Abstract
The proprotein convertases are believed to be responsible for the proteolytic maturation of a large number of peptide hormone precursors. Although potent furin inhibitors have been identified, thus far, no small-molecule prohormone convertase 1/3 or prohormone convertase 2 (PC2) inhibitors have been described. After screening 38 small-molecule positional scanning libraries against recombinant mouse PC2, two promising chemical scaffolds were identified: bicyclic guanidines, and pyrrolidine bis-piperazines. A set of individual compounds was designed from each library and tested against PC2. Pyrrolidine bis-piperazines were irreversible, time-dependent inhibitors of PC2, exhibiting noncompetitive inhibition kinetics; the most potent inhibitor exhibited a Ki value for PC2 of 0.54 μM. In contrast, the most potent bicyclic guanidine inhibitor exhibited a Ki value of 3.3 μM. Cross-reactivity with other convertases was limited: pyrrolidine bis-piperazines exhibited Ki values greater than 25 μM for PC1/3 or furin, whereas the Ki values of bicyclic guanidines for these other convertases were more than 15 μM. We conclude that pyrrolidine bis-piperazines and bicyclic guanidines represent promising initial leads for the optimization of therapeutically active PC2 inhibitors. PC2-specific inhibitors may be useful in the pharmacological blockade of PC2-dependent cleavage events, such as glucagon production in the pancreas and ectopic peptide production in small-cell carcinoma, and to study PC2-dependent proteolytic events, such as opioid peptide production.
Prohormone convertases 1/3 and 2 (PC1/3 and PC2) are subtilisin/kexin-like serine proteases with acidic pH optima, consistent with their function in the calcium-rich acidic secretory granules of neuroendocrine cells (Rouille et al., 1995; Cameron et al., 2001). PC1/3 and PC2 share many biochemical and functional characteristics; both work together to mediate the proteolytic activation of a wide variety of peptide hormone precursors. For example, the processing of proinsulin to insulin is carried out mainly by PC1/3 (Smeekens et al., 1992), whereas glucagon is processed from proglucagon intermediates by PC2 (Rouille et al., 1997). Perhaps the most well studied precursor in terms of proteolytic processing is proopiomelanocortin (POMC), which is processed extensively both by PC1/3 and PC2. In AtT-20 cells, which express mainly PC1/3, adrenocorticotropin and β-lipotropin are generated, along with small amounts of β-endorphin (Benjannet et al., 1991; Bloomquist et al., 1991; Thomas et al., 1991). When PC2 is transfected into these cells, additional cleavages occur, which result in the generation of the adrenocorticotropin cleavage product α-melanocyte stimulating hormone and larger amounts of β-endorphin (Zhou and Mains, 1994); this pattern resembles POMC cleavage in the PC2-expressing intermediate lobe of the pituitary.
Small-molecule enzyme inhibitors constitute the largest class of pharmacologically active compounds. Many small-molecule inhibitors have been reported to date against the proprotein convertase furin (Basak et al., 1999; Podsiadlo et al., 2004; Jiao et al., 2006); these may be useful in blocking many furin-dependent pathological processes (Fugere and Day, 2005). However, no small-molecule inhibitors of proprotein convertases other than furin have yet been identified. PC2-specific inhibitors may be useful in the pharmacological blockade of PC2-dependent cleavage events, such as glucagon production in blood sugar control, or ectopic peptide production in small-cell carcinoma. We present data on promising inhibitors obtained from small-molecule library screens using recombinant PC2.
Materials and Methods
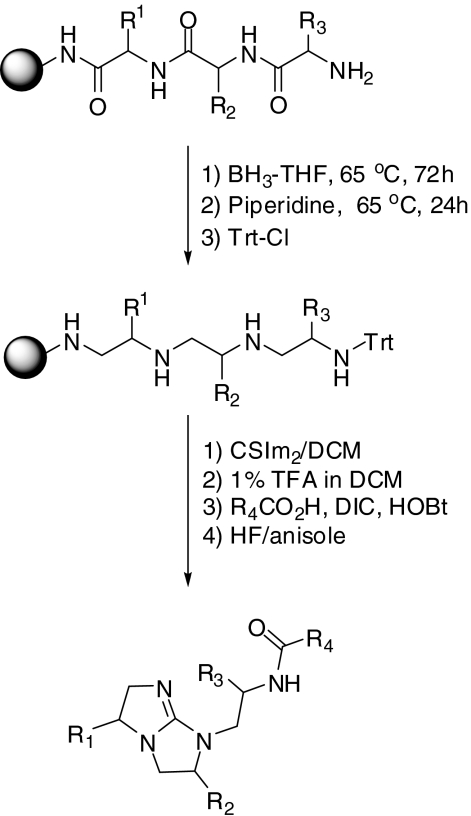
Synthesis of Small-Molecule Compounds and Libraries. All of the libraries and compounds were synthesized at the Torrey Pines Institute for Molecular Studies (San Diego, CA). An N-acylated bicyclic guanidine positional scanning library was screened against purified PC2 at a final library concentration of 0.05 mg/ml. This library was synthesized using the libraries-from-libraries approach (Ostresh et al., 1998), in which a resin-bound tripeptide library is chemically modified using borane-tetrahydrofuran to reduce amide bonds, resulting in a tetra-amine library; cyclized using thiocarbonyldiimidazole; acylated with carboxylic acids; and cleaved from the resin using hydrogen fluoride (Scheme 1). The resulting N-acylated bicyclic guanidine library was made up of four positions of diversity, in which R1 and R2 are synthesized using 34 different l- and d-amino acids, R3 is made up of 17 l- and d-amino acids, and the R4 position is added using 56 different carboxylic acids. The library is made up of mixtures systematically arranged in a positional scanning format (Houghten et al., 1999, 2008), in which each mixture has one defined R position with the other R positions as mixtures. The total number of compounds in this library is 1,100,512. A set of 32 individual compounds (TPI 1267) derived from the combinations of the functionalities defined in the most active mixtures was then synthesized and tested for PC2 inhibition. Two additional bicyclic guanidines (TPI 1669-2 and 7) were also synthesized in an effort to identify improved inhibitors.
Scheme 1.
Synthesis of the N-acylated bicyclic guanidine positional scanning library.
