Abstract
In the past, CYP1A1 protein was known to be located in the endoplasmic reticulum (ER; microsomes). More recently, CYP1A1 was shown also to be targeted to the inner mitochondrial membrane; mitochondrial import is dependent on NH2-terminal processing that exposes a cryptic targeting signal. It is interesting that microsomal and mitochondrial CYP1A1 enzymes exhibit different substrate specificities, electron donors, and inducer properties. To understand the physiological functions of microsomal versus mitochondrial CYP1A1, we have generated three knock-in lines by altering the CYP1A1 NH2 terminus. Cyp1a1(mtt/mtt) mice encode an NH2-terminal 31-amino acid-truncated protein, deleting the ER-targeting signal and exposing the cryptic mitochondrial-targeting signal. Cyp1a1(mtp/mtp) mice encode a protein carrying L7N and L17N mutations; this mutant lacks the signal recognition particle (SRP)-binding site and subsequent ER-targeting, but requires proteolysis by a cytosolic peptidase for mitochondrial import. Cyp1a1(mc/mc) mice encode a microsomal protein having R34D and K39I mutations, which abolish the mitochondrial targeting signal. After dioxin or β-naphthoflavone treatment of these mouse lines, the CYP1A1 protein was shown to be located in the mitochondria of the Cyp1a1(mtp/mtp) and Cyp1a1(mtt/mtt) lines and in microsomes of the Cyp1a1(mc/mc) line. To test for differences in function, we compared the response to dietary benzo[a]pyrene (BaP). After 18 days of daily oral BaP, wild-type and Cyp1a1(mc/mc) mice were completely protected, whereas Cyp1a1(-/-) and Cyp1a1(mtp/mtp) mice showed striking toxicity and compensatory up-regulation of CYP1A2 and CYP1B1 mRNA in several tissues. Our data support the likelihood that it is the microsomal rather than mitochondrial CYP1A1 enzyme that protects against oral BaP toxicity.
The cytochrome P450 monooxygenase superfamily includes 57 and 102 genes in the human and mouse genomes, respectively (Nelson et al., 2004). The CYP1 family is 1 of 18 mammalian cytochrome P450 families and contains three highly conserved members: CYP1A1, CYP1A2, and CYP1B1. The basal and inducible expression of these three genes is regulated by the aryl hydrocarbon receptor (AHR) (Nebert et al., 2004; Hankinson, 2005; Puga et al., 2005; Nebert and Dalton, 2006; Kawajiri and Fujii-Kuriyama, 2007).
In the past, CYP1A1 has been regarded as located only in the endoplasmic reticulum (ER), until a mitochondrial (MT) CYP1A1 was characterized in the liver of rats pretreated with β-naphthoflavone (BNF) (Niranjan et al., 1985; Raza and Avadhani, 1988). Further investigations revealed that ER-versus MT-targeting of the CYP1A1 protein is determined by NH2-terminal signal sequences. Depending on the tissue, animal's age, and inducer pretreatment, varying amounts (from 5 to >50%) of CYP1A1 are directed to the MT inner membrane by means of cryptic MT-targeting signals; a cytosolic peptidase-mediated proteolysis can result in either of two truncated isoforms of mitochondrial CYP1A1 (mt1A1)—one having the first 4 (rats) or 8 (mice), the other having the first 32 (rats and mice) amino acids removed from the NH2 terminus (Addya et al., 1997; Boopathi et al., 2008). In contrast to the NADPH-P450 oxidoreductase (POR)-mediated microsomal CYP1A1 (mc1A1) activity, mt1A1 activity depends on ferredoxin-1 (FDX1, previously named adrenodoxin) and ferredoxin-1 reductase (FDXR); this distinction in the electron-donor complex might explain substrate-specificity differences seen between the mc1A1 and mt1A1 enzymes (Anandatheerthavarada et al., 1999).
Substrates for CYP1A1 include both endogenous compounds and exogenous chemicals (Nebert and Russell, 2002). Many studies have shown that CYP1A1 metabolism plays a major role in detoxication of foreign chemicals and metabolic activation leading to oxidative damage, birth defects, DNA adduct formation, mutagenesis, and carcinogenesis (Nebert, 1989; Park et al., 1996; Nebert et al., 2000b, 2004; Wells et al., 2005; Nebert and Dalton, 2006; Chung et al., 2007). It is tempting to speculate that mc1A1 metabolism might be predominantly involved in some of these processes, whereas mt1A1 metabolism might be more important in others. To test these hypotheses, we generated three knock-in mouse lines by targeted alteration of the Cyp1a1 gene, such that the CYP1A1 protein is expected to be trafficked either to the ER (microsomes) or to the mitochondria. The present report describes the successful creation and characterization of these lines and then tests for functional differences using the oral benzo[a]pyrene (BaP) paradigm described previously (Uno et al., 2004, 2006).
Materials and Methods
Chemicals. BaP and BNF were purchased from Sigma-Aldrich (St. Louis, MO). 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD; dioxin) was purchased from Accustandard, Inc. (New Haven, CT). FDX1 and FDXR were purified from bovine adrenal mitochondria, as described previously (Foster and Wilson, 1975; Raza and Avadhani, 1988). Erythromycin and SKF-525A were purchased from Sigma Chemical Co. All other chemicals and reagents were obtained from either Aldrich Chemical Co. (Milwaukee, WI) or Sigma-Aldrich at the highest available grades. Rabbit anti-human CYP1A1/CYP1A2 (α-1A1/1A2) polyclonal antibody was purchased from BD Gentest (Woburn, MA). Anti-NADPH-P450 oxidoreductase (α-POR) and anti-prohibitin (α-PHB) were bought from Abcam (Cambridge, MA). The polyclonal antibody to bovine ferredoxin (α-FDX1) was raised in the BALB/cJ mouse (Anandatheerthavarada et al., 1997). A polyclonal antibody to mouse glutamate-cysteine ligase modifier subunit (α-GCLM) was generated in the chicken (Chen et al., 2007).
Generation of Targeting Constructs. A 352-bp Cyp1a1 genomic gene fragment, excised by SacI and ApaI from the 7.58-kb EagI-EcoRV fragment (Dalton et al., 2000), was cloned into the pBluescript II KS- vector (Fig. 1A) to use as template DNA for site-directed mutagenesis. Using QuikChange Site-Directed Mutagenesis Kits (Stratagene, La Jolla, CA), we mutated the SacI-ApaI fragment at codons 7 and 17, or we mutated codons 34 and 39; alternatively, we made a 93-bp deletion resulting in the removal of 31 NH2-terminal amino acids (Fig. 1B). Thereafter, these were cloned back into the pBluescript II KS- vector that carried the Cyp1a1 7.58-kb EagI-EcoRV fragment to generate the three Cyp1a1 mutant allele-targeting vectors (Fig. 1, A and B). A NEO minicassette (Mansour et al., 1988), containing an extra EcoRV site flanked by direct-repeat loxP sites (Gu et al., 1994), was inserted into the Cyp1a1 intron 1 unique SacI site. This was followed by cloning of the HSV-TK gene (Capecchi, 1989), flanked by XhoI and SacII at the 3′-end of the EagI-EcoRV fragment, to make the targeting constructs (Fig. 1A, second line). All constructs were linearized by digestion with SacII before microinjection into 129/SvJ mouse embryonic stem (ES) cells by the Mouse Transgenesis Core in the Center for Environmental Genetics (University of Cincinnati, Cincinnati, OH).
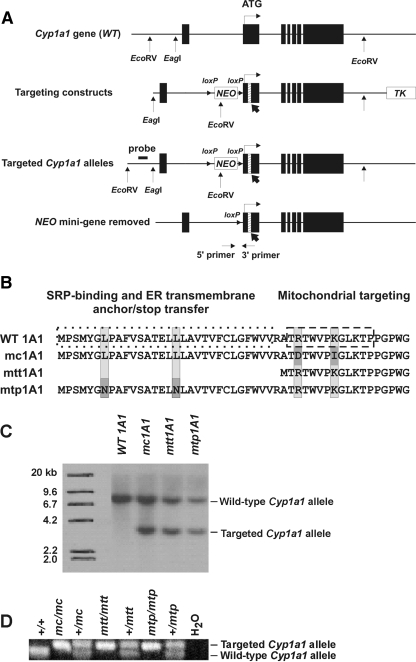
Fig. 1.
Generation of three Cyp1a1 knock-in mouse lines. A, scheme showing the WT Cyp1a1 gene (top line), targeting construct carrying the organelle-specific alterations (second line), targeted Cyp1a1 alleles after removal of the TK gene (third line), and the final targeted alleles (bottom line) after the NEO gene had been removed via Cre-mediated recombination to create the Cyp1a1(mc), Cyp1a1(mtt) and Cyp1a1(mtp) alleles. NEO (G418-resistant minicassette) and TK (thymidine kinase minicassette) represent genes used as selectable markers. ▪ denotes the seven exons; exon 2 (where ATG translation start-site is located) spans 851 bp. The ATG start-site for translation (denoted as vertical line and horizontal arrow) is located 15 nucleotides into exon 2. Exon 2 insertions (bold arrows pointing to hatched rectangles, not drawn completely to scale) represent the organelle-specific variations. EcoRV and EagI sites and the inserted loxP sites (orientation denoted by small arrowheads) are shown. B, NH2-terminal chimeric signal sequences of CYP1A1 proteins encoded by Cyp1a1 genes targeted with organelle-specific variations: WT 1A1, wild-type; mc1A1, ER-specific targeting; mtt1A1, mitochondrial truncated targeting; mtp1A1, mitochondrial proteolysis targeting. The altered residues are outlined in gray. C, Southern blot analysis. Genomic DNA from 129/SvJ mouse ES cell colonies was digested with EcoRV endonuclease followed by hybridization with the probe shown upstream of exon 1 in A (third line). The 8.6- and 3.8-kb bands represent the Cyp1a1 wild-type and targeted (mc, mtt, and mtp) alleles, respectively. D, PCR analysis of genomic DNA for the Cyp1a1 wild-type and targeted (mc, mtt, and mtp) alleles. Positions of the primers in intron 2 are shown in A (bottom line).
