Abstract
A recently developed stable isotope dilution liquid chromatography-multiple reaction/mass spectrometry method to quantify focal adhesion kinase (FAK) activation loop phosphorylation was used to study endogenous Src kinase activity. This revealed that bis-phosphorylated pTyr576/Tyr577-FAK was a biomarker of Src activity and inactivation in vitro and in cell culture. Mouse embryonic fibroblasts (MEFs) expressing endogenous Src family kinases contained 65% unmodified Tyr576/Tyr577, 33% mono-phosphorylated-pTyr576-FAK, and 6% bis-phosphorylated-pTyr576/pTyr577-FAK. In contrast, MEFs expressing oncogenic Y529FSrc contained 38% unmodified Tyr576/Tyr577-FAK, 29% mono-phosphorylated-pTyr576-FAK, and 19% bis-phosphorylated-pTyr576/pTyr577-FAK. This new method has made it possible to accurately determine the absolute amounts of FAK phosphorylation that occur after Src inhibition in cell culture and in vitro with increasing concentrations of the Src inhibitor N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5-(tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine (AZD0530). Phosphorylation of FAK at Tyr576/Tyr577 was inhibited by AZD0530 in a dose-dependent manner both in cell culture and in vitro. However, there was a substantial difference in the ability of AZD0530 to inhibit Src that was constitutively activated in a cellular context (IC50 = 2.12 μM) compared with the isolated enzyme (IC50 = 0.14 μM). When normal MEFs and Y529FSrc-expressing MEFs were treated with pervanadate (a global phosphatase inhibitor), pTyr576/pTyr577-FAK accounted for almost 60% of the total FAK present in the cells. This suggests that activation loop phosphorylation is regulated by tyrosine phosphatases. These results confirm that FAK phosphorylation is a useful biomarker of Src inhibition in vivo. The accuracy and specificity of stable isotope dilution liquid chromatography-mass spectrometry methodology offers significant advantages over current immunochemical approaches for monitoring Src activity.
The Src family kinases comprise 11 structurally related, membrane-associated, nonreceptor protein tyrosine kinases including cellular-Src (c-Src), c-Yes, Fyn, Lyn, Hck, Blk, Brk, Fgr, Frk, Srm, and Yrk (Summy and Gallick, 2003; Yeatman, 2004; Meyn and Smithgall, 2008). Although most Src family members are expressed primarily in cells of hematopoietic origin, c-Src, c-Yes, and Fyn are expressed more ubiquitously with high levels of expression in some epithelial tissues, platelets, and neurons (Thomas and Brugge, 1997). They are rarely mutated in human tumors but are overexpressed or activated in a variety of tumors, including colon, breast, pancreatic, bladder, head and neck, ovarian, and brain (Summy and Gallick, 2003). Although Src is a proto-oncogene, its association with FAK (another nonreceptor tyrosine kinase) is critical for the formation of metastases (Basson, 2008). Therefore, Src is an emerging therapeutic target to prevent metastases from occurring (Sawyer, 2004; Rucci et al., 2008). Preclinical experiments indicate that small-molecule inhibitors of Src block tumor growth, metastasis, and angiogenesis (Hiscox and Nicholson, 2008). The binding of Src to FAK results in phosphorylation of FAK at multiple residues, critical among them being the Tyr576 and Tyr577 sites located on FAK's kinase activation loop (Fig. 1) (Calalb et al., 1995; Owen et al., 1999; Ruest et al., 2000; Ciccimaro et al., 2006). In a global phosphotyrosine proteome analysis, FAK Tyr576 emerged as the most readily detected Src target in cultured nontransformed fibroblasts (Luo et al., 2008). The interplay of Src and FAK is a vital convergence point, integrating signals of cell growth, survival, and migration, making the pharmacological inhibition of Src a unique target for cancer drug therapy (Rucci et al., 2008). The use of recently developed orally available Src kinase inhibitors to reduce tumor growth shows particular promise for preventing metastasis in vivo (Summy et al., 2005; Ischenko et al., 2007; Jallal et al., 2007; Shor et al., 2007; Park et al., 2008). Although the inhibition of Src in the treatment of cancer represents an important molecular target, there remains a need to develop validated biomarkers to monitor the efficacy of this approach. It has been very challenging to accurately assess the function of Src in intact cells because of the complex signal transduction pathways that are involved (Kansra et al., 2005; Mishra et al., 2005; Ozawa et al., 2008; Vultur et al., 2008). Most current approaches use biomarkers for Src activity that rely on quantifying the effects of an isolated enzyme acting on a synthetic peptide substrate (Hennequin et al., 2006) or the use of immunoprecipitation of a downstream protein target followed by Western blot analysis (Summy et al., 2005).
Fig. 1.
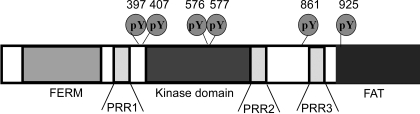
FAK domain structure. Shown is a schematic representation of FAK domains indicating important sites of tyrosine phosphorylation (Tyr397, Tyr407, Tyr576, Tyr577, Tyr861, and Tyr925).
Several studies have been conducted to identify biomarkers of Src activity after treatment of cells with Src inhibitors including those using bosutinib (SKI-606) (Vultur et al., 2008) and AZD0530 (Serrels et al., 2006). These studies were able to correlate tyrosine phosphorylation of Src substrates with drug treatment; autophosphorylation of Src in the former, and paxillin and FAK phosphorylation in the latter. However, they were limited to conducting relative quantification, and so normalization of phosphorylation to the amount of residual unphosphorylated protein was not possible. Therefore, differences in protein expression, immunoprecipitation efficiency, or other intersample variability factors could not be addressed. FAK phosphorylation on Tyr576/Tyr577 most accurately reflects Src activity (Ciccimaro et al., 2006), and so we have developed a new LC-MRM/MS method that quantifies FAK phosphorylation as a robust biomarker of Src activity in vitro, in cell culture, and ultimately in vivo (E. Ciccimaro, S. K. Hanks, and I. A. Blair, submitted).
This new method has made it possible to accurately determine the absolute amounts of FAK phosphorylation that occurs after Src inhibition in vitro and in cell culture with increasing concentrations of the Src inhibitor AZD0530 (Hennequin et al., 2006). Furthermore, because the technique is self-normalizing, problems arising from intersample differences in FAK expression levels, immunoprecipitation efficiency, and assay sensitivity were eliminated.
Materials and Methods
Materials. The gene for human FAK (protein tyrosine kinase 2 α) in the Gateway entry vector pENTER221 was from Invitrogen (Carlsbad, CA). The pDEST26 and pEXP1 plasmids, nickel-chelating resin (Probond), the cell-free expression system, and recombinant full-length c-Src and FAK were also from Invitrogen. Dialysis cassettes were from Pierce Biotechnology (Rockford, IL). Anti-FAK beads (clone 4.47, agarose conjugate beads) were from Millipore (Billerica, MA). Protease inhibitors were from Roche Applied Sciences (Indianapolis, IN). The Src inhibitor AZD0530 was a kind gift from AstraZeneca (Macclesfield, Cheshire, UK). The autosampler was a CTC pal from Leap Technologies (Carrboro, NC), whereas the LC pump was an ExpressLC100 system from Eksigent Technologies (Dublin, CA). LC-grade water and acetonitrile were from Burdick and Jackson (Muskegon, MI), whereas Suprapur formic acid was from EMD Chemical (Gibbstown, NJ). The reverse-phase column (50 × 1 mm internal diameter) was custom-made using extended C18 (300 Å, 3.5 μm) by Agilent Technologies (Santa Clara, CA). All standard peptides were synthesized and quantified using amino acid analysis by AnaSpec, Inc. (San Jose, CA).