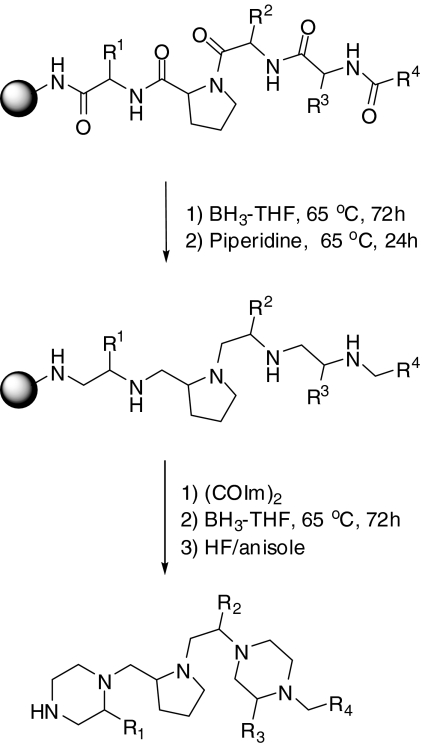
The pyrrolidine bis-piperazine library was screened against purified mouse PC2 at a final inhibitor concentration of 0.1 mg/ml. This library was synthesized using the libraries-from-libraries approach (Hensler et al., 2006), in which a resin-bound proline-containing acylated tetrapeptide is chemically modified into a pyrrolidine bis-piperazine scaffold. The library is made up of four positions of diversity, in which R1, R2, and R3 each are synthesized using 26 different l- and d-amino acids, and the R4 position is added using 42 different carboxylic acids (Scheme 2). The library is made up of 120 mixtures systematically arranged in a positional scanning format in which each mixture has one defined R position with the other R positions as mixtures. The total number of compounds in this library is 738,192. Finally, a set of 20 individual pyrrolidine bis-piperazine compounds (TPI 1435: 1-20), derived from combinations of the functionalities defined in the most active mixtures from the piperazine library, was synthesized and tested for PC2 inhibition.
Scheme 2.
Synthesis of the pyrrolidine bis-piperazine library.
Preparation of mPC1/3, mPC2, and Human Furin. Mouse 87-kDa PC1/3 and activated 66-kDa mouse PC2 were purified from the conditioned medium of stably transfected, methotrexate-amplified CHO cells as described previously (Zhou and Lindberg, 1993; Lamango et al., 1996). Human soluble furin was purified from the conditioned medium of methotrexate-amplified, stably transfected CHO DG44 cells (Kacprzak et al., 2004).
Library Screen and Enzyme Assays. The assay for PC2 was performed in 96-well polypropylene microtiter plates with a final volume of 50 μl, containing 100 mM sodium acetate, pH 5.0, 2 mM CaCl2, 0.2% octyl glucoside, 0.1% NaN3, and 0.1 mg/ml BSA. The substrate pyr-Glu-Arg-Thr-Lys-Arg-methylcoumarinamide (Peptide Institute, Lexington, KY) was used at a final concentration of 50 μM, except where otherwise stated. PC2 was used at a final concentration of 3.1 nM, which generated 0.4 fluorescence units/min (where 1 fluorescence unit corresponds to 3.8 pmol aminomethyl coumarin). Except where indicated differently, inhibitors were preincubated with enzyme for 30 min at 37°C before the addition of substrate. All assays were performed either in duplicate or triplicate (as indicated) for 1 h at 37°C and were quantitated using a Fluoroscan Ascent fluorometer (LabSystems, Waltham, MA) with an excitation/emission wavelength of 380/460 nm. Data were analyzed using GraphPad Prism 4 (GraphPad Inc., San Diego, CA).
The PC1/3 assay was performed at a final concentration of 16 nM PC1/3, 100 mM sodium acetate, pH 5.5, 2 mM CaCl2, 0.2% octyl glucoside, 0.1% NaN3, and 0.1 mg/ml BSA. The furin assay was performed using 1.5 nM furin in 100 mM HEPES, pH 7.0, 2 mM CaCl2, 0.2% octyl glucoside, 0.1% NaN3, and 0.1 mg/ml BSA.
Enzyme Kinetics. Studies of PC2 inhibition kinetics were conducted at the various concentrations of substrate and inhibitors shown in the figures. Bicyclic guanidines (final concentrations, 5-17 μM) were preincubated with the enzyme for 2 h at 37°C before the addition of substrate. For the Ki value determinations of pyrrolidine bis-piperazines, preincubation was carried out for 4 h. To investigate the Ki values of the various pyrrolidine bis-piperazine isomers (range, 2.5-18 μM), a 2-h preincubation preceded the addition of the substrate (final concentration, 100 μM). Ki values were calculated using a least-squares fitting technique (EnzFitter; Biosoft, Cambridge, UK). The Km for PC2, 32 μM, was obtained from the x-intercept of the Lineweaver-Burk double reciprocal plot. The Km values for PC1/3 and furin were 11 and 8 μM, respectively (Cameron et al., 2000a).
Reversibility of Inhibition. Two hundred-microliter samples of PC2 were preincubated with inhibitor in reaction buffer at final concentrations of 6.5 μM (for 1435-6) or 11 μM (for 1267-7) at 37°C for 75 min. Samples were then dialyzed in a Slide-A-Lyzer Mini-Dialysis unit (10,000 molecular weight cutoff; Pierce Chemical, Rockford, IL) against 300 ml of ice-cold buffer containing 100 mM sodium acetate, 2 mM CaCl2, 0.2% octyl glucoside, and 0.1% NaN3, pH 5.0. Enzyme activity was measured in triplicate after 6 h and after 21 h of dialysis at a substrate concentration of 0.2 mM using a Fluoroscan Ascent fluorometer.
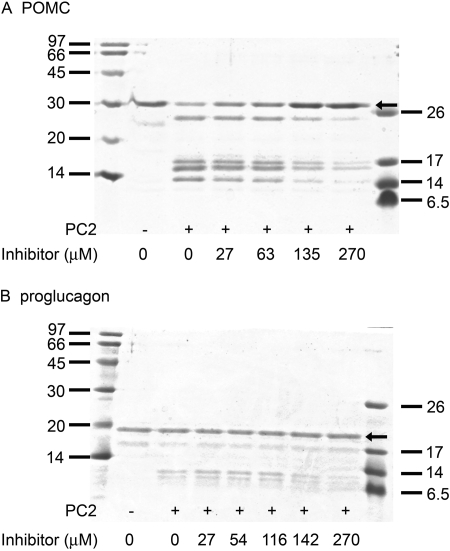
POMC and Proglucagon Cleavage. His-tagged mouse proopiomelanocortin (mPOMC) was prepared via Escherichia coli overexpression, as described in Ozawa et al. (2007). Human proglucagon was prepared as described in Bonic and Mackin (2003). PC2 (15 nM) was preincubated for 2 h at 37°C with various concentrations of the pyrrolidine bis-piperazine 1435-6, as shown in Fig. 9, in 100 mM sodium acetate, pH 5.0, containing 5 mM CaCl2, 0.1% Brij 35, and 20% dimethyl sulfoxide. After this preincubation, 8 μg of either mPOMC or human proglucagon were added. Proteolysis was carried out for 6 h at 37°C; concentrated Laemmli sample buffer was then added, and the samples were boiled. Digestion products were separated on 18% polyacrylamide Tris-HCl gels and Coomassie-stained.
