Abstract
Most cellular functions, including signaling by G protein-coupled receptors (GPCRs), are mediated by protein-protein interactions, making the identification and localization of protein complexes key to the understanding of cellular processes. In complement to traditional biochemical techniques, noninvasive resonance energy transfer (RET) and protein-fragment complementation assays (PCAs) now allow protein interactions to be detected in the context of living cells. In this review, fluorescent and bioluminescent PCAs are discussed and their application illustrated with studies on GPCR signaling. Newly developed techniques combining PCA and RET assays for the detection of ternary and quaternary protein complexes are also presented.
The identification and localization of protein-protein interactions is central to the understanding of biological processes such as extracellular signal integration, gene expression regulation, as well as most cellular metabolic pathways. Immunoprecipitation (IP) and pull-down assays have been used extensively to demonstrate protein-protein interactions. Although these techniques remain very useful, they necessitate the disruption of biological samples and the solubilization of membrane proteins, and they generally provide little information on the subcellular localization of the protein complexes (Milligan and Bouvier, 2005). Fluorescence and bioluminescence resonance energy transfer (FRET and BRET), as well as reporter complementation assays, are noninvasive approaches that overcome some limitations of classic biochemical techniques. They allow the examination of protein-protein interactions in their native context: in living cells or even in living animals. Moreover, information on the subcellular localization of the interaction can be gained with microscopic FRET or bimolecular fluorescence complementation (BiFC) analysis.
Multiple protein-protein interactions play essential roles in signaling events mediated by GPCRs. Oligomerization between GPCRs is now recognized to modulate the pharmacological characteristics of the receptors and influence their coupling to G proteins (Fuxe et al., 2007; Milligan, 2007; Pin et al., 2007). The interaction between GPCRs and G proteins is also well documented. GPCR activation upon ligand binding induces conformational changes in the receptor structure that promotes a GDP-to-GTP nucleotide exchange to the α subunit of the interacting trimeric G protein. GTP-bound Gα subunits then dissociate from (or change conformation relative to) the receptor and the β/γ subunits, allowing interactions between Gα as well as Gβ/γ with effector proteins (Lambert, 2008). In contrast to early models in which individual components of the system were envisioned as free-diffusing and random-colliding (see Rebois and Hébert, 2003, and references therein), other evidence indicates the existence of signaling complexes comprising GPCRs, G proteins, and effector proteins such as adenylyl cyclase, phospholipase C, or ion channels (Rebois and Hébert, 2003; Galés et al., 2006; Rebois et al., 2006). However, the extent to which these observations may be generalized to the highly diverse repertoire of GPCR signaling remains to be determined. This review focuses on the recent application of PCAs to study GPCR oligomerization as well as interactions involving effector or regulatory proteins. Although the principle of RET techniques is not covered (for reviews, see Marullo and Bouvier, 2007; Gandia et al., 2008), new methods combining PCAs and RET techniques for the detection of multiprotein complexes are discussed.
Principle of Fluorescent and Luminescent PCAs
PCAs have been developed in which fluorescent or luminescent protein fragments are used for complementation. These assays are also known as bimolecular fluorescence/luminescence complementation (BiFC/BiLC). They each rely on the ability of nonfluorescent/luminescent protein fragments to reconstitute functional fluorescent/luminescent proteins when brought in close contact by fusion partners (Hu et al., 2006; Kerppola, 2006; Remy and Michnick, 2007; Shyu and Hu, 2008). Several fluorescent proteins (FPs) have been successfully split and used in BiFC assays (Shyu et al., 2006; Fan et al., 2008) (Fig. 1A, Table 1). These include Venus (Nagai et al., 2002), Cerulean (Rizzo et al., 2004), and mCherry (Shaner et al., 2004). The BiFC technique is very straightforward in that protein-protein interactions are revealed by simple fluorescence intensity measurements in live cells or in live animals (Shyu et al., 2008a). These may be performed using fluorescence-activated cell sorting (Giese et al., 2005; Watanabe et al., 2008), fluorometry (Hynes et al., 2008; Vidi et al., 2008a), or microscopy (e.g., Hu et al., 2002), the latter providing insights into the subcellular localization of the interacting proteins.
Fig. 1.
