Abstract
In recent years, sphingolipids have garnered increasing attention for their roles in modulating intracellular signaling events. Circulating factors associated with obesity promote excess accumulation of ceramide or glucosylceramide derivatives, which impair insulin action in peripheral tissues. In this issue, Villa et al. (p. 866) provide evidence that, in yeast, the progestin and adipoQ receptor superfamily of receptors mediate their effects via a novel ceramidase activity, generating sphingoid base as a second messenger.
An epidemic rate of obesity and associated metabolic disorders may soon start to cause a decline in life expectancy, which has steadily increased for the past 2 centuries (Olshansky et al., 2005). Obesity increases the risk for numerous disorders, including insulin resistance, cardiovascular disease, and diabetes. Although it remains unclear exactly how obesity leads to insulin resistance (i.e., the inability to induce a normal anabolic response in peripheral tissues in response to physiological levels of insulin), excess accumulation of deleterious lipids and low levels of chronic inflammation have both been implicated as causal factors. Recent advancements in the ability to target disruptions in sphingolipid production have revealed potential roles for the sphingolipid ceramide in insulin resistance, β-cell failure, cardiomyopathy and vascular dysfunction (for review, see Holland and Summers, 2008). In stark contrast, the adipose-derived factor adiponectin (Acpr30), has emerged as an insulin sensitizing protein with protective effects on the β-cell, cardiomyocyte and vasculature (for review, see Trujillo and Scherer, 2006). In the current issue of Molecular Pharmacology, Villa et al. (2009) provide evidence that suggests that yeast homologs of adiponectin receptors may function, in part, by modulating sphingolipid metabolism.
Adipocyte-specific secreted molecules, termed adipokines, have dispelled the notion of adipose tissue as an inert storage depot for lipids and highlighted its role as an active endocrine organ that monitors and alters whole-body metabolism and maintains energy homeostasis. One of these adipokines, adiponectin (also known as Acrp30, AdipoQ, and GBP28), first identified by our lab in the mid-1990s (Scherer et al., 1995), has gained significant attention recently as a mediator of insulin sensitivity. Three different forms of the protein have been investigated in a number of different laboratories. Full-length adiponectin circulates as a trimer, a hexamer, and a higher order complex (high-molecular-weight form). These three forms represent the complexes found under normal physiological conditions. A fourth form, globular adiponectin, is a simple trimer of head domains only, lacking the wild-type protein's collagenous domain, with potent pharmacological properties in muscle; however, the physiologic relevance of this form remains to be demonstrated (Yamauchi et al., 2002, 2007).
Clinical studies have revealed low serum concentrations of adiponectin in patients with obesity, insulin resistance, and cardiovascular disease (Wang and Scherer, 2008). Studies from adiponectin knockout and transgenic mice have highlighted and confirmed its role in metabolism. Under basal conditions, mice heterozygous for the adiponectin locus display a 60% reduction in adiponectin serum levels and mild insulin resistance, whereas a more severe insulin resistance with glucose intolerance is observed in knockout animals and exacerbated by as little as 2 weeks of a high-fat/high-sucrose diet (Kubota et al., 2002; Maeda et al., 2002). Adenoviral re-expression of full-length adiponectin largely restores plasma glucose and insulin levels, suggesting that the phenotype of these knockout animals is a direct effect of the lack of adiponectin in serum. Likewise, restoration of serum adiponectin partially restores insulin sensitivity in a lipodystrophic mouse model and completely restores insulin sensitivity if leptin is also restored. Transgenic mice have also been developed that promote physiological (2- -3-fold) overexpression of circulating full-length, wild-type adiponectin, resulting in improved hepatic insulin sensitivity and postprandial lipid clearance in an FVB mouse strain (Combs et al., 2004). The elevated adiponectin secretion completely rescued the diabetic phenotype in leptin deficient (ob/ob) mice, preserving β-cell function and insulin sensitivity despite substantially increased fat mass (Kim et al., 2007)
The progestin and adipoQ receptors (PAQR) are a novel family of receptors that includes adiponectin receptors 1 and 2 (AdipoR1 and AdipoR2) and the membrane progesterone receptors γ and β. AdipoR1, identified by its ability to bind the globular form of adiponectin, is ubiquitously distributed with higher expression in muscle (Yamauchi et al., 2003). AdipoR2 is expressed predominantly in liver and seems to display greater affinity for full-length adiponectin (for review, see Kadowaki and Yamauchi, 2005).
