Abstract
Psoralen plus UVA light (PUVA) is commonly used to treat psoriasis, a common skin disorder associated with rapid proliferation of cells. PUVA exerts its antiproliferative activity through formation of DNA monoadducts and interstrand cross-links (ICLs). However, this treatment may lead to skin malignancies as a direct result of inducing carcinogenic DNA damage. Inactivation of the p53 tumor suppressor gene is an important event in the development of skin cancer. p53 is rapidly phosphorylated and stabilized in response to DNA damage, and the induction of apoptosis by p53 is an important mechanism by which p53 exerts its tumor-suppressive activity. To better understand the mechanism by which PUVA treatment induces p53, we exposed human skin fibroblasts with PUVA under conditions that differentially produce monoadducts and ICLs and found that psoralen-induced ICLs induced phosphorylation of the Ser-15 site of p53 and apoptosis much more effectively than psoralen-induced monoadducts. The induction of p53 phosphorylation by psoralen ICLs did not require factors believed to be involved in the repair of psoralen ICLs [xeroderma pigmentosum (XP)-A, XP-C, XP-F, Cockayne's syndrome-B, Fanconi anemia] but did require the ataxia-telangiectasia and Rad3-related but not the ataxia-telangiectasia mutated kinase. Psoralen-induced ICLs blocked transcription and replication more efficiently than monoadducts, and induction of p53 and apoptosis correlated with doses causing interference with transcription rather than DNA replication. Our finding that cells underwent apoptosis preferentially during S-phase suggests that the combined blockade of transcription and DNA replication by psoralen ICLs during S-phase elicits a strong apoptotic response.
Psoralens are planar tricyclic compounds that readily enter cells and intercalate DNA (Cimino et al., 1985). Exposure to UVA light (365 nm) activates psoralen molecules to form covalent bonds with primarily 5,6 double bonds of thymines. The psoralen derivative 4′-hydroxymethyl-4,5′,8-trimethylpsoralen (HMT) has two reactive groups that can form either mono- or difunctional adducts with DNA, whereas angelicin is angular and can only form monoadducts. Induction of an HMT interstrand DNA cross-link (ICL), which requires absorption of two independent photons, effectively halts cell proliferation by inhibiting replication (Song and Tapley, 1979; Akkari et al., 2000). This inhibitory effect on cell proliferation is the basis for the use of psoralens and UVA (PUVA) to treat psoriasis (Parrish et al., 1974). However, PUVA treatment can lead to skin cancer, so a better understanding of the cellular responses to psoralen-induced ICLs is warranted (Stern et al., 1984; Gasparro, 2000).
It has been suggested that psoralen-induced ICLs trigger cellular responses primarily when cells enter S-phase, in which ICLs would be expected to interfere with DNA replication (Akkari et al., 2000). However, psoralen-induced ICLs have been shown to induce DNA damage responses such as phosphorylation of the histone variant H2AX (γH2AX) throughout the cell cycle and induce an arrest at the G1/S border of the cell cycle (Mogi et al., 2008; Viola et al., 2008). We and others have shown that agents that block transcription induce p53 (Yamaizumi and Sugano, 1994; Ljungman and Zhang, 1996; Ljungman and Lane, 2004) and apoptosis in human cells (Ljungman and Zhang, 1996; McKay et al., 1998; Brash et al., 2001). Furthermore, agents that block the elongation step of transcription induce an RPA and ATR-dependent phosphorylation of p53, suggesting that ATR monitors transcription elongation and responds to stalled transcription complexes by activating p53 (Ljungman et al., 2001; Derheimer et al., 2007). Although replication represents a larger target for inactivation by ICLs than transcription, transcription is inhibited by ICLs, and this blockade would occur in all phases of the cell cycle. Thus, it is possible that blockage of transcription is an important mechanism by which cells trigger the induction of p53 and apoptosis in human cells after the formation of psoralen-induced ICLs.
In this study, we investigated the mechanisms by which PUVA-induced ICLs trigger the induction of p53 and apoptosis in human cells. We show that psoralen photo-cross-linking induces p53 in a dose-dependent manner. The induction of p53 correlates with inhibition of RNA synthesis rather than inhibition of DNA replication after PUVA treatment. Phosphorylation of the Ser-15 site of p53 after PUVA treatment was diminished by the PI3-kinase inhibitor wortmannin and by nuclear microinjection of inhibitory anti-ATR antibodies, suggesting that the ATR kinase responds to ICLs and initiates a signal transduction pathway leading to the activation and stabilization of p53. Psoralen-induced ICLs also induced apoptosis in a dose-dependent manner. It is noteworthy that it was found that even though a blockade of DNA replication alone by lower doses of photoactivated psoralen did not seem to induce p53 or apoptosis, induction of apoptosis at higher doses was preferentially induced during S-phase under conditions that lead to the inhibition of RNA synthesis. Our data suggest that the apoptotic response induced by photoactivated psoralen in human fibroblasts may occur as a result of complications when replication forks meet RNA polymerase complexes stalled at sites of psoralen-induced ICLs.
Materials and Methods
Cell Culture. Diploid human fibroblasts and the AT fibroblast cell line GM01588 (Coriell, Camen, NJ) immortalized with human telomerase reverse transcriptase were grown as monolayers on culture dishes in minimal essential medium supplemented with 10% fetal bovine serum and antibiotic/antimycotic (Invitrogen, Carlsbad, CA).
Psoralen and UVA Treatments. Cells were rinsed with PBS and treated for 10 min on ice with HMT or angelicin in PBS. Cells were then irradiated with either 500 J/m2 (10 s) UVA or 30 kJ/m2 (10 min) UVA. In some experiments, cells were rinsed 3 × 5 min in PBS after the initial treatment and exposure to 500 J/m2 UVA to remove unbound HMT or angelicin and then reirradiated with 30 kJ/m2 to convert psoralen monoadducts to ICLs. This treatment will increase the ratio of ICLs to monoadducts without changing the total number of lesions (Vos and Hanawalt, 1987)
Western Blots. After treatment, cells were rinsed with PBS, detached by scraping, and collected by centrifugation. Cell samples were boiled for 5 min in loading buffer (2% SDS, 10% glycerol, 5% 2-mercaptoethanol, 0.05% bromphenol blue and 62.5 mM Tris, pH 6.8) before loading. Protein concentration was quantified using a protein assay (Bio-Rad, Hercules, CA), and approximately 30 μg of protein was loaded per lane. After SDS-polyacrylamide gel electrophoresis, proteins were electrophoretically transferred to Immobilon-P transfer membranes (Millipore, Billerica, MA). Antibodies used for immunoblotting were anti-Ser-15 phospho-specific antibody (Ser-15; Cell Signaling Technology, Danvers, MA), anti-p53 antibody (Calbiochem, San Diego, CA) and monoclonal anti-γH2AX antibodies (Millipore). X-ray film (Biomax AR; GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) and enhanced chemiluminescence (Super Signal CL-HRP Substrate System; Pierce, Rockford, IL) were used to visualize proteins. The quality of total protein transfer was assessed by staining blots with Coomassie Brilliant Blue after exposure of the membranes to X-ray film or by staining with anti-β-actin antibodies.