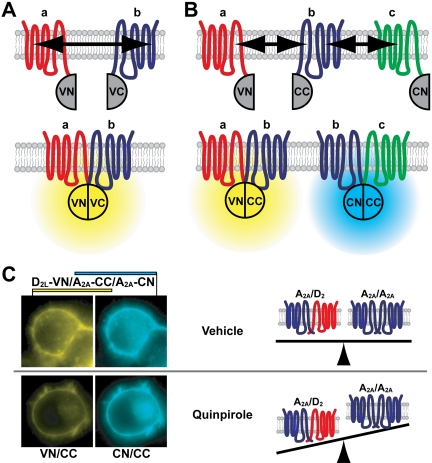
Application of BiFC to monitor GPCR interactions. A, BiFC principle. FP fragments (e.g., Venus N- and C-termini, VN and VC) are fused to the carboxyl-termini of the receptors (a and b). Upon interaction of the tagged receptors, the protein fragments reconstitute a functional FP. B, multicolor BiFC principle. Different receptor interactions lead to the complementation of distinct fluorescent protein variants. Complex formation between receptor a (tagged with VN) and b (tagged with the C-terminal moiety of Cerulean, CC) reconstitutes a yellow fluorescent protein, whereas interaction between b and c reconstitutes Cerulean (see Kerppola, 2006, for detailed protocols and general technical considerations). C, application of multicolor BiFC to the simultaneous detection of A2A homomers and A2A/D2 heteromers in neuronal cells. Prolonged treatment with the D2 agonist quinpirole resulted in decreased Venus (VN/CC) over Cerulean (CN/CC) fluorescence, interpreted as an alteration in GPCR homo-/heteromer abundance (right). [Adapted from Vidi PA, Chemel BR, Hu CD, and Watts VJ (2008a) Ligand-dependent oligomerization of dopamine D2 and adenosine A2A receptors in living neuronal cells. Mol Pharmacol 74:544-551. Copyright © 2008 American Society of Pharmacology and Experimental Therapeutics. Used with permission.]
TABLE 1.
Fluorescent and bioluminescent protein fragments used in PCA assays
| Protein | Split Position | Ex/Em | References | |
|---|---|---|---|---|
| nm | ||||
| BiFC | GFP | 157-158 | 485/500 | Ghosh et al., 2000 |
| YFP, Citrine, Venus | 154-155; 172-173 | 515/528 | Hu et al., 2002; Shyu et al., 2006 | |
| CFP, Cerulean | 154-155; 172-173 | 452/478 | Hu et al., 2002; Shyu et al., 2006 | |
| Venus/Cerulean | 154-155; 172-173 | 504/513 | Hu and Kerppola, 2003; Shyu et al., 2006 | |
| mRFP1-Q66T | 154-155; 168-169 | 549/570 | Jach et al., 2006 | |
| mCherry | 159-160 | 587/610 | Fan et al., 2008 | |
| BiLC | RLuc | 110-111 | Ch/485 | Stefan et al., 2007 |
| GLuc | 93-94 | Ch/480 | Remy and Michnick, 2006 |
Ex/Em, excitation/emission wavelengths; Ch, coelenterazine h
Similar to other recombinant protein tagging approaches, the addition of the BiFC/BiLC fragments may disrupt or alter the function and/or localization of the fusion partner. It is therefore essential to test these possibilities with functional assays (e.g., by measuring second messenger levels upon ligand exposure in the case of GPCR-BiFC/BiLC fusions) and by comparing the subcellular localization of tagged versus untagged proteins. FP complementation has proven to be irreversible so far, and it seems that BiFC essentially locks the protein-protein interaction (Hu et al., 2002). Although this may be an advantage in some cases, it is also a major limitation of the technique because dynamic changes in protein complexes can not be examined. Therefore, when designing experiments in which BiFC is combined with other methods to detect multiple proteins in a complex (see below), BiFC is preferable for the detection of the more stable interaction. Another potential pitfall is that in some conditions certain FP fragments may reconstitute a FP without an interaction of their fusion partners. To rule out the possibility of nonspecific interactions, negative controls included in BiFC experiments need therefore to be carefully chosen (see http://sitemaker.umich.edu/kerppola.lab/kerppola.bifc/bifc_protocols_). For example, transfections omitting one of the two FP-fragment fusion proteins may serve to determine background fluorescence, but this does not indicate specificity of BiFC signals. As a suitable negative control, one of the FP-fragment fusion proteins should be replaced by a functional protein with subcellular distribution and expression levels similar to that of the tested protein.
Several unique split positions have been used for YFP and CFP (or their enhanced versions; see Table 1) and a similar effectiveness of complementation is achieved with different FP fragment combinations (e.g., VN155/VC155, VN173/VC173, or N173/C155) (Shyu et al., 2006). The intensity of the fluorescence complementation signal, however, is consistently weaker than the signal from the corresponding full-length FP under similar transfection conditions, typically 2.5- to 5.5-fold (unpublished observations). This reduction in signal intensity may hinder the detection of receptor-BiFC fusions that are not expressed at sufficient levels. Finally, the composition and length of the linker sequence separating the protein of interest and the FP fragment, as well as the design of the assay (which fragment fused to which partner), may affect the efficiency of fluorescence complementation (Chen et al., 2006; unpublished data).
