Abstract
Homomeric 5-hydroxytryptamine (5-HT)3A and heteromeric 5-HT3AB receptors mediate rapid excitatory responses to serotonin in the central and peripheral nervous systems. The alkaloid morphine, in addition to being a μ-opioid receptor agonist, is a potent competitive inhibitor of 5-HT3 receptors. We examined whether methadone, an opioid often used to treat morphine dependence, also exhibited 5-HT3 receptor antagonist properties. Racemic (R/S)-methadone inhibited currents mediated by human homomeric 5-HT3A receptors (IC50 = 14.1 ± 2.5 μM). Incorporation of the 5-HT3B subunit into heteromeric 5-HT3AB receptors reduced the potency of inhibition by (R/S)-methadone (IC50 = 41.1 ± 0.9 μM). (R/S)-Methadone also increased apparent desensitization of both 5-HT3 receptor subtypes. The inhibition of the 5-HT3A receptor was competitive; however, incorporation of the 5-HT3B subunit caused the appearance of inhibition that was insurmountable by 5-HT. In the absence of rapid desensitization, when dopamine was used as an agonist of 5-HT3AB receptors, the inhibition by (R/S)-methadone was voltage-dependent. The antagonist and desensitization-enhancing effects of (R/S)-methadone were shared by pure (R)- and (S)-methadone enantiomers, which had similar actions on 5-HT-evoked currents mediated by 5-HT3 receptors. However, (R)-methadone exhibited a larger voltage-dependent inhibition of dopamine-evoked currents mediated by 5-HT3AB receptors than did (S)-methadone. Inhibition of 5-HT3A receptors by (R/S)-methadone was not influenced by voltage. Thus, methadone displays multimodal subunit-dependent antagonism of 5-HT3 receptors.
The 5-hydroxytryptamine (5-HT) type 3 receptor is a ligand-gated cation channel that mediates rapid serotonergic excitatory synaptic transmission (Sugita et al., 1992). It contains binding sites for 5-HT and several allosteric modulators. The 5-HT3 receptor is a member of the Cys-loop superfamily of pentameric receptors, which also includes the nicotinic acetylcholine, γ-aminobutyric acid, and glycine receptors, and the Zn2+-activated ion channel (Barnes et al., 2009). The 5-HT3A subunit forms homomeric receptors and can also combine into heteromeric receptors with the 5-HT3B subunit, which is by contrast unable to form homomeric receptors (Davies et al., 1999). Coexpression of the 5-HT3A subunit with the 5-HT3B subunit confers unique properties (Davies et al., 1999; Peters et al., 2005). Genes encoding 5-HT3C, 5-HT3D, and 5-HT3E subunits have also been cloned; however, their functional significance is poorly understood (Niesler et al., 2003).
5-HT3 receptors participate in nausea and vomiting, nociception, gastrointestinal motility, and reward (Allan et al., 2001; Galligan, 2002; Thompson and Lummis, 2006). Many therapeutic drugs structurally distinct from 5-HT affect 5-HT3 receptor function. These include competitive antagonists such as the “setrons” (including ondansetron); the nicotinic drugs curare (Peters et al., 1990), epibatidine, and mecamylamine (Drisdel et al., 2007); cannabinoids (Barann et al., 2002); and some opioids (Fan, 1995; Wittmann et al., 2006). 5-HT3 receptor antagonists are used to treat nausea and vomiting and, to a lesser extent, irritable bowel syndrome (Galligan, 2002). Ondansetron is also effective in the treatment of early onset alcoholism (Kranzler et al., 2003) and seems to aid detoxification of heroin-dependent individuals (Ye et al., 2001).
The alkaloid morphine, the principal active metabolite of heroin, has been known for more than 50 years to have inhibitory effects on specific serotonin receptor subtypes such as those located in the guinea pig ileum (Gaddum and Picarelli, 1957). Morphine-sensitive, so called 5-HTM receptors were later renamed 5-HT3 receptors (Bradley et al., 1986). Morphine directly and competitively inhibits 5-HT3 receptors at low concentrations (Fan, 1995; Wittmann et al., 2006).
We investigated whether the 5-HT3 receptor-inhibiting properties of morphine were shared by methadone, an opioid frequently used to treat morphine dependence. Methadone is a chiral molecule and as such exists as either an R- or S-enantiomer. Compared with (S)-methadone, (R)-methadone binds preferentially to the μ-opioid receptor (Kristensen et al., 1995) and exhibits more potent inhibitory effects at the NMDA subtype of the glutamate receptor (Callahan et al., 2004). By contrast, compared with (R)-methadone, (S)-methadone more potently inhibits cardiac hERG K+ channels (Eap et al., 2007). We tested (R)- and (S)-methadone to determine whether the modulatory effects of (R/S)-methadone on 5-HT3 receptors are enantiomer-specific.
Materials and Methods
Cell Culture and Transfection. Human embryonic kidney (HEK) cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% bovine serum, 50 μg/ml streptomycin, and 50 U/ml penicillin in a humid atmosphere of 5% CO2. HEK cells were transfected by calcium phosphate precipitation with cDNA encoding the human 5-HT3A subunit either alone or in combination with the human 5-HT3B subunit cDNA in the pCDM8 vector at a cDNA ratio of 1:1, as described previously (Davies et al., 1999). All tissue culture reagents were from Invitrogen (Carlsbad, CA). Cells were used 48 to 96 h after transfection for electrophysiological experiments.
Electrophysiological Recording. The whole-cell configuration of the patch-clamp technique was used to record currents from HEK cells expressing recombinant receptors. The electrode solution contained 140 mM CsCl, 2 mM MgCl2, 0.1 mM CaCl2, 1.1 mM EGTA, and 10 mM HEPES (pH 7.4 with CsOH). The extracellular solution contained 140 mM NaCl, 2.8 mM KCl, 2 mM MgCl2, 1 mM CaCl2, 10 mM HEPES, and 10 mM glucose (pH 7.4 with NaOH). Unless otherwise stated, cells were voltage-clamped at an electrode potential of -60 mV. In experiments investigating the voltage dependence of inhibition by methadone, the voltage was adjusted between -60 and +60 mV (in 20-mV increments). No correction was made for the compensation for liquid junction potential. 5-HT3 receptors were activated by locally applying 5-HT or dopamine to the cell by pressure (10 psi) ejection (Picospritzer II; General Valve, Fairfield, NJ). The recording chamber was continuously perfused with extracellular solution (5 ml/min). Methadone was diluted from frozen stocks into the extracellular solution on the day of recording. During experiments examining the concentration dependence of inhibition of 5-HT3 receptors, methadone was bath-applied, whereas 5-HT (30 μM) was applied every 60 s for 100 ms from a micropipette positioned ∼50 μm from the cell. When investigating the concentration dependence of 5-HT and desensitization in the absence and presence of methadone, 5-HT alone or 5-HT plus methadone was applied for 1 s from the micropipette, as described previously (Adodra and Hales, 1995). A period of at least 120 s elapsed between each application to allow recovery from desensitization. The ratio of 5-HT-evoked current amplitudes recorded at -60 and 60 mV was established before applying methadone to an HEK cell transfected with 5-HT3A and 5-HT3B subunit cDNAs. A 5-HT-evoked 60/-60 mV ratio of ∼1 (compared with ∼0.5 for 5-HT3A receptors) was used as an indication of successful 5-HT3B subunit incorporation into heteromeric 5-HT3AB receptors (e.g., Fig. 5). Currents were recorded using an Axopatch 200B amplifier, low-pass filtered at 2 KHz, digitized at 10 KHz using a Digidata 1320A interface, and acquired using pCLAMP8 software (all from Molecular Devices, Sunnyvale, CA) on to the hard drive of a personal computer for off-line analysis. All experiments were performed at room temperature.
