Abstract
The nucleus accumbens (NAc) is a critical brain area for reward and motivated behavior. Accumulating evidence suggests that altered function of the transcription factor cAMP response element binding protein (CREB) within the NAc is involved in depressive behavior. In rats, stress activates CREB within the NAc, and elevation of CREB expression in this region produces depressive-like behaviors that are accompanied by activation of CREB-regulated target genes. The depressive-like behaviors seem to be due, at least in part, to CREB-mediated increases in dynorphin function, because they are mimicked by κ-opioid receptor (KOR) agonists and attenuated by KOR antagonists. We hypothesized that if CREB-mediated dynorphin expression in the NAc contributes to depressive behavior, then antidepressants might reduce dynorphin function in this region. Here, we demonstrate that desipramine (DMI), a norepinephrine reuptake inhibitor that has been used for decades to treat clinical depression, blocks swim stress-induced activation of prodynorphin (encodes dynorphin) in the NAc. In primary cultures of NAc and striatum, DMI decreases basal and stimulated CREB phosphorylation by causing reductions in intracellular calcium (Ca2+) availability that are independent of norepinephrine or other monoaminergic inputs, identifying a potential mechanism for alterations in CREB-mediated gene expression. Fluoxetine (FLX), a selective serotonin reuptake inhibitor, has similar effects in culture, suggesting a common intracellular effect of these antidepressants. These findings raise the possibility that a therapeutically relevant mechanism of action of DMI occurs through attenuation of CREB-mediated gene transcription, which is mediated via previously uncharacterized mechanisms that occur directly within the NAc.
Decreased motivation and reduced ability to experience reward (anhedonia) are prominent signs of clinical depression (American Psychiatric Association, 2000), suggesting that brain reward circuits such as the mesolimbic dopamine system are involved in the neurobiology of depressive behaviors. This system comprises dopamine (DA)-containing neurons originating within the ventral tegmental area and projecting to the nucleus accumbens (NAc). Although the NAc is often associated with the rewarding effects of drugs of abuse, it is also a substrate for natural rewards, including food, sex, and social interaction (Wise, 2004). In rodents, manipulations within the NAc produce behaviors that may model aspects of clinical depression, including anhedonia, dysphoria, and behavioral despair (Harris and Aston-Jones, 1994; Pliakas et al., 2001; Wise, 2004). Although the NAc has not been a major focus of depression research, it innervates—and is innervated by—regions often studied in depressed humans, including the hippocampus, frontal cortex, and amygdala (Nestler and Carlezon, 2006). In addition, norepinephrine (NE) and serotonin inputs modulate the NAc (Pasquier et al., 1977).
Neuroadaptations within the NAc contribute to the development of depressive-like behaviors. Stress elevates activity of the transcription factor cAMP response element binding protein (CREB) within the NAc (Pliakas et al., 2001). Elevated CREB function within the NAc increases depressivelike behavior in the forced swim test (FST) (Pliakas et al., 2001), a procedure often used to study depression (Cryan et al., 2002). Furthermore, elevated CREB reduces the motivational impact of drugs and natural rewards, a sign of anhedonia (Carlezon et al., 1998). The depressive-like behavioral effects that accompany elevated NAc CREB function seem related to altered transcription of dynorphin (Carlezon et al., 1998), an endogenous peptide that acts at κ-opioid receptors (KORs) (Chavkin et al., 1982). Disruption of CREB function within the NAc produces antidepressant-like effects (Pliakas et al., 2001) accompanied by decreases in dynorphin expression (Carlezon et al., 1998). Likewise, KOR antagonists attenuate the behavioral effects of elevated CREB expression within the NAc and have antidepressant-like effects (Pliakas et al., 2001; Newton et al., 2002; Mague et al., 2003; McLaughlin et al., 2003). These findings are consistent with observations that KOR agonists produce depressive signs in humans (Pfeiffer et al., 1986) and rats (Shippenberg and Herz, 1987; Mague et al., 2003; Todtenkopf et al., 2004). Thus, there are strong links between CREB-mediated regulation of dynorphin within the NAc and depressive behavior.
The present studies were designed to test the hypothesis that if CREB function in the NAc contributes to depressive behavior, then desipramine (a NE reuptake inhibitor used for decades to treat clinical depression; Frazer, 1997) might affect CREB-regulated gene expression within this brain region. We first examined the effects of DMI on stress-induced alterations in prodynorphin mRNA expression within the NAc. We then used an in vitro model (primary cell cultures of NAc/striatum) to explore potential intracellular mechanisms of this effect. For comparison, we also examined the in vitro effects of fluoxetine (FLX), a selective serotonin reuptake inhibitor (SSRI) with clinical efficacy similar to DMI (Frazer, 1997).
Materials and Methods
Rats. Thirty-three male Sprague-Dawley rats (325-375 g; Charles River Laboratories, Inc., Wilmington, MA) were used for analysis of prodynorphin mRNA expression. These rats were housed in groups of 4 in a climate-controlled vivarium and maintained on a 12-h light (7:00 AM-7:00 PM)/dark cycle with free access to food and water except during testing. In addition, 16 timed-pregnant Sprague-Dawley rats were used to obtain embryonic day 18 (E18) tissue for the primary cell culture studies. These rats were used on the day of delivery. Experiments were conducted in accordance with the 1996 Guide for the Care and Use of Laboratory Animals (National Institutes of Health), as well as McLean Hospital policies.
Drugs. For in vivo studies in rats, desipramine hydrochloride (DMI; Sigma, St. Louis, MO) was dissolved in distilled water vehicle and administered in a volume of 1.0 ml/kg. For in vitro studies in the NAc/striatal primary cultures, DMI, fluoxetine hydrochloride (FLX; Sigma), potassium chloride (KCl; Fisher Scientific), SKF 82958 (Sigma), and N-methyl-d-aspartic acid (NMDA; Sigma) were dissolved in sterile ultra-pure water. FPL 64176 and thapsigargin (Sigma) were dissolved in dimethyl sulfoxide. Phorbol 12-myristate 13-acetate (PMA; Sigma) was dissolved in 100% ethanol. A 50× stock of SKF 82958 was administered directly to cell culture media (20 μl of 2.5 mM into 1000 μl of media/well) whereas all other drugs were administered at 100× concentration (10 μl of drug into 1000 μl of media/well). Vehicle controls were water for DMI, KCl, SKF 82958, and NMDA, dimethyl sulfoxide for FPL 64176 and thapsigargin, and ethanol for PMA.
Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction. Thirty-three rats were used to determine the effects of DMI and forced swimming on prodynorphin gene expression within the NAc shell. The standard FST is a 2-day procedure in which rats swim under conditions in which escape is not possible. On the first day, rats are forced to swim for 15 min. At first the rats attempt to escape from the water, but eventually they adopt a posture of immobility in which they make only the movements necessary to keep their heads above water. When the rats are retested 24 h later, the latency to immobility is decreased. Treatment with standard antidepressant drugs within the 24 h between the first and second exposures to forced swimming can block facilitated immobility, an effect associated with antidepressant efficacy in humans (Detke et al., 1995). In this study, we sacrificed the rats on day 2, at the time when they would have experienced their second exposure to forced swimming. There were four treatment groups (6-13 rats per group): sham (no swim), sham plus DMI, forced swim (FS), and FS plus DMI. On the first day of the experiment, rats were forced to swim for 15 min as described above. Sham-treated rats were placed on a platform set at a height of 45 cm inside a dry Plexiglas cylinder for 15 min, to mimic as closely as possible the FST without actually exposing the rats to forced swimming. After the first forced swimming or sham session, rats were towel-dried and placed in a warm environment for 30 min. All rats were then injected IP with either saline or DMI (10 mg/kg) at 1, 19, and 23 h after the forced swimming session. At 24 h after the first swimming session, the rats were sacrificed by decapitation. The brains were rapidly removed and frozen in -80°C isopentane. Frozen brains were sectioned on a sliding microtome until the NAc shell was exposed (bregma ∼2.20). Bilateral 1-mm3 punches of tissue were extracted from the NAc shell and placed in Eppendorf tubes on dry ice. Q-PCR methods were similar to those described previously (MacDonald et al., 2005). RNA was extracted from approximately 20 to 30 mg of tissue using the RNAgent kit (Promega, Madison, WI). RNA quality was assessed by analytical gel, and 3.5 μg of total RNA was used for cDNA synthesis with the SuperScript First-Strand Synthesis System for real-time quantitative PCR (Invitrogen, Carlsbad, CA) and oligonucleotide deoxythymidine primer. Primers specific for the prodynorphin and β-actin genes (Genbank accession numbers NM_019374 and NM_031144) were designed with Primer3 software (www.genome.wi.mit.edu/cgi-bin/primer/primer3.cgi). Melt curve analysis and polyacrylamide gel electrophoresis were used to confirm the specificity of the primers. The prodynorphin and β-actin amplicons are 109 and 182 base pairs in length (Fig. 1B), respectively. A Q-PCR kit (Platinum SYBR Green Q-PCR SuperMix UDG; Invitrogen) was used and the reaction was carried out on a DNA Engine Opticon II (MJ Research, Waltham, MA) in a volume of 20 μl, with 4 ml of 1:10 diluted cDNA samples and 0.3 mmol/ml primers. The PCR cycling conditions were 50°C for 2 min, 95°C for 7 min, 39 cycles at 94°C for 10 s, 55°C for 15 s, and 72°C for 30 s. Data were collected at a read temperature of 77°C based on the prodynorphin and β-actin amplicon melt temperature. Standard dilution curves for prodynorphin and β-actin were generated by diluting control (sham) NAc shell cDNA to a final concentration of 1.00, 0.2, 0.04, and 0.008. The log10 of the dilution values was plotted against the cycle values for the standard curves. Opticon Monitor Data Analysis Software version 1.4 (MJ Research) was used to analyze the data. Blanks were run with the dilution curves to control for contamination. All samples were run in duplicate and reported values were normalized to the internal standard β-actin. Data are expressed as mean relative levels of prodynorphin/β-actin mRNA ± S.E.M. and were analyzed using a one-way ANOVA followed by Fisher's protected t tests.
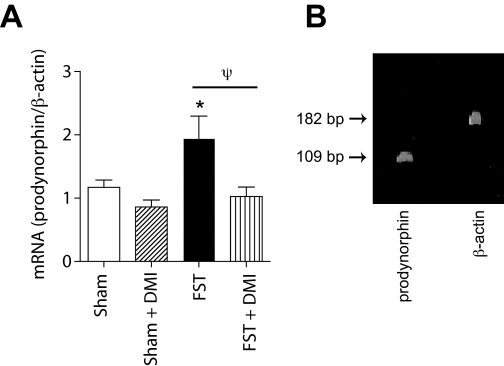
Fig. 1.
DMI regulates molecular consequences of CREB activity within the NAc. A, prodynorphin mRNA levels within the NAc shell were significantly elevated 24 h after exposure to forced swimming. A treatment regimen of DMI (10 mg/kg) that produces antidepressant-like effects in the FST completely blocked the induction of prodynorphin mRNA but had no effect in rats exposed to a sham swim session (“Sham”; see Materials and Methods). Data are expressed as relative levels of prodynorphin mRNA, corrected for β-actin mRNA content. *, P < 0.05 compared with sham-treated rats; ψ, P < 0.05 comparing FST and DMI + FST, Fisher's protected t tests, 6 to 13 rats per group. B, visualization of prodynorphin and β-actin amplicons from Q-PCR.
Primary Cultures of NAc and Striatum. Primary cultures were prepared as described previously (Chartoff et al., 2003). In brief, striata (which contain NAc and striatal tissue) were dissected from E18 Sprague-Dawley rat fetuses in 1× Hank's balanced salt solution (Invitrogen) using a stereomicroscope. Striata were resuspended in 2 ml of defined medium [50% Ham's F12/Dulbecco's modified Eagle's medium and 50% Dulbecco's modified Eagle's medium (Mediatech, Herndon, VA)] that contained the following supplements per liter of medium:4 g of dextrose, 1× B27 (Invitrogen), 10 ml of penicillin-streptomycin liquid (Sigma-Aldrich), and 7.5 mM HEPES (Sigma-Aldrich). Using a Pasteur pipette, the tissue was mechanically dissociated and the cells resuspended to 1 × 106 cells/ml. For Western blot analysis, cells were plated in 12-well plates (1 × 106 cells/well). For the live/dead assay, cells were plated on round glass coverslips (15-mm diameter; Electron Microscopy Sciences, Ft. Washington, PA) that fit inside 12-well plates. Coverslips were sterilized by soaking overnight in concentrated nitric acid, washing three times with sterile H2O, and baking for 30 min. Coverslips and 12-well plates (BD Biosciences, Franklin Lakes, NJ) were pretreated with 1 ml of sterile polyethylenimine (1:500 in H2O; Sigma-Aldrich) for 24 h, washed twice with sterile H2O, and coated with 2.5% fetal bovine serum (Sigma-Aldrich) in PBS for 4 h. The serum was aspirated just before plating of the cells. All experiments were performed with cells in culture for 7 days, and treatments were done in triplicate using cells obtained from at least two independent dissections.