Adenoviral overexpression of AdipoR1 or -2, like the overexpression of the yeast homolog in the current article (Villa et al., 2009), suggests that the receptors may offer some degree of constitutive activity (Yamauchi et al., 2007). When overexpressed in leptin receptor-deficient mice, these receptors seem to improve insulin sensitivity and glucose homeostasis. Genetic ablation of adiponectin receptors has yielded conflicting results. Yamauchi et al. (2007) have demonstrated that ablation of either AdipoR1 or AdipoR2 promotes an insulin resistant phenotype. By contrast, Liu et al. (2007) determined that AdipoR2-deficient mice show superior glucose and lipid metabolism compared with wild-type controls but are ultimately more susceptible to β-cell decompensation and diabetes despite their improved metabolism.
From a mechanistic perspective, adiponectin may stimulate AMP-activated protein kinase (AMPK) in muscle, liver, and β-cells. Yamauchi et al. (2002) provided the first strong evidence for the molecular mechanism of adiponectin action. Using isolated muscle or C2C12 myocytes, they demonstrated that treatment with the globular form of adiponectin leads to a rapid, transient phosphorylation of AMPK peaking at 5 min after treatment (Yamauchi et al., 2002). AMPK phosphorylation leads to acetyl CoA carboxylase inhibition (by 15 min), decreased malonyl CoA, and subsequent derepression of carnitine palmitoyltransferase 1 activity, causing increased fatty acid oxidation in muscle. Adenoviral overexpression of AdipoR1, but not AdipoR2, was sufficient to accentuate AMPK activation, perhaps suggesting distinct signaling mechanisms for the two receptors (Yamauchi et al., 2007). Rather, overexpression of AdipoR2 increased the expression of PPAR-α and its target genes in liver.
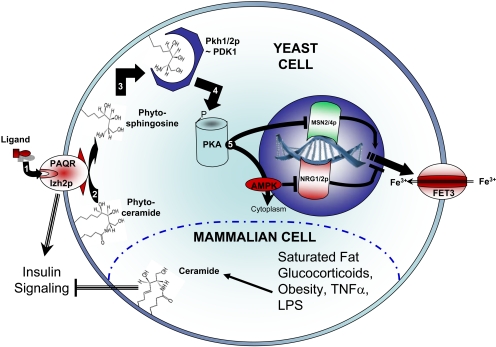
In yeast, overexpression of AdipoR1 or other PAQRs represses expression of the iron transporter FET3, providing a reliable reporter gene. Noting that the PAQR family of receptors contains significant sequence homology with alkaline ceramidase (an enzyme that cleaves ceramide to produce sphingosine and free fatty acids), Villa et al. (2009) evaluated the yeast PAQ receptor Izh2p to determine whether it functions via ceramidase activity. Although it remains uncertain whether the enzyme itself possesses ceramidase activity, their data clearly demonstrate that, at least in yeast, this class of receptors mediates its effect via this enzymatic cleavage of phytoceramide to produce phytosphingosine (Fig. 1). To our knowledge, this is the first report of a ligand activated receptor possessing this type of enzymatic activity.
Figure 1.
PAQR signaling is mediated by sphingolipid metabolism. In yeast, Izh2p leads to repression of the iron transporter FET3. This process is sequentially mediated by 1) PAQ receptors binding to their cognate ligands, 2) the cleavage of phytoceramide to regenerate phytosphingosine and fatty acid, 3) activation of Pkh1/2p by sphingoid base, 4) activation of protein kinase A (PKA) by Pkh1/2p, 5) PKA-induced inhibition of the transcriptional coactivator MSN2/4p and nuclear export of AMP kinase (preventing inhibition of the transcriptional corepressor NRG1/2p). In mammalian cells, circulating factors impair insulin sensitivity via aberrant ceramide accumulation.