Fig. 5.
(R/S)-Methadone affects 5-HT-evoked current-voltage relationships. A, superimposed, leak subtracted 5-HT (30 μM)-evoked currents recorded from an HEK cell expressing 5-HT3A receptors in the absence (left) and presence (right) of (R/S)-methadone (30 μM). (R/S)-Methadone was bath-applied before and during local 5-HT application. The same cell was used to generate all traces at holding potentials between -60 and 60 mV (20-mV increments). Note that 5-HT-evoked currents, recorded in the absence and presence of (R/S)-methadone, are displayed using different scale bars. B, 5-HT current-voltage relationships recorded in the presence and absence of (R/S)-methadone from cells expressing 5-HT3A receptors. Current amplitudes were normalized to 5-HT-evoked current amplitudes recorded from the same cell at -60 mV. Data points are averages of four recordings and vertical lines represent ± S.E.M. C, superimposed, leak subtracted 5-HT (30 μM)-evoked currents recorded from an HEK cell expressing 5-HT3AB receptors in the absence (left) and presence (right) of (R/S)-methadone (100 μM). The same cell was used to generate all traces at holding potentials between -60 and 60 mV (20-mV increments). Note that 5-HT-evoked currents, recorded in the absence and presence of (R/S)-methadone, are displayed using different scale bars. D, 5-HT current-voltage relationships recorded in the presence and absence of (R/S)-methadone. Current amplitudes were normalized to 5-HT current amplitudes recorded from the same cell at -60 mV. Data points are averages of five recordings and vertical lines represent ± S.E.M.
Data Analysis. The peak amplitudes of agonist-activated currents were measured using pCLAMP8 software. Systematic effects of 5-HT-evoked current rundown were corrected using regression analysis, normalizing current amplitudes to that evoked by 100 μM 5-HT. Concentration-response relationships were fitted with a modified logistic function to determine EC50, IC50, and Hill slope values, as described previously (Adodra and Hales, 1995). We used the method of Lew and Angus (1995) to investigate whether (R/S)-methadone had competitive or noncompetitive inhibitory effects on 5-HT3 receptors. The inhibition of 5-HT3AB receptors by (R/S)-methadone exhibited a component that was insurmountable by 5-HT, thus pre-cluding calculation of binding affinity. The pEC50 values for 5-HT [determined in the absence and presence of differing concentrations of (R/S)-methadone] were plotted against (R/S)-methadone concentration. Data points were fitted with the following equation:
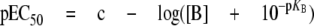 |
(1) |
where [B] is the concentration of (R/S)-methadone and c is a fitting constant. As recommended by Lew and Angus (1995), we also fitted the plots of 5-HT pEC50 values versus [(R/S)-methadone] with formulae that allow deviations equivalent, when using Schild analysis, to either nonlinearity:
 |
(2) |
or a nonunity slope:
 |
(3) |
Whether the interaction was competitive was then determined by comparisons of the goodness of fit. Fitting the data with eqs. 2 and 3 failed to significantly improve the fidelity of the fits (established using the F-test) achieved using eq. 1. A Clarke plot was generated to illustrate the predicted relationship between the 5-HT EC50 values and the concentration of alkaloid, using the value of Kb generated by eq. 1. This approach demonstrated that (R/S)-methadone caused competitive inhibition of the 5-HT3A receptor.
Results
Concentration-Dependent Inhibition of 5-HT3A Receptors by (R/S)-Methadone. In addition to its classic interaction with the μ-opioid receptor, morphine directly and competitively inhibits 5-HT3 receptors (Fan, 1995; Wittmann et al., 2006). We examined whether this property was shared by methadone. We used the whole-cell patch-clamp technique to record currents from voltage-clamped HEK cells transiently expressing human 5-HT3A receptors. 5-HT (30 μM), applied transiently to cells clamped at -60 mV, activated inward currents with a mean peak amplitude of 4.0 ± 0.43 nA (n = 20). Bath-applied racemic (R/S)-methadone hydrochloride inhibited 5-HT-evoked currents in a concentration-dependent manner (Fig. 1A). We fitted the concentration-response relationship for (R/S)-methadone using the logistic equation, yielding an IC50 value of 14.1 ± 2.5 μM.
Fig. 1.
Concentration-dependent inhibition of 5-HT3A and 5-HT3AB receptors by (R/S)-methadone. Inhibition of currents mediated by 5-HT3 receptors activated by 5-HT (30 μM) in the absence and presence of (R/S)-methadone bath-applied before and during local 5-HT (30 μM) application. A, concentration-response relationship for (R/S)-methadone as an inhibitor of 5-HT3A (open symbols) and 5-HT3AB receptors (closed symbols). IC50 values, generated by logistic fits to the data points, were 14.1 ± 2.5 and 41.1 ± 0.9 μM, respectively. Data are expressed as percentage of control 5-HT (30 μM)-evoked current amplitude. Vertical lines are ± S.E.M. B, 5-HT-evoked currents mediated by 5-HT3A receptors recorded from the same cell. Left, 5-HT (100 μM) was applied for 1 s in the absence of (R/S)-methadone (Meth). Middle, 5-HT (100 μM) was applied for 1 s with (R/S)-methadone (10 μM). Right, (R/S)-methadone (10 μM) was preapplied for 5 min before its coapplication with 5-HT (100 μM). Preapplication of (R/S)-methadone enhanced apparent desensitization and caused a reduction in peak current amplitude.