Fig. 9.
Cleavage of mPOMC and human proglucagon is inhibited by a pyrrolidine bis-piperazine in a dose-dependent manner. Digestion of mouse POMC (A) and human proglucagon (B) by PC2 was examined in the presence of the pyrrolidine bis-piperazine 1435-6 at the indicated concentrations. The boldface arrows indicate undigested mPOMC and human proglucagon.
Results
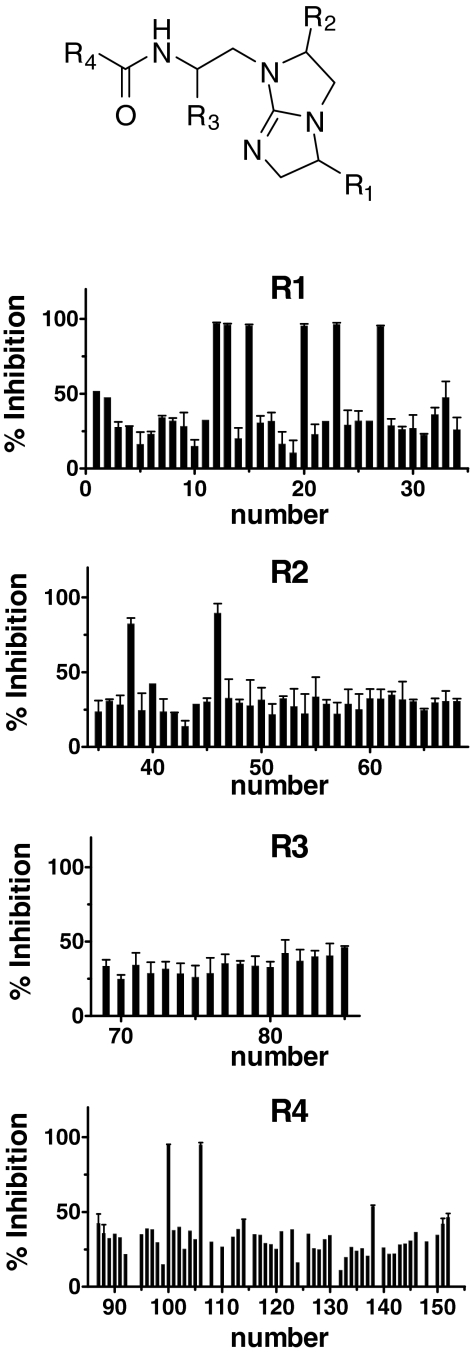
Bicyclic Guanidine and Piperazine Scaffolds Represent Strong Inhibitors of PC2. After screening the positional scanning N-acylated bicyclic guanidine library, six mixtures having an R1 position with greater than 90% inhibition of PC2 were identified. Four of these, namely mixtures composed of R-4-hydroxybenzyl (mixture 12), R-2-napthylmethyl (20), S-4-chlorobenzyl (23), and S-cyclohexyl groups (27), contained large hydrophobic ring moieties. The other two active mixtures, S-ethyl (13) and S-propyl (15) contained short aliphatic functionalities. Only two mixtures were found to be active at the R2 position, namely mixtures 38 (S-isobutyl) and 46 (R-4-hydroxybenzyl). No mixtures at the R3 position were found to have greater than 50% inhibitory activity; mixtures 81 (R-cyclohexylmethyl) and 85 (R-4-methoxybenzyl) were the most inhibitory. With regard to the R4 position, mixtures 100 (3-[3,4-dimethoxyphenyl]-propyl) and 106 (2-methoxyphenylpropyl) were clearly the most active. These data are summarized in Fig. 1.
Fig. 1.
Bicyclic guanidine library screening against PC2. The N-acylated bicyclic guanidine positional scanning library was tested at 0.05 mg/ml against PC2. R1, R2, R3, and R4 represent the four diversity positions of the library. The percentage of inhibition was calculated from (1 - Vi/V0)·100, where Vi and V0 are the enzyme velocities (fluorescence units per minute) in the presence and absence of inhibitor, respectively. The percentage of inhibition is expressed as the mean ± S.D. and is performed in duplicate.
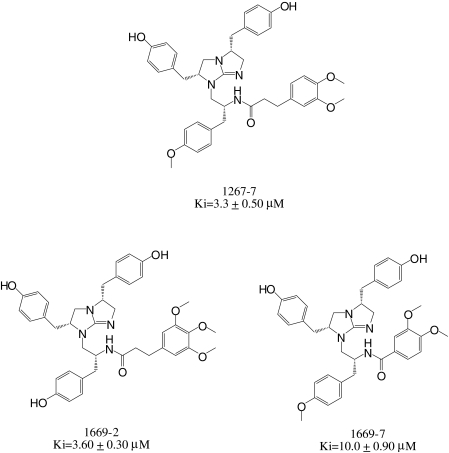
Individual active compounds were identified from positional scanning libraries by synthesizing compounds containing the functionalities defined in the most active mixtures of the various libraries. From the N-acylated bicyclic guanidine library, four mixtures were chosen from R1 (mixtures 12, 13, 20, and 23), and two mixtures each were chosen from R2 (38 and 46), R3 (81 and 85), and R4 (100 and 106) to make individual compounds. Thus, 32 individual N-acylated bicyclic guanidine compounds (TPI 1267) were synthesized based on combinations of the functionalities defined in the most active mixtures and were tested for PC2 inhibition. Figure 2 shows the inhibitory activities of the most active individual N-acylated bicyclic guanidines; the Ki values of these compounds were in the micromolar range. The most active bicyclic guanidine, 1267-7, inhibited PC2 with a Ki value of 3.3 μM. Two additional bicyclic guanidines were synthesized in an effort to identify more potent PC2 inhibitors. Because 1267-7 contained many hydroxybenzyl and methoxybenzyl groups, compound 1669-2 was synthesized by including an additional methoxy group at the R4 position and replacing the 4-methoxybenzyl group at R3 with 4-hydroxybenzyl. This compound had inhibitory activity (Ki = 3.6 μM) against PC2 very similar to that of 1267-7 (Fig. 2). A second compound was prepared by reducing the length between the dimethoxybenzyl group and the amide bond at the R4 position and resulted in the compound 1669-7; this compound was much less active than 1267-7 (Ki = 10 μM).
Fig. 2.