Cell-Free Synthesis of Stable Isotope-Labeled FAK Protein ([13C15N]FAK). A full-length FAK construct (from cDNA clone MGC:34721) correlating to the human gene, protein tyrosine kinase 2 α, was obtained from commercial sources. Creation of an expression clone suitable for cell-free production was accomplished by following the manufacturer's instructions using restriction-free recombination technology (Gateway) and plasmids pDEST26 and then pEXP1. The resultant expression clone contained FAK N-terminally tagged with a 6XHIS epitope downstream of the T7 promoter and ribosomal binding site. FAK protein labeled with [13C6 15N4]arginine, [13C9]tyrosine, [13C6 15N2]lysine, and [13C6 15N]leucine was produced using the expressway cell-free expression system, supplied with heavy isotope-labeled amino acids, similar to published techniques (Torizawa et al., 2004; Hanke et al., 2008). The cell-free reaction was conducted according to the manufacturer's directions, and the subsequent 13C15N-labeled 6XHIS-FAK ([13C15N]FAK) was purified on a 2-ml nickel-chelating resin under nondenaturing conditions. After a wash step, [13C15N]FAK was eluted and stored in the presence of 10% glycerol at -80°C until further use. A portion of this reaction mixture was resolved on an SDS-polyacrylamide gel electrophoresis gel and stained with Coomassie blue. The prominent band corresponding to ∼125 kDa (compared with a protein mass marker) was picked for LC-MS analysis. In gel, trypsin digestion was performed, and the isotopic purity of [13C15N]FAK was determined by LC-tandem mass spectrometry.
In Vitro Tyrosine Phosphorylation of [13C15N]FAK. To phosphorylate [13C15N]FAK using purified recombinant Src in an in vitro kinase reaction, it was first necessary to denature [13C15N]FAK and allow refolding during buffer exchange. To accomplish this, [13C15N]FAK was mixed 1:1 with a denaturing buffer consisting of 6 M urea, 2 M thiourea, and 10 mM dithiothreitol and heated at 37°C for 30 min. After heating, the solution containing denatured [13C15N]FAK was dialyzed for 6 h at 4°C against oxidizing dialysis buffer (1 mM oxidized glutathione, 40 mM Tris-HCl, pH 7.5, 150 mM NaCl, 270 mM sucrose, 100 μM EGTA, 100 μMNa3VO4, and 0.03% Brij-35) using a 10,000 molecular weight cutoff filter dialysis cassette. After 6 h, the dialysis buffer was replaced, and dialysis was allowed to continue for an additional 6 h. The refolded and buffer-exchanged [13C15N]FAK was then split into portions, and each portion was adjusted to contain 1 mM ATP, 1× kinase buffer, and recombinant Src (33 nM full-length recombinant c-Src). After incubation at 37°C for 30 min, all portions were pooled and stored at -80°C. Both full-scanning LC/tandem mass spectrometry and LC-MRM/MS were used to ascertain the extent of Tyr576/Tyr577 phosphorylation on [13C15N]FAK.
In Vitro Src Inhibition Assay. Recombinant full-length human c-Src (12 nM) was incubated in kinase buffer (25 mM Tris-HCl, pH 7.5, 5 mM β-glycerophosphate, 2 mM dithiothreitol, 0.1 mM Na3VO3, and 10 mM MgCl2) containing vehicle (0.1% DMSO) or AZD0530 (0.01, 0.1, 1, and 10 μM) at 4°C for 30 min. After incubation, samples were prepared with recombinant human FAK (120 nM) and 1 mM ATP except for the vehicle (-ATP) sample, which did not receive ATP. Samples were brought to 37°C and allowed to incubate for 10 min, at which point samples were heat-inactivated at 70°C for 10 min and unphosphorylated together with phosphorylated [13C15N]FAK were added as recovery standards. All samples were then trypsin-digested in the presence of 1 pmol [13C15N]peptide standard mixture (E. Ciccimaro, S. K. Hanks, and I. A. Blair, submitted). Results were normalized to total FAK and represent the activation loop form amount per total FAK (picomole per picomole).
LC-MS Conditions. Tryptic peptides were loaded onto a micro-analytical C18 column at 25 μl/min using 100% buffer containing acetonitrile/H2O [0.1:20 (v/v)] with 0.1% formic acid. The mobile phase was diverted to waste for the first 5 min under these conditions to remove salts, after which the flow rate was reduced to 14 μl/min, and peptides were eluted over a 30-min gradient from 0 to 40% buffer containing acetonitrile/H2O [19:1 (v/v)] with 0.1% formic acid. An LTQ mass spectrometer (Thermo Fisher Scientific, San Jose, CA) was operated in the positive electrospray ionization mode with helium as the collision gas.
Analysis of FAK Activation Loop Phosphorylation Status Using LC-MRM/MS. Peptides spanning the tryptic region containing FAK activation loop Tyr576 and Tyr577 (Y570MEDSTYYK578) with the endogenous four-amino acid overhangs at both the amino and carboxyl termini and their 13C15N analogs were synthesized as standards for unmodified FAK, pTyr576-FAK, and pTyr576pTyr577-FAK (E. Ciccimaro, S. K. Hanks, and I. A. Blair, submitted). In addition, a control segment of FAK, representing an unmodified tryptic peptide, was synthesized as labeled and unlabeled forms. After trypsin digestion, LC-MRM/MS was used to monitor a total of 12 peptides resulting from the hydrolysis of endogenous FAK, [13C15N]FAK proteins, and 13C15N-labeled peptide standards in a single LC-MRM/MS analysis. MRM transitions for the unmodified Tyr576/Tyr577 FAK tryptic peptide were as follows: YMEDSTYYK (m/z 600.25→905.40), [13C]YMEDST[13C9]Y[13C9]Y[13C6 15N2]K (m/z 617.25→930.40), and YMEDSTYY[13C6 15N2]K (m/z 604.25→913.40). MRM transitions for the mono-phosphorylated (pTyr576) peptide were as follows: YMEDSTpYYK (m/z 640.25→985.35 and 967.35), [13C9]YMEDST-[13C9]p[13C9]Y[13C6 15N2]K (m/z 657.25→1010.36 and 992.36), and YMEDSTpYY[13C6 15N2]K (m/z 644.25→993.36 and 975.36). MRM transitions for the bis-phosphorylated (pTyr576/pTyr577) peptide were as follows: YMEDSTpYpYK (m/z 680.25→1047.31), [13C9]YMEDST-[13C9]pY[13C9]pY[13C6 15N2]K (m/z 697.25→1072.32), and YMED-STpYpY[13C6 15N2]K (m/z 684.25→1055.32). MRM transitions for the control segment peptide were as follows: EVGLALR (m/z 379.23→ 359.24), EVG[13C6 15N]LA[13C6 15N]L[13C6 15N4]R (m/z 382.23→359.24), and E[13C5 15N]VGLALR (m/z 391.23→376.24).
