Abstract
Nicotinic acetylcholine receptor (nAChR) agonists stimulate the release of GABA from GABAergic nerve terminals, but the nAChR subtypes that mediate this effect have not been elucidated. The studies reported here used synaptosomes derived from the cortex, hippocampus, striatum, and thalamus of wild-type and α4-, α5-, α7-, β2-, and β4-null mutant mice to identify nAChR subtypes involved in acetylcholine (ACh)-evoked GABA release. Null mutation of genes encoding the α4 or β2 subunits resulted in complete loss of ACh-stimulated [3H]GABA release in all four brain regions. In contrast, α5 gene deletion exerted a small but significant decrease in maximal ACh-evoked [3H]GABA release in hippocampus and striatum, with a more profound effect in cortex. Acetylcholine-stimulated [3H]GABA release from thalamic synaptosomes was not significantly affected by α5 gene deletion. No effect was detected in the four brain regions examined in α7- or β4-null mutant mice. Further analysis of ACh-evoked [3H]GABA release revealed biphasic concentration-response relationships in the four brain regions examined from all wild-type animals and in α5 null mutant mice. Moreover, a selective reduction in the maximum response of the high-affinity component was apparent in α5-null mutant mice. The results demonstrate that α4β2-type nAChRs are critical for ACh-stimulated [3H]GABA release from all four brain regions examined. In addition, the results suggest that α5-containing receptors on GABAergic nerve terminals comprise a fraction of the high ACh-sensitivity component of the concentration-response curve and contribute directly to the ability of nicotinic agonists to evoke GABA release in these regions.
The nicotinic acetylcholine receptor (nAChR) arguably represents one of the most evolutionarily conserved and well characterized neurotransmitter receptors, and the majority of high-resolution structural data have been accumulated from studies of the torpedo- and muscle-type nAChR (Millar and Gotti, 2009). It is generally believed that the structures of neuronal nAChRs are very similar to the muscle-type nAChR subunits because the amino acid sequences of neuronal nAChR subunits are similar to the muscle-type subunits (Lindstrom et al., 1998). Assuming that the neuronal nAChRs are pentameric assemblies that resemble the peripheral-type nAChR, the fact that mammalian neurons express mRNAs for nine nAChR genes (designated α2-α7 and β2-β4) suggests that many different nAChR subtypes might be expressed in the central nervous system.
The importance of identifying the sites of expression and subunit compositions of those nAChRs that are actually expressed in brain is demonstrated by observations that the biophysical and pharmacological properties of nAChRs are affected when different subunit combinations are examined in heterologous expression systems (Zwart and Vijverberg, 1998; Moroni et al., 2006; Kuryatov et al., 2008). Of particular importance is the observation that α4α5β2 nAChRs are more cation-permeable than other high-affinity heteromeric nAChRs, and the calcium-permeability of these α4α5β2 nAChRs is exceeded only by α7-type nAChRs and N-methyl-d-aspartate glutamate receptors (Kuryatov et al., 2008). Indeed, previous studies performed in our laboratory (Brown et al., 2007) showed that deletion of the α5 subunit decreased maximal agonist-evoked 86Rb+ efflux in several brain regions without any loss of total receptor binding. These results suggest that the α5 subunit exerts a distinct and neuron phenotype-specific effect on sequelae of nAChR activation, such as neurotransmitter release.
The development of genetically engineered (gene knockout or null mutant) mice has provided a vital tool that has facilitated the identification and characterization of native neuronal nAChRs. For example, the findings that α4 and β2 mRNAs are found together in many brain regions (Marks et al., 1992, Whiteaker et al., 2006) that also express high-affinity [3H]nicotine binding sites (Marks and Collins, 1982; Clarke et al., 1985) led to the suggestion that those nAChRs that bind nicotinic agonists with high affinity are composed of α4 and β2 subunits. Definitive proof that the α4 and β2 subunits are required to form these binding sites was provided by studies that showed the absence of high-affinity [3H]nicotine binding in brain tissue derived from α4- (Marubio et al., 1999) and β2- (Picciotto et al., 1995) null mutant mice. Likewise, the findings that 125I-α-bungarotoxin binding is eliminated in α7-null mutant mice helped establish that α7-type receptors bind 125I-α-bungarotoxin with high affinity (Orr-Urtreger et al., 1997). More recent studies using null mutant mice have demonstrated that the α6 (Champtieux et al., 2001), β2 (Salminen et al., 2005), and β3 (Cui et al., 2003) subunits are required to form the 125I-α-conotoxin MII binding sites expressed in catecholaminergic neurons. In addition, studies from our laboratory (Marks et al., 2007) have used null mutants to identify the subunit compositions of seven different nAChRs that can be measured with the aid of high- and low-affinity [3H]epibatidine binding.