Studies of P-CREB Induction. To study the effects of antide-pressants on phospho-CREB (P-CREB) in NAc tissue, we treated neuronal cultures with DMI or FLX for 1 h (intended to correspond with immediate effects) or 24 h (intended to correspond with longer-term effects). For studies investigating the effects of DMI on basal P-CREB levels, DMI was added to the tissue culture media either 24 or 1 h before cell harvesting. For studies investigating the effects of antidepressant drugs on the activation of P-CREB by cellular depolarization, DMI or FLX was added to the media either 21 or 1 h before KCl (40 mM), and the cells were harvested 3 h later. This time course is based on previously published work (Schwaninger et al., 1995). To determine the mechanisms by which DMI and FLX might be attenuating CREB function, several pathways known to activate CREB were investigated. DMI was administered 1 h before SKF 82958 (DA D1-selective agonist), FPL 64176 (L-type Ca2+ channel activator), PMA (protein kinase C activator), NMDA, or thapsigargin, (sarco-endoplasmic reticulum Ca2+-ATPase inhibitor), which increases levels of free cytosolic Ca2+ (Treiman et al., 1998), and the cells were harvested 15 min later.
Western Blot Analysis. Primary rat NAc/striatal cultures were harvested in Nu-PAGE lithium dodecyl sulfate sample buffer (Invitrogen) and 50 mM dithiothreitol as described previously (Chartoff et al., 2003). Cell lysates were sonicated and centrifuged briefly at 4°C and then heated to 70°C for 10 min before gel electrophoresis. Ten microliters of each sample were loaded onto Nu-PAGE Novex 4 to 12% Bis-Tris gels (Invitrogen) for separation by gel electrophoresis. Proteins were subsequently transferred to polyvinylidene fluoride membrane (PerkinElmer Life and Analytical Sciences, Waltham, MA). Nonspecific binding sites on the membranes were blocked for 2 h at room temperature in blocking buffer [5% nonfat dry milk in PBS and 0.1% Tween 20 (PBS-T)]. Blots were then incubated in primary antibody [1:4000 monoclonal anti-Ser133-phospho-CREB (P-CREB) or 1:4000 anti-CREB (Cell Signaling Technology Inc., Danvers, MA)] in PBS-T overnight at 4°C. Blots were washed four times for 15 min each in PBS-T and then incubated in secondary antibody [1:5000 goat anti-rabbit, or anti-mouse, horseradish peroxidase-linked IgG (Vector Laboratories, Burlingame, CA)] for 2 h at room temperature. Blots were washed four times for 15 min each in PBS-T, followed by immunological detection with Chemiluminescence Reagent Plus (PerkinElmer Life and Analytical Sciences). P-CREB or CREB antibodies were stripped from the blots by incubation with stripping buffer (62.5 mM Tris, 2% SDS, and 100 mM β-mercaptoethanol, pH 6.8) for 15 min at 50°C. Blots were subsequently re-blocked and probed with 1:20,000 anti-β-actin (Sigma). SeeBlue Plus 2 (Invitrogen) prestained standards were run for molecular mass estimation. P-CREB and CREB bands were detected at 43 kDa, and β-actin was detected at 42 kDa.
Protein immunoblots were analyzed using Kodak 1D Image Analysis software (Carestream Health, Rochester, NY). Relative optical densities were determined for each band of interest. To control for loading differences of protein, the optical density of each band was normalized with the corresponding optical density of β-actin. To allow for comparisons of blots from independent dissections, data were normalized to the vehicle-treated controls in each experiment. Data are expressed as the mean -fold induction compared with vehicle control ± S.E.M. and were analyzed using one-way ANOVA followed by Fisher's protected t tests.
Live/Dead Assay. To determine whether DMI is cytotoxic to striatal cultures, a Live/Dead Viability/Cytotoxicity Kit (Invitrogen) was used. Cells were plated on round glass coverslips as described above and treated as described in the figure legends. At the appropriate time after drug treatments, cells were washed twice with PBS. The coverslips containing the cells were then inverted onto 150-μl drops of a dye mixture containing 1 mM calcein AM and 1 mM ethidium homodimer-1 and allowed to incubate for 30 min at room temperature in a darkened chamber containing a water-soaked Kimwipe to prevent the coverslips from drying. Coverslips were then washed briefly in PBS and placed cell-side down onto 20-μl drops of PBS on microscope slides. Visualization (Axioskop 2 plus; Zeiss GmbH, Jena, Germany) and digital image capture (Openlab 3.0.7 software; Improvision, Coventry, UK) of cells were done immediately, because the coverslips dried out after 1 h. Live cells fluoresced green as a result of esterase-mediated cleavage of cell-permeant calcein AM to fluorescent calcein and were detected with an enhanced green fluorescent protein filter. Dead cells fluoresced red as a result of the entry of ethidium homodimer-1 into cells with damaged membranes and subsequent binding to nucleic acid; they were detected with a Texas Red filter. To quantify the percentage of live cells, ImageJ 1.33u software (http://rsb.info.nih.gov/ij/) was used. A 1020 × 760-pixel box was randomly overlaid onto each digital image, and the number of living (green) and dead (red) cells was counted. The number of green plus red cells was used as the total cell population, and data are expressed as the percentage of live cells. The live/dead assay was performed on cells from two separate dissections, and for each dissection, the treatments were administered in duplicate. Data are expressed as the mean percentage of live cells ± S.E.M. and were analyzed using one-way ANOVAs followed by Fisher's protected t tests.
Results
DMI Blocks Stress-Induced Prodynorphin mRNA Expression. To examine the possibility that the antidepressant actions of DMI involve disruption of CREB function within the NAc, we examined its effects on local expression of prodynorphin, a gene known to be regulated by CREB (Douglass et al., 1994; Cole et al., 1995). Stress is a major trigger for depression (Nestler et al., 2002), and it has been shown that FS stress activates CREB within the NAc (Pliakas et al., 2001) and induces the release of dynorphin (McLaughlin et al., 2003). Thus DMI—which reduces FS-induced depressivelike behavior (Cryan et al., 2002)—may act in part to attenuate stress-induced neuroadaptations such as CREB-regulated increases in prodynorphin. We found that prodynorphin mRNA levels within the NAc depended upon treatment (F3,29 = 4.091; P < 0.05) (Fig. 1A): forced swimming induced increased expression of prodynorphin within the NAc that was sustained for at least 24 h (P < 0.05), and this effect was blocked by a treatment regimen of DMI (10 mg/kg injection) that produces antidepressant-like effects in the FST (Carlezon et al., 2002). In contrast, DMI had no effect on prodynorphin mRNA levels in rats not exposed to forced swim stress. Visualization confirmed that the prodynorphin and β-actin amplicons were the expected size on a polyacrylamide gel (Fig. 1B).