Preparation of Standard Curves, Data Processing, and Normalization. Standard curves were prepared by mixing known amounts of unlabeled peptide standards, a known amount of [13C15N]-FAK, and fixed amounts of the 13C15N peptide standards. Concentrations were calculated using Excalibur (version 2.2) software (Thermo Fisher Scientific). For immunoprecipitation experiments, the [13C15N]-FAK standards were used to determine percent recovery over a range of 2 to 50%. Response ratios of endogenous and [13C15N]FAK to their heavy isotope-labeled standards were calculated by interpolation using their respective standard curves to determine the picomole amount of endogenous FAK and immunoprecipitation recovery for [13C15N]FAK. Two corrections for immunoprecipitation efficiency, an unmodified percentage recovery and a phosphorylated percentage recovery were calculated. These correction values were applied to correct for recovery of the relevant FAK form. Results are presented as means (±S.E.M.).
Cell Culture. MEF populations expressing murine Src Y529For vector-only were used (Brabek et al., 2004). Cells were cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, and penicillin/streptomycin. Cultures were kept lower than 90% confluence on 100-mm culture dishes until harvesting, at which point they were allowed to grow to full confluence. Pervanadate treatment was conducted by exposing cell monolayers to 3 mM H2O2/1 mM Na3VO4 for 10 min before harvesting. Src inhibition using AZD0530 was performed by incubating cells in complete Dulbecco's modified Eagle's medium (as above) containing either vehicle (0.1% DMSO) or AZD0530 (0.01, 0.1, 1, 10, or 50 μM) for 3 or 12 h.
Immunoprecipitation Recovery of FAK from Cell Lysates. Recombinant FAK (258.8 nM) and c-Src (12.8 nM) in a total volume of 200 μl was incubated in the presence of ATP (1 mM) at 37°C for 5 min. The reaction mixture was then heated to 70°C for 10 min. A 10-μl portion of this reaction mixture together with 12 μl of [13C15N]FAK solution were added to a lysing dish of FAK-/- MEFs, or a 10-μl portion of this reaction together with 6 μl of the [13C15N]FAK solution were added to two lysing dishes, and pooled. Immunoprecipitation was conducted as described above. In parallel, 12 μl of heat-inactivated [13C15N]FAK solution was mixed with a 10-μl portion of the in vitro kinase reaction in a vial. After the elution of FAK from the immune complex, 13C15N peptide standards were added, the mixtures were digested with trypsin, and the digests were analyzed by LC-MRM/MS as described above.
Analysis of FAK Activation Loop Phosphorylation after Immunoprecipitation. The quantification of FAK Tyr576 and Tyr577 in the unmodified, mono-(pTyr576), and bis-phosphorylated-pTyr576/pTyr577 forms relative to total FAK was carried out as described previously (E. Ciccimaro, S. K. Hanks, and I. A. Blair, submitted). In brief, confluent plates of MEF cells were lysed in the presence of [13C15N]FAK, and total FAK was immunoprecipitated. 13C15N peptide standards were added to the immunoprecipitate elution, and the mixture was digested with trypsin. Tryptic digests were analyzed by LC-MRM/MS to quantify both endogenous FAK and [13C15N]FAK. Endogenous and [13C15N]FAK peptides were quantified using their respective calibration curves. Endogenous FAK peptide levels were then corrected using the calculated immunoprecipitation recovery value (using the [13C15N]FAK standard) and normalized to total FAK (using the amount unmodified control peptide).
Analysis of FAK Activation Loop Phosphorylation in MEFs after Inhibition with Pervanadate. Before cell lysis, confluent plates of normal and Y529FSrc-expressing MEFs were treated with pervanadate for 10 min. Cells were then lysed in the presence of [13C15N]FAK, and total FAK was immunoprecipitated and processed as above.
Analysis of FAK Activation Loop Phosphorylation in MEFs after Src Inhibition. Confluent plates of normal and Y529FSrc-expressing MEFs were treated with 1 μM AZD0530 for 3 h. After this incubation, cells were treated with pervanadate for 10 min. Cells were then lysed in the presence of [13C15N]FAK, and total FAK was immunoprecipitated and processed as described above.
Dose-Response of FAK Activation Loop Phosphorylation after Src Inhibition in Oncogenic Y529FSrc-Expressing MEFs. Confluent plates of normal and Y529FSrc-expressing MEFs were treated with vehicle (0.1% DMSO) or 0.01, 0.1, 1, 10, and 50 μM concentrations of the Src inhibitor AZD0530 for 12 h. Cells were then lysed in the presence of [13C15N]FAK, and total FAK was immunoprecipitated and processed as described above.
Results
In Vitro Inhibition of Src. To evaluate FAK inhibition by AZD0530 in a controlled setting, Src was inhibited by the drug in vitro and then incubated with recombinant FAK. FAK incubated with vehicle-treated Src in the presence of ATP showed 0.170 (± 0.016) pmol of unmodified FAK. This represents consumption of 0.660 pmol or 78% of the starting material, which was found to contain 0.832 (± 0.012) pmol of unmodified FAK. In addition, this condition showed the production of 0.240 (± 0.005) mono-pTyr576-FAK, and 0.422 (± 0.018) pmol of bis-pTyr576/pTyr577-FAK greater than the starting material amount, which contained 0.132 (± 0.009) and 0.036 (± 0.004) pmol of each form, respectively. The values recorded for FAK reacted with vehicle-treated Src in the presence of ATP correspond to maximal kinase activity under these experimental conditions, and therefore, all AZD0530-inhibited Src results are presented as the percentage of maximal activity relative to these amounts.
Analysis of the unmodified activation loop region (YMED-STYYK) showed the activity of Src under assay conditions was not significantly inhibited by 0.01 μM AZD0530. A 10% reduction in Src activity to 89.9% of the maximum was seen in the 0.1 μM AZD0530 treatment, with further reductions to 44.7 and 4.9% of maximum at 1 and 10 μM treatment with AZD0530, respectively (Fig. 2A). The prevention of Src's ability to modify the activation loop region coincided with a dose-dependent decrease in the production of FAK mono-pTyr576 and bis-pTyr576pTyr577 activation loop forms. At 0.1 μM, AZD0530 reduced the bis-phosphorylation of the activation loop to 64.8% of the maximal level; however, this was concurrent with an increase in the amount of the mono-pTyr576 form to 34.0% greater than the maximum. Bis-pTyr576/pTyr577 was further reduced with 1 and 10 μM AZD0530 to 12.5 and 0.4%, respectively. Mono-phosphorylation at Tyr576 was not inhibited at 1 μM but was reduced to 12.6% of the maximal level at a concentration of 10 μM AZD0530 (Fig. 2B). A best-fit IC50 value using the quantification of bis-phosphorylated FAK activation loop domain was found to be 0.14 μM (95% confidence 0.07-0.30 μM) (Fig. 2C).
Fig. 2.