Southern Blot Hybridization. DNA, extracted from ES cell colonies followed sequentially by EcoRV digestion, was examined by Southern blot on Nytran SuperCharge membranes (Schleicher and Schuell, Keene, NH) via capillary transfer. A 792-bp region, which is 53 bp upstream (outside the targeting region) from the 5′-end of the EagI-EcoRV fragment, was used as template DNA for the probe generated by PCR (Fig. 1A, third line). Primers used (flanking the 792-bp probe for Southern blot) were the following: forward, 5′-ACAGGGGAGGGCAGGTGAAGGT-3′; and reverse, 5′-TCACCTCTAAGGGTCACCTTAG-3′. 32P-Labeled random-primed DNA probes (2 × 106 cpm/ml) were hybridized overnight with EcoRV-digested genomic DNA blotted on the membranes after prehybridization at 65°C for 4 h in 6 × SSC, 10× Denhardt's, and 1% SDS. Membranes were washed in 1× SSC + 0.1% SDS at 65°C for 1 h, then washed in 0.2× SSC + 0.1% SDS, and finally washed in 0.1X SSC + 0.1% SDS at 65°C for 20 min twice, followed by a 48-h exposure to Kodak X-OMAT film (Eastman Kodak, Rochester, NY).
Generation of Cyp1a1(mc/mc), Cyp1a1(mtp/mtp), and Cyp1a1-(mtt/mtt) Mouse Lines. Targeted ES cells (agouti) were microinjected into the blastocoele cavity of C57BL/6J embryos (nonagouti), and blastocysts were transferred into pseudopregnant CD-1 foster dams (Li et al., 1994). Identification of chimeric pups was determined by the presence of agouti coat color at 10 days of age. Male chimeric mice were then bred to C57BL/6J female mice, and agouti-colored offspring were screened by PCR for the presence of the NEO gene, denoting germ-line transmission of the targeted Cyp1a1 alleles (Fig. 1A, third line). Heterozygotes carrying the loxP-flanked NEO gene were then bred with mice carrying bacterial Cre driven by the chicken β-actin promoter (Araki et al., 1995) to remove the NEO gene cassette; this resulted in the final three targeted Cyp1a1 alleles without NEO (Fig. 1A, bottom line). Breeding of heterozygotes generated homozygosity of the targeted alleles, which were genotyped by taking advantage of the extra 34-bp loxP site.
Animals. The Cyp1a1(mc/mc), Cyp1a1(mtp/mtp), Cyp1a1(mtt/mtt), and Cyp1a1(-/-) mouse lines were backcrossed into C57BL/6J for eight generations; this ensured that the knock-in genotypes reside in a genetic background that is >99.8% C57BL/6J (Nebert et al., 2000a). Age-matched C57BL/6J mice, purchased from The Jackson Laboratory (Bar Harbor, ME) could thus be used as Cyp1a1(+/+) wild-type (WT 1A1) controls. The Cyp1a1(-/-) knockout mouse line (Dalton et al., 2000) was used as the CYP1A1-null control. All animal experiments were approved by and conducted in accordance with the National Institutes of Health standards for the care and use of experimental animals and the University Cincinnati Medical Center Institutional Animal Care and Use Committee.
Biohazard Precaution. BaP and TCDD are regarded as highly toxic and probable human carcinogens. All personnel were instructed in safe handling procedures. Lab coats, gloves, and masks were worn at all times, and contaminated materials were collected separately for disposal by the Hazardous Waste Unit or by independent contractors. BaP- and TCDD-treated mice were housed separately, and their carcasses were regarded as contaminated biological materials.
Pretreatment. TCDD (5 μg/ml) and BNF (8 mg/ml) were dissolved in corn oil. Cyp1a1(+/+), Cyp1a1(mc/mc), Cyp1a1(mtp/mtp), Cyp1a1(mtt/mtt), and Cyp1a1(-/-) mice were given intraperitoneal TCDD (15 μg/kg for 3 consecutive days) or BNF (80 mg/kg for 10 consecutive days) as described previously (Boopathi et al., 2000; Uno et al., 2004). At 10 days after initiating TCDD or BNF treatment, four tissues (kidney, lung, small intestine, and liver) were collected. These regimens allow optimal accumulation of the CYP1A1 protein in mitochondria (Boopathi et al., 2000; Genter et al., 2006).
For the oral BaP experiments with these new lines, no differences in weight gain or immunosuppression between male and female mice were found after 5 or 15 days on dietary BaP; thus, male mice only were chosen for further studies. BaP, given in corn oil-soaked food, was administered to 6-week-old Cyp1a1(+/+), Cyp1a1(mc/mc), Cyp1a1(mtp/mtp), and Cyp1a1(-/-) male mice. The rodent food (Harlan Teklad, Madison, WI) was soaked at least 24 h in BaP-containing corn oil (10 mg/ml) before being offered to the mice. By knowing the weight of the food ingested daily by a 20-g mouse and by using [3H]benzo[a]pyrene in early experiments (Robinson et al., 1975), we estimated the daily oral BaP dose to be ∼125 mg/kg/day. To start day 1 of the experiment, after an overnight fast, mice were presented with the BaP-laced food; control mice received food soaked in corn oil alone. Mice eagerly eat corn oil-soaked food. The oral BaP experiments were concluded on day 18, which is when some mouse lines exhibit weight loss but no animals are overtly ill (Uno et al., 2004, 2006). All tissues were harvested between 9:00 and 10:00 AM to exclude any circadian rhythm effects.
Reverse Transcription. Total RNA was isolated (liver, kidney, lung, small intestine, and spleen; n = 4 mice per group) using the TRI Reagent total RNA isolation reagent (Molecular Research Center, Cincinnati, OH). Total RNA (1 μg) was added to a final reaction solution of 13 μl containing oligo(dT)20 (3.8 μM) and dNTP (0.77 mM). Reactions were incubated at 65°C for 5 min and chilled on ice for 2 min. To the reaction mixture was added 7 μl of a solution containing 200 U of SuperScript III and 5 μM dithiothreitol (Invitrogen, Carlsbad, CA). Reactions were incubated at 50°C for 50 min, followed by 85°C for 10 min (to inactivate the reverse transcriptase). The cDNA samples were stored at -80°C until further study.
Quantitative Real-Time Polymerase-Chain Reaction. Primers used for detecting mouse mRNA were the following: for CYP1A1: forward (f), 5′-CCTCATGTACCTGGTAACCA-3′, and reverse (r), 5′-AAGGATGAATGCCGGAAGGT-3′; for CYP1A2: f, 5′-AAGACAATGGCGGTCTCATC-3′, and r, 5′-GACGGTCAGAAAGCCGTGGT-3′; for CYP1B1: f, 5′-ACATCCCCAAGAATACGGTC-3′, and r, 5′-TAGACAGTTCCTCACCGATG-3′; and for β-actin: f, 5′-CATCCGTAAAGACCTCTATGCC-3′, and r, 5′-ACGCAGCTCAGTAACAGTCC-3′. The quantitative real-time polymerase-chain reaction (qRT-PCR) was performed with the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA), using iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). Each sample was normalized to β-actin mRNA.
Western Immunoblot Analysis. Kidney, small intestine, and liver were chopped finely and homogenized on ice in 50 mM potassium phosphate buffer containing 0.1 mM EDTA and 1.15% KCl. Small pieces of lung were homogenized in H-medium, as described previously (Bhagwat et al., 1999). The homogenates were centrifuged at 500g for 15 min; supernatant fractions were centrifuged at 1000g for another 15 min, followed by centrifugation of the supernatant at 10,000g for 15 min. The pellets were resuspended and repeatedly centrifuged at 10,000g five more times to acquire relatively pure mitochondrial fractions. The resulting supernatant fractions were centrifuged at 18,000g for 30 min, followed by centrifugation of the resulting supernatants at 100,000g for 60 min to obtain the microsomal fractions. The pellets were resuspended, quantified, and subjected to electrophoresis on a 12% SDS or mixed-alcohol-detergent (MAD) (Brown, 1988) polyacrylamide minigel under denaturing conditions. Proteins were then transferred to nitrocellulose membranes for immunoblotting. The membrane was blocked with 3% bovine serum albumin in 1.5 M sodium chloride and 0.1 M Tris, pH 7.4, containing 0.1% Tween 20, followed by incubation overnight at 4°C with α-1A1/1A2, α-POR, α-PHB, or α-GCLM. After triplicate washes in phosphate-buffered saline-Tween 20, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA; 1:10,000) for 60 min. After triplicate washes, protein bands were visualized using enhanced chemiluminescence (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK).
Enzyme Assays. BaP hydroxylase activity was assayed by the standard spectrophotofluorimetric method (Nebert and Gelboin, 1968), determining the rate of formation of hydroxylated products of BaP (measured in picomoles per minute per milligram of protein). Erythromycin N-demethylase (ERND) activity was assayed spectrophotometrically (Anandatheerthavarada et al., 1999; Boopathi et al., 2000) by following the rate of HCHO formation (measured in nanomoles per minute per milligram of protein).
Detection of BaP in Blood. BaP levels in whole blood were quantified by a modification of methods described previously (Garcia-Falcon et al., 1996; Kim et al., 2000). Whole blood (30 μl) was extracted three times with ethyl acetate/acetone mixture [2:1 (v/v)]. The organic extracts were pooled and dried under argon, and the residue was resuspended in 250 μl of acetonitrile. An aliquot (100 μl) was injected onto a Nova-Pak C18 reverse-phase column (4 μm, 150 × 3.9-mm internal diameter; Waters Associates, Milford, MA). High-performance liquid chromatography analysis was conducted on a Waters model 600 solvent controller, equipped with a fluorescence detector (F-2000; Hitachi, Tokyo, Japan). Isocratic separation was performed using an acetonitrile/water [85:15 (v/v)] mobile phase at a flow rate of 1 ml/min. Excitation and emission wavelengths were 294 and 404 nm, respectively. BaP concentrations in blood were calculated by comparing the peaks of samples with those of control blood that had been spiked with different known concentrations of BaP. The calibration curve for BaP showed excellent linearity (correlation coefficient r > 0.998); four major and several minor BaP metabolites were found to run far ahead of BaP on the column and thus did not interfere. The detection limit (defined as 3 times the signal-to-noise ratio) was 0.05 pg/μl, and the limit of BaP quantification was determined to be 0.20 pg/μl. The intraday and interday precision of repeated analyses (n = 4) gave us coefficients of variation of <12%.