Effects of DMI on CREB Function in Dissociated NAc/Striatal Cultures. The NAc receives relatively minor NE input (Delfs et al., 1998), raising the possibility that the ability of DMI to block FS stress-induced prodynorphin in the NAc is due either to indirect circuit effects or to direct effects on NAc function that are independent of NE reuptake blockade. To test whether DMI can act directly on postsynaptic cells in the NAc to modulate intracellular signaling, we used primary cultures of dissociated E18 rat NAc and striatal tissue. We treated these cultures for 1 h or 24 h with DMI and measured the relative levels of CREB protein or CREB phosphorylated at serine residue 133 (P-CREB). One-hour treatment with DMI decreased basal P-CREB levels (F5,209 = 7.59; P < 0.01) without affecting total CREB protein (F5,21 = 0.15; not significant) (Fig. 2A). Twenty-four-hour treatment with DMI had a bimodal effect on P-CREB levels (F5,171 = 22.0; P < 0.01) (Fig. 2B): intermediate doses of DMI increased (P < 0.01) basal P-CREB, whereas a high dose (3 μM) decreased it (P < 0.01). It is noteworthy that the high dose of 24-h DMI (3 μM) increased levels of CREB protein (F5,57 = 6.79; P < 0.01) (Fig. 2B). Inasmuch as it has been reported previously that long-term treatment with antidepressant drugs increased both CREB mRNA levels and CREB function in the hippocampus (Nibuya et al., 1996), our data indicate that CREB-mediated plasticity is complex and probably brain area-dependent (Carlezon et al., 2005).
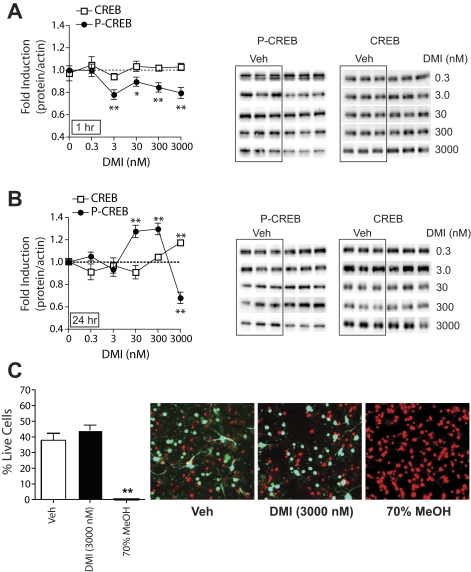
Fig. 2.
DMI affects basal CREB phosphorylation in primary cultures of NAc and striatal tissue. Levels of CREB (□) and P-CREB (•) were quantified by Western blot (representative blots at right). The ratios of CREB/β-actin or P-CREB/β-actin were determined for each sample and normalized to the control group ratio to yield a -fold induction. A, 1-h pretreatment with DMI altered P-CREB but not CREB expression. B, 24-h pretreatment with DMI altered both P-CREB and CREB expression. **, P < 0.01 compared with the corresponding vehicle (Veh) control, Fisher's protected t tests, n = 2 to 4 experiments per dose of DMI with treatments given in triplicate. C, 24-h pretreatment of primary cultures with DMI (3 μM) does not affect cell viability, whereas a 20-min treatment with 70% methanol results in 100% cell death. Representative images are shown from NAc/striatal cultures treated with the respective drugs. Dead cells fluoresce red with ethidium homodimer-1 and live cells fluoresce green with calcein. The number of green plus red cells was used as the total cell population, and data are expressed as the percentage of live cells (± S.E.M.). **, P < 0.01 compared with vehicle-treated cells, Fisher's protected t tests.
The decrease in P-CREB observed with 24-h DMI (3 μM) treatment could be due to cellular toxicity. High concentrations of DMI (20 mM) have been shown to induce cell death in a neural cell line (Post et al., 2000). Although the doses of DMI used in our studies did not approach this level, the propensity of DMI to cause toxicity in primary NAc and striatal cultures is unknown. We found that DMI had no effect on cell viability when cultures were treated for 24 h with the highest concentration of the drug (3 μM), whereas a brief treatment with 70% methanol caused 100% cell death (F2,6 = 35.4; P < 0.01) (Fig. 2C). Likewise, we observed no effects of 1-h DMI (3 μM) treatment on cell viability (data not shown).
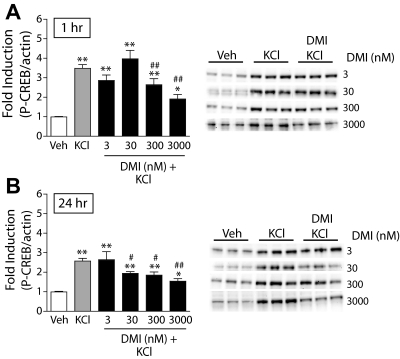
To examine whether DMI regulates CREB under conditions of heightened neuronal activation—similar to what may occur in the brain during periods of stress—we depolarized the cell cultures with KCl (40 mM). This has been shown to activate CRE (cAMP response element)-mediated gene transcription by influx of Ca2+ through L-type voltage-dependent Ca2+ channels (Sheng et al., 1990). Addition of KCl to the cultures induced robust increases in P-CREB, which were dose-dependently attenuated by both 1-h DMI pretreatment (F5,155 = 34.1; P < 0.01) (Fig. 3A; solid black bars) and 24-h DMI pretreatment (F5,128 = 20.4; P < 0.01) (Fig. 3B; solid black bars).
Fig. 3.
DMI reduces KCl-stimulated CREB phosphorylation in primary cultures of NAc and striatal tissue. The ratio of P-CREB/β-actin was determined for each sample and normalized to the control group ratio to yield a -fold induction. Representative Western blots are shown to the right of each panel. A, a 3-h treatment with KCl (40 mM) significantly increased P-CREB; a 1-h pretreatment with DMI dose dependently decreased KCl-stimulated P-CREB. B, likewise, a 24-h pretreatment with DMI decreased KCl-stimulated P-CREB. *, P < 0.05; **, P < 0.01 compared with vehicle (Veh). #, P < 0.05; ##, P < 0.01 compared with KCl alone. Fisher's protected t tests, n = 3 to 4 experiments per dose of DMI with treatments given in triplicate.