Most active bicyclic guanidine inhibitors of PC2. The values shown represent the mean and S.E. of Ki determinations carried out in triplicate in an inhibitor concentration range from 5 to 17 μM, with a 2-h preincubation.
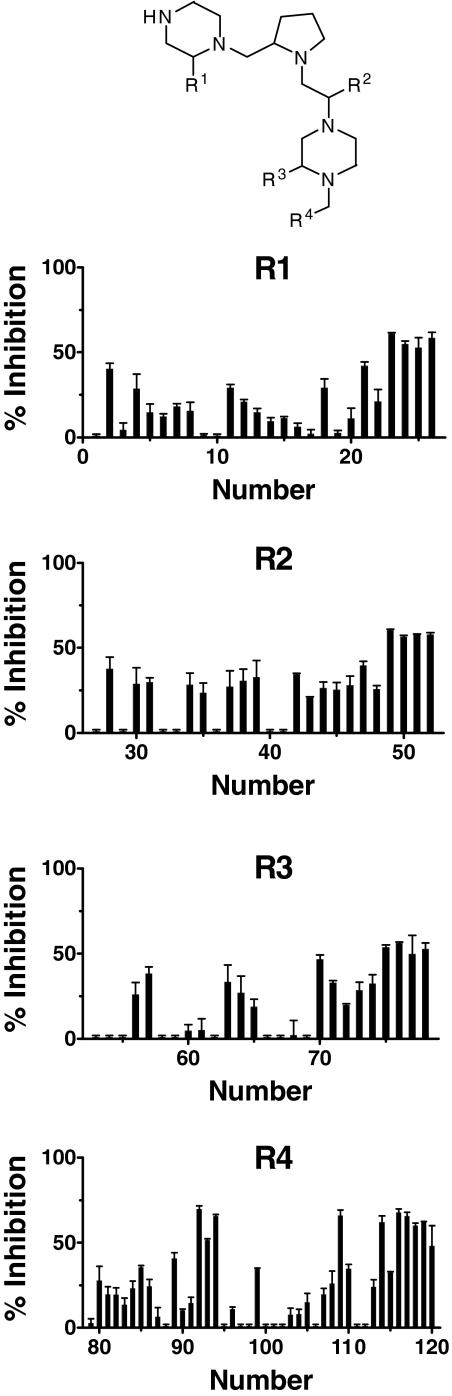
Pyrrolidine Bis-Piperazines are Submicromolar Inhibitors of PC2. A single 96-well plate containing scaffolds of 37 libraries was screened at 0.1 and 0.05 mg/ml against purified PC2 (data not shown). Several scaffolds containing bicyclic guanidines were identified as strong inhibitors, and the pyrrolidine bis-piperazine scaffold was identified as well. The pyrrolidine bis-piperazine positional scanning library was then tested at 0.1 mg/ml against PC2 (Fig. 3), and active functional groups were identified. The R4 position contributed most to the inhibition. Nine mixtures were found to have more than 50% inhibitory activity, and the most active mixtures were 92 (2-[4-isobutyl-phenyl]-propyl), 94 (2-[3,5-bis-trifluoromethyl-phenyl]-ethyl), and 116 (4-tert-butyl-cyclohexyl-methyl). Four R1 mixtures exhibited inhibitory activity between 50 and 60%, namely mixtures 23, 24, 25, and 26. Similar inhibitory activity was found in the R2 and R3 positions. The four mixtures at the R2 position that exhibited at least 50 to 60% were mixtures 49, 50, 51, and 52. Finally, the four most inhibitory mixtures at the R3 position were mixtures 75, 76, 77, and 78. The most effective functionalities defined at the R1, R2, and R3 positions were the large hydrophobic ring moieties of R- or S-2-naphthylmethyl and R- or S-cyclohexyl.
Fig. 3.
Pyrrolidine bis-piperazine library screening against PC2. The pyrrolidine bis-piperazine library positional scanning was tested at 0.1 mg/ml against PC2. R1, R2, R3, and R4 represent the four diversity positions. The percentage of inhibition was calculated from (1 - Vi/V0)·100, where Vi and V0 are the enzyme velocities (fluorescence units per minute) in the presence and absence of inhibitor, respectively. The percentage of inhibition is expressed as the mean ± S.D., with duplicate determinations.
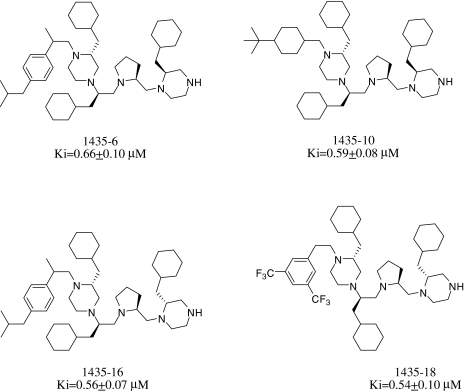
Individual active compounds are identified from positional scanning libraries by synthesizing compounds that contain the functionalities that are defined in the most active mixtures of the libraries. From this pyrrolidine bis-piperazine library, two mixtures each were chosen from R1 (25 and 26) and R2 (49 and 52), one mixture from R3 (85), and five mixtures from R4 (92, 93, 94, 113, and 116) to make individual compounds. Thus, the most inhibitory functionalities were combined to synthesize 20 individual pyrrolidine bispiperazine compounds (TPI 1435). These compounds were tested for inhibition against PC2. It is interesting to note that the isomeric pair of compounds 1435-6 and 1435-16 exhibited similar inhibitory activity (Ki values obtained after 2-h preincubation with PC2 were 3.5 ± 0.5 and 2.7 ± 0.8 μM, respectively), whereas the isomeric pairs 1435-5 (Ki = 6.4 ± 1 μM) and 1435-15 (Ki = 12.8 ± 1 μM), and 1435-8 (8.8 ± 0.7 μM) and 1435-18 (2.2 ± 0.5 μM) were significantly different in inhibitory potency. These data indicate that the spatial arrangement of substituent groups plays a strong role in binding to PC2.
The Ki values for the four most active pyrrolidine bis-piperazines obtained after 4 h of preincubation with PC2 were in the submicromolar range, much better than those of the best bicyclic guanidines (Fig. 4).
Fig. 4.
Most active pyrrolidine bis-piperazine inhibitors of PC2. The values shown represent the mean and S.E. of Ki value determinations carried out at a final inhibitor concentration range from 1.2 to 14 μM, with a 4-h preincubation.