Yeast made an ideal biological system to evaluate a sphingolipid-dependent mechanism, in part because yeast possess only two ceramidase enzymes. By contrast, five human ceramidases have been characterized to date (for review, see Mao and Obeid, 2008). Distinguished by their pH optima, alkaline (three isoforms), acid, and neutral ceramidase are localized to the endoplasmic reticulum/Golgi, plasma membrane, and lysosome, respectively. To date, few studies have analyzed the influence of these enzymes on glucose metabolism; however, in cell culture models, their overexpression protects against lipid-induced insulin resistance in cultured myotubes (Chavez et al., 2005) and cytokine-induced β-cell apoptosis (Zhu et al., 2008).
Lipopolysaccharide, the proinflammatory component of Gram-negative bacteria, and the proinflammatory cytokines interleukin 1β and tumor necrosis factor α (TNFα) all induce ceramide accumulation in a variety of tissues and cell types. It is noteworthy that the death domain of the TNFα receptor contains sphingomyelinase activity that, upon its activation, regenerates ceramide from sphingomyelin via the cleavage of choline (Peraldi et al., 1996). Interleukin-1 has similarly been shown to promote the hydrolysis of sphingomyelin (Santana et al., 1996). The generation of ceramide and glucosylceramide are critical for TNFα-mediated insulin resistance (Grigsby and Dobrowsky, 2001; Tagami et al., 2002). This mechanism of action of the TNF receptors is an excellent example for a hormone-mediated signaling event that affects cellular glucose homeostasis via the modulation of sphingolipid metabolism.
Sphingolipid metabolism has garnered increasing attention for its roles in insulin resistance, β cell failure, and cardiovascular disease. The development of new drugs and novel transgenic animal models have allowed for targeted disruption of sphingolipid metabolism. In vivo, ceramide and certain glucosylceramide derivatives have been shown to impair insulin action in peripheral tissues, promote lipotoxic apoptosis in pancreatic β cells, and impair vascular and cardiac function.
Several factors have suggested a role for ceramide in the impairment of peripheral insulin actions. First, ceramide accumulates in insulin-resistant rodents and humans. Second, circulating factors known to impair glucose homeostasis, such as glucocorticoids, saturated free fatty acids, and TNFα, are known to promote aberrant ceramide accumulation. Third, addition of short-chain ceramide analogs to cultured muscle, liver, or adipose tissue is sufficient to impair insulin stimulated activation of Akt, a key regulator of insulin's anabolic effects. At least two distinct mechanisms link ceramide to the inhibition of Akt, in that ceramide activates protein kinase C-ζ and protein phosphatase 2A to prevent Akt phosphorylation and promote its dephosphorylation, respectively. Important to the context of the current article (Villa et al., 2009), ceramide does not impair insulin signaling upstream of Akt and does not prevent sphingosine-induced activation of PDK1, an important kinase in Akt activation (Stratford et al., 2004).
Noting these effects, Holland et al. (2007) recently evaluated the role of excess ceramide accumulation in the pathogenesis of insulin resistance and type 2 diabetes. Using pharmacologic inhibitors or mice with genetically impaired ceramide synthesis in vivo, glucocorticoid, saturated fat, or obesity-induced insulin resistance was inhibited. Moreover, inhibiting ceramide synthesis prevented diabetes in the ZDF rat, consistent with previous studies implicating ceramide as a key mediator of lipotoxic apoptosis in the β-cell. Using similar approaches, independent groups have prevented or reversed atherosclerotic lesion formation in ApoE-deficient mice (Park et al., 2004; Hojjati et al., 2005). Most recently, the Goldberg group demonstrated that ceramide is essential for cardiac dysfunction in a lipotoxic model of heart disease (Park et al., 2008).
Indeed aberrant accumulation of ceramide produces numerous metabolic insults, whereas the potent adipokine adiponectin protects against them. The potential connection of the two fields prompts a number of interesting and important questions, and validation of these results in mammalian cell culture models and in vivo will provide exciting insights into the mechanisms of obesity and associated comorbidities.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants R01-DK55758, R90-DK081180-02] and the National Institutes of Health National Cancer Institute [Grant R01-CA112023].
Please see the related article on page 866.
ABBREVIATIONS: PAQR, progestin and adipoQ receptor; AMPK, AMP-activated protein kinase; TNF, tumor necrosis factor.