Flow Cytometry of Bromodeoxyuridine and DNA Content (Propidium Iodide). For analysis of cell-cycle distribution and apoptosis after PUVA treatment, cells were treated with different PUVA doses and incubated for 72 h. Cells were then fixed in ethanol and stained with propidium iodide (PI), and samples were analyzed using a flow cytometer as described previously (Hoy et al., 1989; Chang et al., 1999; McKay et al., 2002). In experiments determining whether apoptotic cells arise from cells replicating DNA, the cells were prelabeled for 15 min with 30 μM bromodeoxyuridine (BrdU) (Sigma, St. Louis, MO), followed by treatment with HMT and UVA. Immediately after psoralen and UVA treatment and three rinses in PBS, the growth media containing 30 μM BrdU were added back to the cells, and cells were incubated for 72 h. Assessment of BrdU staining as a function of PI staining was performed as described previously (Hoy et al., 1989; Chang et al., 1999; McKay et al., 2002). We incubated the fixed cells with mouse anti-BrdU antibodies conjugated to Alexa Fluor 647 (Invitrogen) for 30 min at room temperature. The cell samples were analyzed for BrdU (Alexa Fluor 647) and DNA content (PI) using flow cytometry (Coulter Elite ESP Cell Sorter; Beckman Coulter, Fullerton, CA). The percentages of BrdU-positive cells in the apoptotic fraction (subG1) were assessed.
Measurements of RNA Synthesis. Diploid human fibroblasts were prelabeled with [14C]thymidine by the addition of 185 Bq/ml to growth medium 2 days before the experiments to uniformly label genomic DNA. Nascent RNA was labeled for 60 min by adding [3H]uridine (1.5 × 106 Bq/ml). Cells were then rinsed twice in ice-cold PBS, detached by scraping, and collected by centrifugation. Poly-(A)RNA was isolated from cell lysates using the Straight A's mRNA isolation system (Novagen, Madison WI). Total nascent RNA was measured by precipitating cell lysates with an equal volume of 10% ice-cold trichloroacetic acid (TCA). The samples were kept on ice for 30 min, and the TCA-insoluble material was collected on filters (GF/A; Whatman Inc., Newton Center, MA). The filters were washed with 5 × 1 ml of 5% TCA, 5 × 1 ml of distilled H2O, and 2 × 1 ml of 95% ethanol and then dried under a heating lamp. The 3H and 14C counts present on the filters were counted in a scintillator using a dual-counting program. Relative total RNA synthesis or poly(A)RNA synthesis was then determined by calculating the ratio of 3H/14C for each sample and comparing it with the ratio from an untreated control sample. The data are presented as the percentage of the 3H/14C ratio for each treatment compared with this value determined from mock-irradiated controls.
Antibody Microinjections and Immunocytochemistry. Antibodies used to inhibit ATR (rabbit anti-ATR; Abcam Inc., Cambridge, MA) were microinjected into the nuclei of human fibroblasts at a concentration of 2 mg/ml. Microinjection was performed as described previously (O'Hagan and Ljungman, 2004a). To measure the dependence on ATR for Ser-15 phosphorylation of p53, cells were microinjected with either control IgG or anti-ATR antibodies and incubated at 37°C for 1 h. Cells were then treated with HMT (1 μg/ml) and UVA (10 min), incubated for 2 h and then fixed using 3.7% paraformaldehyde followed by permeabilization with PBS containing 0.2% Triton-X and 0.5% bovine serum albumin. To detect Ser-15 phosphorylation of p53, we used mouse monoclonal phosphospecific anti-Ser-15 p53 antibodies (Cell Signaling Technology) and anti-mouse fluorescein isothiocyanate-conjugated secondary antibodies. Cells injected with the rabbit anti-ATR antibodies were identified by coimmunostaining with anti-rabbit Alexa Fluor-555-conjugated secondary antibodies (Invitrogen).
Measurements of Apoptosis. Cells were seeded to approximately 20% confluence 2 days before treatments. After PUVA treatment, cells were allowed to incubate at 37°C for 72 h before both floating and attached cells were collected, and the percentage of apoptotic cells was assessed by identifying cells stained with propidium iodide containing a sub-G1 DNA content using flow cytometry.
Results
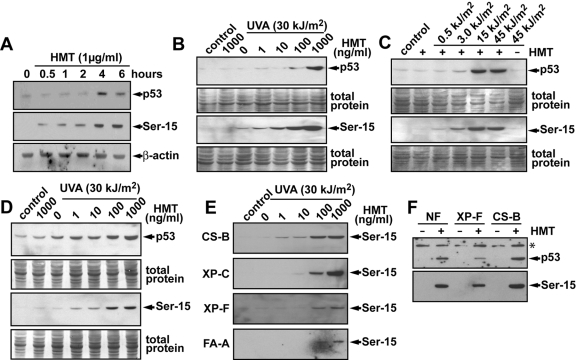
Time Course and Dose-Response for Induction of p53 and Ser-15 Phosphorylated p53 by PUVA. It has been shown that photoactivated psoralen (PUVA) can induce p53, γH2AX, and cell-cycle arrest and apoptosis (Mogi et al., 2008; Viola et al., 2008). The precise mechanism by which psoralen lesions induce these responses is not known. We first investigated the time course of induction of p53 and Ser-15 phosphorylation after exposure to 1 μg/ml (3.8 μM) HMT and 10 min of UVA irradiation (30 kJ/m2) and observed rapid Ser-15 phosphorylation of p53 after 30 min of incubation, whereas the accumulation of total p53 proteins was not detected until 4 h after psoralen photo-cross-linking (Fig. 1A). Thus, the observed induction of Ser-15 phosphorylation was not merely due to an overall accumulation of p53 but indicates that phosphorylation and accumulation of p53 are distinct events as has been suggested previously (O'Hagan and Ljungman, 2004b; Derheimer et al., 2007). Based on the observed kinetics of p53 induction, we used samples collected 6 h after incubation for all other Western blot experiments.