Plasma Enzymes. Peripheral blood was collected after 18 days of dietary BaP. Plasma was isolated from centrifuged whole blood for measuring alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities using kits purchased from Sigma-Aldrich.
Statistical Analysis. Statistics were performed using SigmaStat Statistical Analysis software (SPSS Inc., Chicago, IL). Group means were compared by one-way analysis of variance, followed by Student's t test for pair-wise comparison of means. All data were normally distributed and are reported as the means ± S.E.M. P values of <0.05 were considered statistically significant.
Results
Generation of Knock-In Mouse Lines. In previous studies (Addya et al., 1997), ER-versus MT-targeting of rat and mouse CYP1A1 protein was reported to be due to NH2-terminal chimeric signal sequences in the segment from residues 1 to 44: the amino acid stretch from 1 to 30 provides signals for ER membrane insertion and stop transfer; amino acids 33 to 44 provide MT-targeting signals. Mouse Leu7 and Leu17 are critical for SRP-binding (and thus ER-targeting), whereas positively charged Arg34 and Lys39 function critically for MT-targeting.
Based on this knowledge, by site-directed mutagenesis, we made genetic alterations in the Cyp1a1 wild-type gene (Fig. 1). Mutations at codon-34 (AGA→GAC) and codon-39 (AAA→ATA) to produce R34D and K39I, respectively, should target the CYP1A1 protein only to the ER; this “microsomal-only CYP1A1 line” is called mc1A1 (Fig. 1B, second line). Removal of 93 bp (encoding residues 2-32) replaces Ala32 with methionine, and this truncated CYP1A1 protein should be targeted only to the mitochondria because the entire ER insertion domain is missing; this “mitochondrial-only truncated CYP1A1 line” is called mtt1A1 (Fig. 1B, third line). Mutations at codon-7 (CTT→AAT) and codon-17 (CTC→AAC) to produce L7N and L17N, respectively, should target the CYP1A1 protein exclusively to mitochondria, but only after cytosolic proteolysis; this “mitochondrial-only proteolysis CYP1A1 line” is called mtp1A1 (Fig. 1B, last line).
These organelle-specific variations were cloned into exon 2 of the targeting constructs (Fig. 1A), and then the linearized constructs were independently electroporated into 129/SvJ mouse embryonic stem cells for homologous recombination with the genomic Cyp1a1 gene. The occurrence of homologous recombination between targeting constructs and the genomic Cyp1a1 gene was confirmed by PCR followed by Southern blot hybridization. By use of a primer inside the NEO cassette and a primer 20 bp downstream of the targeting constructs, ∼10% of ES cell colonies showed positive targeting by PCR screening (data not shown). Further testing by Southern blot hybridization (Fig. 1C) by using a probe 53 bp upstream of the targeting constructs (Fig. 1A, third line), we verified the proper incorporation of organelle-specific mutations into the Cyp1a1 locus. Male chimeric mice having partial or complete agouti coat color were then bred to C57BL/6J female mice. Mice with agouti coats and carrying the NEO gene, which represents germ-line transmission of the targeted allele, were crossed with mice carrying the bacterial Cre gene driven by the chicken β-actin promoter (Araki et al., 1995). This removes the NEO gene minicassette from the mouse genome via Cre-loxP-mediated recombination, giving the final targeted Cyp1a1 allele carrying an extra loxP site of 34 bp in intron 1 (Fig. 1A, bottom line). Mice homozygous for the targeted alleles, confirmed by genotyping for the extra loxP site (Fig. 1D), are viable, fertile, and display no overt phenotype.
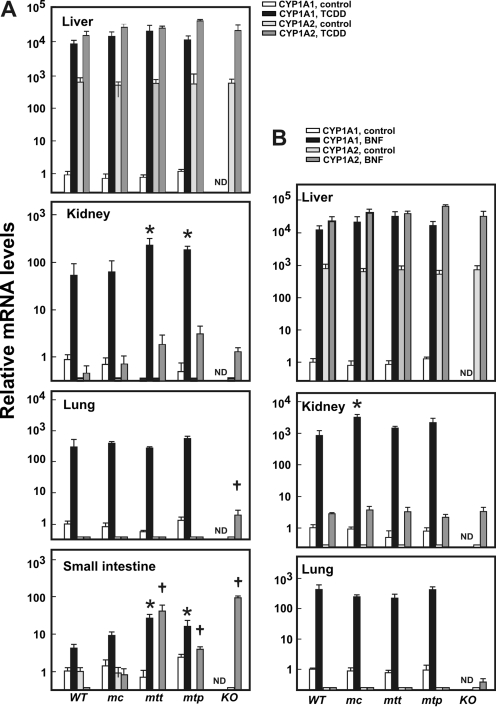
CYP1A mRNA Expression Levels in Knock-In Lines. TCDD and BNF are known AHR ligands, causing CYP1A1 induction and mitochondrial import (Boopathi et al., 2000; Uno et al., 2004, 2006). After TCDD pretreatment (Fig. 2A), qRT-PCR analysis displayed no differences in CYP1A1 mRNA levels among each of the individual organs—liver, kidney, lung, or small intestine—when we compared mc1A1, mtt1A1, and mtp1A1 mice with wild-type mice; exceptions included more highly induced CYP1A1 mRNA in kidney and in small intestine of mtt1A1 and mtp1A1 mice. In contrast and as expected, no CYP1A1 mRNA was detected in the Cyp1a1(-/-) knockout mouse. After BNF pretreatment (Fig. 2B), qRT-PCR analysis displayed significantly higher amounts (5- to 8-fold) of CYP1A1 mRNA only in liver and kidney of mc1A1 than that of wild-type mice. Again, no CYP1A1 mRNA was detected in the KO 1A1 knockout mouse.
Fig. 2.
Tissue mRNA levels. CYP1A1 and CYP1A2 mRNA levels were determined by qRT-PCR in mouse tissues after treatment with TCDD (A, 15 μg/kg i.p. for 3 consecutive days) or BNF (B, 80 mg/kg i.p. for 10 consecutive days). The CYP1A1 mRNA levels from corn-oil treated WT 1A1 mice in each tissue were set as “1.0 control,” and relative mRNA levels are expressed as -fold increases on a logarithmic scale; note values on the y-axis vary over a >10,000-fold range. The mouse lines are noted at the bottom: WT 1A1, mc1A1, mtt1A1, mtp1A1, and KO 1A1. ND, CYP1A1 mRNA was nondetectable. The mRNA levels are expressed relative to β-actin mRNA levels; thus, values within a tissue, but not between tissues, can be compared. Data are reported as means ± S.E.M. of four mice per group. *, P < 0.05, compared with mRNA levels of CYP1A1 in WT mice after the same treatment. †, P < 0.05, compared with mRNA levels of CYP1A2 in WT mice after the same treatment.
Fig. 2A shows that the basal CYP1A2 mRNA level in small intestine was negligible, but TCDD-induced CYP1A2 mRNA was statistically significantly elevated in mtt1A1 (3.2-fold), mtp1A1 (1.3-fold), and KO 1A1 (7.4-fold) mice, compared with that in TCDD-treated WT 1A1 and mc1A1 mice; these intriguing data suggest that the absence of the mc1A1 but not the mt1A1 protein (or presence of the mt1A1 but not the mc1A1 protein) in small intestine might cause up-regulation of the Cyp1a2 gene by TCDD (Uno et al., 2008). Figure 2B illustrates no differences in basal or BNF-induced hepatic CYP1A2 mRNA levels among the wild-type, mc1A1, mtt1A1, or mtp1A1 or KO 1A1 mice. TCDD-induced CYP1A2 mRNA levels are negligible in kidney and lung, except that they are slightly elevated in KO 1A1 (Fig. 2, A and B).
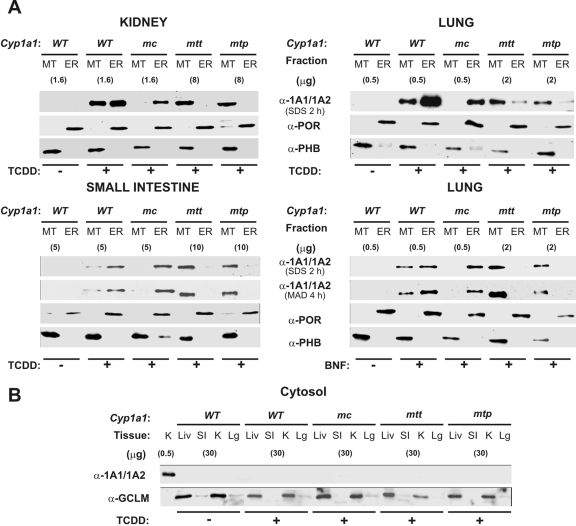
Subcellular Distribution of CYP1A1 Protein. After TCDD or BNF pretreatment (Fig. 3), organelle-targeting of the CYP1A1 protein was examined in WT 1A1, mc1A1, mtt1A1, and mtp1A1 mice. Microsomal and mitochondrial proteins were resolved by SDS-PAGE or mixed-alcohol-detergent in PAGE (Brown, 1988), the latter rendering a superior separation of the 56-kDa CYP1A1 from the 54.5-kDa CYP1A2 protein. Subcellular distribution of the CYP1A1 protein was compared by Western immunoblot using microsomal POR, mitochondrial PHB, and cytosolic GCLM as subcellular-specific markers. Distribution of the CYP1A1 protein (Fig. 3) was consistent with the CYP1A1 mRNA levels in the various tissues (Fig. 2). The CYP1A1 protein in kidney (Fig. 3A, top left) was found: in TCDD-treated but not untreated WT 1A1 mitochondria and microsomes, in TCDD-treated mc1A1 microsomes but not mitochondria, predominantly in TCDD-treated mtt1A1 mitochondria more so than microsomes, and predominantly in TCDD-treated mtp1A1 mitochondria more so than microsomes. The same pattern was seen in lung (Fig. 3A, top right) and small intestine after TCDD treatment (Fig. 3A, bottom left) and lung after BNF treatment (Fig. 3A, bottom right).