Multicolor BiFC assays, in which two FPs with distinct excitation and emission spectra are reconstituted, have been implemented for the detection of multiple protein-protein interactions (Hu and Kerppola, 2003). N-terminal BiFC fragments from green fluorescent protein-derived FPs contain the chromophore, as well as most amino acid residues important for the spectral properties of the FPs (with the notable exception of residue 203 in the C-terminal region). As a consequence, N-terminal fragments from two different FPs, upon complementation with a C-terminal FP fragment (e.g., Cerulean C terminus), reconstitute FPs with distinct excitation and emission maxima, which, as illustrated in Fig. 1B, allows the simultaneous detection of two protein-protein interactions in live cells. Complementation between Venus N-terminal and Cerulean C-terminal fragments (VN/CC) results in green-shifted FPs compared with Venus (Table 1). The absence of tyrosine at position 203, characteristic of Venus and other yellow-shifted green fluorescent protein-variants (Tsien, 1998), in VN/CC is likely to be responsible for this spectral shift.
Luminescence complementation is analogous to BiFC in that split, nonluminescent fragments from Renilla reniformis or Gaussia princeps luciferases (RLuc and GLuc) reconstitute luminescent proteins upon interaction of their fusion partners (Remy and Michnick, 2006; Stefan et al., 2007) (Table 1). In contrast to BiFC, BiLC biosensors do not assemble irreversibly; thus the association as well as the dissociation of protein complexes may be detected (Remy and Michnick, 2006; Stefan et al., 2007). Moreover, because of its low background, the technique is well suited for the detection of protein interactions in living animals (Paulmurugan and Gambhir, 2007). In contrast to BiFC, however, BiLC does not currently provide information on the subcellular localization of protein complexes.
Application of PCA to Study GPCR Oligomerization and Interactions with Modulators
In recent applications of PCAs to GPCR interactions, FP fragments have been genetically fused to the carboxyl end of the receptors (Fig. 1A). Using this approach, homo-oligomers of the α1b adrenoceptor (AR) were detected, consistent with FRET and IP results (Lopez-Gimenez et al., 2007). It is noteworthy that point mutations in the transmembrane domains I and IV of the α1b AR (predicted to constitute the dimer interface) prevented export to the plasma membrane without completely suppressing α1b AR dimerization as monitored by BiFC, in line with the notion that GPCR oligomerization occurs during their synthesis (Bulenger et al., 2005).
The well studied interaction (Fuxe et al., 2007) between the adenosine A2A and the dopamine D2 receptors was also recently examined using BiFC (Vidi et al., 2008a). In this case, multicolor BiFC allowed the simultaneous detection of A2A and D2 homo- and heteromers in neuronal cells. Quantification of fluorescent signals at the cell surface or in intracellular compartments revealed the coexistence and colocalization of A2A/D2 heteromers and A2A homomers. A ratiometric approach was used to quantitate changes in GPCR oligomerization, and it was observed that the relative abundance of A2A/D2 heteromers and A2A or D2 homomers was influenced by prolonged stimulation of either receptor (Vidi et al., 2008a) (Fig. 1C).
Proteins interacting with GPCRs have been shown to modulate receptor expression, membrane targeting, and desensitization. Among these, arrestins are well characterized scaf-folding proteins that are notably involved in receptor internalization after ligand activation (Gurevich et al., 2008). As part of a high-content PCA study based on FP complementation, interaction between β-arrestins and the β2 AR was detected in cells treated with β2 AR agonists, thus providing a measure of receptor activation and internalization (MacDonald et al., 2006). These studies further demonstrate the applicability of fluorescent PCAs for the detection of drug-induced changes in GPCR interactions. Likewise, nonfluorescent PCAs based on β-galactosidase fragment complementation (PathHunter β-Arrestin Assay; DiscoveRx Corporation, Fremont, CA) can also be used for the detection of GPCR-β-arrestin interactions.
Single transmembrane domain receptor activity-modifying proteins (RAMPs) are essential modulators of the calcitonin receptor-like receptor (CRLR) functional activity. The association between RAMP1 and CRLR produces the calcitonin gene-related peptide receptor. Both RAMP1 and CRLR homomers were shown to accumulate at the endoplasmic reticulum (ER) in BiFC assay, whereas CRLR/RAMP1 heteromers were localized at the plasma membrane (Héroux et al., 2007), consistent with the involvement of RAMP1 in receptor trafficking (McLatchie et al., 1998). This work highlights the power of fluorescent PCAs to identify the subcellular localization of protein-protein interactions. Further insight into the composition of the calcitonin gene-related peptide receptor was gained by combining BiFC with BRET (see below).