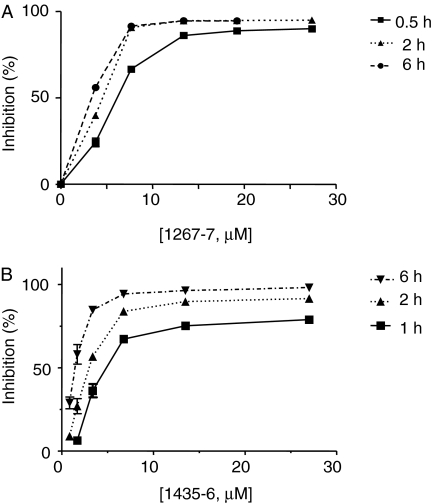
Pyrrolidine Bis-Piperazines and Bicyclic Guanidines Are Time-Dependent Inhibitors. To explore the mechanism of inhibition, the preincubation time and inhibitor concentration were varied. Various concentrations of the pyrrolidine bis-piperazines 1435-6 (from 0.85 to 27 μM) and 1267-7 (from 3.8 to 27 μM) were preincubated for various times with PC2; substrate was then added, and the residual activity was measured (Fig. 5B). The IC50 value of compound 1435-6 was progressively reduced with increasing time of preincubation, indicating that pyrrolidine bis-piperazines act as slow-binding, time-dependent inhibitors of PC2. The ability of bicyclic guanidines to inhibit PC2 was also time-dependent, although less so than bis-piperazines (Fig. 5A).
Fig. 5.
Bicyclic guanidines and pyrrolidine bis-piperazines are concentration-dependent inhibitors of PC2. The most active bicyclic guanidine, 1267-7, was preincubated with PC2 for 30 min, 2 h, or 6 h at the concentrations shown (top). The pyrrolidine bis-piperazine 1435-6 was preincubated with PC2 for 1, 2, or 6 h at the concentrations shown (bottom). The values shown represent triplicate determinations ± S.D.
To determine whether inhibition was reversible, inhibited and noninhibited PC2 was extensively dialyzed against reaction buffer. Enzyme inhibited with the pyrrolidine bis-piperazine 1435-6 still exhibited 87% inhibition after 6 h and 80% inhibition after 21 h, compared with parallel samples containing uninhibited PC2. For the bicyclic guanidine 1267-7, these values were 37 and 29% inhibition, respectively. These data indicate that inhibition by pyrrolidine bis-piperazines, and to a lesser extent bicyclic guanidines, is not readily reversible.
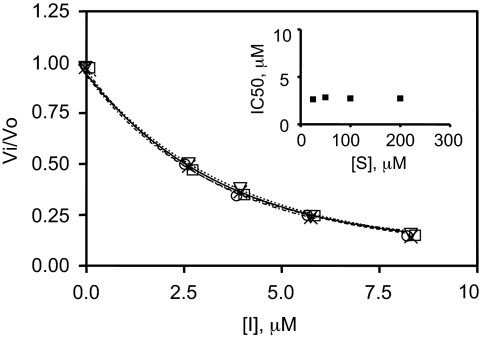
Analysis of Inhibition Mechanism Reveals Noncompetitive Kinetics. Lineweaver-Burk plots were generated to obtain information on the inhibition mechanism of bicyclic guanidines and pyrrolidine bis-piperazines (Fig. 6). Data for the best bicyclic guanidine, 1267-7, are shown in Fig. 6A; these data exhibit a better fit to the equation for noncompetitive inhibition than to that of competitive inhibition (r2 = 0.99 versus 0.90, respectively). Similar results were found for pyrrolidine bis-piperazines (r2 = 0.96 for fit to the noncompetitive and 0.90 for competitive inhibition equations) (Fig. 6B). To confirm a strictly noncompetitive mechanism of inhibition for pyrrolidine bis-piperazines, Vi/V0 values were plotted against inhibitor concentration at four different substrate concentrations (Fig. 7). IC50 values obtained from this graph (inhibitor concentrations for which Vi/V0 = 0.5) were 2.6, 2.9, 2.7, and 2.7 μM for the substrate concentrations 200, 100, 50, and 25 μM, respectively. The fact that these values are independent of [S] (Fig. 7, inset) strongly supports a noncompetitive mechanism for PC2 inhibition for pyrrolidine bis-piperazines.
Fig. 6.
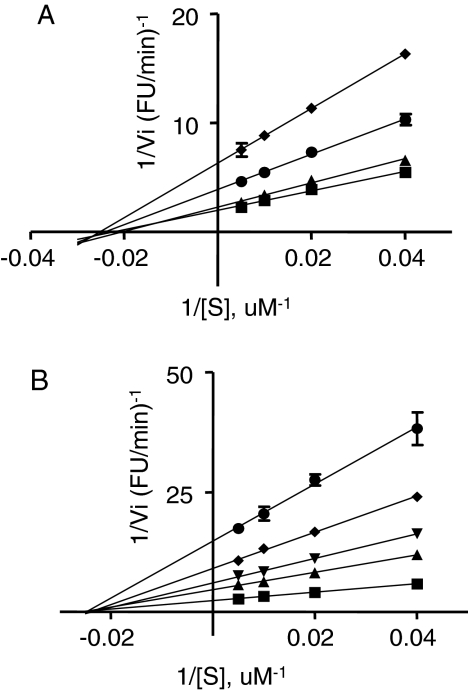
Lineweaver-Burk plots of pyrrolidine bis-piperazine and bicyclic guanidine compounds indicate noncompetitive inhibition kinetics. Lineweaver-Burk plots of bicyclic guanidine 1669-2 (A) were generated using the following inhibitor concentrations: 15 μM (♦), 13 μM (•), 9 μM (▴), and 0 μM (▪). Lineweaver-Burk plots of pyrrolidine bis-piperazine 1435-6 (B) were generated using the following inhibitor concentrations: 8.45 μM (•), 5.85 μM (♦), 4.02 μM (▾), 2.68 μM (▴), and 0 μM (▪). The values shown represent the means ± S.D. of triplicate determinations.
Fig. 7.
Confirmation of noncompetitive inhibition of pyrrolidine bis piperazines using a plot of fractional velocity as a function of inhibitor concentration. The following substrate concentrations were used: 25 μM (○), 50 μM (▿), 100 μM (×), and 200 μM (□). Inset, the lack of effect of substrate concentration on IC50 values.
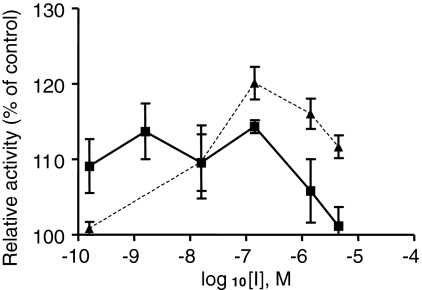
Low Concentrations of Bicyclic Guanidines Stimulate PC2. We observed that low concentrations of certain bicyclic inhibitors paradoxically stimulated enzyme activity (Fig. 8), similarly to the known stimulation of PC2 by various polyarginines (Cameron et al., 2000a). Because of this paradoxical stimulation effect, these lower concentrations were not included in kinetic calculations.