Lack of Agonist Action of (R/S)-Methadone on the 5-HT3A Receptor. A previous study demonstrated that the alkaloid apomorphine acts as a weak partial agonist at 5-HT3 receptors (van Hooft and Vijverberg, 1998). In keeping with this action, when applied simultaneously with 5-HT, apomorphine competitively inhibits 5-HT-evoked currents. We therefore examined the possibility that (R/S)-methadone (300 μM) is a partial agonist by locally administering the opioid alkaloid to HEK cells expressing recombinant 5-HT3A receptors by pressure application. Five cells tested that responded robustly to 5-HT (30 μM) failed to exhibit currents in response to (R/S)-methadone application (data not shown). Therefore, methadone is a 5-HT3 receptor antagonist that lacks efficacy as an agonist at concentrations that cause near-maximal inhibition of 5-HT3A receptor-mediated currents (Fig. 1A).
Inhibition of Heteromeric 5-HT3AB Receptors by (R/S)-Methadone. When expressed with the 5-HT3B subunit the 5-HT3A subunit forms heteromeric receptors with characteristic functional properties (Davies et al., 1999; Das and Dillon, 2005). For example, heteromeric 5-HT3AB receptors are less sensitive to inhibition by the plant alkaloids curare and picrotoxin than are homomeric 5-HT3A receptors. We tested the effects of (R/S)-methadone on 5-HT (30 μM)-activated currents mediated by heteromeric 5-HT3AB receptors. Application of 5-HT to HEK cells transiently transfected with cDNAs encoding 5-HT3A and 5-HT3B subunits activated inward currents recorded at a holding potential of -60 mV. Bath application of (R/S)-methadone (Fig. 1A) caused concentration-dependent inhibition of 5-HT-activated currents mediated by heteromeric 5-HT3AB receptors. We fitted the concentration-response relationship with the logistic equation, yielding an estimate of the IC50 value (41.1 ± 0.9 μM). (R/S)-Methadone was significantly (p < 0.001; Student's t test) less potent as an inhibitor of 5-HT3AB receptors compared with 5-HT3A receptors. The Hill slope values (1.2 ± 0.2 and 1.9 ± 0.1) for the inhibition by (R/S)-methadone of 5-HT3A and 5-HT3AB receptors, respectively, also differed significantly (p < 0.001; Student's t test).
Competitive Antagonism of 5-HT3 Receptors by (R/S)-Methadone. Previous studies demonstrate that morphine competitively inhibits 5-HT3 receptor-mediated currents (Fan, 1995; Wittmann et al., 2006). We examined the nature of 5-HT3 receptor antagonism by (R/S)-methadone of currents mediated by 5-HT3A receptors. Experiments examining the concentration dependence of the inhibition of 5-HT-evoked currents revealed that (R/S)-methadone caused an increase in the apparent desensitization of 5-HT3A receptors. Rapid apparent desensitization of 5-HT-evoked currents after preapplication of (R/S)-methadone probably compromised our ability to measure the peak 5-HT-evoked current amplitude (Fig. 1B, right). Apparent desensitization was enhanced to a lesser extent when 5-HT and (R/S)-methadone were applied simultaneously and the reduction in peak current amplitude was negligible (Fig. 1B, middle). Therefore, to minimize desensitization, we applied 5-HT and (R/S)-methadone simultaneously in subsequent experiments examining their competitive interactions.
5-HT (1-1000 μM) caused a concentration-dependent activation of recombinant 5-HT3A receptors expressed in HEK cells (Fig. 2A; Table 1). (R/S)-Methadone (30-1000 μM) applied simultaneously with 5-HT, caused concentration-dependent dextral shifts of the 5-HT (1-1000 μM) concentration-response relationships of 5-HT3A receptors (Fig. 2A), reducing the apparent potency of 5-HT (Table 1). Increasing the concentration of 5-HT overcame most of the inhibition by (R/S)-methadone even when high concentrations of (R/S)-methadone were used (Fig. 2A). Only at the highest (R/S)-methadone concentration (1 mM) tested was there a small reduction in the maximal efficacy of 5-HT (Table 1).
Fig. 2.
Competitive and insurmountable antagonism of 5-HT3 receptors by (R/S)-methadone. A, superimposed traces are exemplar 5-HT (1-300 μM)-evoked currents recorded, from the same HEK cell expressing 5-HT3A receptors, in the absence (left) and presence (right) of (R/S)-methadone (300 μM) applied simultaneously with 5-HT. The graph depicts mean current amplitudes activated by 5-HT in the presence of (R/S)-methadone expressed as a percentage of control 5-HT (100 μM)-evoked current amplitudes recorded from each cell. Open and closed symbols represent 5-HT-evoked current amplitudes recorded in the absence and presence of (R/S)-methadone, respectively. The 5-HT concentration-response relationship was shifted to the right by increasing concentrations of (R/S)-methadone: 30 (•), 55 (▾), 300 (♦), and 1000 (▪) μM. Vertical lines represent ± S.E.M. 5-HT EC50, maximum current, and Hill slope values for the activation of 5-HT3A receptors in the absence and presence of (R/S)-methadone were determined from logistic fits to the data points (Table 1). The inset graph is a Clark plot of the 5-HT EC50 values (log10) versus log ([methadone] + Kb), where Kb is the apparent binding affinity calculated as described under Materials and Methods (Lew and Angus, 1995). The straight line is the idealized relationship between 5-HT EC50 value and [methadone], with the calculated Kb value (see Results). B, superimposed traces are exemplar 5-HT-evoked currents recorded from an HEK cell expressing 5-HT3AB receptors in the absence (left) and presence (right) of (R/S)-methadone (300 μM) applied simultaneously with 5-HT. The graph represents mean 5-HT-evoked current amplitudes recorded in the presence of (R/S)-methadone expressed as a percentage of control 5-HT (100 μM)-evoked current amplitudes recorded from each cell. 5-HT EC50, maximum current, and Hill slope values for the activation of 5-HT3AB receptors by 5-HT in the presence of (R/S)-methadone were determined from the logistic fits (Table 1). Vertical lines represent ± S.E.M.
TABLE 1.
Parameters of 5-HT concentration-response relationships in the presence and absence of (R/S)-methadone
5-HT concentration-response relationships in the presence and absence of (R/S)-methadone at the concentrations indicated. Current amplitudes were normalized to those recorded from the same cells activated by 5-HT (100 μM) in the absence of (R/S)-methadone. Concentration-response relationships were fitted with a logistic equation (Fig. 2), yielding the parameters provided in this table.
|
5-HT3A Receptor
|
5-HT3AB Receptor
|
|||||
|---|---|---|---|---|---|---|
| EC50 | Imax | Hill Slope | EC50 | Imax | Hill Slope | |
| μM | % | μM | % | |||
| 5-HT alone | 5.0 ± 1.0 | 104 ± 5 | 1.3 ± 0.3 | 15.3 ± 1.4 | 109 ± 3 | 1.2 ± 0.1 |
| 5-HT + methadone (30 μM) | 7.3 ± 0.5* | 94.0 ± 2.1 | 1.7 ± 0.2 | 15.8 ± 2.4 | 92.4 ± 4.4* | 1.2 ± 0.2 |
| 5-HT + methadone (100 μM) | 14.8 ± 1.1* | 97.0 ± 2.0 | 1.5 ± 0.2 | 53.1 ± 7.4* | 85.2 ± 3.4* | 1.0 ± 0.1 |
| 5-HT + methadone (300 μM) | 61.6 ± 8.8* | 95.2 ± 4.5 | 1.2 ± 0.2 | 77.8 ± 2.7* | 63.2 ± 0.8* | 1.2 ± 0.04 |
| 5-HT + methadone (1000 μM) | 127 ± 11* | 80.6 ±* | 1.4 ± 0.1 | 249 ± 52* | 40.4 ± 3.0* | 1.3 ± 0.3 |
Significantly different from equivalent 5-HT alone value, p < 0.01, ANOVA, post hoc Dunnett's test.