Dose-response of Src inhibition on FAK activation loop Tyr576 and Tyr577 modification by Src in vitro. After incubation with varying concentrations of AZD0530 (0.01, 0.1, 1, and 10 μM), recombinant Src was incubated with recombinant FAK for 15 min at 37°C. LC-MRM/MS was then used to absolutely quantify the amount of FAK activation loop modification. A, the amount of modified Tyr576/Tyr577 FAK activation loop (⋄). B, the amount of FAK activation loop in the mono-phosphorylated pTyr576 (□) and bis-phosphorylated pTyr576/pTyr577 (▵) forms. C, an IC50 value of Src inhibition calculated from the amount of pTyr576/pTyr577 (▵) determined at each dose. Data points represent the means of three separate experiments, where error bars are ± S.E.M.
Immunoprecipitation Recovery of FAK from Cell Lysates. To confirm that our methodology correctly quantified FAK phosphorylation after protein immunoprecipitation, we measured the amount of FAK phosphorylation on a known amount of recombinant FAK spiked into and then immunoprecipitated from a FAK-null cell lysate. The amounts of unmodified, pTyr576, and pTyr576/pTyr577 FAK present in the kinase reaction mixture before the immunoprecipitation were 0.707 (± 0.029), 1.893 (± 0.025), and 1.080 (± 0.021) pmol, respectively. After immunoprecipitation, the amounts quantified were 0.160 (± 0.011) pmol unmodified, 0.338 (± 0.018) pmol pTyr576, and 0.187 (± 0.011) pmol pTyr576/pTyr577 FAK. The immunoprecipitation recovery based on [13C15N]FAK was 24.5% for unmodified FAK and 15.1% for pTyr576-FAK. Applying these correction values for each sample to the initial immunoprecipitated amounts resulted in adjusted amounts of 0.669 (± 0.030) pmol for unmodified FAK, 2.123 (± 0.191) pmol for pTyr576-FAK, and 1.221 (± 0.063) pmol for pTyr576/pTyr577-FAK. The total amount of activation loop peptides (corrected for losses during immunoprecipitation) was 4.025 (± 0.298) pmol and the amount control peptide was 3.891 (± 0.278) pmol. The total FAK amount based on the control peptide was therefore 3.891 (± 0.278) pmol [i.e., 486 ng of FAK/1.65 mg of total protein (single-dish background) and 486 ng of FAK/3.30 mg of total protein (double-dish background)].
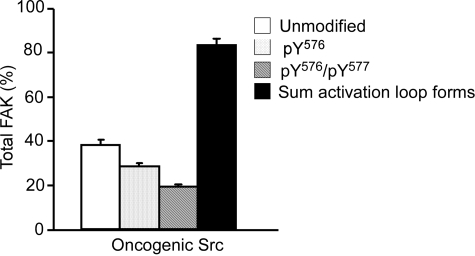
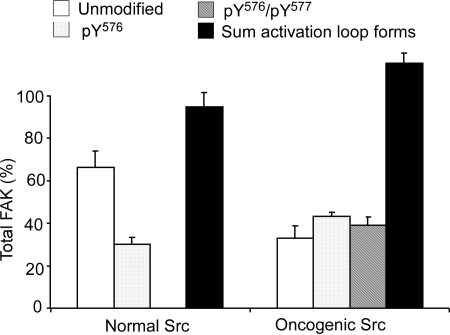
Analysis of FAK Activation Loop Phosphorylation in MEFs. After in vitro experimentation, the ability of the assay to monitor Src activity was investigated in a cell culture system containing normal and oncogenic Src. MEFs expressing normal Src were found previously to contain 0.654 (± 0.034) pmol of unmodified FAK, 0.331 (± 0.052) pmol of pTyr576-FAK, and 0.059 (± 0.010) pmol of pTyr576/pTyr577-FAK (E. Ciccimaro, S. K. Hanks, and I. A. Blair, submitted). The total amount of activation loop peptides normalized to control peptide for normal Src in the basal state was 1.044 (± 0.087) pmol. In contrast, MEFs expressing Y529FSrc were found to express 0.381 (± 0.026) pmol of unmodified FAK, 0.287 (± 0.005) pmol of pTyr576-FAK, and 0.193 (± 0.006) pmol of pTyr576/pTyr577-FAK (Fig. 3). The total amount of FAK activation loop peptides in the Y529FSrc-expressing MEFs was 0.836 (± 0.027) pmol (Fig. 3). These values were obtained by using calculated immunoprecipitation recoveries of 25% for unmodified FAK and 14% for phosphorylated FAK in the Y529FSrc-expressing MEFs. The average total FAK amount (corrected for losses during immunoprecipitation) was 1.5 pmol/plate (i.e., 187.5 ng of FAK/2.47 mg of total protein).
Fig. 3.
Quantification of FAK activation loop Tyr576 and
Tyr577 in MEFs expressing oncogenic Y529FSrc. Shown are
amounts of unmodified Tyr576/Tyr577 (□),
mono-pTyr576 ( ),
and bis-phosphorylated pTyr576/pTyr577
(
),
and bis-phosphorylated pTyr576/pTyr577
( ) activation loop peptides,
and the sum amount of activation loop peptides (▪) from a FAK trypsin
digest after immunoprecipitation from confluent monolayers of MEFs expressing
oncogenic Y529FSrc. The mounts of each peptide are shown after
correction for FAK immunoprecipitation recoveries and normalization to the
amount total FAK. Data points represent the means of three separate
experiments, where error bars are ± S.E.M.
) activation loop peptides,
and the sum amount of activation loop peptides (▪) from a FAK trypsin
digest after immunoprecipitation from confluent monolayers of MEFs expressing
oncogenic Y529FSrc. The mounts of each peptide are shown after
correction for FAK immunoprecipitation recoveries and normalization to the
amount total FAK. Data points represent the means of three separate
experiments, where error bars are ± S.E.M.