Fig. 1.
Induction of Ser-15 phosphorylation and p53 protein levels by PUVA. A, human diploid fibroblasts were treated with HMT (1 μg/ml = 3.8 μM) and irradiated with 30 kJ/m2 with UVA (10 min). Cells were incubated in fresh media at 37°C and harvested at different times, and the levels of total p53 protein and Ser-15 phosphorylated p53 were determined by Western blotting. β-Actin was used as a loading control. B, human diploid fibroblasts were treated with different doses of HMT followed by UVA irradiation (30 kJ/m2). Cells were collected 6 h later, and the levels of total p53 protein and Ser-15 phosphorylated p53 were determined by Western blotting. After exposure to film, the membranes were stained with Coomassie blue stain to visualize total protein loading and transfer in the different lanes. C, human diploid fibroblasts were treated with HMT (1 μg/ml = 3.8 μM) and irradiated with different doses of UVA. Cells were harvested 6 h later, and the levels of total p53 protein and Ser-15 phosphorylated p53 were determined by Western blotting. Note that neither HMT nor UVA (45 kJ/m2) alone induced p53 or phosphorylation of p53, whereas HMT with the higher exposure of UVA light induced high levels of both. D, diploid fibroblasts derived from a patient with xeroderma pigmentosum complementation group A (XP-A) were treated as in B. E, diploid fibroblasts from various DNA repair-deficiency syndromes were treated as described in B and analyzed for Ser-15 phosphorylation. F, diploid normal, XP-F, and CS-B fibroblasts were treated with 100 ng/ml (380 nM) HMT and 30 kJ/m2 UVA and incubated for 24 h before the cellular levels of p53 and Ser-15 phosphorylated p53 were determined by Western blot. *, a nonspecific band was used to assess loading.
Psoralen induces both DNA monoadducts and ICLs when activated by UVA light (Friedberg et al., 2006). We first treated human diploid fibroblasts with different concentrations of HMT followed by a fixed dose of UVA (30 kJ/m2). Neither HMT nor UVA light alone induced significant amounts of p53 or Ser-15 phosphorylated p53 (Fig. 1B). However, when 100 ng/ml HMT treatment was followed by a dose of 30 kJ/m2 UVA irradiation, high levels of both p53 and Ser-15 phosphorylation were detected. A slight induction of Ser-15 phosphorylation was detected at 10 ng/ml HMT. Because the formation of ICLs involves the absorption of two photons, it is a dose-dependent phenomenon in which low (short) exposure will lead primarily to the formation of monoadducts, whereas a strong (long) exposure is needed for the formation of ICLs. To assess whether cells induce p53 in response to monoadducts and/or ICLs formed by HMT and UVA light, we exposed cells to 1 μg/ml (3.8 μM) HMT and then irradiated them for various times with UVA. It was found that p53 accumulation and Ser-15 phosphorylation occurred in cells after being irradiated with UVA for 5 min or more (>15 kJ/m2), whereas only minor induction was observed in cells exposed for 1 min or less (Fig. 1C).
To investigate the potential mechanism for the induction of Ser-15 phosphorylation after psoralen photo-cross-linking, we exposed cells derived from patients with various genetic defects in NER, TCR, or the Fanconi anemia damage response pathway to HMT and UVA and assessed Ser-15 phosphorylation. We reasoned that strand breaks formed as a result of DNA damage-processing may be involved in the activation of ATM and/or ATR signaling, leading to p53 phosphorylation. Furthermore, the Fanconi anemia complex may be involved in regulating the phosphorylation of p53 after psoralen-induced inhibition of replication. It was found that XP-A (Fig. 1D), XP-C, XP-F, CS-B, and FA-A cells (Fig. 1E) were all capable of inducing Ser-15 phosphorylation after photoactivation of HMT. This induction of p53 and Ser-15 phosphorylation of p53 was still evident 24 h after PUVA treatment (Fig. 1F). These results suggest that the triggering of Ser-15 phosphorylation of p53 after HMT and UVA treatment does not seem to require processing or recognition of psoralen DNA adducts by NER, TCR, or Fanconi anemia factors.
Psoralen-Induced ICLs Are More Potent Inducers of p53 Compared with Psoralen Monoadducts. To specifically test whether ICLs are responsible for p53 induction after PUVA treatment, the amount of p53 and Ser-15-phosphorylated p53 was measured in cells either exposed to 500 J/m2 (10 sec) of UVA alone or in cells that had received a short pulse, had unbound psoralen removed through extensive washing, and then reirradiated with 30 kJ/m2. The latter protocol converts monoadducts into ICLs without changing the total number of DNA lesions (Vos and Hanawalt, 1987). We observed only marginal phosphorylation of the Ser-15 site of p53 6 h after an exposure to 500 J/m2 (10 s) of UVA irradiation of HMT-treated cells. However, reirradiation of the cells after removal of unbound psoralen molecules induced a robust increase of Ser-15 phosphorylation levels, suggesting that conversion of monoadducts into ICLs promotes phosphorylation of p53 (Fig. 2A). We also measured the induction of γH2AX as a surrogate marker of DNA damage and found a very similar pattern of induction as for phosphorylation of p53.
Fig. 2.
PUVA-induced ICLs induce a stronger phosphorylation of p53 and H2AX than psoralen monoadducts. A, human diploid fibroblasts were treated with HMT (0.5 or 1 μg/ml) and irradiated with 500 J/m2 (low; lanes 2-4) or 30 kJ/m2 UVA (high; lane 5). Two samples (lanes 6 and 7) were washed after exposure to the low 500 J/m2 UVA dose to remove unbound HMT, and the cells were reirradiated with 30 kJ/m2 UVA to convert monoadducts into ICLs. Cells in lane 8 were irradiated with 30 kJ/m2 in the presence of 0.5 μg/ml HMT. After UVA irradiation, cells were incubated at 37°C for 6 h before cells were harvested and levels of Ser-15 phosphorylation (top) and γH2AX (bottom) were determined by Western blot. The top bands denoted with * are nonspecific bands that serve as surrogate loading control. B, the same as in A, but angelicin was used instead of HMT. 0.5 μg/ml HMT = 1.9 μM; 0.5 μg/ml angelicin = 2.7 μM.