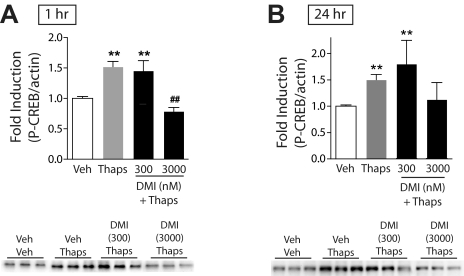
One of the ways in which DMI might block KCl-stimulated increases in P-CREB is by disruption of Ca2+ signaling. It has been shown previously that antidepressant drugs inhibit KCl- and voltage-stimulated calcium channel-induced increases in intracellular Ca2+ levels in neuronal cultures (Cai and McCaslin, 1992; Choi et al., 1992; Shimizu et al., 1994). We found that 1-h pretreatment with DMI had no effect on the induction of P-CREB by the L-type voltage-dependent Ca2+ channel activator FPL 64176 (F2,18 = 38.1; P < 0.01), NMDA (F2,26 = 13.4; P < 0.01), or the protein kinase C (PKC) activator PMA (F2,15 = 21.7; P < 0.01) (Table 1). In contrast, 1-h (F3,29 = 18.9; P < 0.01) pretreatment with DMI inhibited P-CREB induced by thapsigargin (100 nM; blocks uptake of Ca2+ into intracellular stores), and 24-h (F3,74 = 5.713; P < 0.01) pretreatment with DMI (3 μM) attenuated (trend, P = 0.091) thapsigargin-induced P-CREB (Fig. 4, A and B; black bars). Finally, 1-h pretreatment with DMI had no effect on P-CREB induced by the D1 agonist SKF 82958 (F2,9 = 13.9; P < 0.01) (Table 1), further suggesting a specific role for Ca2+ in mediating the effects of DMI.
TABLE 1.
Effect of 1-h DMI pretreatment on agonist-stimulated P-CREB
Values are means (± standard errors) from two to four separate experiments with treatments done in triplicate.
|
Agonist
|
-Fold Induction
|
||
|---|---|---|---|
| Vehicle | Agonist | DMI + Agonist | |
| SKF 82958 (50 μM) | 1.00 (0.092) | 1.86(0.077)** | 1.86(0.123)** |
| FPL 64176 (0.5 μM) | 1.00 (0.032) | 1.61(0.081)** | 1.77(0.052)** |
| PMA (0.1 μM) | 1.00 (0.031) | 1.92(0.115)** | 1.81(0.145)** |
| NMDA (10 μM) | 1.00 (0.044) | 1.86(0.199)** | 1.71(0.066)** |
P < 0.01 compared with corresponding vehicle group.
Fig. 4.
Effects of DMI on Ca2+-mediated signaling in primary cultures of NAc and striatal tissue. The ratio of P-CREB/β-actin was determined for each sample and normalized to the control group ratio to yield a -fold induction. Representative Western blots are shown below each panel. A, a 15-min treatment with thapsigargin (100 nM) significantly increased P-CREB; a 1-h pretreatment with DMI dose dependently blocked thapsigargin-induced P-CREB. B, 24-h pretreatment with DMI dose dependently decreased, but not significantly, thapsigargin-induced P-CREB. **, P < 0.01 compared with vehicle (Veh); ##, P < 0.01 compared with thapsigargin alone (Thaps). Fisher's protected t tests, n = 2 to 3 experiments with treatments given in triplicate.
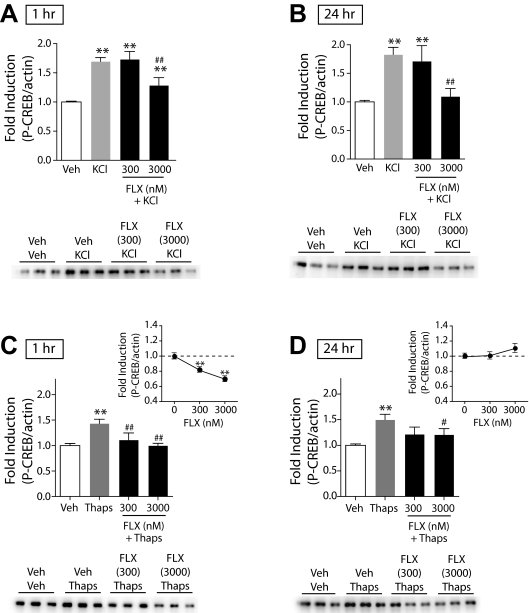
Effects of FLX on CREB Function in Dissociated NAc/Striatal Cultures. To determine whether the ability of DMI to reduce CREB function within the NAc and striatum through inhibition of signaling pathways activated by intracellular Ca2+ release was specific to DMI or generalizable to other classes of antidepressant drugs, we tested the effects of FLX, an SSRI, on KCl- and thapsigargin-stimulated P-CREB in primary cultures of the NAc and striatum. We found that both 1-h (F3,62 = 30.897; P < 0.01) and 24-h (F3,62 = 13.389; P < 0.01) pretreatments with FLX attenuated KCl-induced P-CREB (Fig. 5, A and B; black bars). Likewise, both 1-h (F3,44 = 10.428; P < 0.01) and 24-h (F3,83 = 7.032; P < 0.01) pretreatments with FLX inhibited thapsigargin-induced P-CREB (Fig. 5, C and D; black bars). Similar to our findings with DMI, 1-h FLX reduced basal P-CREB (Fig. 5C, inset) (F2,24 = 12.30; P < 0.001). Twenty-four-hour FLX, however, slightly increased basal P-CREB at the highest dose tested (Fig. 5D, inset) but did not show the bimodal effect on P-CREB observed with DMI (F2,27 = 1.25; ns). Similar to DMI, 1-h FLX had no effect on FPL 64176-induced P-CREB (data not shown). Hence, despite distinct pharmacological profiles, DMI and FLX both seem to act via a common mechanism within the NAc and striatum.
Fig. 5.
Effects of FLX on KCl- and thapsigargin-induced P-CREB in primary cultures of NAc and striatal tissue. The ratio of P-CREB/β-actin was determined for each sample and normalized to the control group ratio to yield a -fold induction. Representative Western blots are shown below each panel. A, a 3-h treatment with KCl (40 mM) significantly increased P-CREB; a 1-h pretreatment with FLX dose-dependently decreased KCl-stimulated P-CREB. B, likewise, a 24-h pretreatment with FLX decreased KCl-stimulated P-CREB. **, P < 0.01 compared with vehicle (Veh); ##, P < 0.01 compared with KCl alone. Fisher's protected t tests, n = 2 to 3 experiments with treatments given in triplicate. C, a 15-min treatment with thapsigargin (100 nM) significantly increased P-CREB; a 1-h pretreatment with FLX blocked thapsigargin-induced P-CREB. A 1-h pretreatment with FLX 1 h before Veh dose-dependently decreased basal P-CREB (inset). B, 24-h pretreatment with FLX also decreased thapsigargininduced P-CREB. A 24-h pretreatment with FLX 24 h before Veh had no significant effect on basal P-CREB (inset). **, P < 0.01 compared with vehicle (Veh); #, P < 0.05; ##, P < 0.01 compared with thapsigargin alone (Thaps), Fisher's protected t tests, n = 2 to 3 experiments with treatments given in triplicate.