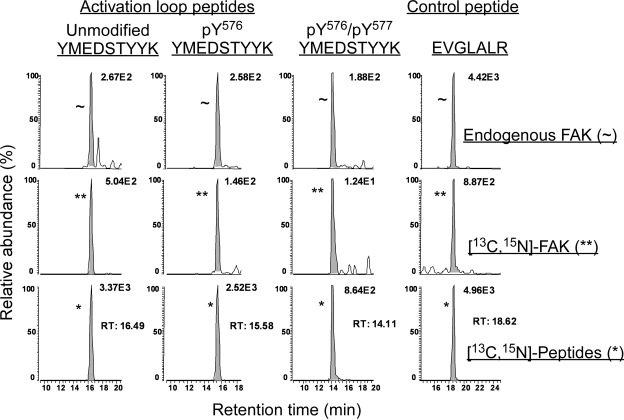
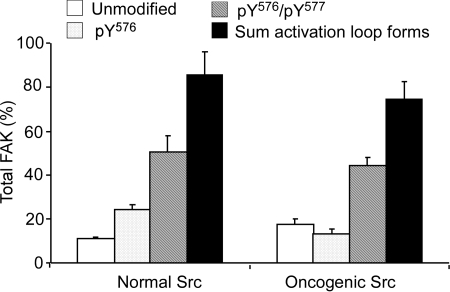
Analysis of FAK Activation Loop Phosphorylation in MEFs after Inhibition with Pervanadate. The ability of AZD0530 to inhibit Src phosphorylation of FAK's activation loop was studied under conditions of FAK hyperphosphorylation. This was accomplished by broadly inhibiting tyrosine phosphatases with pervanadate. A typical LC-MRM/MS chromatogram for digested endogenous and [13C15N]FAK immunoprecipitated from the normal MEFs treated with pervanadate and their heavy isotope-labeled internal standards is shown in Fig. 4. Y529FSrc-expressing MEFs treated with pervanadate gave similar chromatograms (data not shown). After data normalization, as above, the pervanadate-treated normal MEFs contained 0.109 (± 0.008) pmol of unmodified FAK, 0.242 (± 0.023) pmol of pTyr576-FAK, and 0.504 (± 0.080) pmol of pTyr576/pTyr577-FAK. Phosphatase-inhibited Y529FSrc-expressing MEFs were found to express similar amounts, with 0.175 (± 0.026) pmol of unmodified FAK, 0.132 (± 0.019) pmol of pTyr576-FAK, and 0.440 (± 0.040) pmol of pTyr576/pTyr577-FAK. The total amount of activation loop peptides (normalized to control peptide) for pervanadate-treated normal and Y529FSrc-expressing MEFs were 0.855 (± 0.106) pmol and 0.747 (± 0.081) pmol, respectively (Fig. 5). These values were corrected using calculated immunoprecipitation recoveries of 26.0% for unmodified FAK and 14.0% phosphorylated FAK from normal MEFs and 28.0% for unmodified-FAK and 16.0% for phosphorylated from Y529FSrc-expressing MEFs. The average total FAK amounts (based on the immunoprecipitation corrected amount of control peptide) after pervanadate treatment were 2.70 pmol/plate (i.e., 337.5 ng of FAK/2.62 mg of total protein) for normal Src-expressing MEFs and 1.05 pmol/plate (i.e., 125 ng of FAK/2.62 mg of total protein) for Y529FSrc-expressing MEFs.
Fig. 4.
LC-MRM/MS analysis of activation loop and control peptides from digested endogenous and [13C15N]FAK immunoprecipitated from pervanadate-treated MEFs expressing normal Src and their corresponding heavy isotope-labeled internal standards. Shown are ion chromatograms for peptides resulting from the trypsin hydrolysis of immunoprecipitated endogenous (∼) and [13C15N]FAK (**), spiked with [13C15N]labeled peptide standards (*). MRM transitions monitored the unmodified activation loop peptide (YMEDSTY576Y577K, tR = 16.49 min) in the unlabeled (m/z 600.25 → 905.40), 13C33 15N2-labeled (m/z 617.25 → 930.40), and 13C6 15N2-labeled (m/z 604.25 → 913.40) species. Mono-phosphorylated (pY576) peptides (tR = 15.58 min) were monitored by transitions for the unlabeled (m/z 640.25 → 985.35 and 967.35), 13C33 15N2-labeled (m/z 657.25 → 1010.36 and 992.36), and 13C6 15N2-labeled (m/z 644.25 → 993.36 and 975.36) species. Bis-phosphorylated (pTyr576/pTyr577) peptides (tR = 14.11) were monitored by transitions for the unlabeled (m/z 680.25 → 1047.31), 13C33 15N2-labeled (m/z 697.25 → 1072.32), and 13C6 15N2-labeled (m/z 684.25 → 1055.32) species. Finally, the control segment peptides (E956VGLALR962, tR = 18.62) were monitored by transitions for the unlabeled (m/z 379.23 → 359.24), 13C18 15N6-labeled (m/z 382.23 → 359.24), and 13C5 15N-labeled (m/z 391.23 → 376.24) species.
Fig. 5.
Quantification of FAK activation loop Tyr576 and Tyr577 in MEFs expressing normal Src or oncogenic Y529FSrc after pervanadate treatment. Legends and experimental conditions are as for Fig. 3 except that both normal and Y529FSrc-expressing MEFs were used.
Analysis of FAK Activation Loop Phosphorylation in MEFs after Treatment with 1 μM Src Inhibitor. To demonstrate the sensitivity and selectivity of the assay to monitor Src activity, Src kinase function was inhibited in cell culture. MEFs expressing oncogenic Y529FSrc treated with Src inhibitor before pervanadate showed similar chromatograms to those recorded for fibroblasts treated with pervanadate alone (Fig. 4). After data normalization, the Src-inhibited and pervanadate-treated normal MEFs contained 0.667 (± 0.074) pmol of unmodified FAK and 0.302 (± 0.032) pmol of pTyr576-FAK, whereas pTyr576/pTyr577-FAK was not detected. Src-inhibited and phosphatase-treated Y529FSrc-expressing MEFs were found to contain 0.332 (± 0.057) pmol of unmodified FAK, 0.431 (± 0.021) pmol of pTyr576-FAK, and 0.392 (± 0.037) pmol of pTyr576/pTyr577-FAK. The total amount of activation loop peptides (normalized to control peptide) for Src-inhibited and pervanadate-treated normal and Y529FSrc-expressing MEFs were 0.951 (± 0.065) and 1.160 (± 0.047) pmol, respectively (Fig. 6). These values were corrected using calculated immunoprecipitation recoveries of 27.8% unmodified and 20.5% phosphorylated for normal MEFs and 27.3% unmodified and 18.0% phosphorylated for Y529FSrc-expressing MEFs, on average. The average total FAK amounts (based on the immunoprecipitation-corrected amount of control peptide) after pervanadate treatment were 2.00 pmol/plate for normal Src-expressing MEFs and 1.15 pmol/plate for Y529FSrc-expressing MEFs.
Fig. 6.
Quantification of FAK activation loop Tyr576 and Tyr577 phosphorylation in MEFs expressing normal Src or oncogenic Y529FSrc after treatment with 1 μM Src inhibitor. Legends and experimental conditions are as for Fig. 3 except that both normal and Y529FSrc-expressing MEFs were used.
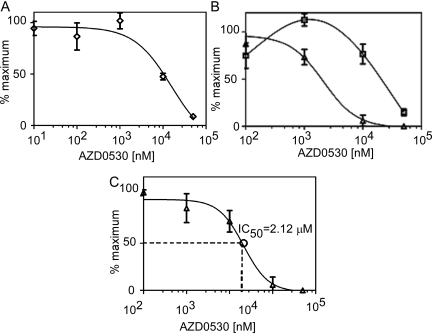
Dose-Response of FAK Activation Loop Phosphorylation after Src Inhibition in Oncogenic Y529FSrc-Expressing MEFs. Treatment with 1 μM Src inhibitor failed to significantly alter FAK activation loop phosphorylation in oncogenic Src-expressing MEFs. Therefore, FAK activation loop was quantified in oncogenic Src-expressing MEFs treated with a broad range of inhibitor concentrations. Culturing Y529FSrc-expressing MEFs in the presence of vehicle or 0.01 or 0.1 μM AZD0530 did not alter FAK activation loop phosphorylation levels compared with those observed in the basal state. The amount of FAK activation loop forms observed in vehicle-treated Y529FSrc-expressing MEFs was considered the maximal level, and all other values are presented as a percentage of these levels. At 1 μM, AZD0530 treatment reduced pTyr576/pTyr577-FAK amounts to 73.2% (± 11.4%), whereas it increased pTyr576-FAK amounts to 112.5% (± 9.0%) of maximum. At this treatment concentration, the amount of unmodified FAK was approximately 100% of the maximal level at 101.8% (± 10.9%). Treatment with 10 μM AZD0530 further reduced pTyr576/pTyr577-FAK amounts to 5.9% (± 8.3%), whereas it reduced pTyr576-FAK amounts to 76.4% (± 14.0%) of maximum. Concurrently, this dose increased the amount of unmodified FAK, and decreased the amount of phosphorylated-FAK activation loop to 47.5% (± 4.5%) of maximum. At 50 μM AZD0530, no pTyr576/pTyr577-FAK was detected, and pTyr576-FAK was reduced to only 14.8% (± 5%) of maximum. At this dose, nearly all FAK recovered was in the unmodified form, reducing the amount of modified forms to 8.7% (± 3.0%) of their maximum levels (Figs. 7, A and B). A best-fit IC50 value using the quantification of bis-phosphorylated FAK activation loop was found to be 2.12 μM (95% confidence 0.74-6.07 μM) (Fig. 7C).