PCA as Measure of G Protein Subunit Composition and Trafficking
The functional roles of G proteins in GPCR recognition and effector regulation are largely determined by their α subunits. In addition, specific combinations among the 5 mammalian G protein β and 12 γ subunits may be required for certain GPCR signaling events (Robishaw and Berlot, 2004). Further insight into G protein subcellular localization and subunit association preferences was recently gained with the application of BiFC and multicolor BiFC techniques (see Hynes et al., 2008, for methodological aspects). Dynamic trafficking studies of GPCRs and G protein subunits revealed that both the β2 AR and the Gαs/β1/γ7 complex are internalized as a consequence of receptor activation; however, the mechanisms and vesicular compartments to which they were trafficked differed (Hynes et al., 2004). The requirement of G protein subunit association for plasma membrane targeting was also addressed using BiFC. Coexpression of BiFC-tagged β1/γ7 dimers lead to plasma membrane localization of CFP-tagged Gαs that otherwise accumulated in the cytosol (Hynes et al., 2004; Mervine et al., 2006). Conversely, expression of Gαo or Gαq was required for plasma membrane targeting of BiFC-tagged β5/γ2 dimers (Yost et al., 2007).
The formation and composition of β/γ complexes was also analyzed using BiFC. Different efficiencies of β5 complex formation with different γ subunits were observed that correlated with signal transduction efficiencies as measured by phospholipase C activity (Yost et al., 2007). The β5/γ2 interaction was found to be the strongest, a finding corroborated with multicolor BiFC competition experiments used to measure the association preference of β and γ subunits (Yost et al., 2007). A similar approach was taken to examine interactions between G protein subunits and alternative binding partners such as the R7 family regulator of G protein signaling 7 (RGS7). A preference of β5 for γ2 over RGS7 was observed that was abolished upon coexpression of the R7 family binding protein (R7BP) (Yost et al., 2007). These experiments highlight the usefulness of BiFC for the detection of less stable protein complexes such as β5/γ2 and to address the role of coexpressed proteins on G protein subunit association.
Combining Techniques to Monitor Interactions between Multiple Proteins
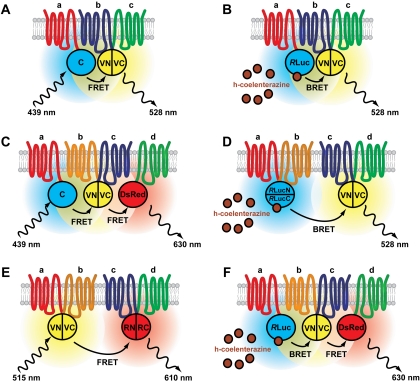
A large number of protein-protein interactions involving GPCRs have been documented, but little is known about the relative stoichiometry of GPCR oligomers or GPCR/G protein/effector complexes. Whereas only two interacting proteins can be detected with most established methods, combining them allows in principle the detection of multiple proteins within a complex. YFP, which has frequently been used in BiFC assays, also constitutes a very popular FRET pair with CFP and serves as an acceptor for RLuc in BRET assays, thus allowing BiFC and RET assays to be combined (Fig. 2, A and B). In BiFC-BRET assays (Rebois et al., 2006), RET between RLuc and complemented YFP indicates a very close proximity of three tagged proteins that can be interpreted as a trivalent protein complex. Likewise, BiFC can be combined with FRET (Shyu et al., 2008b); in this case, the subcellular localization of trivalent complexes can be determined using microscopic FRET measurements.
Fig. 2.
Combination of PCA and RET techniques. Trimeric protein complexes (a, b, c) detected by BiFC-FRET (A) or BiFC-BRET (B). In both approaches, a reconstituted FP serves as donor or acceptor in a RET pair. In this case, complemented Venus serves as acceptor for Cerulean (A) or RLuc (B). Coelenterazine is used as a substrate for RLuc. Maximum excitation and emission wavelengths are indicated. C to F, experimental approaches to detect tetrameric protein complexes (a, b, c, d; see text for examples). Complemented fluorescent (or luminescent) proteins replace a donor or acceptor in sequential FRET (C) or BRET-FRET (F) assays. Bioluminescence (D) or fluorescence (E) resonance energy transfer between two complemented luminescent or fluorescent proteins also allows the detection of tetramers. The approaches illustrated in C, E, and F are theoretical and, to our knowledge, have not yet been implemented.
A BiFC-BRET approach implemented by the Bouvier lab was used to examine the composition of CRLR/RAMP1 complexes and provided evidence for the association of a single RAMP1 molecule with a CRLR homomer (Héroux et al., 2007). BiFC-BRET measurements also demonstrated the existence of A2A homo-oligomers with at least three receptors (Gandia et al., 2008). Subsequent localization studies used BiFC-FRET to demonstrate that higher-order oligomers of A2A receptors occur at their site of action, the plasma membrane (Vidi et al., 2008b). Moreover, inhibiting anterograde protein transport from the ER to the Golgi apparatus by treatment with brefeldin A or by expression of a dominant-negative SarI GTPase led to the accumulation of A2A dimers but not of higher A2A oligomers in intracellular compartments (unpublished observations). These observations suggest the possibility that GPCR dimers are trafficked from the ER to the plasma membrane followed by higher-order oligomerization at the plasma membrane.