Fig. 3.
Western immunoblot analysis of subcellular localization of CYP1A1 proteins. MT and ER (i.e., microsomes) fractions (A) and cytosolic fractions (B) were isolated and resolved by SDS-PAGE or mixed-alcohol-detergent in PAGE. Designation of the mouse lines, with or without TCDD or BNF treatment, noted at the top, is the same as that in Fig. 2. Across each row, CYP1A1, POR (ER marker), PHB (MT marker), and GCLM (cytosolic marker) proteins were detected using antibodies. Protein loadings (micrograms per lane) for the CYP1A1 immunoblots are noted in parentheses; 20 μg of protein from subcellular fractions was loaded for the detection of the ER and MT markers; 30 μg of protein was loaded for detection of the cytosolic marker. K, kidney; Liv, liver; S.I., small intestine. Lg, lung.
In TCDD-treated mtt1A1 and mtp1A1 mice, trace amounts of CYP1A1 protein were detectable in the ER fractions of lung (Fig. 3A, top right) and especially of liver (data not shown). The liver contains so much ER that we always faced some degree of contamination of mitochondrial fragments by microsomes, and this was confirmed using the specific organelle markers. However, when specific subcellular markers are included—POR for microsomes, PHB for mitochondria, and GCLM for cytosol—no detectable contamination was seen on the Western blots (Fig. 3). In our experience, kidney and lung clearly provided more robust separations of mitochondria from microsomes (and vice versa) than small intestine and especially more robust than liver.
CYP1A2 protein cannot be detected on Western immunoblots from kidney or lung and cannot be detected in small intestine after TCDD pretreatment [whereas CYP1A2 protein is detectable after oral BaP treatment (Uno et al., 2008)]. On the other hand, CYP1A2 protein levels are very high in the liver of control and TCDD- or BNF-pretreated mice. These are the reasons why in Fig. 3 we chose to show immunoblots probed by α-1A1/1A2 from kidney, lung, and intestine of TCDD-treated mice and from lung of BNF-treated mice.
It is noteworthy that no CYP1A1 protein was detected in any of the cytosolic fractions (Fig. 3B), indicating that those CYP1A1 proteins without microsomal targeting signals do not inherently accumulate in cytoplasm. In summary, the data in Fig. 3 confirm that the CYP1A1 protein has been successfully targeted in the tissues examined and into the expected subcellular compartment: mc1A1 protein in the mc1A1 line, and mt1A1 protein in the mtt1A1 and mtp1A1 lines.
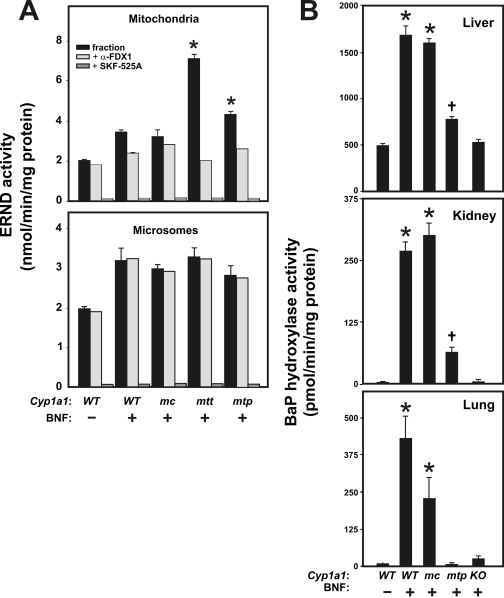
Enzyme Activities in the Cyp1a1 Knock-In Lines. Differences in substrate specificities for mt1A1 versus mc1A1 in metabolizing particular drugs, such as erythromycin and psychotropic drugs including morphine (Anandatheerthavarada et al., 1999; Dasari et al., 2006), have been reported. Because drug metabolism is far more robust in liver than in nonhepatic tissues, we compared ERND and BaP hydroxylase activities in hepatic mitochondria versus microsomes. Mitochondrial fractions in the BNF-treated mtt1A1 and mtp1A1 lines displayed significantly higher ERND activity than that in WT 1A1 or mc1A1 mice (Fig. 4A, top); in microsomal fractions, no differences in ERND activity were seen among the groups of BNF-treated mice (Fig. 4A, bottom), although ERND was induced by BNF to levels ∼1.5-fold higher than basal ERND activity.
Fig. 4.
Mitochondrial versus microsomal enzyme activities in BNF-treated and untreated mice. A, ERND is expressed as the rate of HCHO formation in nanomoles per minute per milligram of protein. Inhibition of ERND activity by prior addition of α-FDX1 or SKF-525A was carried out as described previously (Anandatheerthavarada et al., 1997, 1999). *, P < 0.05, when hepatic mitochondrial ERND activity from BNFtreated mtt1A1 or mtp1A1 mice is compared with that from BNFtreated WT 1A1 mice. B, BaP hydroxylase activity is expressed in picomoles of 3plus 9-hydroxyBaP per minute per milligram of protein. Note the different values on the y-axis in each panel. Data are reported as means ± S.E.M. (n = 3 or 4 mice per group). Designation of mouse lines is same as that in Fig. 2. *, P < 0.01, when microsomal BaP hydroxylase from BNF-treated WT 1A1 or mc1A1 is compared with that from untreated WT 1A1 mice. †, P < 0.05, when microsomal BaP hydroxylase from BNFtreated mtp1A1 is compared with that from untreated WT 1A1 mice.
Microsomal CYP1A1, in the presence of POR added in vitro, is known to exhibit virtually no ERND activity, whereas mt1A1 in the presence of added FDX1 and FDXR shows very high ERND activity (Anandatheerthavarada et al., 1997, 1999; Boopathi et al., 2000). BNF-induced ERND thus represents the mt1A1 protein, whereas mitochondrial basal ERND activity may reflect either CYP1A2 or CYP2D, both of which exist in mitochondria (Boopathi et al., 2000). In all likelihood, microsomal basal and BNF-inducible ERND activities reflect one or more of the CYP3A enzymes (Xu et al., 2006); this most likely explains why we see nearly equal ERND activity in microsomes of all the BNF-treated mouse lines.
Fig. 4A (top) shows that in mitochondria of BNF-treated animals, an antibody to FDX1 inhibited ERND activity ∼70% in mtt1A1 and ∼45% in mtp1A1 mice, compared with ∼30% in WT 1A1 mice and negligible amounts in mc1A1 mice. These findings confirm further that the mt1A1 protein is the principal contributor to mitochondrial ERND activity in the mtt1A1 and mtp1A1 lines.
Fig. 4A (top and bottom) illustrates that ERND activity can be completely inhibited by SKF-525A in both mitochondria and microsomes of all mice. These data confirm that cytochrome P450 monooxygenases are responsible for virtually all of the detectable ERND activity in these assays.
Hepatic microsomes from BNF-treated WT 1A1 and mc1A1 mice (Fig. 4B, top) showed a ∼3.7- and 3.5-fold induction of BaP hydroxylase activity, respectively, compared with untreated WT 1A1 mice; this finding confirms that BNF-induced BaP hydroxylase in the mc1A1 line indeed reflects mc1A1 protein. BNF-treated KO 1A1 mice showed no significant increases in BaP hydroxylase activity over that seen in the untreated wild-type mouse; these data are consistent with numerous previous reports describing the Cyp1a1(-/-) mouse treated with various CYP1A1 inducers (Dalton et al., 2000; Uno et al., 2001, 2004, 2006, 2008; Cheung et al., 2005; Derkenne et al., 2005; Jiang et al., 2005; Genter et al., 2006; Dragin et al., 2007; Ma et al., 2007). BaP hydroxylase activity, which exists in the liver of untreated mice, is known to reflect a CYP2C enzyme (Meehan et al., 1988). On the other hand, hepatic microsomes from BNF-treated mtp1A1 mice produced ∼1.6-fold greater induced BaP hydroxylase activity compared with untreated WT 1A1 mice; we believe that this reflects the degree of contamination of liver mitochondria in the microsomal fractions.
The differences in microsomal BaP hydroxylase activity are much more easily visualized in kidney and lung than in liver for two reasons: 1) basal BaP metabolism is negligible in untreated WT 1A1 mice; and 2) because of the abundance of microsomes in liver and therefore contamination of the mitochondrial fraction with microsomes (and vice versa), separation of the two organelles is much less problematic in kidney and lung. Hence, relative to the untreated WT 1A1 (Fig. 4B, middle), a 50- to 60-fold induction of kidney BaP hydroxylase activity was seen in BNF-treated WT 1A1 and mc1A1 mice compared with a ∼10-fold induction of enzyme activity in BNF-treated mtp1A1 mice. A ∼40- and ∼20-fold induction of lung BaP hydroxylase activity (Fig. 4B, bottom) is seen in BNF-treated WT 1A1 and mc1A1 mice, respectively, compared with no detectable induction of enzyme activity in BNF-treated mtp1A1 mice. These differences in microsomal BaP hydroxylase activity, comparing BNF-treated WT1A1 with the mc1A1 and mtp1A1 lines, are therefore consistent with the qRT-PCR (Fig. 2B) and Western immunoblot data (Fig. 3A). In the Cyp1a1(-/-) mouse, a compensatory up-regulation of CYP1B1 has been noted previously (Uno et al., 2006, 2008), which would explain the small increases in BaP hydroxylase activity observed in liver, kidney, and especially lung.