Fig. 7.
Dose-response of Src inhibitor AZD0530 on FAK activation loop Tyr576 and Tyr576 isolated from oncogenic Src-expressing MEFs. Oncogenic Y529FSrc-expressing MEFs were cultured in the presence of varying concentrations of AZD0530 (0.01, 0.1, 1, 10, and 50 μM) for 12 h. FAK was isolated from cellular lysate by immunoprecipitation. LC-MRM/MS was then used to absolutely quantify the amount of FAK activation loop modification. A, the amount of modified Tyr576/Tyr577 FAK activation loop (⋄). B, the amount of FAK activation loop in the mono-phosphorylated pTyr576 (□) and bis-phosphorylated pTyr576/pTyr577 (▵) forms. C, an IC50 value of Src inhibition was calculated using the amount of pTyr576/pTyr577 (▵) determined at each dose. Data points represent the means of duplicate experiments, where error bars are ± S.D.
Discussion
Inhibition of Src with AZD0530 in vitro prevented modification on the activation loop region as reflected in both the amount of unmodified FAK preserved and the amount of pTyr576-FAK and pTyr576/pTyr577-FAK produced. It is note-worthy that the dose-response curve obtained for pTyr576-FAK showed an initial increase at 0.1 μM AZD0530 (Fig. 2B). This result can be explained by the prevention of Src from converting the monophosphorylated activation loop domain into the bis-phosphorylated form at this concentration without preventing Tyr576-FAK phosphorylation. Concurrently, there was a dose-dependent decrease in pTyr576/pTyr577-FAK with increasing AZD0530 concentrations with an IC50 value of 0.14 μM (Fig. 2C). The initial description of AZD0530 inhibition of Src activity toward the poly(GluTyr) peptide by Hennequin et al. (2006) found an IC50 value of 0.0027 (± 0.0005) μM. The discrepancy between IC50 values can be explained by differences between assay methodologies. Both Src concentrations and ATP concentrations could alter the amount of inhibitor required to inhibit Src activity, because AZD0530 will compete for binding at the ATP binding pocket of Src proteins. Assay conditions in the present study involved the use of 12 nM Src and 1 mM ATP, whereas studies by other investigators used 2 nM Src and 0.04 mM ATP (Ple et al., 2004). The differences in assay conditions reflect the goal of the respective experiments. Our studies involve Src inhibition in cell culture systems; therefore, we chose to use 1 mM ATP as found in cell models (Beis and Newsholme, 1975). Conversely, the goal of previous studies was to develop small compound inhibitors of Src, which required the assay to be sensitive to even weakly inhibiting compounds (Hennequin et al., 2006).
Two cell models were used in the present study: vector control MEFs that expressed only the endogenous Src-family kinases and oncogenic MEFs that expressed the Y529FSrc variant (Brabek et al., 2004). The Tyr529-to-Phe529 amino acid substitution in Src results in a constitutively active form as a result of the inability of the kinase to achieve its normal autoinhibited and inactive conformation. Y529FSrc extensively phosphorylates multiple substrate proteins (including FAK), resulting in cellular transformation (Cooper et al., 1986; Okada and Nakagawa, 1989; Brabek et al., 2004; Luo et al., 2008). Our results showed a distinct increase of pTyr576/pTyr577-FAK in the MEFs expressing Y529FSrc (0.193 pmol/pmol of total FAK), relative to that found previously in normal MEFs (0.059 pmol/pmol of total FAK) (E. Ciccimaro, S. K. Hanks, and I. A. Blair, submitted). A large amount of unmodified FAK and pTyr576-FAK was present in the Y529FSrc-expressing MEFs, calculated at 0.381 pmol and 0.287 pmol/pmol of total FAK, respectively (Fig. 3). This indicates that a significant fraction of total FAK exists in a subcellular locale sequestered from the action of Src and/or that Tyr577 is dephosphorylated by a phosphatase at a greater rate than Src phosphorylates it. To address these issues, the consequences of global tyrosine phosphatase inhibition using pervanadate were explored.
Pervanadate is known to broadly inhibit tyrosine phosphatases, thus dramatically increasing the extent of tyrosine phosphorylation on multiple proteins and, more relevantly, increasing the extent that FAK Tyr576 and Tyr577 are phosphorylated (Maa and Leu, 1998). Pervanadate treatment of either normal or Y529FSrc-expressing MEFs led to the majority of total FAK in the cell existing as the pTyr576/pTyr577-FAK form, with approximately 0.4 to 0.5 pmol/pmol of total FAK, leaving only 0.1 to 0.2 pmol/pmol of total FAK in the unmodified form, regardless of which form of Src was present (Fig. 5). After the 10-min pervanadate treatment, almost 20% of FAK remained in the unmodified form, indicating that a portion of the cellular protein was resistant to phosphorylation by Src; however, almost 60% of FAK existed as the pTyr576/pTyr577-FAK form, suggesting that activation loop phosphorylation is potently regulated by a tyrosine phosphatase(s). We considered the possibility that FAK phosphorylation might be increased in pervanadate-treated MEFs as a result of increased Src activity. This is unlikely because oncogenic Y529F Src lacks regulation by C-terminal tail phosphorylation. In addition, the observation that FAK activation loop phosphorylation was similar in both pervanadate-treated normal MEFs and oncogenic Src-expressing MEFs confirmed that phosphatase regulation of the C-terminal tail of Src was not an important factor in these experiments. We cannot rule out the possibility that phosphatase inhibition caused a decrease in the dephosphorylation of Tyr416, which could then have led to increased Src activity in both experimental cell models. However, it is clear that the methodology we have developed is able to accurately quantify changes in FAK activation loop phosphorylation.
The sum of activation loop forms after pervanadate treatment for normal and Y529FSrc-expressing MEFs were 0.855 (± 0.106) and 0.744 (± 0.081) pmol/pmol of total FAK, respectively. This indicated that a small portion of this region was modified to a form(s) undetected in our assay. The most likely explanation for this observation is that the hydrogen peroxide exposure during the pervanadate treatment resulted in some endogenous methionine sulfoxide formation of Met571 within the activation loop segment tryptic peptide (YMEDSTYYK). Alternatively, it is possible that Tyr570 became partially phosphorylated during the pervanadate treatment. It is noteworthy that normal Src-expressing MEFs were consistently found to have an almost 3-fold increase in total FAK/plate compared with Y529FSrc-expressing MEFs, whereas the total protein amounts per plate between the MEFs were equivalent.
