear factor-κB; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; AG490, α-cyano-(3,4-dihydroxy)-N-benzylcinnamide; PD180970, pyrido[2,3-d]pyrimidine derivative.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
References
- Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, and Ichikawa H (2006) Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci 1091 151-169. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, Nair AS, Shishodia S, and Aggarwal BB (2007) Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemo-resistance through down-regulation of STAT3 and nuclear factor-kappa B-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood 109 2293-2302. [DOI] [PubMed] [Google Scholar]
- Bowman T, Garcia R, Turkson J, and Jove R (2000) STATs in oncogenesis. Oncogene 19 2474-2488. [DOI] [PubMed] [Google Scholar]
- Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernández-Luna JL, Nuñez G, et al. (1999) Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 10 105-115. [DOI] [PubMed] [Google Scholar]
- Cavo M (2006) Proteasome inhibitor bortezomib for the treatment of multiple myeloma. Leukemia 20 1341-1352. [DOI] [PubMed] [Google Scholar]
- Chim CS, Fung TK, Cheung WC, Liang R, and Kwong YL (2004) SOCS1 and SHP1 hypermethylation in multiple myeloma: implications for epigenetic activation of the Jak/STAT pathway. Blood 103 4630-4635. [DOI] [PubMed] [Google Scholar]
- Danial NN, Pernis A, and Rothman PB (1995) Jak-STAT signaling induced by the v-abl oncogene. Science 269 1875-1877. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M and Lipton SA (2001) Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature 412 641-647. [DOI] [PubMed] [Google Scholar]
- Han Y, Amin HM, Franko B, Frantz C, Shi X, and Lai R (2006) Loss of SHP1 enhances JAK3/STAT3 signaling and decreases proteosome degradation of JAK3 and NPM-ALK in ALK+ anaplastic large-cell lymphoma. Blood 108 2796-2803. [DOI] [PubMed] [Google Scholar]
- Irie-Sasaki J, Sasaki T, Matsumoto W, Opavsky A, Cheng M, Welstead G, Griffiths E, Krawczyk C, Richardson CD, Aitken K, et al. (2001) CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature 409 349-354. [DOI] [PubMed] [Google Scholar]
- Iwashita K, Kobori M, Yamaki K, and Tsushida T (2000) Flavonoids inhibit cell growth and induce apoptosis in B16 melanoma 4A5 cells. Biosci Biotechnol Biochem 64 1813-1820. [DOI] [PubMed] [Google Scholar]
- Jain N, Zhang T, Kee WH, Li W, and Cao X (1999) Protein kinase Cδ associates with and phosphorylates Stat3 in an interleukin-6-dependent manner. J Biol Chem 274 24392-24400. [DOI] [PubMed] [Google Scholar]
- Jang HS, Kook SH, Son YO, Kim JG, Jeon YM, Jang YS, Choi KC, Kim J, Han SK, Lee KY, et al. (2005) Flavonoids purified from Rhus verniciflua Stokes actively inhibit cell growth and induce apoptosis in human osteosarcoma cells. Biochim Biophys Acta 1726 309-316. [DOI] [PubMed] [Google Scholar]
- Kawano M, Hirano T, Matsuda T, Taga T, Horii Y, Iwato K, Asaoku H, Tang B, Tanabe O, and Tanaka H (1988) Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature 332 83-85. [DOI] [PubMed] [Google Scholar]
- Kim NY, Pae HO, Oh GS, Kang TH, Kim YC, Rhew HY, and Chung HT (2001) Butein, a plant polyphenol, induces apoptosis concomitant with increased caspase-3 activity, decreased Bcl-2 expression and increased Bax expression in HL-60 cells. Pharmacol Toxicol 88 261-266. [DOI] [PubMed] [Google Scholar]
- Lee JC, Lee KY, Kim J, Na CS, Jung NC, Chung GH, and Jang YS (2004) Extract from Rhus verniciflua Stokes is capable of inhibiting the growth of human lymphoma cells. Food Chem Toxicol 42 1383-1388. [DOI] [PubMed] [Google Scholar]
- Lee SH, Seo GS, Kim HS, Woo SW, Ko G, and Sohn DH (2006) 2′,4′,6′-Tris(methoxymethoxy) chalcone attenuates hepatic stellate cell proliferation by a heme oxygenase-dependent pathway. Biochem Pharmacol 72 1322-1333. [DOI] [PubMed] [Google Scholar]