Proof-of-Principle Experiments in mc1A1 versus mt1A1 after Oral BaP. We wanted to show a clinical difference between mc1A1- and mt1A1-containing mice. Previous studies (Raza and Avadhani, 1988) have shown that the mt1A1 protein is ∼10% as efficient as the mc1A1 protein in BaP metabolism in vitro. Previous oral BaP studies have shown that intestinal (and perhaps hepatic) CYP1A1 functions primarily in the process of detoxication, to protect the mouse against oral BaP-induced immunosuppression (Uno et al., 2004, 2006). We therefore evaluated the role of mc1A1 versus mt1A1 in animals receiving oral BaP. Because of the numbers of mice needed and expenses, we completed all studies with only mtp1A1 mice, although preliminary data with mtt1A1 mice (data not shown) were very similar.
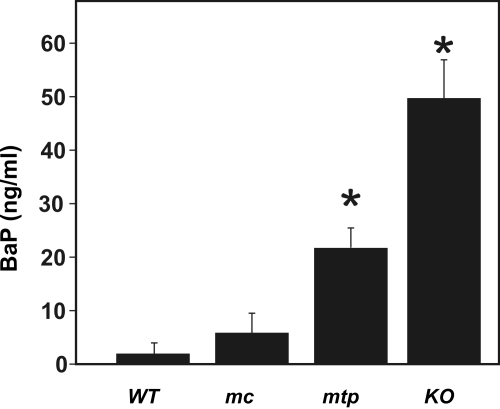
After 5 days of continuously administered oral BaP, whole-blood BaP levels in mc1A1 mice were not different from that of WT 1A1 mice; by contrast, BaP levels were ∼11- and ∼25-fold higher in mtp1A1 and KO 1A1, respectively, than in WT 1A1 mice (Fig. 5). Thus, the mtp1A1 line is more similar to the Cyp1a1(-/-) knockout line than the mc1A1 line; these data support our hypothesis that, in the wild-type mouse, it is mc1A1 rather than mt1A1 that is responsible for detoxication of oral BaP.
Fig. 5.
Whole-blood BaP levels after continuous oral BaP. Whole blood was collected as described from mice receiving oral BaP 125 mg/kg/day for 5 days. Data are reported as means ± S.E.M. of four mice per group. Designation of mouse lines is the same as that in Fig. 2. *, P < 0.01, compared with levels in mc1A1 or WT 1A1 mice. BaP levels in KO 1A1 mice are significantly greater (P < 0.01) than those in mtp1A1 mice.
The data in Table 1 are consistent with previous studies (Uno et al., 2004, 2006). The Cyp1a1(-/-) knockout mouse treated with oral BaP for 18 days exhibits striking oral BaP-induced damage, including 1) the wasting syndrome, as seen by lowered body weight; 2) immunosuppression, as shown by decreased spleen and thymus weight, lymphocytopenia, and bone marrow hypocellularity accompanied by a decreased ratio of lymphoid to myeloid series of cells; 3) anemia, as revealed by decreased hematocrit and hemoglobin; 4) enhanced oxidative stress, as evidenced by elevated methemoglobin; and 5) liver toxicity, as depicted by increased liver weight and elevated plasma ALT and AST activities. These parameters are associated with mortality in Cyp1a1(-/-) mice that is seen after ∼28 days of continuous oral BaP exposure (Uno et al., 2004, 2006).
TABLE 1.
Effect of oral BaP on body weight, organ weight, liver enzymes, hematocrit, hemoglobin, and peripheral blood cell types
Mice (n = 4 per group) were fed corn oil-soaked food or BaP-soaked food (125 mg/kg/day) for 18 days. Values are expressed as means ± S.E.M.
|
Cyp1a1
|
Corn Oil Only
|
BaP
|
||||||
|---|---|---|---|---|---|---|---|---|
| WT | mc | mtp | KO | WT | mc | mtp | KO | |
| Body weight (g, gained or lost) | +0.8 ± 0.5 | +0.6 ± 0.3 | +1.6 ± 0.5 | +1.0 ± 0.4 | +0.7 ± 0.2 | +1.1 ± 0.3 | −1.3 ± 0.3* | −2.7 ± 0.5* |
| Liver weight (mg/g body weight) | 47 ± 4.1 | 46 ± 2.0 | 47 ± 0.9 | 47 ± 2.2 | 41 ± 1.1 | 42 ± 1.3 | 53 ± 2.2* | 55 ± 2.3* |
| Spleen weight (mg/g body weight) | 3.8 ± 0.2 | 3.9 ± 0.1 | 3.6 ± 0.1 | 3.5 ± 0.1 | 3.4 ± 0.1 | 3.1 ± 0.2 | 2.0 ± 0.1* | 1.2 ± 0.1*† |
| Thymus weight (mg/g body weight) | 1.6 ± 0.3 | 1.6 ± 0.1 | 1.6 ± 0.4 | 2.3 ± 0.4 | 1.5 ± 0.1 | 1.5 ± 0.2 | 0.5 ± 0.04* | 0.2 ± 0.04*† |
| ALT (IU/l) | 33 ± 6.9 | 26 ± 6.1 | 18 ± 3.2 | 21 ± 2.1 | 21 ± 4.2 | 41 ± 3.1 | 48 ± 5.1* | 53 ± 3.7* |
| AST (IU/l) | 105 ± 18 | 115 ± 16 | 105 ± 16 | 81 ± 6.8 | 76 ± 1.9 | 84 ± 16 | 98 ± 1.5* | 93 ± 1.8* |
| Hematocrit (%) | 52 ± 1.0 | 52 ± 0.5 | 53 ± 1.1 | 52 ± 0.5 | 53 ± 2.8 | 52 ± 1.1 | 45 ± 1.2* | 38 ± 1.0*† |
| Total hemoglobin (mM) | 6.9 ± 0.6 | 7.3 ± 0.3 | 7.3 ± 0.4 | 6.8 ± 0.3 | 6.9 ± 0.2 | 7.2 ± 0.6 | 6.2 ± 0.3* | 6.1 ± 0.1* |
| Methemoglobin (%) | 2.2 ± 0.9 | 1.0 ± 0.2 | 1.2 ± 0.5 | 1.4 ± 0.4 | 1.3 ± 0.2 | 0.9 ± 0.2 | 2.4 ± 0.2* | 4.9 ± 0.5* |
| Peripheral blood | ||||||||
| Neutrophils (%) | 24 ± 4.7 | 24 ± 1.8 | 21 ± 4.0 | 22 ± 4.1 | 20 ± 4.2 | 19 ± 3.2 | 43 ± 4.2* | 42 ± 3.3* |
| Lymphocytes (%) | 78 ± 5.3 | 73 ± 5.4 | 77 ± 4.6 | 78 ± 4.4 | 76 ± 4.4 | 82 ± 4.0 | 52 ± 5.7* | 53 ± 2.1* |
P <0.05, compared with BaP-treated WT 1A1 wild-type mice.
P <0.05, compared with BaP-treated mtp 1A1 mice.
At 125 mg/kg/day of oral BaP for 18 days, WT 1A1 and mc1A1 mice did not show any of these abnormalities (Table 1). On the other hand, mtp1A1 mice displayed moderate degrees of toxicity in each of the above-mentioned parameters. The data in Table 1 are thus consistent with the whole-blood BaP levels in these lines (Fig. 5) and suggest that absence of the mc1A1 protein in the mtp1A1 mouse leads to oral BaP-induced whole-body damage, albeit to a lesser extent compared with that in the Cyp1a1(-/-) knockout mouse.
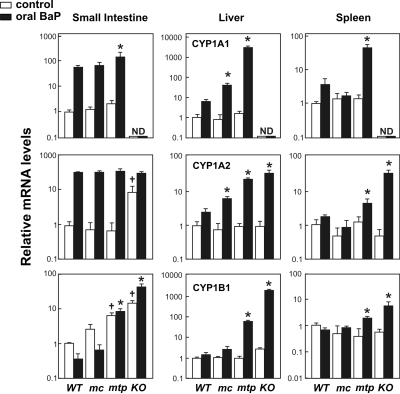
CYP1 mRNA Levels in Oral BaP-Treated Mice. To understand Cyp1 expression in several organs after 18 days of oral BaP administration, we measured mRNA levels from all three Cyp1 genes in small intestine, liver, and spleen. CYP1A1 mRNA was not detectable in any of the three tissues from the Cyp1a1(-/-) mouse. Oral BaP-induced CYP1A1 mRNA levels (Fig. 6, top row) in small intestine were >2-fold greater in mtp1A1 than in mc1A1 or WT 1A1. In liver, CYP1A1 mRNA in mtp1A1 was ∼70-fold higher than that in mc1A1 and ∼560-fold greater than that in WT 1A1. In spleen of oral BaP-treated mice, CYP1A1 mRNA concentrations were >10-fold higher in mtp1A1 than in mc1A1 or WT 1A1.
Fig. 6.
CYP1 mRNA levels after 18 days of continuous oral BaP (125 mg/kg/day). The CYP1A1 mRNA levels from cornoil treated WT 1A1 mice in each tissue were set as “1.0 control,” and relative mRNA levels are expressed as -fold increases on a logarithmic scale; note values on the y-axis vary over a >1000-fold range. ND, nondetectable. The mRNA levels are expressed relative to β-actin mRNA levels; thus, values within a tissue, but not between tissues, can be compared. Data are reported as means ± S.E.M. of four mice per group. Designation of mouse lines is the same as that in Fig. 2. *, P < 0.05, compared with mRNA levels for that gene in BaP-treated WT 1A1 mice. †, P < 0.05, compared with mRNA levels for that gene in corn oiltreated WT 1A1 mice.
Oral BaP-induced CYP1A2 mRNA levels (Fig. 6, middle row) in small intestine were not different among the four oral BaP-treated groups examined; however, basal CYP1A2 mRNA concentrations were ∼10-fold elevated in the untreated KO 1A1 mouse, suggesting that complete absence of intestinal CYP1A1 causes a compensatory up-regulation of the basal CYP1A2 mRNA. This finding (in small intestine, but not in liver or spleen) is consistent with what has been found previously (Uno et al., 2006, 2008). In liver, oral BaP-induced CYP1A2 mRNA levels in mtp1A1 and KO 1A1 mice were >3-fold greater than that in mc1A1, and ∼9-fold greater than that in WT 1A1 mice. In spleen, oral BaP-induced CYP1A2 mRNA concentrations were ∼7-fold higher in KO 1A1 than in mtp1A1, which in turn were ∼3-fold greater than that in mc1A1 or WT 1A1.