We used the method of Lew and Angus (1995) to evaluate the nature of (R/S)-methadone's inhibition of the 5-HT3A receptor. Plots of (R/S)-methadone concentration versus 5-HT pEC50 values were well fitted by eq. 1 (see Materials and Methods), and the fidelity of the fit was not improved significantly by modifications incorporated into either eqs. 2 or 3 (data not shown). The results of this analysis are illustrated in the Clark plot (Fig. 2A). The line represents the predicted relationship between the 5-HT EC50 values and the concentration of (R/S)-methadone, with the value of Kb (34.2 μM) generated by eq. 1. This Kb value reflects the affinity of (R/S)-methadone when it is simultaneously applied with 5-HT. Under these conditions, there is effectively a “race” for occupation of the agonist binding site. Binding affinity seems to be somewhat enhanced by applying (R/S)-methadone before 5-HT3A receptor activation as evidenced from the IC50 value for (R/S)-methadone of 14.1 ± 2.5 μM.
(R/S)-Methadone (30-300 μM) also caused concentration-dependent inhibition of 5-HT (1-1000 μM)-evoked currents recorded from HEK cells expressing recombinant 5-HT3AB receptors (Fig. 2B). Consistent with previous reports (Davies et al., 1999; Stewart et al., 2003), 5-HT3AB receptors were less potently activated by 5-HT (Table 1) and desensitized more rapidly than 5-HT3A receptors (Fig. 2B). (R/S)-Methadone induced dextral shifts of the 5-HT concentration-response relationships mediated by 5-HT3AB receptors (Fig. 2B; Table 1). These data also reveal that all concentrations of (R/S)-methadone that inhibited 5-HT-evoked current amplitudes caused a component of inhibition that could not be surmounted by increasing the concentration of 5-HT (Fig. 2B; Table 1). The presence of an insurmountable component to the inhibition of 5-HT3AB receptors precludes the determination of a binding affinity for (R/S)-methadone. The insurmountable block of 5-HT3AB receptors by (R/S)-methadone could represent either noncompetitive or uncompetitive antagonism. The term uncompetitive antagonism describes inhibition that occurs less effectively at low agonist concentrations compared with high agonist concentrations. Such an effect of methadone would probably cause a systematic change in the Hill coefficients for the 5-HT concentration-response relationships. Because this did not occur, the inhibition by methadone does not seem to be uncompetitive (Table 1).
R- and S-Enantiomers Cause Similar Shifts of the 5-HT Concentration-Response Relationship. Methadone is a chiral molecule and as such exists in two isomeric forms (Fig. 3A). Methadone used thus far in this study was the racemic mixture of R- and S-enantiomers. (R)-Methadone binds preferentially to the μ-opioid receptor (Kristensen et al., 1995). By contrast, (S)-methadone exerts the most potent inhibitory effect on cardiac hERG K+ channels (Eap et al., 2007). We compared the abilities of pure (R)- and (S)-methadone to reduce the potency of 5-HT at 5-HT3A (Fig. 3B) and 5-HT3AB (Fig. 3C) receptors. There was no difference between the 5-HT concentration-response relationships of either 5-HT3A or 5-HT3AB receptors recorded in the presence of 100 μM (R)- or (S)-methadone, suggesting that competitive antagonism by methadone of 5-HT3 receptors is not stereoisomer-specific (Fig. 3, B and C).
Fig. 3.
Antagonism of 5-HT3 receptors by (R)- and (S)-methadone. A, structural diagrams of (R)- and (S)-methadone illustrate the chiral carbon responsible for the stereoisomerization. The graphs illustrate 5-HT concentration-response relationships for recombinant 5-HT3A (B) and 5-HT3AB (C) receptors, evaluated in the absence (○) or presence of either (R)-methadone (▴) or (S)-methadone (▾) (100 μM). (R)- and (S)-Methadone caused similar reductions in the potency of 5-HT.
Methadone Increases Apparent Desensitization of 5-HT3 Receptors. An increased rate of 5-HT-evoked current desensitization is a well documented effect of incorporation of the 5-HT3B subunit (Dubin et al., 1999; Stewart et al., 2003). Inspection of 5-HT3A and 5-HT3AB receptor-mediated currents (Figs. 2 and 4) confirms these previous findings. After 1 s, 5-HT (100 μM)-evoked currents mediated by 5-HT3A and 5-HT3AB receptors declined from initial peak amplitudes by 32 ± 5% (n = 18) and 80 ± 3% (n = 21), respectively. (R/S)-Methadone caused a striking increase in the apparent desensitization of currents mediated by both 5-HT3A (Figs. 1B and 4A) and 5-HT3AB receptors (Fig. 4B). After 1 s, 5-HT (100 μM)-evoked currents mediated by 5-HT3A and 5-HT3AB receptors in the presence of (R/S)-methadone (100 μM) had declined by 99 ± 0.1% (n = 3) and 99 ± 0.2% (n = 4), respectively. Similar increases in apparent desensitization were observed for both (R)- and (S)-methadone (Fig. 4). The time courses of currents mediated by 5-HT3 receptors in the presence of (R/S)-, (R)-, or (S)-methadone were indistinguishable (n = 4). These results suggest that increased apparent desensitization of 5-HT3 receptors by methadone is not stereoisomer-specific.
Fig. 4.
(R)- and (S)-Methadone increase 5-HT3 receptor apparent desensitization. Superimposed and normalized 5-HT (100 μM)-evoked currents recorded from cells expressing either 5-HT3A (A) or 5-HT3AB (B) receptors. In each case, currents were recorded from the same HEK cell in the absence and presence of (R/S)-, (R)-, or (S)-methadone (100 μM).