In further support that ICLs more potently induce p53 and γH2AX compared with monoadducts, we treated cells with angelicin, a psoralen-derivative, which exclusively forms monoadducts at rates similar to those of other psoralens (Kaye et al., 1980). As expected, reirradiation with 30 kJ/m2 of cells treated with angelicin after a short pulse of UVA (500 J/m2) did not increase phosphorylation of p53 or H2AX (Fig. 2B). However, after a long exposure to UVA, angelicin-treated cells did induce phosphorylation of both p53 and H2AX. Taken together, our results suggest that psoralen-induced DNA cross-links trigger a stronger stress response measured as phosphorylation of p53 and H2AX compared with the same amounts of DNA monoadducts.
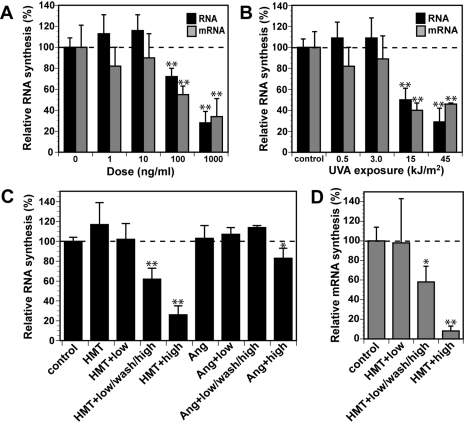
PUVA-Induced DNA Cross-Links Inhibit mRNA Synthesis. We have shown previously that the induction of p53 and Ser-15 phosphorylation of p53 after treatment with photoactivated psoralen is not dependent on replication (Derheimer et al., 2007). To test whether induction and phosphorylation of p53 after PUVA treatment correlates to inhibition of transcription, we measured nascent RNA and mRNA synthesis by [3H]uridine pulse-labeling and mRNA isolation using poly(dT)-conjugated magnetic beads (Ljungman et al., 1999). We found that both total RNA and mRNA synthesis was inhibited in an HMT and UVA dose-dependent manner (Fig. 3, A and B). Significant inhibition of mRNA and total RNA synthesis was achieved only after doses of 100 ng/ml (380 nM) or greater and after exposure to at least 15 kJ/m2 UVA irradiation.
Fig. 3.
PUVA-induced ICLs inhibit transcription preferentially compared with psoralen monoadducts. A, diploid human fibroblasts prelabeled with [14C]thymidine were treated with different doses of HMT followed by UVA (30 kJ/m2). The cells were then incubated for 1 h in fresh media followed by incubation with for 1 h in the presence of [3H]uridine to label nascent RNA. The ratios of 3H/14Cin total RNA or isolated mRNA of the different samples are expressed relative to mock-treated control cells (=100%). B, same as in A, but cells were treated with 1 μg/ml (3.8 μM) HMT followed by irradiation with different doses of UVA. C, ICLs induce a stronger inhibition of RNA synthesis than monoadducts. Diploid human fibroblasts were treated with 1 μg/ml HMT or angelicin alone or with combinations of 500 J/m2 UVA (low) and/or 30 kJ/m2 UVA (high) with a washout in between irradiations. Total nascent RNA synthesis values were compared with untreated control cells as described in A. D, ICLs inhibit mRNA synthesis. Cells were treated with 1 μg/ml HMT and irradiated with 500 J/m2 UVA and/or 30 kJ/m2 UVA with a washout in between, and nascent mRNA synthesis was determined as in A. Error bars show S.D.; *, p < 0.05; **, p < 0.001.
To further evaluate the relative contribution of monoadducts versus cross-links to the inhibition of transcription, we assessed RNA synthesis in cells treated with either HMT + UVA or angelicin + UVA. We also compared short exposure of UVA verses short exposure of UVA followed by removal of unbound HMT and then a 10-min reirradiation with UVA to convert monoadducts to DNA cross-links. We found that a 10-s irradiation was not sufficient to result in any measurable inhibition of RNA (Fig. 3C) or mRNA (Fig. 3D) synthesis. However, when these cells were reirradiated with 10 min of UVA, synthesis of both total RNA and mRNA was reduced by approximately 40%. In contrast, reirradiation of cells treated with angelicin did not cause the inhibition of RNA synthesis, whereas a 10-min irradiation of angelicin-exposed cells did result in a significantly reduced rate of RNA synthesis in accordance with a previous study (Nocentini, 1978). These results strongly suggest that when assessing similar levels of DNA lesions, ICLs more profoundly inhibit RNA synthesis compared with monoadducts, and inhibition of transcription correlates to a more pronounced induction of p53 and γH2AX.
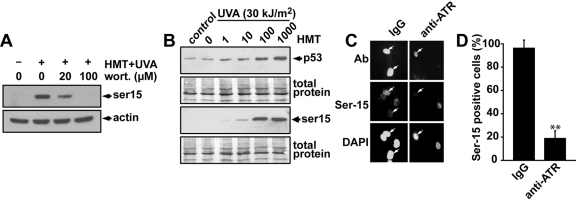
Induction of Ser-15 Phosphorylation of p53 by PUVA Treatment Is ATR-Dependent. The Ser-15 site of p53 has been shown to be important for the activation and stabilization of p53 and can be phosphorylated by the kinases ATM, ATR, DNA-PK, suppressor of morphogenesis in genitalia-1, ERK, and p38 (Canman et al., 1998; Bode and Dong, 2004; Gehen et al., 2008). To investigate which kinase(s) may be responsible for the phosphorylation of the Ser-15 site of p53 after psoralen photo-cross-linking, we first assessed the effect that the PI3 kinase inhibitor wortmannin may have on Ser-15 phosphorylation of p53 after treatment of the cells with HMT and UVA. Cells were pretreated with different doses of wortmannin for 30 min before the cells were treated with HMT and UVA and then incubated at 37°C for 6 h in the presence of wortmannin. A concentration of 20 μM wortmannin is expected to inhibit the activities of ATM and DNA-PK, whereas ATR should be relatively unaffected until higher doses such as 100 μM are used (Sarkaria et al., 1998). The results show that 20 μM wortmannin was not sufficient to abolish the induction of Ser-15 phosphorylation, suggesting that ATM and DNA-PK may play only minor roles in this phosphorylation after PUVA-induced ICLs (Fig. 4A). However, at the 100 μM dose of wortmannin, the induction of Ser-15 phosphorylation of p53 was abolished, suggesting that the ATR, ERK, and/or p38 kinases may be responsible for Ser-15 phosphorylation of p53 after psoralen photo-cross-linking.