Oral BaP-induced CYP1B1 mRNA levels (Fig. 6, bottom row) in intestine were >5-fold higher in KO 1A1 than in mtp1A1 mice, and CYP1B1 mRNA was ∼12-fold greater in mtp1A1 than that in mc1A1 or WT 1A1. A compensatory up-regulation of basal CYP1B1 mRNA was observed in small intestine of both the KO 1A1 and mtp1A1 mice; this finding (seen in small intestine, but not in liver or spleen) is consistent with what has been reported previously (Uno et al., 2006, 2008). In the liver, oral BaP-induced CYP1B1 mRNA levels in the KO 1A1 and mtp1A1 mice were >700- and ∼25-fold higher, respectively, than that in mc1A1 or WT 1A1 mice. In spleen, oral BaP-induced CYP1B1 mRNA concentrations in the KO 1A1 and mtp1A1 mice were ∼7- and ∼2-fold greater, respectively, than that in mc1A1 or WT 1A1 mice.
Our findings in the two knock-in lines (Figs. 5 and 6; Table 1) follow the pattern that the mc1A1 line is more capable of detoxication of oral BaP than the mtp1A1 line: BaP is cleared more readily from the mc1A1 gastrointestinal tract (similar to what is seen in the wild-type tract), leading to lower BaP blood levels and less BaP inducer reaching the liver or spleen and, hence, lower CYP1 mRNA accumulation in the mc1A1 than the mtp1A1 line in these two organs.
Discussion
For the past decade it has been appreciated that the mammalian CYP1A1 protein is located in both the ER and the inner membrane of mitochondria; relative amounts vary depending on the species (rat, mouse, or human), age, and the organ or tissue being studied (Raza and Avadhani, 1988; Addya et al., 1997; Anandatheerthavarada et al., 1997). Moreover, differences in inducer and substrate and electron donor specificity exist between mc1A1 and mt1A1 (Raza and Avadhani, 1988; Addya et al., 1997; Anandatheerthavarada et al., 1997). The obvious questions include why have these two different subcellular CYP1A1 proteins evolved during the past 70 million years of the mammalian radiation, and what special critical life function(s) is specific to one or the other?
It therefore seemed reasonable to genetically engineer mouse lines carrying either an exclusively ER-targeted or an exclusively MT-targeted CYP1A1 protein. Although the proposed feat had no guarantee of success, the present study (Figs. 1 and 3) confirms that we have accomplished our aim. Given this success, a proof-of-principle experiment was then performed to show the differences in function by mc1A1 versus mt1A1 in the intact animal; this was done by using the oral BaP paradigm studied previously in these laboratories, and we show (Fig. 5 and Table 1) that the mc1A1 seems to be more important than the mt1A1 protein in the detoxication of oral BaP.
ER-Specificity of the Targeted CYP1A1 Protein. After stringent centrifugation, it was rare to see microsomes contaminated by MT fractions (as judged by our finding of no MT-specific marker PHB in microsomal fractions, especially TCDD-treated kidney and BNF-treated lung); we therefore believe that the CYP1A1 protein detected in microsomes in mc1A1 mice is indeed the microsomal form. By way of Western immunoblot analysis (Fig. 3), the ER-targeted mc1A1 protein seemed to be localized ∼100% to the microsomes (and not mitochondria) in lung, kidney, and small intestine from TCDD-induced mc1A1 mice; these data indicate that our site-directed mutagenesis experiments, to efficiently block the MT-targeting signal (Fig. 1), were successful in preventing the import of CYP1A1 protein into mitochondria.
In the liver from TCDD or BNF-treated mc1A1 mice, however, there were always small but detectable amounts of anti-CYP1A1-reactive protein in mitochondrial fractions, whether isolated via repeated differential centrifugations or via digitonin-stripped mitoplasts (data not shown). Because there was always the concomitant presence of small but detectable anti-POR reactive proteins also in these same fractions, we believe this finding reflects some degree of contamination by microsomes—in hepatocytes, which are very abundant in rough and smooth ER—rather than some “liver-specific difference in organelle-targeting,” which was not seen in lung, kidney, or small intestine. By inducing CYP1A1 via TCDD or BNF pretreatment, this simply elevated the ratio of CYP1A1 to POR in liver mitochondria but did not decrease the amount of contamination of mitochondria by microsomes.
MT-Specificity of the Targeted CYP1A1 Protein. In the mtp1A1 and mtt1A1 lines, the MT-targeted CYP1A1 protein was detected only in mitochondrial fractions of kidney and small intestine after TCDD and in lung after BNF pretreatment. In mtp1A1 and mtt1A1 mice, trace amounts (∼1-10%) of CYP1A1 could always be detected in microsomes from lung or liver after TCDD and from small intestine after BNF pretreatment (data not shown). The low level of CYP1A1 present in microsomes from TCDD- or BNF-pretreated mtp1A1 and mtt1A1 mice varied as a function of different inducers and tissues, suggesting that the NH2-terminal signal sequence might not be absolute in affecting MT-targeting of the CYP1A1 protein in the intact animal. This conclusion is consistent with the finding that the protein kinase C (PKC)-mediated phosphorylation of Thr35 in the nascent CYP1A1 peptide chain lowers the affinity for SRP binding, thus decreasing the amount of ER targeting (Dasari et al., 2006). Considering the highly variable expression of PKC isoforms in different tissues and cell types (Yoshida et al., 1988; Webb et al., 2000; Blay et al., 2004) and the involvement of PKC in AHR-signaling pathways (Carrier et al., 1992; Long et al., 1998; Webb et al., 2000), PKC activity might contribute to the incomplete ablation of ER-targeted CYP1A1 in microsomes of some tissues, as seen in our MT-targeted mouse lines. Further studies are needed to understand the underlying mechanisms of tissue-specific CYP1A1 subcellular-targeting in the intact mouse and its physiological significance.
Prevention of Oral BaP-Induced Immunosuppression Is mc1A1-Dependent. Previous studies of mice receiving daily dietary BaP in our laboratories have demonstrated the importance of CYP1A1 in detoxication. In Cyp1a1(-/-) knockout mice having no functional CYP1A1 protein in any tissue, daily oral BaP produced striking abnormalities within 18 days: loss in body weight, increased liver weight per total body weight, atrophy of the spleen and thymus, hypocellularity of the bone marrow with the lymphoid series particularly affected, elevated plasma ALT and AST levels, and evidence of anemia and increases in methemoglobin (Uno et al., 2004, 2006). These abnormalities were observed in the mtp1A1 line (Table 1) but not in the mc1A1 or WT 1A1 mice, strongly suggesting that the absence of microsomal CYP1A1 protein is the critical determinant in not allowing oral BaP detoxication to occur normally. Therefore, this study has established that the mc1A1 protein, and not the mt1A1 protein, is necessary and sufficient to support detoxication of BaP given orally to the intact mouse.
It should also be noted that after daily oral BaP, absolute CYP1A1 mRNA levels in mtp1A1 are higher than those in mc1A1 or wild-type mice to a minor degree in small intestine and more strikingly in both liver and spleen (Fig. 6). The significance of these observations is not known.
Differences in Up-Regulation of CYP1A2 and CYP1B1 mRNA. It is interesting that the subcellular location of the CYP1A1 protein seemed to have an effect on CYP1A2 or CYP1B1 mRNA inducibility; moreover, this effect was tissue-specific. CYP1A2 mRNA levels are higher in mtt1A1 and mtp1A1 (compared with that in mc1A1) mice in small intestine after intraperitoneal TCDD pretreatment; in this regard, the MT-targeted lines are more similar to the Cyp1a1(-/-) knockout than the wild-type mouse (Fig. 2A, bottom). CYP1A2 mRNA levels are higher in mtp1A1 (compared with that in mc1A1) mice in liver and spleen after administration of daily oral BaP; again, the MT-targeted line is more similar to the Cyp1a1(-/-) knockout mouse (Fig. 6, middle, right). CYP1B1 mRNA levels are higher in mtp1A1 (compared with that in mc1A1) mice in small intestine, liver, and spleen after daily oral BaP; yet again, the MT-targeted line is more similar to the Cyp1a1(-/-) knockout mouse (Fig. 6, bottom). Hence, CYP1A2 and/or CYP1B1 mRNA accumulation requires either the presence of mitochondrial CYP1A1 (unlikely, because WT 1A1 mice have mt1A1) or the absence of microsomal CYP1A1. Reasons for these fascinating observations are not understood and will require further experimentation.
How can we explain these “compensatory increases” in CYP1A2 and/or CYP1B1 mRNA when CYP1A1 is absent in one or the other organelle? Our analysis of basal CYP1 mRNA levels in the organelle-specific Cyp1a1 knock-in mouse lines suggests that some interesting cross-talk exists between the Cyp1 genes. This is particularly evident in the small intestine, in which the CYP1B1 basal mRNA level is elevated in both KO 1A1 and mtp1A1 mice, whereas the CYP1A2 basal mRNA level is elevated only in KO 1A1 mice. In previous studies (Uno et al., 2006, 2008), we have noted that compensatory up-regulation of one Cyp1 family member often occurs in the absence of one or more of the other members. In the present study, we now find that the mitochondrial isoform of CYP1A1 apparently can compensate for CYP1A2 but not for CYP1B1 in the small intestine. The reason for this is unclear. Recent genomics research has uncovered multiple (currently poorly understood) mechanisms of genomic cross-talk. Perhaps the dynamic genome should be considered to be like a “community”: when one member of that community suddenly becomes absent (e.g., due to gene ablation), the rest of the genome decides which other members should increase or decrease their levels of expression to compensate for that missing member. In the liver of the untreated Cyp1a2(-/-) knockout mouse, for example, 11 genes became significantly up-regulated, and 21 genes were down-regulated (Smith et al., 2003).