Sequential RET approaches have been developed as an alternative to BiFC-RET and applied to GPCR oligomerization. In these approaches, the acceptor from a first RET pair serves as donor for a second RET pair (Fig. 2, C and F). By implementing a three-chromophore FRET (3-FRET) protocol (Galperin et al., 2004), Lopez-Gimenez et al. (2007) showed that recombinant α1b AR exists as higher-order oligomers in HEK293 cells. Likewise, complexes of A2A, D2, and cannabinoid CB1 receptors were identified using sequential BRET-FRET assays (Carriba et al., 2008).
A proof of concept for the detection of tetravalent protein complexes with a BiFC-BiLC combination (Fig. 2D) was given by Rebois et al. (Rebois et al., 2008): the β2 AR was fused to N- or C-terminal fragments from Venus or GLuc, and BRET signals were detected in cells expressing the four-receptor fusion constructs (Rebois et al., 2008). A similar approach was used to demonstrate D2 homo-oligomeric complexes with four protomers (Guo et al., 2008), a finding supported by modeling of the two interaction interfaces and confirmed by cross-linking experiments (Guo et al., 2008). In line with earlier pharmacological and biochemical data (Wreggett and Wells, 1995; Nimchinsky et al., 1997; Lee et al., 2003; Park and Wells, 2004), these results suggest that higher-order GPCR oligomers, likely constituted of four protomers, may be a general rule. The physiological and pharmacological significance of tetrameric GPCR complexes, however, remains largely to be determined.
New evidence for GPCR signaling complexes has also emerged from recent BiFC-BRET studies. The assembly of trimeric G proteins was shown in cells expressing Gαi1-RLuc and Gβ1/Gγ2 fusions to YFP fragments (Dupré et al., 2006). Likewise, the association of the type II adenylyl cyclase (ACII) or the inward-rectifier potassium channel Kir3.1 with Gβ1/γ2 was demonstrated in cells expressing ACII-RLuc or Kir3.1-RLuc and Gβ1/γ2 fusions to YFP fragments (Rebois et al., 2006; Rebois et al., 2008). In cells expressing ACII-RLuc and Gβ1/γ2 fusions to YFP fragments, activation of cotransfected β2 AR resulted in stronger BRET signals, possibly reflecting conformational changes within the complex (Rebois et al., 2006). The association between a GPCR, a G protein subunit, and an effector was also revealed in BiFC-BRET assays using cells coexpressing ACII-RLuc with FP-fragment fusions to β2 AR and Gγ2 (Rebois et al., 2008). These experiments highlight the use of BiFC-BRET as a novel method to study and examine the existence of GPCR signaling complexes.
Despite the strengths of the studies described above, some caution is warranted in the interpretation of results from PCA, RET, or combined assays for a variety of reasons. All of these approaches are proximity indicators and do not provide direct proof of physical contact between proteins. Such methods do not define the total number of interacting proteins, leaving open the possibility of higher-order oligomers. Finally, in combined RET/PCA assays, increasing the number of interacting partners increases the number of combinations for possible interactions (many of which may be “nonproductive”; i.e., not leading to RET or PCA readouts) and possibly weaker overall RET/PCA signals. This consideration highlights the need for more sensitive RET/PCA techniques and the importance to develop brighter and more stable fluorescent/luminescent proteins.
Conclusions and Outlook
Recent studies applying PCA to GPCR signaling highlight the usefulness of the technique and new investigations using PCA, alone or in combination with other approaches are soon to follow. As discussed above, PCA can be readily combined with RET measurements, but also with fluorescence correlation spectroscopy (Briddon et al., 2008), or with biochemical techniques such as IP (Héroux et al., 2007). In addition, a score of unexplored possibilities can be envisaged. The incorporation of BiFC-tagged proteins in sequential RET experiments would allow the detection of tetrameric complexes (Fig. 2, C and F). Moreover, dynamic interactions between GPCR dimers and their localization may be followed with a BiFC-BiFC-FRET experimental setup (Fig. 2E). We also predict the usefulness of BiFC and multicolor BiFC, not only for the visualization and quantification of multiple GPCR interactions (Vidi et al., 2008a) but also for the isolation of oligomeric complexes, because the complementation of fluorescent proteins is likely to stabilize protein-protein interactions (Hu et al., 2002). These efforts are likely to bring new insights into the functional and pharmacological significance of GPCR interactions. Finally, we anticipate a growing importance of PCAs in drug screening efforts (MacDonald et al., 2006) and propose that multicolor BiFC assays may be used to screen for compounds that selectively alter the composition of GPCR oligomers in living cells.