References
- Chavez JA, Holland WL, Bär J, Sandhoff K, and Summers SA (2005) Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem 280 20148-20153. [DOI] [PubMed] [Google Scholar]
- Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, et al. (2004) A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 145 367-383. [DOI] [PubMed] [Google Scholar]
- Grigsby RJ and Dobrowsky RT (2001) Inhibition of ceramide production reverses TNF-induced insulin resistance. Biochem Biophys Res Commun 287 1121-1124. [DOI] [PubMed] [Google Scholar]
- Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S, and Jiang XC (2005) Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J Biol Chem 280 10284-10289. [DOI] [PubMed] [Google Scholar]
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, et al. (2007) Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5 167-179. [DOI] [PubMed] [Google Scholar]
- Holland WL and Summers SA (2008) Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev 29 381-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T and Yamauchi T (2005) Adiponectin and adiponectin receptors. Endocr Rev 26 439-451. [DOI] [PubMed] [Google Scholar]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, et al. (2007) Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117 2621-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, et al. (2002) Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277 25863-25866. [DOI] [PubMed] [Google Scholar]
- Liu Y, Michael MD, Kash S, Bensch WR, Monia BP, Murray SF, Otto KA, Syed SK, Bhanot S, Sloop KW, et al. (2007) Deficiency of adiponectin receptor 2 reduces diet-induced insulin resistance but promotes type 2 diabetes. Endocrinology 148 683-692. [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, et al. (2002) Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8 731-737. [DOI] [PubMed] [Google Scholar]
- Mao C and Obeid LM (2008) Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim Biophys Acta 1781 424-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, and Ludwig DS (2005) A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 352 1138-1145. [DOI] [PubMed] [Google Scholar]
- Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, et al. (2008) Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res 49 2101-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TS, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, Kindt EK, Homan R, Karathanasis SK, and Rekhter MD (2004) Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 110 3465-3471. [DOI] [PubMed] [Google Scholar]
- Peraldi P, Hotamisligil GS, Buurman WA, White MF, and Spiegelman BM (1996) Tumor necrosis factor (TNF)-α inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem 271 13018-13022. [DOI] [PubMed] [Google Scholar]
- Santana P, Llanes L, Hernandez I, Gonzalez-Robayna I, Tabraue C, Gonzalez-Reyes J, Quintana J, Estevez F, Ruiz de Galarreta CM, and Fanjul LF (1996) Interleukin-1 beta stimulates sphingomyelin hydrolysis in cultured granulosa cells: evidence for a regulatory role of ceramide on progesterone and prostaglandin biosynthesis. Endocrinology 137 2480-2489. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, and Lodish HF (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270 26746-26749. [DOI] [PubMed] [Google Scholar]
- Stratford S, Hoehn KL, Liu F, and Summers SA (2004) Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem 279 36608-36615. [DOI] [PubMed] [Google Scholar]
- Tagami S, Inokuchi Ji J, Kabayama K, Yoshimura H, Kitamura F, Uemura S, Ogawa C, Ishii A, Saito M, Ohtsuka Y, et al. (2002) Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem 277 3085-3092. [DOI] [PubMed] [Google Scholar]
- Trujillo ME and Scherer PE (2006) Adipose tissue-derived factors: impact on health and disease. Endocr Rev 27 762-778. [DOI] [PubMed] [Google Scholar]
- Villa NY, Kupchak BR, Garitaonandia I, Smith JL, Alonso E, Alford C, Cowart LA, Hannun YA, and Lyons TJ (2009) Sphingolipids function as downstream effectors of a fungal PAQR. Mol Pharmacol 75 866-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZV and Scherer PE (2008) Adiponectin, cardiovascular function, and hypertension. Hypertension 51 8-14. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et al. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423 762-769. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. (2002) Adiponectin stimulates glucose utilization and fattyacid oxidation by activating AMP-activated protein kinase. Nat Med 8 1288-1295. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, et al. (2007) Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13 332-339. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Jin JF, Shan XH, Liu CP, Mao XD, Xu KF, and Liu C (2008) Chronic activation of neutral ceramidase protects beta-cells against cytokine-induced apoptosis. Acta Pharmacol Sin 29 593-599. [DOI] [PubMed] [Google Scholar]