Since publication of the Encyclopedia of DNA Elements (ENCODE) pilot project (Birney et al., 2007), our lack of knowledge about the genome is more apparent than ever before. It is now clear how little is known about “what `a gene' is,” what mechanisms are involved in the cross-talk of cis- and trans-regulatory factors, how chromatin-remodeling and epigenetics affect gene expression, and why 60% of all (generich and gene-desert) regions of the DNA chosen for the ENCODE study are incredibly conserved between puffer fish and mammals (Gerstein et al., 2007; Nebert et al., 2008). We can only conclude that there is much more to learn about compensatory up- and down-regulation of gene expression.
Conclusions
The generation of three knock-in mouse lines carrying CYP1A1 protein targeted principally to the ER or the MT was successful. Whether there are tissue- or cell-specific differences in the success of CYP1A1-protein organelle-targeting or whether the tissue-specific dissimilarities represent minor problems in our ability to separate microsomes from mitochondrial (and vice versa) with absolute certainty remains open to further study. The administration of daily dietary BaP to these knock-in mouse lines provided us with proof-in-principle; we conclude that the mc1A1 and not the mt1A1 is important in oral BaP detoxication and thus protection from the various forms of BaP-induced damage. We have also found striking increases in CYP1A2 and CYP1B1 mRNA up-regulation by oral BaP in the mt1A1 (similar to that found previously in the KO 1A1) but not the mc1A1 lines.
It would be desirable also to show a clinical effect that happens in mtt1A1 and mtp1A1 mice but not in mc1A1 mice. Knowing that the LD50 for erythromycin in mice is 280 mg/kg, we gave doses of 28, 56, and 140 mg/kg to WT 1A1, mc1A1, mtt1A1, and mtp1A1 and watched for overt toxicity during the next 2 weeks; no differences were seen clinically, and no histological differences were observed in liver, lung, kidney, thymus, spleen, or bone marrow. Because one or more enzymes of the CYP3A family are responsible for most erythromycin metabolism (Tanaka, 1998), it is likely that mt1A1-mediated erythromycin metabolism is relatively minor compared with CYP3A-mediated metabolism. This is also likely to be true of other psychotropic drugs metabolized by mt1A1 (Anandatheerthavarada et al., 1999; Dasari et al., 2006).
Our reasons for the construction of these knock-in mouse lines include several hypotheses. First, our overriding assumption is that both ER- and MT-specific CYP1A1 function primarily for endogenous regulatory reasons (Nebert and Karp, 2008). ER-specific CYP1A1 might be important principally for the synthesis and degradation of critical life endogenous compounds and detoxication, as well as metabolic activation of various foreign chemicals entering the cell; this metabolism might lead to genotoxicity and mutagenesis of the genomic DNA, carcinogenesis, and perhaps organ-specific toxicity. On the other hand, MT-specific CYP1A1 might be important principally for the synthesis and degradation of mitochondrial critical life endogenous compounds and the detoxication, as well as metabolic activation of various foreign chemicals entering the mitochondrion; such metabolism might lead to genotoxicity, mutagenesis of the mitochondrial DNA, apoptosis by way of the cytochrome c release-signaling pathway, and perhaps birth defects. One postulate, that mc1A1 rather than mt1A1 is important in the detoxication of oral BaP, has been demonstrated in the present study. The remaining hypotheses require additional work. These knock-in mouse lines are available to all interested colleagues.
Acknowledgments
We thank our colleagues for discussions and careful reading of this manuscript.
These studies were supported, in part, by the National Institutes of Health National Institute of Environmental Health Sciences [Grants R01-ES08147, R01-ES014403, P30-ES06096] and the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM034883].
These data were presented in part at the 26th (March 5-9, 2006; San Diego, CA) and 27th (March 25-29, 2007; Charlotte, NC) Annual Meetings of the Society of Toxicology.
ABBREVIATIONS: AHR, aryl hydrocarbon receptor; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BaP, benzo[a]pyrene; BNF, β-naphthoflavone; ER, endoplasmic reticulum; MT, mitochondrial; POR, NADPH-P450 oxidoreductase; PHB, prohibitin; GCLM, glutamate-cysteine ligase modifier subunit; SRP, signal-recognition particle; ERND, erythromycin N-demethylase; FDX1, ferredoxin-1; FDXR, ferredoxin reductase; CYP1A1, full-length translated protein from the mouse Cyp1a1 gene; mc1A1, microsomal (endoplasmic reticulum)-targeted CYP1A1 protein; mt1A1, mitochondrial-targeted CYP1A1 protein; Cyp1a1(+/+) or WT 1A1, wild-type (C57BL/6J) mouse; Cyp1a1(-/-) or KO 1A1, mouse line having global knockout of the Cyp1a1 gene; Cyp1a1(mc/mc) or mc1A1, line carrying endoplasmic reticulum-targeted CYP1A1 protein; Cyp1a1(mtp/mtp) or mtp1A1, line carrying mitochondrial-targeted CYP1A1 protein via proteolysis; Cyp1a1(mtt/mtt) or mtt1A1, line carrying mitochondrial-targeted CYP1A1 protein via truncation; TCDD, dioxin, 2,3,7,8-tetrachlorodibenzo-p-dioxin; PAGE, polyacrylamide gel electrophoresis; PKC, protein kinase C; KO, knockout; bp, base pair(s); kb, kilobase pair(s); TK, thymidine kinase; ES, embryonic stem; PCR, polymerase chain reaction; SSC, standard saline citrate; WT, wild type; qRT-PCR, quantitative real-time polymerase-chain reaction; SKF-525A, 2-diethyl-aminoethyl-2,2-diphenylvalerate hydrochloride; f, forward; r, reverse.
References
- Addya S, Anandatheerthavarada HK, Biswas G, Bhagwat SV, Mullick J, and Avadhani NG (1997) Targeting of NH2-terminal-processed microsomal protein to mitochondria: a novel pathway for the biogenesis of hepatic mitochondrial P450MT2. J Cell Biol 139 589-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandatheerthavarada HK, Addya S, Dwivedi RS, Biswas G, Mullick J, and Avadhani NG (1997) Localization of multiple forms of inducible cytochromes P450 in rat liver mitochondria: immunological characteristics and patterns of xenobiotic substrate metabolism. Arch Biochem Biophys 339 136-150. [DOI] [PubMed] [Google Scholar]
- Anandatheerthavarada HK, Vijayasarathy C, Bhagwat SV, Biswas G, Mullick J, and Avadhani NG (1999) Physiological role of the N-terminal processed P450 1A1 targeted to mitochondria in erythromycin metabolism and reversal of erythromycin-mediated inhibition of mitochondrial protein synthesis. J Biol Chem 274 6617-6625. [DOI] [PubMed] [Google Scholar]
- Araki K, Araki M, Miyazaki J, and Vassalli P (1995) Site-specific recombination of a transgene in fertilized eggs by transient expression of Cre recombinase. Proc Natl Acad Sci U S A 92 160-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwat SV, Mullick J, Raza H, and Avadhani NG (1999) Constitutive and inducible cytochromes P450 in rat lung mitochondria: xenobiotic induction, relative abundance, and catalytic properties. Toxicol Appl Pharmacol 156 231-240. [DOI] [PubMed] [Google Scholar]
- Birney E, Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, et al. (2007) Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447 799-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blay P, Astudillo A, Buesa JM, Campo E, Abad M, García-García J, Miquel R, Marco V, Sierra M, Losa R, et al. (2004) Protein kinase-Cq is highly expressed in gastrointestinal stromal tumors, but not in other mesenchymal neoplasias. Clin Cancer Res 10 4089-4095. [DOI] [PubMed] [Google Scholar]
- Boopathi E, Anandatheerthavarada HK, Bhagwat SV, Biswas G, Fang JK, and Avadhani NG (2000) Accumulation of mitochondrial P450MT2, NH2-terminal truncated cytochrome P450 1A1 in rat brain during chronic treatment with b-naphthoflavone. A role in the metabolism of neuroactive drugs. J Biol Chem 275 34415-34423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boopathi E, Srinivasan S, Fang JK, and Avadhani NG (2008) Bimodal targeting of proteins with chimeric signals and activation of cryptic mitochondrial-targeting signals by an inducible cytosolic endoprotease. Mol Cell 32 32-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EG (1988) Mixed anionic detergent/aliphatic alcohol-polyacrylamide gel electrophoresis alters the separation of proteins relative to conventional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem 174 337-348. [DOI] [PubMed] [Google Scholar]
- Capecchi MR (1989) The new mouse genetics: altering the genome by gene targeting. Trends Genet 5 70-76. [DOI] [PubMed] [Google Scholar]
- Carrier F, Owens RA, Nebert DW, and Puga A (1992) Dioxin-dependent activation of murine Cyp1a1 gene transcription requires protein kinase C-dependent phosphorylation. Mol Cell Biol 12 1856-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yang Y, Miller ML, Shen D, Shertzer HG, Stringer KF, Wang B, Schneider SN, Nebert DW, and Dalton TP (2007) Hepatocyte-specific Gclc gene deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology 45 1118-1128. [DOI] [PubMed] [Google Scholar]
- Cheung C, Ma X, Krausz KW, Kimura S, Feigenbaum L, Dalton TP, Nebert DW, Idle JR, and Gonzalez FJ (2005) Differential metabolism of 2-amino-1-methyl-6phenylimidazo[4,5-b]pyridine (PhIP) in mice humanized for CYP1A1 and CYP1A2. Chem Res Toxicol 18 1471-1478. [DOI] [PubMed] [Google Scholar]
- Chung JY, Kim JY, Kim WR, Lee SG, Kim YJ, Park JE, Hong YP, Chun YJ, Park YC, Oh S, et al. (2007) Abundance of aryl hydrocarbon receptor potentiates benzo[a]pyrene-induced apoptosis in Hepa-1c1c7 cells via CYP1A1 activation. Toxicology 235 62-72. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Dieter MZ, Matlib RS, Childs NL, Shertzer HG, Genter MB, and Nebert DW (2000) Targeted knockout of Cyp1a1 gene does not alter hepatic constitutive expression of other genes in the mouse [Ah] battery. Biochem Biophys Res Commun 267 184-189. [DOI] [PubMed] [Google Scholar]