Fig. 4.
The ATR kinase is required for Ser-15 phosphorylation after PUVA treatment. A, diploid human fibroblasts were pretreated with different concentrations of wortmannin for 30 min followed by treatment with HMT (1 μg/ml) and UVA (30 kJ/m2). Cells were incubated for 6 h in the presence of wortmannin before the cells were harvested, and Ser-15 phosphorylation of p53 was analyzed by Western blot. β-Actin was used as loading control. B, diploid AT fibroblasts were treated with different concentrations of HMT followed by UVA light (30 kJ/m2). Cells were harvested 6 h later, and the levels of total p53 protein and Ser-15 phosphorylated p53 were determined by Western blotting. C, human diploid fibroblasts were microinjected into the nucleus with either IgG or anti-ATR antibodies. After 1-h incubation, the cells were treated with HMT (1 μg/ml) and UVA (30 kJ/m2), and 2 h later, the cells were fixed and stained for Ser-15 phosphorylation of p53. Arrows indicate microinjected cells. D, quantification of the percentage of cells staining positive for phosphorylated Ser-15 of p53 treated as in C. The total numbers of injected cells were 100 and 102 for IgG and anti-ATR, respectively. The error bars represent the S.D. of results from at least 5 different experiments with approximately 20 microinjected cells for each.
Although the wortmannin experiment described above suggested that the ATM kinase may not be responsible for the Ser-15 phosphorylation after psoralen photo-cross-linking, we wanted to test this in a more direct way. Because PUVA-induced ICLs can result in double-strand break induction in a replication-mediated fashion (Bessho, 2003) and the ATM kinase is known to phosphorylate the Ser-15 site of p53 after induction of double-strand breaks (Canman et al., 1998), it would be conceivable that the ATM kinase is activated by psoralen DNA cross-links. To test the potential role of ATM in Ser-15 phosphorylation, we treated fibroblasts derived from a patient with ataxia telangiectasia with different concentrations of HMT and assessed the levels of p53 and Ser-15 phosphorylated p53. It was found that the induction of p53 and Ser-15 phosphorylation by HMT and UVA was indistinguishable from that induced in wild-type cells, suggesting that the Ser-15 site of p53 is phosphorylated in an ATM-independent manner after psoralen photo-cross-linking (Fig. 4B).
We next directly tested whether the ATR kinase is involved in the Ser-15 phosphorylation of p53 after psoralen photo-cross-linking. ATR is an essential protein necessary for regulating DNA replication (Brown and Baltimore, 2000, 2003; Cha and Kleckner, 2002), and it is therefore difficult to apply short interfering RNA-mediated knockdown approaches because cells become stressed and lose viability. We showed recently that microinjections of ATR-specific polyclonal antibodies into the nuclei of cells effectively and rapidly inhibited ATR kinase activity (Derheimer et al., 2007). Here we used this approach and compared HMT + UVA-mediated induction of Ser-15 phosphorylation in single cells that had been microinjected with either anti-ATR antibodies or IgG as a control. We show that microinjection of anti-ATR antibodies, but not IgG, abolished the induction of Ser-15 phosphorylation after psoralen photo-cross-linking (Fig. 4, C and D). Injection of IgG alone did not induce Ser-15 phosphorylation above background (Derheimer et al., 2007). Taken together, these results strongly suggest that the ATR kinase is activated by DNA cross-links and is responsible for the induction of Ser-15 phosphorylation of p53 after psoralen photo-cross-linking, which is in agreement with previous studies (Hovest et al., 2006; Derheimer et al., 2007).
PUVA Treatment Triggers Apoptosis Preferentially in S-Phase. We next examined whether PUVA treatments induce apoptosis in human fibroblasts. Cells were treated with different concentrations of HMT followed by exposure to 30 kJ/m2 UVA irradiation. The cells were then incubated at 37°C for 72 h, at which time both floating and attached cells were collected for flow cytometric analysis. It was found that neither HMT nor UVA irradiation alone was sufficient to induce apoptosis, measured as the population of cells with a sub-G1 DNA content (Fig. 5A). Although no increase in apoptosis was evident after treatment with 10 ng/ml (38 nM) HMT + UVA, this dose resulted in a significant increase in the percentage of cells in late S- and G2/M-phases of the cell cycle, suggesting that these cells either had difficulties progressing through S-phase or that an S-phase checkpoint had been activated. At the 100 ng/ml (380 nM) dose, no such increase of cells in late S-phase was seen, but rather a dramatic increase in sub-G1 apoptotic cells was evident. Taken together, although cells accumulated in S-phase after exposure to 10 ng/ml HMT, they did not undergo apoptosis suggesting that interference with DNA replication or arrest in S-phase is not sufficient on its own for the induction of apoptosis.
Fig. 5.
PUVA-induced ICLs induce apoptosis preferentially in cells progressing through S-phase. A, diploid human fibroblasts were treated with different concentrations of HMT followed by UVA (30 kJ/m2). Cells were harvested 72 h later, and the percentages of cells undergoing apoptosis were determined by the sub-G1 fraction of cells detected using flow cytometry. Note that after a dose of 10 ng/ml, cells are accumulating in the S- and G2/M-phases of the cell cycle without inducing any significant amounts of apoptosis. B, diploid fibroblasts were treated as described in Fig. 2A and were harvested 72 h later, and the percentages of cells undergoing apoptosis were determined by the sub-G1 fraction of cells detected using flow cytometry. Note that the cell population reirradiated with 30 kJ/m2 UVA to convert monoadducts into ICLs induced 13% of apoptotic cells compared with 3% in the cell population that was only exposed to 0.5 kJ/m2 of UVA. C, diploid human fibroblasts pretreated with BrdU for 15 min were treated with different concentrations of HMT and UVA (30 kJ/m2). Cells were then incubated in the presence of BrdU and harvested 72 h later, and the percentages of BrdU-containing cells undergoing apoptosis (sub-G1 fraction) were assessed. It was found that 63 and 68% of the apoptotic cells detected after treatment with 100 and 1000 ng/ml, respectively, stained positive for BrdU.