Discussion
The NE reuptake inhibitor DMI decreases CREB function and prodynorphin mRNA expression in the NAc, supporting the hypothesis that increased CREB function in the NAc contributes to depressive-like behaviors (Carlezon et al., 2005). We demonstrate this in two distinct but complementary ways. First, we found that swim stress-induced activation of prodynorphin within the NAc is blocked by a DMI regimen that produces antidepressant-like effects in the FST. Second, we used primary cultures of embryonic NAc and striatal tissue to show that DMI reduced CREB function. The SSRI FLX had similar effects in studies designed to determine whether this in vitro effect was shared by other types of antidepressant drugs. These findings support behavioral and molecular evidence suggesting that activation of CREB within the NAc is critically involved in depressive-like behavior (Pliakas et al., 2001; Conti et al., 2002; Newton et al., 2002).
The observation that forced swimming increases prodynorphin expression within the NAc extends our previous finding that swim stress activates CREB in this region (Pliakas et al., 2001), and provides a potential explanation for why stressors might cause subsequent increases in depressive-like behavior. Dynorphin seems to play an important role in regulating mood via actions at KORs. There is substantial evidence that KORs are expressed on DA terminals within the NAc (Svingos et al., 2001). Stimulation of these receptors reduces mesolimbic system function and decreases levels of extracellular DA, effects thought to contribute to depressivelike states (Wise, 1982; Carlezon et al., 2006). Indeed, KOR agonists produce depressive-like effects (Bals-Kubik et al., 1993; Mague et al., 2003; Todtenkopf et al., 2004; Carlezon et al., 2006), whereas KOR antagonists have antidepressant-like effects (Pliakas et al., 2001; Newton et al., 2002; Mague et al., 2003), even when restricted to the NAc (Newton et al., 2002). Our finding that DMI blocks stress-induced activation of prodynorphin within the NAc provides new links among the antidepressant-like effects of this drug, the antidepressant-like effects of disrupting CREB function (and subsequent prodynorphin expression) in this region (Pliakas et al., 2001), and the antidepressant-like effects of NAc microinjections of KOR antagonists (Newton et al., 2002).
Effects of DMI and FLX in Vitro. To understand the mechanism by which DMI blocks swim stress-induced prodynorphin expression, we used dissociated cultures of NAc and striatal tissue to examine the effects of DMI on CREB and CREB phosphorylation—a marker of CREB activation (Mayr and Montminy, 2001). In humans and in animal models of depression, the therapeutic effects of antidepressant drugs occur primarily after prolonged administration. To be sensitive to this important issue, we assessed CREB function in response to 1 h (corresponds to short-term effects) and 24 h (corresponds to long-term effects) treatments. We show that both regimens of DMI treatment reduce baseline and KCl-stimulated CREB phosphorylation in primary cultures of NAc and striatal tissue. We demonstrate that DMI can have direct, NE-independent actions within this novel in vitro model that are not due to toxicity. Our data further support the hypothesis that DMI reduces CREB function and CREB-regulated target gene expression within the NAc. Considering that CREB protein levels remain unaltered by DMI at doses that significantly affect P-CREB, our in vivo finding that DMI reduces stress-activated prodynorphin expression within the NAc is probably due to attenuated CREB phosphorylation rather than reduced CREB levels.
Twenty-four-hour pretreatment with DMI had bimodal effects on basal P-CREB: intermediate doses of DMI increased P-CREB, whereas a high dose decreased it. This is consistent with the observation that DMI alone slightly increases CRE-mediated transcription in cultured cell lines (Schwaninger et al., 1995). However, it does not fit well with our in vivo finding that DMI reduces stress-activated prodynorphin expression within the NAc. Because these findings were from cell cultures in a basal or “normal” state, we induced depolarization in the cultures with KCl to more closely approximate physiological states induced by forced swim stress. In this putative model of stress, DMI dose dependently decreased KCl-induced P-CREB. These data are the first to demonstrate an antidepressant-induced reduction of CREB function in cultured neurons obtained directly from the NAc and striatum. Similar findings have been reported in engineered cell lines derived from non-neuronal sources (Schwaninger et al., 1995), indicating that DMI can attenuate the intracellular signaling events that lead to depolarization-induced P-CREB in a variety of cell phenotypes.
The ability of DMI to disrupt CREB function within the NAc tissue seems to involve, at least in part, effects on intracellular Ca2+ storage. DMI attenuates the ability of thapsigargin-induced increases in intracellular Ca2+ to phosphorylate CREB. Thapsigargin inhibits sarco-endoplasmic reticulum Ca2+-AT-Pase (SERCA) pumps, which actively pump Ca2+ into intracellular stores (SER). SERCA inhibition results in increased cytosolic Ca2+ levels, which in turn can trigger waves of Ca2+-induced Ca2+ release through activation of ryanodine receptors on the SER. The resultant calcium “waves” can activate calcium-regulated signal transduction pathways that lead to CREB phosphorylation. In our in vitro studies with NAc and striatal neuronal cultures, DMI had no effect on P-CREB stimulated by the L-type Ca2+ channel agonist FPL 64176 or NMDA, suggesting that DMI does not modulate Ca2+ entry through these specific ion channels. This is in apparent contrast to several previous studies showing that tricyclic antidepressants suppress Ca2+ entry through voltage-sensitive Ca2+ channels and inhibit KCl-stimulated increases in intracellular Ca2+ levels that originate from extracellular sources (Cai and McCaslin, 1992; Choi et al., 1992; Shimizu et al., 1994). However, DMI did not completely block KCl-stimulated P-CREB, which is consistent with a partial role for intracellular stores in CREB activation. In addition, our studies are unique in that we are examining P-CREB regulation rather than Ca2+ entry, and our findings suggest that DMI-mediated decreases in P-CREB depend, at least in part, on targeting of intracellular Ca2+ stores. We also show that DMI does not affect P-CREB stimulated by the PKC activator PMA. PKC is activated by Ca2+ and has been shown to phosphorylate CREB (Mao et al., 2007). In addition, it has been shown previously that DMI does not block CaM kinase IV-induced CRE-mediated gene transcription (Schwaninger et al., 1995). Finally, we show that DMI has no effect on P-CREB stimulated by the D1 receptor agonist SKF 82958, which is consistent with the previous finding that DMI did not alter forskolin-stimulated CRE-mediated gene transcription (Schwaninger et al., 1995). Together, these findings raise the possibility that the effects of DMI are not due to general inhibition of Ca2+- or cAMP-mediated signaling cascades but rather to direct actions at SERCA pumps or at receptors that control release of Ca2+ from the SER (e.g., ryanodine receptors).