- Dasari VR, Anandatheerthavarada HK, Robin MA, Boopathi E, Biswas G, Fang JK, Nebert DW, and Avadhani NG (2006) Role of protein kinase C-mediated protein phosphorylation in mitochondrial translocation of mouse CYP1A1, which contains a non-canonical targeting signal. J Biol Chem 281 30834-30847. [DOI] [PubMed] [Google Scholar]
- Derkenne S, Curran CP, Shertzer HG, Dalton TP, Dragin N, and Nebert DW (2005) Theophylline pharmacokinetics: comparison of Cyp1a1(-/-) and Cyp1a2(-/-) knockout mice, humanized hCYP1A1_1A2 knock-in mice lacking either the mouse Cyp1a1 or Cyp1a2 gene, and Cyp1(+/+) wild-type mice. Pharmacogenet Genomics 15 503-511. [DOI] [PubMed] [Google Scholar]
- Dragin N, Uno S, Wang B, Dalton TP, and Nebert DW (2007) Generation of `humanized' hCYP1A1_1A2_Cyp1a1/1a2(-/-) mouse line. Biochem Biophys Res Commun 359 635-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RP and Wilson LD (1975) Purification and characterization of adrenodoxin reductase from bovine adrenal cortex. Biochemistry 14 1477-1484. [DOI] [PubMed] [Google Scholar]
- García Falcón MS, González Amigo S, Lage Yusty MA, López de Alda Villaizán MJ, and Simal Lozano J (1996) Determination of benzo[a]pyrene in lipid-soluble liquid smoke (LSLS) by HPLC-FL. Food Addit Contam 13 863-870. [DOI] [PubMed] [Google Scholar]
- Genter MB, Clay CD, Dalton TP, Dong H, Nebert DW, and Shertzer HG (2006) Comparison of mouse hepatic mitochondrial versus microsomal cytochromes P450 following TCDD treatment. Biochem Biophys Res Commun 342 1375-1381. [DOI] [PubMed] [Google Scholar]
- Gerstein MB, Bruce C, Rozowsky JS, Zheng D, Du J, Korbel JO, Emanuelsson O, Zhang ZD, Weissman S, and Snyder M (2007) What is a gene, post-ENCODE? History and updated definition. Genome Res 17 669-681. [DOI] [PubMed] [Google Scholar]
- Gu H, Marth JD, Orban PC, Mossmann H, and Rajewsky K (1994) Deletion of a DNA polymerase-b gene segment in T cells using cell type-specific gene targeting. Science 265 103-106. [DOI] [PubMed] [Google Scholar]
- Hankinson O (2005) Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor. Arch Biochem Biophys 433 379-386. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Dalton TP, Jin L, Wang B, Tsuneoka Y, Shertzer HG, Deka R, and Nebert DW (2005) Toward the evaluation of function in genetic variability: characterizing human SNP frequencies and establishing BAC-transgenic mice carrying the human CYP1A1_CYP1A2 locus. Hum Mutat 25 196-206. [DOI] [PubMed] [Google Scholar]
- Kawajiri K and Fujii-Kuriyama Y (2007) Cytochrome P450 gene regulation and physiological functions mediated by the aryl hydrocarbon receptor. Arch Biochem Biophys 464 207-212. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kwack SJ, and Lee BM (2000) Lipid peroxidation, antioxidant enzymes, and benzo[a]pyrene-quinones in the blood of rats treated with benzo[a]pyrene. Chem Biol Interact 127 139-150. [DOI] [PubMed] [Google Scholar]
- Li H, Witte DP, Branford WW, Aronow BJ, Weinstein M, Kaur S, Wert S, Singh G, Schreiner CM, and Whitsett JA (1994) Gsh4 encodes a LIM-type homeodomain, is expressed in the developing central nervous system, and is required for early postnatal survival. EMBO J 13 2876-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long WP, Pray-Grant M, Tsai JC, and Perdew GH (1998) Protein kinase C activity is required for aryl hydrocarbon receptor pathway-mediated signal transduction. Mol Pharmacol 53 691-700. [DOI] [PubMed] [Google Scholar]
- Ma X, Idle JR, Malfatti MA, Krausz KW, Nebert DW, Chen CS, Felton JS, Waxman DJ, and Gonzalez FJ (2007) Mouse lung CYP1A1 catalyzes the metabolic activation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Carcinogenesis 28 732-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour SL, Thomas KR, and Capecchi MR (1988) Disruption of the proto-oncogene Int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature 336 348-352. [DOI] [PubMed] [Google Scholar]
- Meehan RR, Speed RM, Gosden JR, Rout D, Hutton JJ, Taylor BA, Hilkens J, Kroezen V, Hilgers J, and Adesnik M (1988) Chromosomal organization of the cytochrome Cyp2c gene family in the mouse: a locus associated with constitutive aryl hydrocarbon hydroxylase activity. Proc Natl Acad Sci U S A 85 2662-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW (1989) The [Ah] locus: genetic differences in toxicity, cancer, mutation, and birth defects. Crit Rev Toxicol 20 153-174. [DOI] [PubMed] [Google Scholar]
- Nebert DW and Dalton TP (2006) The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer 6 947-960. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, Okey AB, and Gonzalez FJ (2004) Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem 279 23847-23850. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, Stuart GW, and Carvan MJ 3rd (2000a) “Gene-swap knockin” cassette in mice to study allelic differences in human genes. Ann N Y Acad Sci 919 148-170. [DOI] [PubMed] [Google Scholar]
- Nebert DW and Gelboin HV (1968) Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. I. Assay and properties of induced enzyme. J Biol Chem 243 6242-6249. [PubMed] [Google Scholar]
- Nebert DW and Karp CL (2008) Endogenous functions of the aryl hydrocarbon receptor: intersection of cytochrome P450 (CYP1)-metabolized eicosanoids and AHR biology. J Biol Chem 283 36061-36065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, and Dalton TP (2000b) Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol 59 65-85. [DOI] [PubMed] [Google Scholar]
- Nebert DW and Russell DW (2002) Clinical importance of the cytochromes P450. Lancet 360 1155-1162. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Zhang G, and Vesell ES (2008) From human genetics and genomics to pharmacogenetics and pharmacogenomics: past lessons, future directions. Drug Metab Rev 40 187-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, and Nebert DW (2004) Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14 1-18. [DOI] [PubMed] [Google Scholar]
- Niranjan BG, Avadhani NG, and DiGiovanni J (1985) Formation of benzo[a]pyrene metabolites and DNA adducts catalyzed by a rat liver mitochondrial monooxygenase system. Biochem Biophys Res Commun 131 935-942. [DOI] [PubMed] [Google Scholar]
- Park JY, Shigenaga MK, and Ames BN (1996) Induction of cytochrome P450 1A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin or indolo[2,3-b]carbazole is associated with oxidative DNA damage. Proc Natl Acad Sci U S A 93 2322-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga A, Tomlinson CR, and Xia Y (2005) Ah receptor signals cross-talk with multiple developmental pathways. Biochem Pharmacol 69 199-207. [DOI] [PubMed] [Google Scholar]
- Raza H and Avadhani NG (1988) Hepatic mitochondrial cytochrome P-450 system. Purification and characterization of two distinct forms of mitochondrial cytochrome P-450 from b-naphthoflavone-induced rat liver. J Biol Chem 263 9533-9541. [PubMed] [Google Scholar]
- Robinson JR, Felton JS, Levitt RC, Thorgeirsson SS, and Nebert DW (1975) Relationship between “aromatic hydrocarbon responsiveness” and the survival times in mice treated with various drugs and environmental compounds. Mol Pharmacol 11 850-865. [PubMed] [Google Scholar]
- Smith AG, Davies R, Dalton TP, Miller ML, Judah D, Riley J, Gant T, and Nebert DW (2003) Intrinsic hepatic phenotype associated with the Cyp1a2 gene as shown by cDNA expression microarray analysis of the knockout mouse. EHP Toxico-genomics 111 45-51. [PubMed] [Google Scholar]
- Tanaka E (1998) Clinical importance of non-genetic and genetic cytochrome P450 function tests in liver disease. J Clin Pharm Ther 23 161-170. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Derkenne S, Curran CP, Miller ML, Shertzer HG, and Nebert DW (2004) Oral exposure to benzo[a]pyrene in the mouse: detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol Pharmacol 65 1225-1237. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Dragin N, Curran CP, Derkenne S, Miller ML, Shertzer HG, Gonzalez FJ, and Nebert DW (2006) Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol Pharmacol 69 1103-1114. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Shertzer HG, Genter MB, Warshawsky D, Talaska G, and Nebert DW (2001) Benzo[a]pyrene-induced toxicity: paradoxical protection in Cyp1a1(-/-) knockout mice having increased hepatic BaP-DNA adduct levels. Biochem Biophys Res Commun 289 1049-1056. [DOI] [PubMed] [Google Scholar]
- Uno S, Dragin N, Miller ML, Dalton TP, Gonzalez FJ, and Nebert DW (2008) Basal and inducible CYP1 mRNA quantitation and protein localization throughout the mouse gastrointestinal tract. Free Radic Biol Med 44 570-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb BL, Hirst SJ, and Giembycz MA (2000) Protein kinase C isoenzymes: review of their structure, regulation and role in regulating airways, smooth muscle tone, and mitogenesis. Br J Pharmacol 130 1433-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells PG, Bhuller Y, Chen CS, Jeng W, Kasapinovic S, Kennedy JC, Kim PM, Laposa RR, McCallum GP, Nicol CJ, et al. (2005) Molecular and biochemical mechanisms in teratogenesis involving reactive oxygen species. Toxicol Appl Pharmacol 207 354-366. [DOI] [PubMed] [Google Scholar]
- Xu DX, Wang JP, Sun MF, Chen YH, and Wei W (2006) Lipopolysaccharide down-regulates the expression of intestinal pregnane X receptor and cytochrome P450 Cyp3a11. Eur J Pharmacol 536 162-170. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Huang FL, Nakabayashi H, and Huang KP (1988) Tissue distribution and developmental expression of protein kinase C isozymes. J Biol Chem 263 9868-9873. [PubMed] [Google Scholar]