We next explored whether the induction of apoptosis observed after psoralen photo-cross-linking is due to monoadducts or whether formation of ICLs is necessary. Cells were treated with HMT and irradiated for 10 s with UVA light to induce psoralen monoadducts and then either incubated directly or washed and reirradiated for 10 min to allow for the conversion of monoadducts into ICLs. Analysis of the cell-cycle distribution 72 h later revealed that the conversion of monoadducts into ICLs caused a substantial increase in cells occupying the S-phase of the cell cycle (Fig. 5B). This result probably reflects the inability to replicate DNA templates containing ICLs. In contrast, no accumulation of cells in the S-phase was observed in the samples containing predominantly monoadducts, although we did observe an increase of cells in the G2/M-phase of the cell cycle. Conversions of psoralen monoadducts into ICLs by reirradiation increased the induction of apoptosis from 3 to 13%, suggesting that ICLs are more potent inducers of apoptosis than monoadducts.
To examine whether the induction of apoptosis after psoralen photo-cross-linking requires DNA replication, we pretreated cells for 15 min with BrdU to label cells in S-phase followed by HMT treatment and UVA irradiation at 4°C. Cells were then incubated at 37°C for 72 h in the presence of BrdU to mark any cells progressing through S-phase during the incubation. Induction of apoptosis was analyzed using flow cytometry, and the results are expressed as log fluorescein isothiocyanate (BrdU) versus log PI (DNA content) (Fig. 5C). It was found that of the cells that had undergone apoptosis (sub-G1 DNA content) the majority (63 and 68%) stained positive for BrdU, suggesting that the induction of apoptosis is preferentially triggered in cells that are attempting to replicate their genomes. Taken together, the results suggest that PUVA-induced ICLs trigger apoptosis preferentially in cells progressing through S-phase.
Discussion
PUVA is used in the clinic to treat psoriasis, but there is concern that this treatment may induce skin cancer (Stern et al., 1984; Gasparro, 2000). Thus, it is of importance to better understand the mechanisms by which cells respond to PUVA-induced DNA damage. In this study, we investigated the potential mechanisms of induction of DNA damage stress responses by PUVA in human cells and found that this treatment induces p53 and phosphorylation of p53 in a dose-dependent fashion. The initial triggering of phosphorylation of the Ser-15 site of p53 did not require functional NER or TCR, nor was a functional Fanconi anemia pathway necessary. Furthermore, although induction of phosphorylation of the Ser-15 site of p53 by psoralen photo-cross-linking did not require DNA replication, induction of apoptosis occurred preferentially in cells progressing through S-phase. Finally, the induction of p53 phosphorylation at the Ser-15 site did not require the ATM kinase but was dependent on ATR and correlated with inhibition of transcription.
Psoralen induces both monoadducts and ICLs after activation by UVA light (Cimino et al., 1985). Because the formation of ICLs requires two activation events, monoadducts are predominantly formed after short UVA irradiations, whereas ICLs only form after longer irradiations. In this study, we found that p53 was activated primarily after longer UVA irradiations, suggesting that ICLs were triggering p53 activation (Fig. 1). The repair of DNA containing ICLs is believed to require a combination of enzymes from different repair pathways such as nucleotide excision repair, mismatch repair, the Fanconi anemia pathway, homologous recombination, and translesion DNA polymerases (Friedberg et al., 2006; Raschle et al., 2008). To explore whether repair-induced processing of ICLs is responsible for the triggering of p53 activation, we investigated Ser-15 phosphorylation of p53 in various DNA repair-deficient cells after treatment with HMT and UVA. The results from these studies do not provide any evidence that intermediates of NER or TCR or the action of the FA pathway are required for the triggering of Ser-15 phosphorylation of p53.
Because ICLs are believed to block both replication and transcription by interfering with the separation of DNA strands, it is possible that p53 induction is triggered by the stalling of either DNA replication or transcription. We have shown recently that DNA replication is not a requirement for the induction of Ser-15 phosphorylation after HMT and UVA treatment because p53 was phosphorylated in serum-starved cells and in non-S-phase cells in asynchronously growing cell populations (Derheimer et al., 2007). Instead, the dose-response for the induction of Ser-15 phosphorylation closely correlated to the blockage of transcription in an RPA- and ATR-dependent manner, suggesting that this blockage by PUVA-induced ICLs may trigger phosphorylation of p53. This finding is in concert with previous reports showing a connection between blocked transcription and p53 induction (Yamaizumi and Sugano, 1994; Ljungman and Zhang, 1996; Dumaz et al., 1997; McKay et al., 1998; Ljungman et al., 1999, 2001; Brash et al., 2001; Ljungman and Lane, 2004; Derheimer et al., 2007). We also show that induction of γH2AX at lower doses of photoactivated psoralen correlates with induction of ICLs rather than monoadducts and correlates with the inhibition of transcription.
The Ser-15 site of p53 has been shown to be phosphorylated by PI3 kinases such as ATM, ATR, DNA-PK, and suppressor of morphogenesis in genitalia-1 (SMG-1), as well as by ERK and p38 (Ljungman, 2000; Bode and Dong, 2004; Brumbaugh et al., 2004). By using the PI3 kinase inhibitor wortmannin, we show that phosphorylation of the Ser-15 site after treatment with HMT and UVA involves a PI3 kinase (Fig. 4A). ATM was ruled out as a potential kinase because ATM-defective AT cells were able to induce phosphorylation of the Ser-15 site of p53 after PUVA treatment to similar levels as wild-type cells (Fig. 4B). However, when using a nuclear microinjection approach with anti-ATR antibodies to specifically and rapidly inhibit ATR, psoralen-induced phosphorylation of the Ser-15 site of p53 was abolished (Fig. 4, C and D). Thus, our results suggest a model in which formation of psoralen ICLs lead to the activation of the ATR kinase that in turn phosphorylates the Ser-15 site of p53. A similar role of ATR in response to PUVA-induced DNA damage has been reported recently for the induction of senescence (Hovest et al., 2006). We have shown recently that blockage of transcription elongation, even in the absence of DNA damage, triggers Ser-15 phosphorylation of p53 in an ATR- and RPA-dependent manner. Thus, it is plausible that the ATR-mediated phosphorylation of p53 after treatment with PUVA is triggered by the blockage of transcription elongation (Derheimer et al., 2007).