Acknowledgments
We acknowledge Dr. Chang-Deng Hu, Julie A. Przybyla, and the reviewers for providing helpful comments on the manuscript.
This work was supported by the National Institutes of Health National Institute of Mental Health [Grant MH060397] and by Purdue University.
ABBREVIATIONS: FRET, fluorescence (Förster) resonance energy transfer; BRET, bioluminescence resonance energy transfer; BiFC, bimolecular fluorescence complementation; GPCR, G protein-coupled receptor; PCA, protein complementation assay; RET, resonance energy transfer; BiLC, bimolecular luminescence complementation; FP, fluorescent protein; YFP, yellow fluorescent protein; CFP, cyan fluorescent protein; VN, Venus N-terminal fragment; VC, Venus C-terminal fragment; CN, Cerulean N-terminal fragment; CC, Cerulean C-terminal fragment; AR, adrenoceptor; IP, Immunoprecipitation; CRLR, calcitonin receptor-like receptor; ER, endoplasmic reticulum; RAMP, receptor activity-modifying protein; ACII, type II adenylyl cyclase.
References
- Briddon SJ, Gandía J, Amaral OB, Ferré S, Lluís C, Franco R, Hill SJ, and Ciruela F (2008) Plasma membrane diffusion of g protein-coupled receptor oligomers. Biochim Biophys Acta 1783 2262-2268. [DOI] [PubMed] [Google Scholar]
- Bulenger S, Marullo S, and Bouvier M (2005) Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci 26 131-137. [DOI] [PubMed] [Google Scholar]
- Carriba P, Navarro G, Ciruela F, Ferré S, Casadó V, Agnati L, Cortés A, Mallol J, Fuxe K, Canela EI, et al. (2008) Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat Methods 5 727-733. [DOI] [PubMed] [Google Scholar]
- Chen CD, Oh SY, Hinman JD, and Abraham CR (2006) Visualization of APP dimerization and APP-Notch2 heterodimerization in living cells using bimolecular fluorescence complementation. J Neurochem 97 30-43. [DOI] [PubMed] [Google Scholar]
- Dupré DJ, Robitaille M, Ethier N, Villeneuve LR, Mamarbachi AM, and Hébert TE (2006) Seven transmembrane receptor core signaling complexes are assembled prior to plasma membrane trafficking. J Biol Chem 281 34561-34573. [DOI] [PubMed] [Google Scholar]
- Fan JY, Cui ZQ, Wei HP, Zhang ZP, Zhou YF, Wang YP, and Zhang XE (2008) Split mCherry as a new red bimolecular fluorescence complementation system for visualizing protein-protein interactions in living cells. Biochem Biophys Res Commun 367 47-53. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Canals M, Torvinen M, Marcellino D, Terasmaa A, Genedani S, Leo G, Guidolin D, Diaz-Cabiale Z, Rivera A, et al. (2007) Intramembrane receptor-receptor interactions: a novel principle in molecular medicine. J Neural Transm 114 49-75. [DOI] [PubMed] [Google Scholar]
- Galés C, Van Durm JJ, Schaak S, Pontier S, Percherancier Y, Audet M, Paris H, and Bouvier M (2006) Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol 13 778-786. [DOI] [PubMed] [Google Scholar]
- Galperin E, Verkhusha VV, and Sorkin A (2004) Three-chromophore FRET microscopy to analyze multiprotein interactions in living cells. Nat Methods 1 209-217. [DOI] [PubMed] [Google Scholar]
- Gandia J, Galino J, Amaral OB, Soriano A, Lluís C, Franco R, and Ciruela F (2008) Detection of higher-order G protein-coupled receptor oligomers by a combined BRET-BiFC technique. FEBS Lett 582 2979-2984. [DOI] [PubMed] [Google Scholar]
- Ghosh I, Hamilton AD, and Regan L (2000) Antiparallel leucine zipper-directed protein reassembly: application to the green fluorescent protein. J Am Chem Soc 122 5658-5659. [Google Scholar]
- Giese B, Roderburg C, Sommerauer M, Wortmann SB, Metz S, Heinrich PC, and Müller-Newen G (2005) Dimerization of the cytokine receptors gp130 and LIFR analysed in single cells. J Cell Sci 118 5129-5140. [DOI] [PubMed] [Google Scholar]
- Guo W, Urizar E, Kralikova M, Mobarec JC, Shi L, Filizola M, and Javitch JA (2008) Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J 27 2293-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV, and Cleghorn WM (2008) Arrestins as multi-functional signaling adaptors. Handb Exp Pharmacol 186 15-37. [DOI] [PubMed] [Google Scholar]
- Héroux M, Hogue M, Lemieux S, and Bouvier M (2007) Functional calcitonin generelated peptide receptors are formed by the asymmetric assembly of a calcitonin receptor-like receptor homo-oligomer and a monomer of receptor activity-modifying protein-1. J Biol Chem 282 31610-31620. [DOI] [PubMed] [Google Scholar]