Many nAChRs are expressed on presynaptic nerve terminals, where they modulate the release of acetylcholine (ACh), GABA, glutamate, serotonin, and dopamine (Wonnacott, 1997). We (Salminen et al., 2004) used α4-, α5-, α7-, β2-, β3-, and β4-null mutant mice, along with an assay that measures [3H]dopamine release from striatal synaptosomes, to identify the subunit compositions of the native receptors that are expressed in dopaminergic nerve terminals. Our results indicate that four different nAChR subtypes (α4β2, α4α5β2, α4α6β2β3, and α6β2β3) are expressed in dopaminergic nerve terminals derived from mouse striatum. An identical conclusion was drawn by Gotti et al. (2005) using immunological methodologies.
The studies reported here used synaptosomes obtained from wild-type and α4-, α5-, α7-, β2-, and β4-null mutant mice in an attempt to identify the role of these subunits in modulating GABA release. ACh-stimulated [3H]GABA release was studied using synaptosomes prepared from the hippocampus, striatum, cortex, and thalamus. Immunological (Gahring et al., 2004) and reverse-transcription polymerase chain reaction (Klink et al., 2001) data have demonstrated that these subunits may be found in GABAergic neurons in several mouse brain regions. The results indicate that ACh-evoked [3H]GABA release in all four brain regions requires α4 and β2 subunits and that the α5 subunit contributes to the formation of functional receptors in three of the four brain regions examined, but to differing degrees.
Materials and Methods
Materials. [3H]GABA (33.4 Ci/mmol) was purchased from Perkin-Elmer Life and Analytical Sciences (Waltham, MA). Sucrose and HEPES were obtained from Roche Diagnostics (Indianapolis, IN). The following compounds were products of Sigma Chemical Co. (St. Louis, MO): ACh, atropine sulfate, NO-711, aminooxyacetic acid, GABA, sodium chloride, potassium chloride, calcium chloride, magnesium sulfate, potassium dihydrogen phosphate, d-glucose, and diisopropylfluorophosphate. Econosafe scintillation cocktail was purchased from Research Products International Corp. (Mt. Prospect, IL), and OptiPhase Supermix scintillation cocktail was obtained from PerkinElmer.
Mice. Male and female mice carrying gene deletions (knockouts) for Chrna4, Chrna5, Chrna7, Chrnb2, and Chrnb4, which encode α4, α5, α7, β2, and β4 nAChR subunits, respectively, were used in these studies. All nAChR subunit-null mutant mice were bred onto the C57BL/6 strain for the indicated number of generations: α4 (Ross et al., 2000), 5 generations; α5 (Salas et al., 2003), 8 generations; α7 (Orr-Urtreger et al., 1997), 10 generations; β2 (Picciotto et al., 1995), 10 generations; and β4 (Xu et al., 1999), 10 generations. All of the animals were produced by heterozygous (+/-) matings maintained at the Institute for Behavioral Genetics (University of Colorado, Boulder, CO).
The mice were weaned and separated by gender at 25 days of age and were housed in groups of five to a cage. The animals were maintained on a 12-h light/dark cycle (lights on from 7:00 AM to 7:00 PM), were given unlimited access to food (Teklad Rodent Diet) and water and were used when they were 60 to 120 days old. All animal care and experimental procedures were performed in accordance with the National Institutes of Health's Guide for Care and Use of Laboratory Animals and were approved by the University of Colorado's Animal Care and Use Committee.