Voltage-Dependent Inhibition of 5-HT3AB Receptors by Methadone. The apparent reduction by (R/S)-methadone of the efficacy of 5-HT as an activator of 5-HT3AB receptors could be caused by enhanced desensitization. Indeed, currents rapidly decay in the presence of high concentrations of (R/S)-methadone and 5-HT potentially compromising measurements of peak current amplitude (Fig. 4). This is particularly likely in the case of the 5-HT3AB receptor, which desensitizes rapidly even in the absence of (R/S)-methadone. However, in addition to its ability to increase desensitization, (R/S)-methadone may also reduce efficacy of 5-HT by exerting a negative allosteric effect and/or a direct channel block. The latter can be identified by the presence of voltage-dependent inhibition. Therefore we examined the current-voltage relationship of currents mediated by 5-HT3A and 5-HT3AB receptors in the presence and absence of (R/S)-methadone. (R/S)-Methadone was bath-applied at approximately similarly effective concentrations in experiments examining 5-HT3A -and 5-HT3AB receptors (30 and 100 μM, respectively). 5-HT (30 μM)-evoked currents mediated by 5-HT3A receptors were inhibited by (R/S)-methadone (30 μM) at potentials between -60 and 60 mV (Fig. 5, A and B). The 5-HT current-voltage relationship exhibited characteristic inward rectification both in the absence and presence of (R/S)-methadone (Fig. 5B). There was no significant (p > 0.05, ANOVA) change in the inhibition of the peak current amplitude by (R/S)-methadone at each potential (Fig. 6).
Fig. 6.
Voltage-dependent inhibition of 5-HT3AB receptors by (R/S)-methadone. The graph depicts the effect of voltage on inhibition by (R/S)-methadone (30 and 100 μM) of 5-HT-evoked currents mediated by 5-HT3A and 5-HT3AB receptors, open and gray bars, respectively. (R/S)-Methadone was bath-applied before and during local 5-HT (30 μM) application (Fig. 5). Bars are mean inhibitions recorded from at least four cells in each case; vertical lines represent ± S.E.M. Asterisks indicate that inhibitions of currents mediated by 5-HT3AB receptors, at 40 and 60 mV, were significantly smaller than those at equivalent negative holding potentials, p < 0.05 and 0.01, respectively.
As reported previously (Davies et al., 1999), incorporation of the human 5-HT3B subunit caused the 5-HT-evoked current-voltage relationship to become linear (Fig. 5, C and D). It is noteworthy that the presence of (R/S)-methadone (100 μM) caused the appearance of marked outward rectification (Fig. 5, C and D), with more inhibition of peak current amplitude at negative potentials compared with the equivalent positive potentials (Fig. 6).
It is possible that the voltage-dependence of the inhibition of 5-HT3AB receptors by (R/S)-methadone results from an effect of voltage on desensitization. To address this possibility, we simultaneously applied 5-HT (100 μM) alone and with (R/S)-methadone (100 μM) and investigated the voltage dependence of inhibition and desensitization. Currents recorded from the same cell, activated by 5-HT (100 μM) at -60 and 60 mV exhibited similar kinetics (Fig. 7A). At a holding potential of -60 mV, (R/S)-methadone speeded up apparent desensitization as demonstrated previously (Fig. 4). The effect of (R/S)-methadone on the rate of current decay was most marked at -60 mV (Fig. 7A). Likewise, when (R/S)-methadone and 5-HT were applied simultaneously, the current amplitude was significantly larger at 60 than at -60 mV (Fig. 7B). These data could either be explained by a voltage-dependent increase in 5-HT3AB receptor desensitization by (R/S)-methadone or alternatively the apparent desensitization in the presence of (R/S)-methadone could seem faster at -60 mV because of open channel block.
Fig. 7.
Voltage-dependent inhibition by methadone of 5-HT3AB receptors is independent of desensitization. A, 5-HT3AB receptor-mediated currents evoked by 5-HT (100 μM) applied for 1 s either alone (in black) or simultaneously with (R/S)-methadone (100 μM; in gray) at holding potentials of -60 and 60 mV. Inset, currents recorded at -60 and 60 were normalized and superimposed to compare kinetics. The time course of desensitization of 5-HT-evoked currents recorded at both potentials in the absence of (R/S)-methadone (black traces) was similar. In the presence of (R/S)-methadone (gray traces), 5-HT-evoked currents decayed faster at -60 mV. B, percentage of inhibition at -60 and 60 mV (n = 5). The inhibition by (R/S)-methadone was significantly smaller at 60 mV than at -60 mV (*, p < 0.05; paired t test). Vertical lines are ± S.E.M. C, 5-HT3AB receptor-mediated currents evoked by dopamine (DA; 3 mM) applied for 1 s either alone (in black) or simultaneously with 100 μM (R/S)-(medium gray), (R)-(light gray), or (S)-(dark gray) methadone at holding potentials of -60 and 60 mV. D, percentage of inhibition at -60 and 60 mV (n = 5). The inhibitions by all three methadone formulations were significantly smaller at 60 mV than at -60 mV (*, p < 0.05; **, p < 0.01; paired t test). Furthermore, inhibition by (S)-methadone was significantly smaller than (R)-methadone at -60 mV (#, p < 0.05) and both (R/S)- and (R)-methadone at 60 mV (##, p < 0.001) as determined by ANOVA with post hoc Dunnett's test. Vertical lines are ± S.E.M.
We attempted to reduce desensitization and test whether this diminished the voltage-dependent blockade when (R/S)-methadone was applied simultaneously with 5-HT. 5-Hydroxyindole attenuates desensitization of 5-HT3 receptors (Kooyman et al., 1993). However, as reported previously (Hu and Peoples, 2008), 5-hydoxyindole (10 mM) had little effect on desensitization of 5-HT-evoked currents mediated by 5-HT3AB receptors relative to 5-HT3A receptors (data not shown). Therefore, we adopted an alternative strategy using the partial agonist dopamine to activate slowly desensitizing currents. Dopamine (3 mM) activated 9.8 ± 3.2% (n = 6) of the current amplitude evoked by 5-HT (100 μM) when applied to cells expressing 5-HT3AB receptors. Administration of 10 mM dopamine failed to increase the 5-HT3AB receptor-mediated current amplitude (n = 6; data not shown), demonstrating that at 3 mM, dopamine had reached its maximal efficacy. Dopamine-evoked currents exhibited little desensitization after 1 s (Fig. 7C). At -60 mV 82 ± 3% (n = 4) of the dopamine-evoked current remained after 1 s of its application to 5-HT3AB receptors. By contrast, after 1 s of application of 5-HT (100 μM) to the same cells only 12 ± 3% (n = 4) of current remained. (R/S)-Methadone (100 μM) reduced the peak amplitude of dopamine-evoked currents mediated by 5-HT3AB receptors at -60 and 60 mV by 44 ± 5 and 27 ± 3% (n = 5), respectively (Fig. 7D). The inhibition was significantly (p < 0.05) reduced at a holding potential of 60 mV.