- Li Z, Xu M, Xing S, Ho WT, Ishii T, Li Q, Fu X, and Zhao ZJ (2007) Erlotinib effectively inhibits JAK2V617F activity and polycythemia vera cell growth. J Biol Chem 282 3428-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A, et al. (1996) Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature 379 645-648. [DOI] [PubMed] [Google Scholar]
- Nelson KL, Rogers JA, Bowman TL, Jove R, and Smithgall TE (1998) Activation of STAT3 by the c-Fes protein-tyrosine kinase. J Biol Chem 273 7072-7077. [DOI] [PubMed] [Google Scholar]
- Nilsson K, Bennich H, Johansson SG, and Pontén J (1970) Established immunoglobulin producing myeloma (IgE) and lymphoblastoid (IgG) cell lines from an IgE myeloma patient. Clin Exp Immunol 7 477-489. [PMC free article] [PubMed] [Google Scholar]
- Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A, Chang A, Kraker A, Jove R, and Yu H (2002) Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene 21 7001-7010. [DOI] [PubMed] [Google Scholar]
- Oka T, Ouchida M, Koyama M, Ogama Y, Takada S, Nakatani Y, Tanaka T, Yoshino T, Hayashi K, Ohara N, et al. (2002) Gene silencing of the tyrosine phosphatase SHP1 gene by aberrant methylation in leukemias/lymphomas. Cancer Res 62 6390-6394. [PubMed] [Google Scholar]
- Otero DC, Poli V, David M, and Rickert RC (2006) Cutting edge: inherent and acquired resistance to radiation-induced apoptosis in B cells: a pivotal role for STAT3. J Immunol 177 6593-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey MK, Sandur SK, Sung B, Sethi G, Kunnumakkara AB, and Aggarwal BB (2007) Butein, a tetrahydroxychalcone, inhibits nuclear factor (NF)-κB and NF-κB-regulated gene expression through direct inhibition of IκBα kinase β on cysteine 179 residue. J Biol Chem 282 17340-17350. [DOI] [PubMed] [Google Scholar]
- Shuai K, Stark GR, Kerr IM, and Darnell JE Jr (1993) A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science 261 1744-1746. [DOI] [PubMed] [Google Scholar]
- Simonian PL, Grillot DA, and Nuñez G (1997) Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood 90 1208-1216. [PubMed] [Google Scholar]
- Tu Y, Renner S, Xu F, Fleishman A, Taylor J, Weisz J, Vescio R, Rettig M, Berenson J, Krajewski S, et al. (1998) BCL-X expression in multiple myeloma: possible indicator of chemo-resistance. Cancer Res 58(2): 256-262. [PubMed] [Google Scholar]
- Wang Y, Chan FL, Chen S, and Leung LK (2005) The plant polyphenol butein inhibits testosterone-induced proliferation in breast cancer cells expressing aromatase. Life Sci 77 39-51. [DOI] [PubMed] [Google Scholar]
- Willett WC (1994) Diet and health: what should we eat? Science 264 532-537. [DOI] [PubMed] [Google Scholar]
- Woetmann A, Nielsen M, Christensen ST, Brockdorff J, Kaltoft K, Engel AM, Skov S, Brender C, Geisler C, Svejgaard A, et al. (1999) Inhibition of protein phosphatase 2A induces serine/threonine phosphorylation, subcellular redistribution, and functional inhibition of STAT3. Proc Natl Acad Sci U S A 96 10620-10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Sun M, Liu L, and Zhou GW (2003) The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene 306 1-12. [DOI] [PubMed] [Google Scholar]
- Yit CC and Das NP (1994) Cytotoxic effect of butein on human colon adenocarcinoma cell proliferation. Cancer Lett 82 65-72. [DOI] [PubMed] [Google Scholar]
- Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J, and Jove R (1995) Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science 269 81-83. [DOI] [PubMed] [Google Scholar]
- Yu Z, Zhang W, and Kone BC (2002) Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor kappaB. Biochem J 367 97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Turkson J, Carter-Su C, Smithgall T, Levitzki A, Kraker A, Krolewski JJ, Medveczky P, and Jove R (2000) Activation of Stat3 in v-Src-transformed fibroblasts requires cooperation of Jak1 kinase activity. J Biol Chem 275 24935-24944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.