The removal of psoralen ICLs is remarkably slow in human fibroblasts, with only approximately 11 ICLs estimated to be removed per hour per cell (Akkari et al., 2000). Because ICLs interfere with replication, progression through S-phase will be impeded even after fairly low doses of PUVA. Consistent with this concept, we found that after exposure of human fibroblasts to 10 ng/ml HMT + UVA, cells accumulated in the S-phase of the cell cycle (Fig. 5A). However, we could not detect any significant increase in the induction of apoptosis and only marginal induction of p53 and Ser-15 phosphorylation of p53. This suggests that interference with DNA replication by ICLs alone is not sufficient for the induction of apoptosis or p53 (Luftl et al., 1998). Treatment with 100 ng/ml HMT + UVA induced approximately 50% apoptosis in cells and a strong induction of p53 levels and phosphorylation. At this dose, we observed a robust inhibition of transcription (Fig. 3A). At a dose of 10 ng/ml HMT, approximately 25,000 ICLs are expected to be formed per genome or ∼1 ICL/220 kilobase pairs of DNA (Akkari et al., 2000). This dose would be sufficient to inhibit replication, whereas many transcription units would be expected to be lesion-free, and only limited inhibition would be detected. Taken together, our results and results from others (Luftl et al., 1998) show a strong dose-response relationship between the inhibition of transcription and the induction of apoptosis and p53.
We have shown previously that UV-induced apoptosis is closely linked to blockage of transcription (Ljungman and Zhang, 1996) and to inhibition of S-phase progression (McKay et al., 2002). In the present study, we observed that apoptosis induced by photoactivated psoralen correlated more closely with the inhibition of transcription rather than with replication but yet occurred preferentially in cells attempting to traverse S-phase (Fig. 5C). How can apoptosis be linked to S-phase traversal when inhibition of DNA replication after exposure to lower doses of psoralen did not seem to be sufficient for the induction of apoptosis (Fig. 5A) (Akkari et al., 2000)? One possible explanation is that a stalled transcription complex constitutes a barrier that a replication elongation complex cannot bypass, resulting in apoptosis (Ljungman and Lane, 2004; Rudolph et al., 2007). At lower levels of ICLs in the DNA template, replication is preferentially affected because of its larger target size of inactivation. However, at these lower doses, neither p53 nor apoptosis is induced significantly, and cells will enter S-phase with damaged DNA templates, a scenario that may contribute to carcinogenesis and the formation of skin tumors in patients treated with PUVA.
Acknowledgments
We are grateful to Gabriel Nunez and the Flow Cytometry Core at the University of Michigan Comprehensive Cancer Center for excellent technical assistance.
This work was supported by the National Institutes of Health National Cancer Institute [Grant P30-CA46592]; the National Institutes of Health National Institute of General Medical Sciences [Grant GM007767] [Program in Cellular and Molecular Biology, the University of Michigan Comprehensive Cancer Center Core] and by the Department of Radiation Oncology, University of Michigan.
ABBREVIATIONS: HMT, 4′-hydroxymethyl-4,5′,8-trimethylpsoralen; PUVA, psoralen + ultraviolet A; ICL, DNA interstrand cross-link; ATM, ataxia-telangiectasia mutated; ATR, ataxia-telangiectasia and Rad3-related; XP, xeroderma pigmentosum; CS, Cockayne's syndrome; FA, Fanconi anemia; NER, nucleotide excision repair; TCR, transcription-coupled repair; RPA, replication protein A; ERK, extracellular signal-regulated kinase; PBS, phosphate-buffered saline; PI3, phosphatidylinositol-3; PI, propidium iodide; BrdU, bromodeoxyuridine; TCA, trichloroacetic acid; DNA-PK, DNA-dependent protein kinase.
References
- Akkari YM, Bateman RL, Reifsteck CA, Olson SB, and Grompe M (2000) DNA replication is required to elicit cellular responses to psoralen-induced DNA interstrand cross-links. Mol Cell Biol 20 8283-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessho T (2003) Induction of DNA replication-mediated double strand breaks by psoralen DNA interstrand cross-links. J Biol Chem 278 5250-5254. [DOI] [PubMed] [Google Scholar]
- Bode AM and Dong Z (2004) Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 4 793-805. [DOI] [PubMed] [Google Scholar]
- Brash DE, Wikonkal NM, Remenyik E, van der Horst GT, Friedberg EC, Cheo DL, van Steeg H, Westerman A, and van Kranen HJ (2001) The DNA damage signal for Mdm2 regulation, Trp53 induction, and sunburn cell formation in vivo originates from actively transcribed genes. J Invest Dermatol 117 1234-1240. [DOI] [PubMed] [Google Scholar]
- Brown EJ and Baltimore D (2000) ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev 14 397-402. [PMC free article] [PubMed] [Google Scholar]
- Brown EJ and Baltimore D (2003) Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev 17 615-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbaugh KM, Otterness DM, Geisen C, Oliveira V, Brognard J, Li X, Lejeune F, Tibbetts RS, Maquat LE, and Abraham RT (2004) The mRNA surveillance protein hSMG-1 functions in genotoxic stress response pathways in mammalian cells. Mol Cell 14 585-598. [DOI] [PubMed] [Google Scholar]
- Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan MB, and Siliciano JD (1998) Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281 1677-1679. [DOI] [PubMed] [Google Scholar]
- Cha RS and Kleckner N (2002) ATR homolog Mec1 promotes fork progression, thus averting breaks in replication slow zones. Science 297 602-606. [DOI] [PubMed] [Google Scholar]
- Chang D, Chen F, Zhang F, McKay BC, and Ljungman M (1999) Dose-dependent effects of DNA-damaging agents on p53-mediated cell cycle arrest. Cell Growth Differ 10 155-162. [PubMed] [Google Scholar]
- Cimino GD, Gamper HB, Isaacs ST, and Hearst JE (1985) Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem 54 1151-1193. [DOI] [PubMed] [Google Scholar]
- Derheimer FA, O'Hagan HM, Krueger HM, Hanasoge S, Paulsen MT, and Ljungman M (2007) RPA and ATR link transcriptional stress to p53. Proc Natl Acad Sci U S A 104 12778-12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumaz N, Duthu A, Ehrhart JC, Drougard C, Appella E, Anderson CW, May P, Sarasin A, and Daya-Grosjean L (1997) Prolonged p53 protein accumulation in trichothiodystrophy fibroblasts dependent on unrepaired pyrimidine dimers on the transcribed strands of cellular genes. Mol Carcinog 20 340-347. [PubMed] [Google Scholar]
- Friedberg E, Walker G, Siede W, Wood R, Schultz R and Ellenberger T (2006) DNA Repair and Mutagenesis. ASM Press, Washington, DC.