We compared the voltage-dependent blockade of dopamine-evoked currents by (R)- and (S)-methadone (100 μM). The inhibition of dopamine-evoked currents by (R)-methadone at -60 mV was 48 ± 6% (n = 5). Inhibition by (R)-methadone was reduced to 32 ± 4% at 60 mV (Fig. 7D). By contrast (S)-methadone caused a smaller inhibition of dopamine-evoked currents (26 ± 4%; n = 5) than did either (R/S)-or (R)-methadone (Fig. 7C). This weaker inhibition was essentially reversed (3.1 ± 2.7%) by a holding potential of 60 mV.
Taken together, these recordings of dopamine-evoked currents demonstrate that there is a voltage-dependent component to the inhibition of 5-HT3AB receptors that is present despite diminution of receptor desensitization and therefore represents open channel blockade. The noncompetitive block by methadone is influenced by the identity of its stereoisomer, with (R)-methadone causing a stronger block than (S)-methadone.
Discussion
The opioid alkaloid methadone inhibited 5-HT-evoked currents mediated by homomeric 5-HT3A receptors in a concentration-dependent manner. Increasing concentrations of 5-HT surmounted the inhibitory effect of (R/S)-methadone. The inhibition was predominantly competitive; increasing concentrations of (R/S)-methadone caused a linear dextral shift in the 5-HT concentration-response relationship. The incorporation of the 5-HT3B subunit reduced the potency of inhibition by (R/S)-methadone and caused the appearance of a component of antagonism that could not be overcome by 5-HT. Methadone also increased 5-HT3A and 5-HT3AB receptor apparent desensitization. The insurmountable inhibition of 5-HT3AB receptors by (R/S)-methadone was influenced by voltage. Inhibition was significantly larger at negative voltages than corresponding positive voltages. By contrast, the inhibition of 5-HT3A receptors by (R/S)-methadone was independent of voltage.
The voltage-dependent nature of the insurmountable inhibition of 5-HT3AB receptors by (R/S)-methadone could reflect attenuation of desensitization at positive voltages or relief from open channel block of outward currents. There was no effect of voltage on the rate of desensitization of 5-HT-evoked currents mediated by 5-HT3AB receptors in the absence of (R/S)-methadone. However, apparent desensitization in the presence of (R/S)-methadone was faster at -60 mV compared with 60 mV. A voltage-dependent open channel block by methadone could contribute to the apparent desensitization of 5-HT-evoked currents mediated by 5-HT3AB receptors. To investigate this further, we used dopamine, a partial agonist of 5-HT3 receptors. Maximal dopamine-evoked current mediated by 5-HT3AB receptors exhibited little desensitization. In the presence of (R/S)-methadone, dopamine-evoked currents also exhibited slower apparent desensitization than did 5-HT-evoked currents. Nevertheless, (R/S)-methadone caused voltage-dependent inhibition of dopamine-evoked currents mediated by 5-HT3AB receptors. These data suggest that the voltage-dependent inhibition of currents mediated by 5-HT3AB receptors is independent of desensitization. Instead, it is likely that the voltage-dependent inhibition reflects a block by (R/S)-methadone within the channel of heteromeric 5-HT3AB receptors, an action that is lacking from homomeric 5-HT3A receptors. Compared with other opioid alkaloids, methadone has a high pKa value (∼9.3) and is therefore predominantly cationic at physiological pH (Carr and Cousins, 1998) and, as such, it may most effectively access the channel of the 5-HT3AB receptor at negative membrane potentials.
Methadone can exist as either R- or S-enantiomers. (R)-Methadone binds to μ-opioid receptors with higher affinity than (S)-methadone. We used pure (R)- and (S)-methadone to examine whether competitive inhibition, increased desensitization, voltage-dependent inhibition of 5-HT3 receptors, or a combination are also stereoselective actions. The shifts by (R)- and (S)-methadone of the 5-HT concentration-response relationships for both 5-HT3A and 5-HT3AB receptors were similar, as were the levels of apparent desensitization that the isomers induced. Nor was there an apparent selectivity of methadone enantiomers for the insurmountable inhibition of 5-HT-evoked currents mediated by 5-HT3AB receptors. However, when dopamine was used as an agonist to minimize desensitization, the voltage-dependent inhibition by (R)-methadone of 5-HT3AB receptors was significantly greater than was the equivalent inhibition by (S)-methadone. Taken together, our findings suggest that (R)-methadone has a stronger interaction than (S)-methadone with a binding site on the 5-HT3B subunit located within the second transmembrane domain lining the channel pore. Future studies will be required to identify the residues involved.
Despite the lack of voltage-dependent inhibition of homomeric 5-HT3A receptors, (R/S)-methadone in the low micromolar concentration range caused increased desensitization. This effect was accentuated by preapplication of (R/S)-methadone and may contribute to the clinical actions of the drug. The increased rate of desensitization makes measurement of equilibrium binding affinity problematic. The IC50 value for inhibition of 5-HT-evoked currents, determined by preapplying (R/S)-methadone, was somewhat lower than the calculated binding affinity derived from shifts of the 5-HT concentration-response relationships by simultaneously applied (R/S)-methadone. It is possible that greater desensitization, induced by prolonged application, may increase (R/S)-methadone's affinity for the 5-HT3A receptor. Alternatively (R/S)-methadone may not reach equilibrium-binding conditions when applied simultaneously with 5-HT. The route to the binding site is probably tortuous and 5-HT may win the race to access its site. However, the explanation that we favor is that preapplication induces greater desensitization, which compromises the ability to resolve the peak current amplitude when (R/S)-methadone is preapplied. Under these conditions, the IC50 value reflects the affinity of (R/S)-methadone for its site of desensitization and the 5-HT binding site. Our data suggest that these two sites are distinct. First, (R/S)-methadone does not act as a partial agonist and therefore would be unlikely to increase desensitization through occupancy of the agonist binding site. Second, (R/S)-methadone causes increased 5-HT3 receptor desensitization even in the presence of saturating concentrations of 5-HT, which would completely displace (R/S)-methadone from the agonist binding site. Therefore, we propose that there are at least three binding sites for (R/S)-methadone on 5-HT3 receptors: one site that overlaps with the agonist binding site and accounts for the observed competitive antagonism; a second that is outside the agonist binding site, occupancy of which enhances desensitization; and a third located within the channel pore of the heteromeric 5-HT3AB receptor, which is responsible for voltage-dependent blockade by (R/S)-methadone.