Fig. 8.
Stimulation of PC2 activity at low concentrations of bicyclic guanidines. PC2 was preincubated for 2 h with bicyclic guanidines at the concentrations indicated (▴, 1669-7; ▪, 1267-7).
Pyrrolidine Bis-Piperazines Inhibit PC2 More Potently than PC1/3 and Furin. The inhibitory specificity of pyrrolidine bis-piperazines and bicyclic guanidines were tested against mPC1/3 and human furin, other prohormone convertase family members. The Ki values against PC1/3, PC2, and furin are summarized in Table 1. The Ki value of the best bis-piperazine against PC1/3 was greater than 40 μM, at least 74 times higher than the Ki value of this compound against PC2. When tested against furin, these Ki values were greater than 25 μM, at least 45 times higher than those against PC2. The Ki values for bicyclic guanidines against PC1/3 were greater than 20 μM, approximately 6 times higher than those against PC2. The Ki values for bicyclic guanidines against furin were greater than 15 μM, approximately 4.5 times higher than against PC2. These cross-inhibition data indicate that pyrrolidine bis-piperazines exhibit much greater specificity for PC2 than do bicyclic guanidines.
TABLE 1.
Inhibition of PC1/3, PC2, and furin by individual pyrrolidine bispiperazines and bicyclic guanidines
Values represent the mean ± S.D. of Ki determinations carried out in triplicate.
|
Compound
|
Ki
|
||
|---|---|---|---|
| PC1/3 | PC2 | Furin | |
| μM | |||
| 1435-6 | >40 | 0.66 ± 0.10 | >25 |
| 1435-10 | >40 | 0.59 ± 0.08 | >25 |
| 1435-16 | >40 | 0.56 ± 0.07 | >25 |
| 1435-18 | >40 | 0.54 ± 0.10 | >25 |
| 1669-2 | >20 | 3.60 ± 0.30 | >15 |
| 1669-7 | >20 | 10.0 ± 0.90 | >15 |
| 1267-7 | >20 | 3.30 ± 0.50 | >15 |
POMC and Proglucagon Cleavage Is Inhibited by Pyrrolidine Bis-Piperazines. To determine whether these PC2 inhibitors can block PC2-mediated cleavage of physiologically relevant substrates, one of the most potent inhibitors, the pyrrolidine bis-piperazine 1435-6, was preincubated with POMC or proglucagon at various concentrations. Figure 9 shows that 1435-6 can block the processing of both POMC (Fig. 9A) and proglucagon (Fig. 9B). Unexpectedly high inhibitor concentrations were required to block prohormone processing compared with inhibition of fluorogenic substrate hydrolysis. It is interesting to note that all bands were equally inhibited, suggesting no preference of inhibition at particular sites.
Discussion
Proprotein convertases, maturation enzymes in the secretory pathway, represent known targets for both natural and exogenous inhibitors. Natural inhibitors of PC2 consist of the 7B2 and its carboxy-terminal peptide (Martens et al., 1994) and the cystatin-related epididymal protein (Cornwall et al., 2003). proSAAS and its derived peptides represent endogenous inhibitors of PC1/3 (Cameron et al., 2000b; Qian et al., 2000). The endogenous inhibitor of furin has not yet been identified; based on peptide library screening, we have predicted that it should contain a stretch of positively charged amino acids (Kacprzak et al., 2004).
Convertase inhibitors represent potential therapeutic targets for cancer and many other diseases (Fugere and Day, 2005); therefore, increasing attention has been paid to the development of specific and potent synthetic convertase inhibitors. Using both in vitro and cell-based assays, several protein or peptide-based furin inhibitors with excellent inhibitory potency have been identified (Angliker et al., 1993; Jean et al., 1998; Cameron et al., 2000a; Komiyama and Fuller, 2000; Fugere et al., 2002). However, the large molecular weight, potential immunogenicity, and instability of many protein- or peptide-based inhibitors is a clear limitation in therapeutic applications. Nonpeptide small molecules are needed to generate nonimmunogenic, stable, and easily diffusible enzyme inhibitors. Andrographolide paniculata diterpines of the labdane family were the first example of such nonpeptide convertase inhibitors (Basak et al., 1999); however, inhibition was quite weak and nonspecific. Much more potent furin inhibitors have been generated recently by derivatization of 2,5-dideoxytryptamine with the addition of guanidinylated aryl groups (Jiao et al., 2006) and by using cell-based assays for screening (Coppola et al., 2007). For example, guanidinylated aryl compounds exhibit nanomolar Ki values and possess excellent specificity (Jiao et al., 2006). These data support the idea that convertases can be specifically and potently inhibited by small molecules.
In this work, we screened 38 chemical scaffold libraries containing more than 30 million compounds. Two specific chemical scaffolds, namely pyrrolidine bis-piperazines and bicyclic guanidines, were identified as potential inhibitors of the proprotein convertase PC2. Positional scanning libraries for these two chemical scaffolds were then tested. Deconvolution of each library resulted in two sets of inhibitory compounds that were identified as submicromolar (pyrrolidine bis-piperazines) and micromolar (bicyclic guanidines) inhibitors of PC2. Both pyrrolidine bis-piperazines and bicyclic guanidines are positively charged, reminiscent of polyarginine inhibition of furin (Cameron et al., 2000a). We previously used a decapeptide positional scanning library to show that an increase in the number of positive charges results in improved inhibition of PC2 (Kacprzak et al., 2004); we also found that increasing the number of positive charges in PC2 substrates was correlated with an increase in the specificity constant (Henrich et al., 2005). Our finding of two positively charged scaffolds is consistent with these previous peptide results. Interestingly, low concentrations of one set of positively charged inhibitors, the bicyclic guanidines, stimulated PC2 activity in a manner reminiscent of the stimulation of PC2 by polyarginines, although not as potently (Cameron et al., 2000a). The mechanism for such stimulation is not known but represents an interesting area for future study.
Because the crystal structure of PC2 is not yet available, molecular docking of pyrrolidine bis-piperazines into the PC2 structure is not possible; however, models of PCs have been constructed (Henrich et al., 2005). The substrate binding region seems to be characterized by the clustering of an extremely large number of negatively charged residues. We surmise that the positively charged scaffold in pyrrolidine bis-piperazines interacts with the highly acidic substrate binding groove of PC2 and is anchored by Asp278 (Henrich et al., 2005). Pyrrolidine bis-piperazines 1435-6 and 1435-10, which both contain a cyclohexylmethyl group at the R1, R2, and R3 positions, exhibited much greater inhibition of PC2 than inhibitors containing a naphthylmethyl group at R2 (data not shown), implying that certain hydrophobic groups are favored at the R1, R2, and R3 positions. This is most likely due to complementary hydrophobic sites in and around the substrate binding pocket; however, an actual PC2 crystal structure will be required to determine the precise location of these subsites. This finding implies that the three-dimensional arrangement of substituent groups plays an important role in binding to PC2, an important feature for specific inhibitor development.