Genotyping. All genotyping was performed from tail clip samples (∼1 cm). QIAGEN (Valencia, CA) DNEasy Tissue Kits were used to extract DNA from the tail clippings. The α4, α5, α7, β2, and β4 genotypes were determined by polymerase chain reaction with oligonucleotide probes specific for the Chrna4, Chrna5, Chrna7, Chrnb2, and Chrnb4 sequences, respectively, as described in Salminen et al. (2004). The gene products were electrophoresed on 1.5% agarose gels and stained with ethidium bromide. Two independent observers then scored the genotypes.
Synaptosome Preparation. Each mouse was sacrificed by cervical dislocation, and the brain was removed from the skull. The cortex, hippocampus, striatum, and thalamus were then dissected on ice. Crude synaptosomes were prepared from each dissected brain region by hand homogenization in 4 volumes of 0.5 ml of ice-cold (4°C) Percoll medium I (320 mM sucrose and 5 mM HEPES, pH 7.5) with a glass Teflon homogenizer. The homogenates were then centrifuged at 12,000g for 20 min at 4°C. The pellets of each homogenate were resuspended in uptake buffer (128 mM NaCl, 2.4 mM KCl, 3.2 CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4·7H2O, 25 mM HEPES, and 10 mM glucose, pH 7.5). The volume of perfusion buffer used for resuspending the synaptosomes was 0.8 ml for each brain region.
[3H]GABA Release. These experiments used a protocol adapted from that developed by Lu et al. (1998) with modifications as specified below. Synaptosomal preparations were incubated for 10 min at 37°C in uptake buffer containing 1.25 mM aminooxyacetic acid, a GABA transaminase inhibitor. [3H]GABA (final concentration, 0.3 μM), unlabeled GABA (final concentration, 0.25 μM), and diisopropylfluorophosphate (final concentration, 10 μM), an irreversible cholinesterase inhibitor, were then added to the suspension, and the suspension was incubated for another 10 min. Aliquots (80 μl) were collected with gentle suction onto 6-mm diameter A/E glass-fiber filters (Gelman Science, Ann Arbor, MI) on the perfusion apparatus and then perfused with buffer containing 1 g/l bovine serum albumin (1.8 ml/min) for 10 min before fraction collection was started. ACh (0.03-1000 μM) was added to the perfusate for 12 s in the middle of each fraction collection run. The amount of radioactivity in each fraction was then determined with scintillation spectrometry (45% counting efficiency). Atropine (1 μM) was included in the perfusion buffer to block any possible muscarinic responses. NO-711 (100 nM), a potent (Kd ∼6 nM) and selective antagonist of the neuronal GABA transporter (Borden, 1996), was also added to the perfusion buffer to prevent calcium-independent [3H]GABA release due to reversal of the GABA transporter by large concentrations of ACh (data not shown).
Data Analysis. The amount of ACh-stimulated [3H]GABA release was determined in 12-s fractions. The data were normalized using Sigma Plot 5.0 (Systat Software, Inc., San Jose, CA) as described by Grady et al. (1992). In short, the fractions recorded in the absence of agonist are fit to a single-phase exponential decay equation to obtain a measure of baseline release. The stimulated release was divided by the calculated baseline release during the time of stimulation to calculate “response units,” where one unit is defined as twice basal release. The responses at each time interval of stimulation were then summed to yield the total response for a given agonist exposure. For comparisons between genotypes, the mean value of a near-maximal concentration of ACh (30 μM) for each brain region was used as a further normalizing factor, and all were responses plotted as a percentage of this control value. Concentration-effect curves for [3H]-GABA release were plotted and fit with the four-parameter hyperbolic equation f = VS/(K + S) + vS/(k + S), where S is agonist concentration, maximum response for high and low-sensitivity components are V and v, respectively, and half-maximal agonists concentrations for high- and low-sensitivity components are represented by K and k, respectively. Curve-fits were performed using SigmaPlot (SigmaPlot DOS or SigmaPlot 2001), and an F test was conducted on each data set to ensure that the four-parameter equation was statistically preferred. To obtain concentration-response parameters, initial curve-fits were calculated from mean concentration-response curves, and the theoretical values were applied to each individual experiment to generate a series of individual experiment curve-fits. Significant differences (p < 0.05) between two-group data sets were determined with univariate analysis of variance (SPSS 16.2). Both raw data values and calculated curve-fit parameters were analyzed with ANOVA to assess genotype differences that may be evident across a concentration-response curve but less apparent when calculated from theoretical curve-fit parameters. When EC50 values were compared, their log values were used. This was done because log(EC50) values are normally distributed, but EC50 values are not (Hancock et al., 1988).