In addition to 5-HT3 receptors, methadone directly interacts with several other ion channels, including hERG K+ channels (Katchman et al., 2002; Eap et al., 2007), inwardly rectifying K+ channels (Rodriguez-Martin et al., 2008), the NMDA subtype of the glutamate receptor (Ebert et al., 1995; Callahan et al., 2004), and the α3β4 and α7 nicotinic receptors (Xiao et al., 2001; Pakkanen et al., 2005). (R)-Methadone, the isomer that preferentially binds to μ-opioid receptors is also more potent than (S)-methadone as an inhibitor of NMDA receptors (Kristensen et al., 1995; Callahan et al., 2004), whereas (S)-methadone has a higher potency than (R)-methadone as an inhibitor of hERG channels (Eap et al., 2007). In keeping with its preferential effect at μ-opioid and NMDA receptors, (R)-methadone caused a greater voltage-dependent inhibition of 5-HT3AB receptors than did (S)-methadone.
Patients undergoing treatment for morphine dependence often receive high doses of methadone (Eap et al., 2007). Methadone has a long half-life and can reach micromolar concentrations equivalent to those that inhibit 5-HT3 receptors, particularly in individuals who are slow metabolizers of the compound, raising the possibility that antagonism of 5-HT3 receptors may be clinically relevant. 5-HT3 receptors are distributed throughout the central and peripheral nervous systems, with dense expression in the dorsal vagal complex, an area that coordinates the vomiting reflex (Barnes et al., 2009). The antiemetic actions of 5-HT3 receptor antagonists are likely to be mediated through this region of the brainstem. 5-HT3 receptors are also expressed at lower levels elsewhere in the central nervous system, including the forebrain. In humans, there are 5-HT3 binding sites in the caudate nucleus and putamen, two regions associated with drug craving (Harlan and Garcia, 1999; Thompson and Lummis, 2006). The use of in situ hybridization and immunohistochemistry demonstrated 5-HT3A subunit expression in the brain. By contrast, the distribution of the 5-HT3B subunit in the brain is somewhat controversial (van Hooft and Yakel, 2003). Some studies suggest that there is an absence of 5-HT3B subunit transcript from the rodent brain (Morales and Wang, 2002), whereas others report labeling of rodent central neurons with antibodies to the 5-HT3B subunit (Reeves and Lummis, 2006). 5-HT3B subunit transcripts have consistently been detected in human brain tissue (Davies et al., 1999; Tzvetkov et al., 2007). However, there is a lack of functional studies implicating a role of the 5-HT3B subunit in central neurons.
Recombinant studies of homomeric 5-HT3A and heteromeric 5-HT3AB receptors reveal that they have distinct functional properties. Homomeric 5-HT3A receptors have a single channel conductance <1 pS and a similar permeability to Ca2+ and Na+ (Davies et al., 1999). By contrast, heteromeric 5-HT3AB receptors are less permeable to divalent cations and have a single channel conductance of ∼15 pS. Single channels mediated by 5-HT3 receptors in enteric neurons have conductances similar to those of 5-HT3AB receptors (Galligan, 2002). Therefore 5-HT3AB receptors are located peripherally where they are likely to participate in the gastrointestinal effects of the setrons, which cause increased colonic transit time (Talley et al., 1990). Thus, antagonists with selectivity for homomeric 5-HT3A receptors may have fewer peripheral side effects. Because (R/S)-methadone exhibits subunit selective inhibitory actions, modification of this opioid alkaloid structure may provide a strategy for designing new 5-HT3 receptor antagonists that act either centrally or peripherally.
The list of drugs that have modulatory effects on 5-HT3 receptors has expanded to include the alkaloid methadone. The complex multimodal actions of methadone on 5-HT3 receptors reveals sites, distinct from the agonist binding site, through which alkaloids can affect desensitization and block the channel pore. The rich variety of alkaloids available for pharmacophore analysis provides probes for modeling the structure of competitive and noncompetitive antagonist binding sites in 5-HT3 receptors.
Acknowledgments
Pure (R)- and (S)-methadone enantiomers were provided by the National Institute on Drug Abuse.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA05010] and the National Science Foundation [Grant 0447156].
ABBREVIATIONS: 5-HT, 5-hydroxytryptamine; HEK, human embryonic kidney; hERG, human ether-à-go-go-related gene; ANOVA, analysis of variance; NMDA, N-methyl-d-aspartate; Meth, methadone.
References
- Adodra S and Hales TG (1995) Potentiation, activation and blockade of GABAA receptors of clonal murine hypothalamic GT1-7 neurones by propofol. Br J Pharmacol 115 953-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan AM, Galindo R, Chynoweth J, Engel SR, and Savage DD (2001) Conditioned place preference for cocaine is attenuated in mice over-expressing the 5-HT3 receptor. Psychopharmacology (Berl) 158 18-27. [DOI] [PubMed] [Google Scholar]
- Barann M, Molderings G, Brüss M, Bönisch H, Urban BW, and Göthert M (2002) Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br J Pharmacol 137 589-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Hales TG, Lummis SC, and Peters JA (2009) The 5-HT3 receptor - the relationship between structure and function. Neuropharmacology 56 273-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PB, Engel G, Feniuk W, Fozard JR, Humphrey PP, Middlemiss DN, Mylecharane EJ, Richardson BP, and Saxena PR (1986) Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology 25 563-576. [DOI] [PubMed] [Google Scholar]
- Callahan RJ, Au JD, Paul M, Liu C, and Yost CS (2004) Functional inhibition by methadone of N-methyl-d-aspartate receptors expressed in Xenopus oocytes: stereospecific and subunit effects. Anesth Analg 98 653-659. [DOI] [PubMed] [Google Scholar]
- Carr DB and Cousins MJ (1998) Spinal route of analgesia: opioids and future options, in Neural Blockade in Clinical Anesthesia and Management of Pain (Cousins MJ and Bridenbaugh PO eds), pp. 915-984, Lippincott Williams & Wilkins, Baltimore, MD.