To further investigate the relationship between Src activity and FAK activation loop phosphorylation, the small molecule AZD0530 was used to inhibit Src tyrosine kinases in cell culture. The recommended concentration of AZD0530 for inhibition of Src kinases in cell culture is 1 μM. At this concentration, AZD0530 has been shown by Western blot to reduce the phosphorylation of Src substrates such paxillin (Hennequin et al., 2006; Jones et al., 2008). AZD0530 is a potent inhibitor of FAK phosphorylation in vitro. However, it is a poor inhibitor of a number of other tyrosine- and serine/threonine protein kinases (Hennequin et al., 2006). Furthermore, the specificity and potency of AZD0530 toward Src compares very favorably with other inhibitors such as the pyrazolo[3,4-d]pyrimidines PP1 and PP2, CGP-77675, dasatinib, and bosutinib (Hanke et al., 1996; Missbach et al., 1999; Serrels et al., 2006; Vultur et al., 2008). Although the phosphorylation of Src substrates is reduced by AZD0530 treatment, FAK autophosphorylation of Tyr397 is not altered, indicating that FAK kinase activity is unaffected by treatment with AZD0530. Our results showed that 1 μM AZD0530 reduced the amount of pTyr576/pTyr577-FAK to undetectable levels when normal fibroblasts were incubated in the presence of the drug for 3 h before pervanadate treatment. Surprisingly, the amount of pTyr576/pTyr577-FAK in Src-transformed fibroblasts expressing Y529FSrc was not significantly affected by 1 μM AZD0530 pretreatment. Although the amount of pTyr576/pTyr577-FAK in Src-transformed fibroblasts was unchanged when the pervanadate treatment was compared with Src inhibition and pervanadate treatment (Figs. 5 and 6), the amounts of unmodified and pTyr576-FAK forms were slightly increased. However, the sum of FAK activation loop forms for Src inhibited and pervanadate-treated transformed fibroblasts was in agreement with calculated amounts of the control peptide. This suggests that oncogenic Y529FSrc expression can cause further post-translational modifications to FAK's activation loop domain during pervanadate treatment and that these additional modifications are inhibited by 1 μM AZD0530 pretreatment.
Src inhibitors will be targeted to cancers in which elevated Src activity is driving disease progression; therefore, the Src-transformed MEF model was chosen to conduct dose-response experiments. More importantly, pTyr576/pTyr577-FAK was found to contribute only 6.0% to the total FAK in MEFs expressing normal Src, compared with 23.0% in oncogenic Src expressing MEFs. This limited the possible observable response to Src inhibition in the normal MEFs. A dose-response curve to the Src inhibitor AZD0530 conducted in cell culture showed a similar trend (Fig. 7C) to that observed in vitro (Fig. 2C), although the dose-response was shifted to the right by more than an order of magnitude. In comparison with in vitro Src inhibition, which showed a decrease in the amount of pTyr576/pTyr577-FAK with a drug concentration as low as 0.1 μM, a decrease in pTyr576/pTyr577-FAK was not observed in Src-transformed fibroblasts until the cells had been treated with 1 μM concentration of the drug (Fig. 7C). A reduction of 50% in bis-phosphorylation of Tyr576/Tyr577-FAK was calculated to occur at 2.12 μM AZD0530 (IC50 value). Inhibition of paxillin (a substrate for Src phosphorylation at focal adhesions) phosphorylation by 70% was observed with 1 μM AZD0530 when studies were conducted in the human lung tumor cell line (A549 non-small-cell lung cancer) using Western blot analysis (Hennequin et al., 2006). Therefore, these previous experiments showed a similar trend to those we have obtained using bis-phosphorylation of FAK as a biomarker of cellular Src activity.
In summary, our new stable isotope dilution LC-MRM/MS assay showed that the Src inhibitor AZD0530 caused inhibition of pTyr576/pTyr577-FAK formation in a dose-dependent manner (Figs. 2B and 7B). From these data, it was possible to determine AZD0530 IC50 values for Src inhibition both in vitro (Fig. 2C) and in cell culture (Fig. 7C). There was a substantial difference in the ability of AZD0530 to inhibit Src that was constitutively activated in a cellular context (IC50 = 2.12 μM) compared with the isolated enzyme (IC50 = 0.14 μM). This suggests that higher concentrations of Src inhibitors such as AZD0530 might be required for in vivo efficacy than has been considered previously (Jones et al., 2008). Results from the present study can be considered as the proof-of-concept for using FAK phosphorylation as a biomarker to determine the efficacy of Src inhibition in vivo. The increased accuracy and specificity that can be obtained by quantitative stable isotope dilution LC-MRM/MS methodology to quantify FAK phosphorylation offers significant advantages over current immunochemical approaches. Furthermore, elaboration of this methodology to other kinase targets will make it possible to rigorously assess the efficacy and specificity of novel targeted kinase inhibitors that are developed in the future.
Acknowledgments
We thank Dr. Weifeng Luo (Vanderbilt University School of Medicine) for technical assistance and AstraZeneca (Macclesfield, Cheshire, UK) for the kind gift of AZD0530.
This work was supported by the National Institutes of Health National Cancer Institute [Grant R01-CA95586]; the National Institutes of Health National Institute of Environmental Health Sciences [Grant P30-ES013508]; the National Institutes of Health National Heart, Lung, and Blood Institute [Grant T32-HL007954]; and the National Institutes of Health National Institute of General Medical Sciences [Grant GM49882].
ABBREVIATIONS: c-Src, cellular Src; FAK, focal adhesion kinase; LC-MRM/MS, liquid chromatography-multiple reaction monitoring/mass spectrometry; MEF, mouse embryonic fibroblast; AZD0530, N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5-(tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine; DMSO, dimethyl sulfoxide; SKI-606, bosutinib; CGP-77675, substituted 5,7-diphenyl-pyrrolo(2,3-d)pyrimidine.