- Hu CD, Chinenov Y, and Kerppola TK (2002) Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol Cell 9 789-798. [DOI] [PubMed] [Google Scholar]
- Hu CD, Grinberg AV and Kerppola TK (2006) Visualization of protein interactions in living cells using bimolecular fluorescence complementation (BiFC) analysis. Curr Protoc Cell Biol Chapter 21:Unit 21.3. [DOI] [PubMed]
- Hu CD and Kerppola TK (2003) Simultaneous visualization of multiple protein interactions in living cells using multicolor fluorescence complementation analysis. Nat Biotechnol 21 539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes TR, Mervine SM, Yost EA, Sabo JL, and Berlot CH (2004) Live cell imaging of Gs and the beta2-adrenergic receptor demonstrates that both alphas and beta1gamma7 internalize upon stimulation and exhibit similar trafficking patterns that differ from that of the beta2-adrenergic receptor. J Biol Chem 279 44101-44112. [DOI] [PubMed] [Google Scholar]
- Hynes TR, Yost E, Mervine S, and Berlot CH (2008) Multicolor BiFC analysis of competition among G protein beta and gamma subunit interactions. Methods 45 207-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jach G, Pesch M, Richter K, Frings S, and Uhrig JF (2006) An improved mRFP1 adds red to bimolecular fluorescence complementation. Nat Methods 3 597-600. [DOI] [PubMed] [Google Scholar]
- Kerppola TK (2006) Visualization of molecular interactions by fluorescence complementation. Nat Rev Mol Cell Biol 7 449-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NA (2008) Dissociation of heterotrimeric g proteins in cells [published erratum appears in Sci Signal 1:er3, 2008]. Sci Signal 1 re5. [DOI] [PubMed] [Google Scholar]
- Lee SP, O'Dowd BF, Rajaram RD, Nguyen T, and George SR (2003) D2 dopamine receptor homodimerization is mediated by multiple sites of interaction, including an intermolecular interaction involving transmembrane domain 4. Biochemistry 42 11023-11031. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Canals M, Pediani JD, and Milligan G (2007) The alpha1b-adrenoceptor exists as a higher-order oligomer: effective oligomerization is required for receptor maturation, surface delivery, and function. Mol Pharmacol 71 1015-1029. [DOI] [PubMed] [Google Scholar]
- MacDonald ML, Lamerdin J, Owens S, Keon BH, Bilter GK, Shang Z, Huang Z, Yu H, Dias J, Minami T, et al. (2006) Identifying off-target effects and hidden phenotypes of drugs in human cells. Nat Chem Biol 2 329-337. [DOI] [PubMed] [Google Scholar]
- Marullo S and Bouvier M (2007) Resonance energy transfer approaches in molecular pharmacology and beyond. Trends Pharmacol Sci 28 362-365. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, and Foord SM (1998) RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393: 333-339. [DOI] [PubMed] [Google Scholar]
- Mervine SM, Yost EA, Sabo JL, Hynes TR, and Berlot CH (2006) Analysis of G protein betagamma dimer formation in live cells using multicolor bimolecular fluorescence complementation demonstrates preferences of beta1 for particular gamma subunits. Mol Pharmacol 70 194-205. [DOI] [PubMed] [Google Scholar]
- Milligan G (2007) G protein-coupled receptor dimerisation: molecular basis and relevance to function. Biochim Biophys Acta 1768 825-835. [DOI] [PubMed] [Google Scholar]
- Milligan G and Bouvier M (2005) Methods to monitor the quaternary structure of G protein-coupled receptors. FEBS J 272 2914-2925. [DOI] [PubMed] [Google Scholar]
- Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, and Miyawaki A (2002) A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 20 87-90. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Hof PR, Janssen WG, Morrison JH, and Schmauss C (1997) Expression of dopamine D3 receptor dimers and tetramers in brain and in transfected cells. J Biol Chem 272 29229-29237. [DOI] [PubMed] [Google Scholar]
- Park PS and Wells JW (2004) Oligomeric potential of the M2 muscarinic cholinergic receptor. J Neurochem 90 537-548. [DOI] [PubMed] [Google Scholar]