In addition to pyrrolidine bis-piperazines, bicyclic guanidines represent another promising subtype of PC2 inhibitor. During the screening of the 38 chemical scaffold libraries, all guanidine-containing scaffolds showed strong inhibitory activity against PC2. These data imply that building upon the positively charged guanidine backbone could represent a promising strategy for the synthesis of future inhibitors. Among the various guanidine scaffolds, the bicyclic guanidines showed the strongest inhibition, with micromolar Ki values against PC2. The R1 hydrophobic groups of bicyclic guanidines may bind to hydrophobic residues within the PC2 catalytic domain, especially within the S6, S2, and S2′ subsites, modeled previously as occupied by hydrophobic residues (proline, phenylalanine, and leucine) (Henrich et al., 2005). The molecular mass of bicyclic guanidine 1267 previously 7 is 679 Da, which makes it an attractive small-molecule lead compound for further chemical modification in attempts to increase inhibitory potency.
Kinetic studies showed that pyrrolidine bis-piperazines exhibited slow, irreversible binding with noncompetitive inhibition kinetics, supporting the idea that these compounds represent promising initial leads. The Ki values of pyrrolidine bis-piperazines are in the submicromolar range, in contrast to the low nanomolar Ki inhibitors, which have been described for furin (Jiao et al., 2006). Although we were able to block the conversion of the prohormone substrates POMC and proglucagon to smaller products using the most potent pyrrolidine bis-piperazine, very high concentrations were required compared with inhibition of the hydrolysis of fluorogenic substrates. The reason for this is unknown but may reflect the complexity of using large substrates with multiple cleavage sites. Clearly, further chemical modifications of these pyrrolidine bis-piperazines will be required to obtain the potency that previous groups have obtained for furin and to be able to achieve cellular inhibition of prohormone processing.
It is interesting to note that pyrrolidine bis-piperazines were found to be relatively selective, with at least 74-fold higher Ki values against PC1/3 and 45-fold higher Ki values against furin than against PC2. We believe that these pyrrolidine bis-piperazine selectivity data bode well for the future development of more specific convertase inhibitors. Obtaining convertase-specific inhibitors is paramount in directing inhibitory potency only to the desired convertase but often can be difficult to achieve: for example, convertase prodomains, which represent low nanomolar in trans inhibitors, exhibit significant convertase cross-reaction (Fugere et al., 2002).
In summary, we have identified here novel lead compounds for the design of specific PC2 inhibitors; this is the first description of synthetic inhibitors against this enzyme and should eventually lead to more potent and specific compounds. It is important to note that even the total loss of PC2 activity is not necessarily deleterious. Although PC2-null mice are hypoglycemic because of their reduced ability to process proglucagon to glucagon (Furuta et al., 1997), they are essentially healthy, and their hypoglycemia is correctable with the addition of exogenous glucagon (Furuta et al., 2001), suggesting that the loss of PC2 can act as a functional glucagon antagonist. The compounds identified here may be of eventual therapeutic use in lowering blood sugar by similarly blocking glucagon production. PC2 inhibitors could also be useful to study PC2-specific processing pathways in vivo, for example to study local production of enkephalins, small opioid-active products known to be specifically generated by PC2 (Johanning et al., 1996; Peinado et al., 2003).
Acknowledgments
We are very grateful to J. Hassen for library screens, and we thank C. Dinkins for purifying PC1/3, PC2, and furin.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA05084].
ABBREVIATIONS: PC, prohormone convertase; POMC, proopiomelanocortin; BSA, bovine serum albumin.
References
- Angliker H, Wikstrom P, Shaw E, Brenner C, and Fuller RS (1993) The synthesis of inhibitors for processing proteinases and their action on the Kex2 proteinase of yeast. Biochem J 293 75-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak A, Cooper S, Roberge AG, Banik UK, Chrétien M, and Seidah NG (1999) Inhibition of proprotein convertases-1, -7 and furin by diterpines of Andrographis paniculata and their succinoyl esters. Biochem J 338 107-113. [PMC free article] [PubMed] [Google Scholar]
- Benjannet S, Rondeau N, Day R, Chrétien M, and Seidah NG (1991) PC1/3 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci U S A 88 3564-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomquist BT, Eipper BA, and Mains RE (1991) Prohormone-converting enzymes: regulation and evaluation of function using antisense RNA. Mol Endocrinol 5 2014-2024. [DOI] [PubMed] [Google Scholar]
- Bonic A and Mackin RB (2003) Expression, purification, and PC1/3-mediated processing of human proglucagon, glicentin, and major proglucagon fragment. Protein Expr Purif 28 15-24. [DOI] [PubMed] [Google Scholar]
- Cameron A, Apletalina EV and Lindberg I (2001) The enzymology of PC1/3 and PC2, in The Enzymes XXII (Dalbey RE and Sigman DS eds) pp 291-331, Academic Press, San Diego.