References
- Basson MD (2008) An intracellular signal pathway that regulates cancer cell adhesion in response to extracellular forces. Cancer Res 68 2-4. [DOI] [PubMed] [Google Scholar]
- Beis I and Newsholme EA (1975) The contents of adenine nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates. Biochem J 152 23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brábek J, Constancio SS, Shin NY, Pozzi A, Weaver AM, and Hanks SK (2004) CAS promotes invasiveness of Src-transformed cells. Oncogene 23 7406-7415. [DOI] [PubMed] [Google Scholar]
- Calalb MB, Polte TR, and Hanks SK (1995) Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src-family kinases. Mol Cell Biol 15 954-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccimaro E, Hevko J, and Blair IA (2006) Analysis of phosphorylation sites on focal adhesion kinase using nanospray liquid chromatography/multiple reaction monitoring mass spectrometry. Rapid Commun Mass Spectrom 20 3681-3692. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Gould KL, Cartwright CA, and Hunter T (1986) Tyr527 is phosphorylated in Pp60c-Src: implications for regulation. Science 231 1431-1434. [DOI] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, and Connelly PA (1996) Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem 271 695-701. [DOI] [PubMed] [Google Scholar]
- Hanke S, Besir H, Oesterhelt D, and Mann M (2008) Absolute SILAC for accurate quantitation of proteins in complex mixtures down to the attomole level. J Proteome Res 7 1118-1130. [DOI] [PubMed] [Google Scholar]
- Hennequin LF, Allen J, Breed J, Curwen J, Fennell M, Green TP, Lambert-van der Brempt C, Morgentin R, Norman RA, Olivier A, et al. (2006) N-(5-chloro-1,3-benzodioxol-4-Yl)-7-[2-(4-methylpiperazin-1-Yl)ethoxy]-5-(tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific C-Src/Abl kinase inhibitor. J Med Chem 49 6465-6488. [DOI] [PubMed] [Google Scholar]
- Hiscox S and Nicholson RI (2008) Src inhibitors in breast cancer therapy. Expert Opin Ther Targets 12 757-767. [DOI] [PubMed] [Google Scholar]
- Ischenko I, Guba M, Yezhelyev M, Papyan A, Schmid G, Green T, Fennell M, Jauch KW, and Bruns CJ (2007) Effect of Src kinase inhibition on metastasis and tumor angiogenesis in human pancreatic cancer. Angiogenesis 10 167-182. [DOI] [PubMed] [Google Scholar]
- Jallal H, Valentino ML, Chen G, Boschelli F, Ali S, and Rabbani SA (2007) A Src/Abl kinase inhibitor, SKI-606, blocks breast cancer invasion, growth, and metastasis in vitro and in vivo. Cancer Res 67 1580-1588. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Young O, Renshaw L, Jacobs V, Fennell M, Marshall A, Green TP, Elvin P, Womack C, Clack G, et al. (2008) Src inhibitors in early breast cancer: a methodology, feasibility and variability study. Breast Cancer Res Treat doi: 10.1007/s10549-008-9997-1. [DOI] [PubMed]
- Kansra S, Stoll SW, Johnson JL, and Elder JT (2005) Src family kinase inhibitors block amphiregulin-mediated autocrine ErbB signaling in normal human keratinocytes. Mol Pharmacol 67 1145-1157. [DOI] [PubMed] [Google Scholar]
- Luo W, Slebos RJ, Hill S, Li M, Brábek J, Amanchy R, Chaerkady R, Pandey A, Ham AJ, and Hanks SK (2008) Global impact of oncogenic Src on a phosphotyrosine proteome. J Proteome Res 7 3447-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maa MC and Leu TH (1998) Vanadate-dependent FAK activation is accomplished by the sustained FAK Tyr-576/577 phosphorylation. Biochem Biophys Res Commun 251 344-349. [DOI] [PubMed] [Google Scholar]
- Meyn MA 3rd and Smithgall TE (2008) Small molecule inhibitors of Lck: the search for specificity within a kinase family. Mini Rev Med Chem 8 628-637. [DOI] [PubMed] [Google Scholar]
- Mishra R, Wang Y, and Simonson MS (2005) Cell cycle signaling by endothelin-1 requires Src nonreceptor protein tyrosine kinase. Mol Pharmacol 67 2049-2056. [DOI] [PubMed] [Google Scholar]
- Missbach M, Jeschke M, Feyen J, Müller K, Glatt M, Green J, and Susa M (1999) A novel inhibitor of the tyrosine kinase Src suppresses phosphorylation of its major cellular substrates and reduces bone resorption in vitro and in rodent models in vivo. Bone 24 437-449. [DOI] [PubMed] [Google Scholar]
- Okada M and Nakagawa H (1989) A protein tyrosine kinase involved in regulation of Pp60c-Src function. J Biol Chem 264 20886-20893. [PubMed] [Google Scholar]
- Owen JD, Ruest PJ, Fry DW, and Hanks SK (1999) Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol Cell Biol 19 4806-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa Y, Williams AH, Estes ML, Matsushita N, Boschelli F, Jove R, and List AF (2008) Src family kinases promote AML cell survival through activation of signal transducers and activators of transcription (STAT). Leuk Res 32 893-903. [DOI] [PubMed] [Google Scholar]
- Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, Gelovani JG, Kim SJ, Wang Z, and Gallick GE (2008) Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res 68 3323-3333. [DOI] [PubMed] [Google Scholar]
- Plé PA, Green TP, Hennequin LF, Curwen J, Fennell M, Allen J, Lambert-Van Der Brempt C, and Costello G (2004) Discovery of a new class of anilinoquinazoline inhibitors with high affinity and specificity for the tyrosine kinase domain of c-Src. J Med Chem 47 871-887. [DOI] [PubMed] [Google Scholar]
- Rucci N, Susa M, and Teti A (2008) Inhibition of protein kinase C-Src as a therapeutic approach for cancer and bone metastases. Anticancer Agents Med Chem 8 342-349. [DOI] [PubMed] [Google Scholar]
- Ruest PJ, Roy S, Shi E, Mernaugh RL, and Hanks SK (2000) Phosphospecific antibodies reveal focal adhesion kinase activation loop phosphorylation in nascent and mature focal adhesions and requirement for the autophosphorylation site. Cell Growth Differ 11 41-48. [PubMed] [Google Scholar]
- Sawyer TK (2004) Cancer metastasis therapeutic targets and drug discovery: emerging small-molecule protein kinase inhibitors. Expert Opin Investig Drugs 13 1-19. [DOI] [PubMed] [Google Scholar]
- Serrels A, Macpherson IR, Evans TR, Lee FY, Clark EA, Sansom OJ, Ashton GH, Frame MC, and Brunton VG (2006) Identification of potential biomarkers for measuring inhibition of Src kinase activity in colon cancer cells following treatment with dasatinib. Mol Cancer Ther 5 3014-3022. [DOI] [PubMed] [Google Scholar]
- Shor AC, Keschman EA, Lee FY, Muro-Cacho C, Letson GD, Trent JC, Pledger WJ, and Jove R (2007) Dasatinib inhibits migration and invasion in diverse human sarcoma cell lines and induces apoptosis in bone sarcoma cells dependent on SRC kinase for survival. Cancer Res 67 2800-2808. [DOI] [PubMed] [Google Scholar]
- Summy JM and Gallick GE (2003) Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev 22 337-358. [DOI] [PubMed] [Google Scholar]
- Summy JM, Trevino JG, Baker CH, and Gallick GE (2005) C-Src regulates constitutive and EGF-mediated VEGF expression in pancreatic tumor cells through activation of phosphatidyl inositol-3 kinase and P38 MAPK. Pancreas 31 263-274. [DOI] [PubMed] [Google Scholar]
- Thomas SM and Brugge JS (1997) Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13 513-609. [DOI] [PubMed] [Google Scholar]
- Torizawa T, Shimizu M, Taoka M, Miyano H, and Kainosho M (2004) Efficient production of isotopically labeled proteins by cell-free synthesis: a practical protocol. J Biomol NMR 30 311-325. [DOI] [PubMed] [Google Scholar]
- Vultur A, Buettner R, Kowolik C, Liang W, Smith D, Boschelli F, and Jove R (2008) SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol Cancer Ther 7 1185-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman TJ (2004) A renaissance for SRC. Nat Rev Cancer 4 470-480. [DOI] [PubMed] [Google Scholar]