Antidepressant drugs have been shown to inhibit P-CREB and CREB-mediated transcription in vitro (Schwaninger et al., 1995). Considering the present data demonstrating the ability of Ca2+-stimulating agents to increase P-CREB and the known ability to P-CREB to regulate prodynorphin (Cole et al., 1995), our finding that DMI inhibits Ca2+-mediated activation of CREB strongly suggests that prodynorphin expression would be inhibited in NAc/striatal tissue (as it is in vivo). However, there is some evidence in the literature that Ca2+ can have mixed effects on CREB-regulated gene expression. Work done in cultured cortical neurons has shown that Ca2+ can sometimes act to inhibit CREB-mediated transcription by parallel induction of phosphorylation at Ser142,143, which would tend to offset the stimulatory effects of phosphorylation at Ser133 (Kornhauser et al., 2002). In contrast, work done in culture spinal cord neurons has shown that Ca2+ can potentiate prodynorphin expression by binding to and removing the transcriptional repressor DREAM (Cheng et al., 2002). In addition, in cultured myocardial cells 4 h after KCl administration, prodynorphin expression is dramatically increased in a Ca2+-dependent manner (Ventura et al., 1994). Together, these findings indicate that Ca2+-stimulated signal transduction pathways are complex and can have a variety of effects on transcription that probably depend on cell type and brain region (see Carlezon et al., 2005).
We hypothesized that if the effects of DMI on intracellular Ca2+ function within the NAc contributes to the antidepressant actions of the drug, then other classes of antidepressants might have similar effects. We found that 1-h and 24-h treatments with FLX also attenuated KCl-stimulated P-CREB in the cell cultures. This effect can be attributed, at least in part, to a decrease in intracellular Ca2+ function: FLX reduced thapsigargin-stimulated P-CREB. Although DMI and FLX had similar effects on P-CREB, there were minor differences. One-hour, but not 24-hour, FLX reduced basal P-CREB levels in the absence of stimulation. In addition, KCl-stimulated P-CREB was less sensitive to FLX than DMI, whereas thapsigargin-stimulated P-CREB appeared more sensitive to FLX than DMI. These differences might be related to the pharmacokinetics of these drugs. In humans, steady-state serum concentrations of DMI and FLX can reach approximately 1000 and 1500 nM (Baldessarini, 2006), which are within the range of doses we have shown to have effects on P-CREB. It is possible that, relative to DMI, slightly higher concentrations of FLX are required to modulate basal P-CREB over the 24-h time course as well as attenuate KCl-stimulated P-CREB. Likewise, the mechanism of action of FLX might be more dependent on regulation of intracellular Ca2+. Future work might identify the mechanism by which these drugs act upon intracellular Ca2+ stores and determine whether this is a point of convergence for all antidepressants.
Conclusions
We identify a novel mechanism through which DMI has direct effects on Ca2+-mediated signaling that lead to decreased CREB function and prodynorphin expression within the NAc. These findings are congruent with evidence that KOR blockade and Ca2+ channel inhibitors produce antidepressant-like effects, and that inhibition of Ca2+ influx within the NAc has reward-facilitating effects (Pucilowski, 1992; Pliakas et al., 2001; Newton et al., 2002; Mague et al., 2003; Chartoff et al., 2006). They are also consistent with work demonstrating that deletion of CREB isoforms throughout the brain produces antidepressant-like effects (Conti et al., 2002), and activation of CREB-regulated target genes within the mesolimbic dopamine system contributes to induction of depressive-like states (Berton et al., 2006). Considering the growing sentiment that altered monoaminergic function alone is unlikely to explain depression or the therapeutic effects of antidepressants (Castrén, 2005; Nestler and Carlezon, 2006), our findings have important implications. First, the therapeutic effects of antidepressants including DMI might involve diminished activity of CREB-regulated target genes within the NAc. Second, such effects may occur independently of—or in addition to—their ability to regulate monoaminergic systems. These data provide early evidence that antidepressants produce some of their therapeutic effects directly within the NAc.
Acknowledgments
We thank S. Mague for technical assistance and A. Dow for comments on the manuscript.
This work was supported by the National Institutes of Health National Institute of Mental Health [Grant MH063266] and donations from John A. Kaneb.
ABBREVIATIONS: DA, dopamine; NAc, nucleus accumbens; NE, norepinephrine; CREB, cAMP response element binding protein; FST, forced swim test; KOR, κ-opioid receptors; E18, embryonic day 18; DMI, desipramine hydrochloride; FLX, fluoxetine hydrochloride; SKF 82958, (±)-6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetra-hydro-1H-benzazepine; NMDA, N-methyl-d-aspartic acid; FPL 64176, methyl 2,5-dimethyl-4-(2-(phenylmethyl)benzoyl)-1H-pyrrole-3-carboxylate; PMA, phorbol 12-myristate 13-acetate; FS, forced swim; Q-PCR, quantitative real-time reverse transcriptase polymerase chain reaction; PBS, phosphate-buffered saline; PBS-T, PBS/0.1% Tween 20; P-CREB, phospho-CREB; ANOVA, analysis of variance; PKC, protein kinase C; SERCA, sarcoendoplasmic reticulum calcium-ATPase; SSRI, selective serotonin reuptake inhibitor.
References
- American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders, 4th ed. rev. American Psychiatric Press, Washington DC.
- Baldessarini RJ (2006) Drug therapy of depression and anxiety disorders, in The Pharmacological Basis of Therapeutics (Brunton LL, Lazo JS and Parker KL eds) pp 429-459, McGraw-Hill, New York.