- Paulmurugan R and Gambhir SS (2007) Combinatorial library screening for developing an improved split-firefly luciferase fragment-assisted complementation system for studying protein-protein interactions. Anal Chem 79 2346-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Neubig R, Bouvier M, Devi L, Filizola M, Javitch JA, Lohse MJ, Milligan G, Palczewski K, Parmentier M, et al. (2007) International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomen-clature of G protein-coupled receptor heteromultimers. Pharmacol Rev 59 5-13. [DOI] [PubMed] [Google Scholar]
- Rebois RV and Hébert TE (2003) Protein complexes involved in heptahelical receptor-mediated signal transduction. Receptors Channels 9 169-194. [PubMed] [Google Scholar]
- Rebois RV, Robitaille M, Galés C, Dupré DJ, Baragli A, Trieu P, Ethier N, Bouvier M, and Hébert TE (2006) Heterotrimeric G proteins form stable complexes with adenylyl cyclase and Kir3.1 channels in living cells. J Cell Sci 119 2807-2818. [DOI] [PubMed] [Google Scholar]
- Rebois RV, Robitaille M, Pétrin D, Zylbergold P, Trieu P, and Hébert TE (2008) Combining protein complementation assays with resonance energy transfer to detect multipartner protein complexes in living cells. Methods 45 214-218. [DOI] [PubMed] [Google Scholar]
- Remy I and Michnick SW (2006) A highly sensitive protein-protein interaction assay based on Gaussia luciferase. Nat Methods 3 977-979. [DOI] [PubMed] [Google Scholar]
- Remy I and Michnick SW (2007) Application of protein-fragment complementation assays in cell biology. Biotechniques 42: 137, 139, 141 passim. [DOI] [PubMed] [Google Scholar]
- Rizzo MA, Springer GH, Granada B, and Piston DW (2004) An improved cyan fluorescent protein variant useful for FRET. Nat Biotechnol 22 445-449. [DOI] [PubMed] [Google Scholar]
- Robishaw JD and Berlot CH (2004) Translating G protein subunit diversity into functional specificity. Curr Opin Cell Biol 16 206-209. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, and Tsien RY (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22 1567-1572. [DOI] [PubMed] [Google Scholar]
- Shyu YJ, Hiatt SM, Duren HM, Ellis RE, Kerppola TK, and Hu CD (2008a) Visualization of protein interactions in living Caenorhabditis elegans using bimolecular fluorescence complementation analysis. Nat Protoc 3 588-596. [DOI] [PubMed] [Google Scholar]
- Shyu YJ and Hu CD (2008) Fluorescence complementation: an emerging tool for biological research. Trends Biotechnol 26 622-630. [DOI] [PubMed] [Google Scholar]
- Shyu YJ, Liu H, Deng X, and Hu CD (2006) Identification of new fluorescent protein fragments for bimolecular fluorescence complementation analysis under physiological conditions. Biotechniques 40 61-66. [DOI] [PubMed] [Google Scholar]
- Shyu YJ, Suarez CD, and Hu CD (2008b) Visualization of AP-1 NF-kappaB ternary complexes in living cells by using a BiFC-based FRET. Proc Natl Acad Sci U S A 105 151-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan E, Aquin S, Berger N, Landry CR, Nyfeler B, Bouvier M, and Michnick SW (2007) Quantification of dynamic protein complexes using Renilla luciferase fragment complementation applied to protein kinase A activities in vivo. Proc Natl Acad Sci U S A 104 16916-16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY (1998) The green fluorescent protein. Annu Rev Biochem 67 509-544. [DOI] [PubMed] [Google Scholar]
- Vidi PA, Chemel BR, Hu CD, and Watts VJ (2008a) Ligand-dependent oligomerization of dopamine D2 and adenosine A2A receptors in living neuronal cells. Mol Pharmacol 74 544-551. [DOI] [PubMed] [Google Scholar]
- Vidi PA, Chen J, Irudayaraj JM, and Watts VJ (2008b) Adenosine A(2A) receptors assemble into higher-order oligomers at the plasma membrane. FEBS Lett 582 3985-3990. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Bodin L, Pandey M, Krause M, Coughlin S, Boussiotis VA, Ginsberg MH, and Shattil SJ (2008) Mechanisms and consequences of agonist-induced talin recruitment to platelet integrin alphaIIbbeta3. J Cell Biol 181 1211-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreggett KA and Wells JW (1995) Cooperativity manifest in the binding properties of purified cardiac muscarinic receptors. J Biol Chem 270 22488-22499. [DOI] [PubMed] [Google Scholar]
- Yost EA, Mervine SM, Sabo JL, Hynes TR, and Berlot CH (2007) Live cell analysis of G protein beta5 complex formation, function, and targeting. Mol Pharmacol 72 812-825. [DOI] [PubMed] [Google Scholar]