- Das P and Dillon GH (2005) Molecular determinants of picrotoxin inhibition of 5-hydroxytryptamine type 3 receptors. J Pharmacol Exp Ther 314 320-328. [DOI] [PubMed] [Google Scholar]
- Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, and Kirkness EF (1999) The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature 397 359-363. [DOI] [PubMed] [Google Scholar]
- Drisdel RC, Sharp D, Henderson T, Hales TG, and Green WN (2008) High affinity binding of epibatidine to 5-HT3 receptors. J Biol Chem 283 9659-9665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin AE, Huvar R, D'Andrea MR, Pyati J, Zhu JY, Joy KC, Wilson SJ, Galindo JE, Glass CA, Luo L, et al. (1999) The pharmacological and functional characteristics of the serotonin 5-HT3A receptor are specifically modified by a 5-HT3B receptor subunit. J Biol Chem 274 30799-30810. [DOI] [PubMed] [Google Scholar]
- Eap CB, Crettol S, Rougier JS, Schläpfer J, Sintra Grilo L, Déglon JJ, Besson J, Croquette-Krokar M, Carrupt PA, and Abriel H (2007) Stereoselective block of hERG channel by (S)-methadone and QT interval prolongation in CYP2B6 slow metabolizers. Clin Pharmacol Ther 81 719-728. [DOI] [PubMed] [Google Scholar]
- Ebert B, Andersen S, and Krogsgaard-Larsen P (1995) Ketobemidone, methadone and pethidine are non-competitive N-methyl-d-aspartate (NMDA) antagonists in the rat cortex and spinal cord. Neurosci Lett 187 165-168. [DOI] [PubMed] [Google Scholar]
- Fan P (1995) Nonopioid mechanism of morphine modulation of the activation of 5-hydroxytryptamine type 3 receptors. Mol Pharmacol 47 491-495. [PubMed] [Google Scholar]
- Gaddum JH and Picarelli ZP (1957) Two kinds of tryptamine receptor. Br J Pharmacol 12 323-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan JJ (2002) Ligand-gated ion channels in the enteric nervous system. Neurogastroenterol Motil 14 611-623. [DOI] [PubMed] [Google Scholar]
- Harlan RE and Garcia MM (1999) Brain regions and drug addiction. Science 284 1124-1125. [DOI] [PubMed] [Google Scholar]
- Hu XQ and Peoples RW (2008) The 5-HT3B subunit confers spontaneous channel opening and altered ligand properties of the 5-HT3 receptor. J Biol Chem 283 6826-6831. [DOI] [PubMed] [Google Scholar]
- Jackson DA, Kischka U, and Wurtman RJ (1995) The mu1, mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci 56 45-49. [DOI] [PubMed] [Google Scholar]
- Katchman AN, McGroary KA, Kilborn MJ, Kornick CA, Manfredi PL, Woosley RL, and Ebert SN (2002) Influence of opioid agonists on cardiac human ether-a-go-go-related gene K+ currents. J Pharmacol Exp Ther 303 688-694. [DOI] [PubMed] [Google Scholar]
- Kooyman AR, van Hooft JA, and Vijverberg HP (1993) 5-Hydroxyindole slows desensitization of the 5-HT3 receptor-mediated ion current in N1E-115 neuroblastoma cells. Br J Pharmacol 108 287-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Pierucci-Lagha A, Feinn R, and Hernandez-Avila C (2003) Effects of ondansetron in early-versus late-onset alcoholics: a prospective, open-label study. Alcohol Clin Exp Res 27 1150-1155. [DOI] [PubMed] [Google Scholar]
- Lew MJ and Angus JA (1995) Analysis of competitive agonist-antagonist interactions by nonlinear regression. Trends Pharmacol Sci 16 328-337. [DOI] [PubMed] [Google Scholar]
- Morales M and Wang SD (2002) Differential composition of 5-hydroxytryptamine3 receptors synthesized in the rat CNS and peripheral nervous system. J Neurosci 22 6732-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesler B, Frank B, Kapeller J, and Rappold GA (2003) Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. Gene 310 101-111. [DOI] [PubMed] [Google Scholar]
- Pakkanen JS, Nousiainen H, Yli-Kauhaluoma J, Kylänlahti I, Möykkynen T, Korpi ER, Peng JH, Lukas RJ, Ahtee L, and Tuominen RK (2005) Methadone increases intracellular calcium in SH-SY5Y and SH-EP1-halpha7 cells by activating neuronal nicotinic acetylcholine receptors. J Neurochem 94 1329-1341. [DOI] [PubMed] [Google Scholar]
- Peters JA, Hales TG, and Lambert JJ (2005) Molecular determinants of single-channel conductance and ion selectivity in the Cys-loop family: insights from the 5-HT3 receptor. Trends Pharmacol Sci 26 587-594. [DOI] [PubMed] [Google Scholar]
- Peters JA, Malone HM, and Lambert JJ (1990) Antagonism of 5-HT3 receptor mediated currents in murine N1E-115 neuroblastoma cells by (+)-tubocurarine. Neurosci Lett 110 107-112. [DOI] [PubMed] [Google Scholar]
- Reeves DC and Lummis SC (2006) Detection of human and rodent 5-HT3B receptor subunits by anti-peptide polyclonal antibodies. BMC Neurosci 7 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Martin I, Braksator E, Bailey CP, Goodchild S, Marrion NV, Kelly E, and Henderson G (2008) Methadone: does it really have low efficacy at μ-opioid receptors? Neuroreport 19 589-593. [DOI] [PubMed] [Google Scholar]
- Stewart A, Davies PA, Kirkness EF, Safa P, and Hales TG (2003) Introduction of the 5-HT3B subunit alters the functional properties of 5-HT3 receptors native to neuroblastoma cells. Neuropharmacology 44 214-223. [DOI] [PubMed] [Google Scholar]
- Sugita S, Shen KZ, and North RA (1992) 5-Hydroxytryptamine is a fast excitatory transmitter at 5-HT3 receptors in rat amygdala. Neuron 8 199-203. [DOI] [PubMed] [Google Scholar]
- Talley NJ, Phillips SF, Haddad A, Miller LJ, Twomey C, Zinsmeister AR, MacCarty RL, and Ciociola A (1990) GR 38032F (ondansetron), a selective 5HT3 receptor antagonist, slows colonic transit in healthy man. Dig Dis Sci 35 477-480. [DOI] [PubMed] [Google Scholar]
- Thompson AJ and Lummis SC (2006) 5-HT3 receptors. Curr Pharm Des 12 3615-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzvetkov MV, Meineke C, Oetjen E, Hirsch-Ernst K, and Brockmöller J (2007) Tissue-specific alternative promoters of the serotonin receptor gene HTR3B in human brain and intestine. Gene 386 52-62. [DOI] [PubMed] [Google Scholar]
- van Hooft JA and Vijverberg HP (1998) Agonist and antagonist effects of apomorphine enantiomers on 5-HT3 receptors. Neuropharmacology 37 259-264. [DOI] [PubMed] [Google Scholar]
- van Hooft JA and Yakel JL (2003) 5-HT3 receptors in the CNS: 3B or not 3B? Trends Pharmacol Sci 24 157-160. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Peters I, Schaaf T, Wartenberg HC, Wirz S, Nadstawek J, Urban BW, and Barann M (2006) The effects of morphine on human 5-HT3A receptors. Anesth Analg 103 747-752. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Smith RD, Caruso FS, and Kellar KJ (2001) Blockade of rat α3β4 nicotinic receptor function by methadone, its metabolites, and structural analogs. J Pharmacol Exp Ther 299 366-371. [PubMed] [Google Scholar]
- Ye JH, Ponnudurai R, and Schaefer R (2001) Ondansetron: a selective 5-HT3 receptor antagonist and its applications in CNS-related disorders. CNS Drug Rev 7 199-213. [DOI] [PMC free article] [PubMed] [Google Scholar]