- Bals-Kubik R, Ableitner A, Herz A, and Shippenberg TS (1993) Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther 264 489-495. [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, et al. (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311 864-868. [DOI] [PubMed] [Google Scholar]
- Cai Z and McCaslin PP (1992) Amitriptyline, desipramine, cyproheptadine and carbamazepine, in concentrations used therapeutically, reduce kainate- and N-methyl-d-aspartate-induced intracellular Ca2+ levels in neuronal culture. Eur J Pharmacol 219 53-57. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr, Béguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, and Cohen BM (2006) Depressive-like effects of the kappa-opioid receptor agonist salvinorin a on behavior and neurochemistry in rats. J Pharmacol Exp Ther 316 440-447. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr, Duman RS, and Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28 436-445. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, and Nestler EJ (1998) Regulation of cocaine reward by CREB. Science 282 2272-2275. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Pliakas AM, Parow AM, Detke MJ, Cohen BM, and Renshaw PF (2002) Antidepressant-like effects of cytidine in the forced swim test in rats. Biol Psychiatry 51 882-889. [DOI] [PubMed] [Google Scholar]
- Castrén E (2005) Is mood chemistry?. Nat Rev Neurosci 6 241-246. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Papadopoulou M, Konradi C, and Carlezon WA Jr (2003) Dopamine-dependent increase in phosphorylation of cAMP response element binding protein (CREB) during precipitated morphine withdrawal in primary cultures of rat striatum. J Neurochem 87 107-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Pliakas AM, and Carlezon WA Jr (2006) Microinjection of the L-type calcium channel antagonist diltiazem into the ventral nucleus accumbens shell facilitates cocaine-induced conditioned place preferences. Biol Psychiatry 59 1236-1239. [DOI] [PubMed] [Google Scholar]
- Chavkin C, James IF, and Goldstein A (1982) Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 215 413-415. [DOI] [PubMed] [Google Scholar]
- Cheng HY, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, et al. (2002) DREAM is a critical transcriptional repressor for pain modulation. Cell 108 31-43. [DOI] [PubMed] [Google Scholar]
- Choi JJ, Huang GJ, Shafik E, Wu WH, and McArdle JJ (1992) Imipramine's selective suppression of an L-type calcium channel in neurons of murine dorsal root ganglia involves G proteins. J Pharmacol Exp Ther 263 49-53. [PubMed] [Google Scholar]
- Cole RL, Konradi C, Douglass J, and Hyman SE (1995) Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron 14 813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti AC, Cryan JF, Dalvi A, Lucki I, and Blendy JA (2002) cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci 22 3262-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Markou A, and Lucki I (2002) Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23 238-245. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, and Aston-Jones GS (1998) Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res 806 127-140. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, and Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 121 66-72. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, and Pollock KM (1994) Identification of multiple DNA elements regulating basal and protein kinase A-induced transcriptional expression of the rat prodynorphin gene. Mol Endocrinol 8 333-344. [DOI] [PubMed] [Google Scholar]
- Frazer A (1997) Pharmacology of antidepressants. J Clin Psychopharmacol 17 (Suppl 1): 2S-18S. [DOI] [PubMed] [Google Scholar]
- Harris GC and Aston-Jones G (1994) Involvement of D2 dopamine receptors in the nucleus accumbens in the opiate withdrawal syndrome. Nature 371 155-157. [DOI] [PubMed] [Google Scholar]
- Kornhauser JM, Cowan CW, Shaywitz AJ, Dolmetsch RE, Griffith EC, Hu LS, Haddad C, Xia Z, and Greenberg ME (2002) CREB transcriptional activity in neurons is regulated by multiple, calcium-specific phosphorylation events. Neuron 34 221-233. [DOI] [PubMed] [Google Scholar]
- MacDonald ML, Eaton ME, Dudman JT, and Konradi C (2005) Antipsychotic drugs elevate mRNA levels of presynaptic proteins in the frontal cortex of the rat. Biol Psychiatry 57 1041-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC Jr, Jones RM, Portoghese PS, and Carlezon WA Jr (2003) Antidepressant-like effects of κ-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther 305 323-330. [DOI] [PubMed] [Google Scholar]
- Mao LM, Tang Q, and Wang JQ (2007) Protein kinase C-regulated cAMP response element-binding protein phosphorylation in cultured rat striatal neurons. Brain Res Bull 72(4-6) 302-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr B and Montminy M (2001) Transcriptional regulation by the phosphorylationdependent factor CREB. Nat Rev Mol Cell Biol 2 599-609. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, and Chavkin C (2003) Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci 23 5674-5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, and Monteggia LM (2002) Neurobiology of depression. Neuron 34 13-25. [DOI] [PubMed] [Google Scholar]
- Nestler EJ and Carlezon WA Jr (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59 1151-1159. [DOI] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve R, Nestler EJ, and Duman RS (2002) Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci 22 10883-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, and Duman RS (1996) Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci 16 2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier DA, Kemper TL, Forbes WB, and Morgane PJ (1977) Dorsal raphe, substantia nigra and locus coeruleus: interconnections with each other and the neostriatum. Brain Res Bull 2 323-339. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, and Emrich HM (1986) Psychotomimesis mediated by kappa opiate receptors. Science 233 774-776. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, and Carlezon WA Jr (2001) Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci 21 7397-7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post A, Crochemore C, Uhr M, Holsboer F, and Behl C (2000) Differential induction of NF-kappaB activity and neural cell death by antidepressants in vitro. Eur J Neurosci 12 4331-4337. [DOI] [PubMed] [Google Scholar]
- Pucilowski O (1992) Psychopharmacological properties of calcium channel inhibitors. Psychopharmacology (Berl) 109 12-29. [DOI] [PubMed] [Google Scholar]
- Schwaninger M, Schöfl C, Blume R, Rössig L, and Knepel W (1995) Inhibition by antidepressant drugs of cyclic AMP response element-binding protein/cyclic AMP response element-directed gene transcription. Mol Pharmacol 47 1112-1118. [PubMed] [Google Scholar]
- Sheng M, McFadden G, and Greenberg ME (1990) Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron 4 571-582. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Nishida A, Fukuda H, Saito H, and Yamawaki S (1994) Inhibitory effect of imipramine on depolarization-induced increases in intracellular Ca2+ of rat cortical neurons. Eur J Pharmacol 268 65-71. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS and Herz A (1987) Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of mu- and kappaopioid agonists. Brain Res 436 169-172. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Chavkin C, Colago EE, and Pickel VM (2001) Major coexpression of kappa-opioid receptors and the dopamine transporter in nucleus accumbens axonal profiles. Synapse 42 185-192. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, and Carlezon WA Jr (2004) Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 172 463-470. [DOI] [PubMed] [Google Scholar]
- Treiman M, Caspersen C, and Christensen SB (1998) A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases. Trends Pharmacol Sci 19 131-135. [DOI] [PubMed] [Google Scholar]
- Ventura C, Guarnieri C, Vaona I, Campana G, Pintus G, and Spampinato S (1994) Dynorphin gene expression and release in the myocardial cell. J Biol Chem 269 5384-5386. [PubMed] [Google Scholar]
- Wise RA (1982) Neuroleptics and operant behavior: the anhedonia hypothesis. Behav Brain Sci 5 39-87. [Google Scholar]
- Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5 483-494. [DOI] [PubMed] [Google Scholar]