- Gasparro FP (2000) The role of PUVA in the treatment of psoriasis. Photobiology issues related to skin cancer incidence. Am J Clin Dermatol 1 337-348. [DOI] [PubMed] [Google Scholar]
- Gehen SC, Staversky RJ, Bambara RA, Keng PC, and O'Reilly MA (2008) hSMG-1 and ATM sequentially and independently regulate the G1 checkpoint during oxidative stress. Oncogene 27 4065-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovest MG, Brüggenolte N, Hosseini KS, Krieg T, and Herrmann G (2006) Senescence of human fibroblasts after psoralen photoactivation is mediated by ATR kinase and persistent DNA damage foci at telomeres. Mol Biol Cell 17 1758-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy CA, Seamer LC, and Schimke RT (1989) Thermal denaturation of DNA for immunochemical staining of incorporated bromodeoxyuridine (BrdUrd): critical factors that affect the amount of fluorescence and the shape of BrdUrd/DNA histogram. Cytometry 10 718-725. [DOI] [PubMed] [Google Scholar]
- Kaye J, Smith CA, and Hanawalt PC (1980) DNA repair in human cells containing photoadducts of 8-methoxypsoralen or angelicin. Cancer Res 40 696-702. [PubMed] [Google Scholar]
- Ljungman M (2000) Dial 9-1-1 for p53: Mechanisms of p53 activation by cellular stress. Neoplasia 2 208-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman M and Lane DP (2004) Transcription—guarding the genome by sensing DNA damage. Nat Rev Cancer 4 727-737. [DOI] [PubMed] [Google Scholar]
- Ljungman M, O'Hagan HM, and Paulsen MT (2001) Induction of ser15 and lys382 modifications of p53 by blockage of transcription elongation. Oncogene 20 5964-5971. [DOI] [PubMed] [Google Scholar]
- Ljungman M and Zhang F (1996) Blockage of RNA polymerase as a possible trigger for UV light-induced apoptosis. Oncogene 13 823-831. [PubMed] [Google Scholar]
- Ljungman M, Zhang F, Chen F, Rainbow AJ, and McKay BC (1999) Inhibition of RNA polymerase II as a trigger for the p53 response. Oncogene 18 583-592. [DOI] [PubMed] [Google Scholar]
- Lüftl M, Röcken M, Plewig G, and Degitz K (1998) PUVA inhibits DNA replication, but not gene transcription at nonlethal dosages. J Invest Dermatol 111 399-405. [DOI] [PubMed] [Google Scholar]
- McKay BC, Becerril C, Spronck JC, and Ljungman M (2002) Ultraviolet light-induced apoptosis is associated with S-phase in primary human fibroblasts. DNA Repair 1 811-820. [DOI] [PubMed] [Google Scholar]
- McKay BC, Ljungman M, and Rainbow AJ (1998) Persistent DNA damage induced by ultraviolet light inhibits p21waf1 and bax expression: implications for DNA repair, UV sensitivity and the induction of apoptosis. Oncogene 17 545-555. [DOI] [PubMed] [Google Scholar]
- Mogi S, Butcher CE, and Oh DH (2008) DNA polymerase eta reduces the gamma-H2AX response to psoralen interstrand crosslinks in human cells. Exp Cell Res 314 887-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocentini S (1978) Impairment of RNA synthesis and its recovery in angelicin photosensitized mammalian cells. A probe for DNA damage and repair. Biochim Biophys Acta 521 160-168. [DOI] [PubMed] [Google Scholar]
- O'Hagan HM and Ljungman M (2004a) Efficient NES-dependent protein nuclear export requires ongoing synthesis and export of mRNAs. Exp Cell Res 297 548-559. [DOI] [PubMed] [Google Scholar]
- O'Hagan HM and Ljungman M (2004b) Phosphorylation and nuclear accumulation are distinct events contributing to the activation of p53. Mutat Res 546 7-15. [DOI] [PubMed] [Google Scholar]
- Parrish JA, Fitzpatrick TB, Tanenbaum L, and Pathak MA (1974) Photochemotherapy of psoriasis with oral methoxsalen and longwave ultraviolet light. N Engl J Med 291 1207-1211. [DOI] [PubMed] [Google Scholar]
- Räschle M, Knipsheer P, Enoiu M, Angelov T, Sun J, Griffith JD, Ellenberger TE, Schärer OD, and Walter JC (2008) Mechanism of replication-coupled DNA interstrand crosslink repair. Cell 134 969-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph CJ, Dhillon P, Moore T, and Lloyd RG (2007) Avoiding and resolving conflicts between DNA replication and transcription. DNA Repair (Amst) 6 981-993. [DOI] [PubMed] [Google Scholar]
- Sarkaria JN, Tibbetts RS, Busby EC, Kennedy AP, Hill DE, and Abraham RT (1998) Inhibition of phosphoinositide 3-kinase related kinases by the radiosenzitizing agent wortmannin. Cancer Res 58 4375-4382. [PubMed] [Google Scholar]
- Song PS and Tapley KJ Jr (1979) Photochemistry and photobiology of psoralens. Photochem Photobiol 29 1177-1197. [DOI] [PubMed] [Google Scholar]
- Stern RS, Laird N, Melski J, Parrish JA, Fitzpatrick TB, and Bleich HL (1984) Cutaneous squamous-cell carcinoma in patients treated with PUVA. N Engl J Med 310 1156-1161. [DOI] [PubMed] [Google Scholar]
- Viola G, Fortunato E, Cecconet L, Del Giudice L, Dall'Acqua F, and Basso G (2008) Central role of mitochondria and p53 in PUVA-induced apoptosis in human keratinocytes cell line NCTC-2544. Toxicol Appl Pharmacol 227 84-96. [DOI] [PubMed] [Google Scholar]
- Vos JM and Hanawalt PC (1987) Processing of psoralen adducts in an active human gene: repair and replication of DNA containing monoadducts and interstrand cross-links. Cell 50 789-799. [DOI] [PubMed] [Google Scholar]
- Yamaizumi M and Sugano T (1994) UV-induced nuclear accumulation of p53 is evoked through DNA damage of actively transcribed genes independent of the cell cycle. Oncogene 9 2775-2784. [PubMed] [Google Scholar]