- Cameron A, Appel J, Houghten RA, and Lindberg I (2000a) Polyarginines are potent furin inhibitors. J Biol Chem 275 36741-36749. [DOI] [PubMed] [Google Scholar]
- Cameron A, Fortenberry Y, and Lindberg I (2000b) The SAAS granin exhibits structural and functional homology to 7B2 and contains a highly potent hexapeptide inhibitor of PC1/3. FEBS Lett 473 135-138. [DOI] [PubMed] [Google Scholar]
- Coppola JM, Hamilton CA, Bhojani MS, Larsen MJ, Ross BD, and Rehemtulla A (2007) Identification of inhibitors using a cell-based assay for monitoring Golgi-resident protease activity. Anal Biochem 364 19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall GA, Cameron A, Lindberg I, Hardy DM, Cormier N, and Hsia N (2003) The cystatin-related epididymal spermatogenic protein inhibits the serine protease prohormone convertase 2. Endocrinology 144 901-908. [DOI] [PubMed] [Google Scholar]
- Fugère M and Day R (2005) Cutting back on pro-protein convertases: the latest approaches to pharmacological inhibition. Trends Pharmacol Sci 26 294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugère M, Limperis PC, Beaulieu-Audy V, Gagnon F, Lavigne P, Klarskov K, Leduc R, and Day R (2002) Inhibitory potency and specificity of subtilase-like pro-protein convertase (SPC) prodomains. J Biol Chem 277 7648-7656. [DOI] [PubMed] [Google Scholar]
- Furuta M, Yano H, Zhou A, Rouillé Y, Holst JJ, Carroll R, Ravazzola M, Orci L, Furuta H, and Steiner DF (1997) Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci U S A 94 6646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta M, Zhou A, Webb G, Carroll R, Ravazzola M, Orci L, and Steiner DF (2001) Severe defect in proglucagon processing in islet A-cells of prohormone convertase 2 null mice. J Biol Chem 276 27197-27202. [DOI] [PubMed] [Google Scholar]
- Henrich S, Lindberg I, Bode W, and Than ME (2005) Proprotein convertase models based on the crystal structures of furin and kexin: explanation of their specificity. J Mol Biol 345 211-227. [DOI] [PubMed] [Google Scholar]
- Hensler ME, Bernstein G, Nizet V, and Nefzi A (2006) Pyrrolidine bis-cyclic guanidines with antimicrobial activity against drug-resistant Gram-positive pathogens identified from a mixture-based combinatorial library. Bioorg Med Chem Lett 16 5073-5079. [DOI] [PubMed] [Google Scholar]
- Houghten RA, Pinilla C, Appel JR, Blondelle SE, Dooley CT, Eichler J, Nefzi A, and Ostresh JM (1999) Mixture-based synthetic combinatorial libraries. J Med Chem 42 3743-3778. [DOI] [PubMed] [Google Scholar]
- Houghten RA, Pinilla C, Giulianotti MA, Appel JR, Dooley CT, Nefzi A, Ostresh JM, Yu Y, Maggiora GM, Medina-Franco JL, et al. (2008) Strategies for the use of mixture-based synthetic combinatorial libraries: scaffold ranking, direct testing in vivo, and enhanced deconvolution by computational methods. J Comb Chem 10 3-19. [DOI] [PubMed] [Google Scholar]
- Jean F, Stella K, Thomas L, Liu G, Xiang Y, Reason AJ, and Thomas G (1998) α1-Antitrypsin Portland, a bioengineered serpin highly selective for furin: application as an antipathogenic agent. Proc Natl Acad Sci U S A 95 7293-7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao GS, Cregar L, Wang J, Millis SZ, Tang C, O'Malley S, Johnson AT, Sareth S, Larson J, and Thomas G (2006) Synthetic small molecule furin inhibitors derived from 2,5-dideoxystreptamine. Proc Natl Acad Sci U S A 103 19707-19712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanning K, Mathis JP, and Lindberg I (1996) Role of PC2 in proenkephalin processing: antisense and overexpression studies. J Neurochem 66 898-907. [DOI] [PubMed] [Google Scholar]
- Kacprzak MM, Peinado JR, Than ME, Appel J, Henrich S, Lipkind G, Houghten RA, Bode W, and Lindberg I (2004) Inhibition of furin by polyarginine-containing peptides: nanomolar inhibition by nona-D-arginine. J Biol Chem 279 36788-36794. [DOI] [PubMed] [Google Scholar]
- Komiyama T and Fuller RS (2000) Engineered eglin c variants inhibit yeast and human proprotein processing proteases, Kex2 and furin. Biochem. 39 15156-15165. [DOI] [PubMed] [Google Scholar]
- Lamango NS, Zhu X, and Lindberg I (1996) Purification and enzymatic characterization of recombinant prohormone convertase 2: stabilization of activity by 21 kDa 7B2. Arch Biochem Biophys 330 238-250. [DOI] [PubMed] [Google Scholar]
- Martens GJ, Braks JA, Eib DW, Zhou Y, and Lindberg I (1994) The neuroendocrine polypeptide 7B2 is an endogenous inhibitor of prohormone convertase PC2. Proc Natl Acad Sci U S A 91 5784-5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostresh JM, Schoner CC, Hamashin VT, Nefzi A, Meyer JP, and Houghten RA (1998) Solid-phase synthesis of trisubstituted bicyclic guanidines via cyclization of reduced N-acylated dipeptides. J Org Chem 63 8622-8623. [Google Scholar]
- Ozawa A, Cai Y, and Lindberg I (2007) Production of bioactive peptides in an in vitro system. Anal Biochem 366 182-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado JR, Li H, Johanning K, and Lindberg I (2003) Cleavage of recombinant proenkephalin and blockade mutants by prohormone convertases 1 and 2: an in vitro specificity study. J Neurochem 87 868-878. [DOI] [PubMed] [Google Scholar]
- Podsiadlo P, Komiyama T, Fuller RS, and Blum O (2004) Furin inhibition by compounds of copper and zinc. J Biol Chem 279 36219-36227. [DOI] [PubMed] [Google Scholar]
- Qian Y, Devi LA, Mzhavia N, Munzer S, Seidah NG, and Fricker LD (2000) The C-terminal region of proSAAS is a potent inhibitor of prohormone convertase 1. J Biol Chem 275 23596-23601. [DOI] [PubMed] [Google Scholar]
- Rouillé Y, Bianchi M, Irminger JC, and Halban PA (1997) Role of prohormone convertase PC2 in the processing of proglucagon to glucagon. FEBS Lett 413 119-123. [DOI] [PubMed] [Google Scholar]
- Rouillé Y, Duguay SJ, Lund K, Furuta M, Gong Q, Lipkind G, Oliva AA Jr, Chan SJ, and Steiner DF (1995) Proteolytic processing mechanisms in the biosynthesis of neuroendocrine peptides: the subtilisin-like proprotein convertases. Front Neuroendocrinol 16 322-361. [DOI] [PubMed] [Google Scholar]
- Smeekens SP, Montag AG, Thomas G, Albiges-Rizo C, Carroll R, Benig M, Phillips LA, Martin S, Ohagi S, and Gardner P (1992) Proinsulin processing by the subtilisin-related proprotein convertases furin, PC2, and PC3. Proc Natl Acad Sci U S A 89 8822-8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L, Leduc R, Thorne BA, Smeekens SP, Steiner DF, and Thomas G (1991) Kex2-like endoproteases PC2 and PC3 accurately cleave a model prohormone in mammalian cells: evidence for a common core of neuroendocrine processing enzymes. Proc Natl Acad Sci U S A 88 5297-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A and Mains RE (1994) Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 or 2. J Biol Chem 269 17440-17447. [PubMed] [Google Scholar]
- Zhou Y and Lindberg I (1993) Purification and characterization of the prohormone convertase PC1/3(PC3). J Biol Chem 268 5615-5623. [PubMed] [Google Scholar]