Results
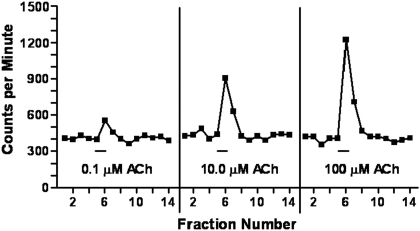
Concentration-Dependent Nature of ACh-Stimulated [3H]GABA Release. Figure 1 presents a typical data trace. The data are presented as cpm of radioactivity obtained when hippocampal synaptosomes loaded with [3H]GABA were perfused with increasing concentrations of ACh. As higher ACh concentrations were used, more [3H]GABA release (units above baseline) was observed. Similar results were obtained with synaptosomes obtained from the other brain regions. These data are consistent with those reported by Lu et al. (1998).
Fig. 1.
Representative data of ACh-stimulated [3H]GABA release from hippocampal synaptosomes. A total of 23 10-s fractions were taken from each synaptosomal sample, and the radioactivity in each fraction was measured in a scintillation spectrometer. Thus, these data are presented as cpm. The synaptosomes were stimulated with a range of ACh concentrations (0.03-1000 μM) for 12 s in the middle of each run (hash marks beneath each trace). The fractions before and after the stimulation were then used to determine the baseline [3H]GABA release, which was subsequently used to determine the size of the response (see Materials and Methods for details). The size of the responses increased in a concentration-dependent manner.
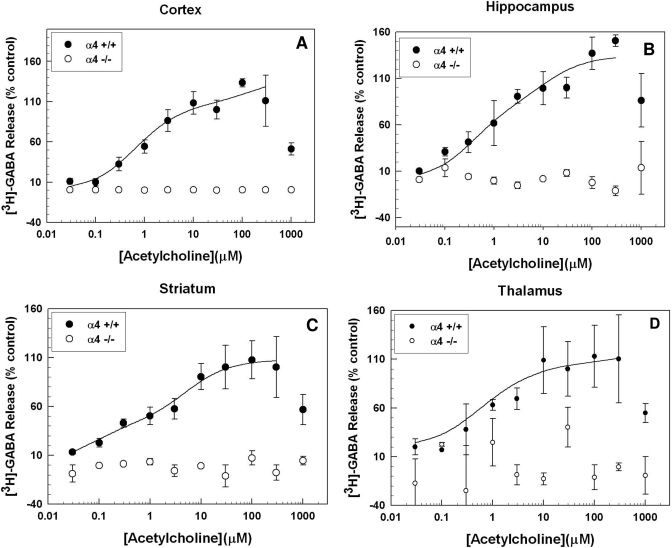
Effects of α4, α5, and β2 Gene Deletion on ACh-Stimulated [3H]GABA Release. Figure 2 illustrates that null mutation of Chrna4, the gene encoding the α4 subunit, resulted in a complete loss of ACh-stimulated GABA release from synaptosomes prepared from all four of the brain regions. Acetylcholine produced a concentration-dependent increase in GABA release in all four of the brain regions, but the maximal responses of the brain regions differed. High concentrations (0.3 and 1 mM) often produced a marked decrease in release from that seen at the determined maximally efficacious concentration (i.e., the concentration effects were of the inverted “U” type). These effects were most likely caused by agonist “channel block,” in which the agonist accumulates in the ion channel and prevents cation flux, even though the receptor is in its active conformation (Zwart and Vijverberg, 1998). In all cases, a minimum of three to four log units of ACh was required to progress from minimal to maximal release. This suggests that more than one receptor subtype may be mediating this response. Therefore, the data were analyzed to determine whether they fit one- or two-site models. The ACh concentration-effect curves in wild-type animals were best fit by a two-site model in all brain regions examined (F test, p < 0.05). The calculated curve-fit parameters [Rmax (maximal release) and EC50 (concentration that elicited half-maximal release)] for the cortex, hippocampus, striatum, cortex, and thalamus are presented in Table 1.
Fig. 2.
Effect of α4 nAChR subunit gene deletion on ACh-evoked [3H]GABA release. Chrna4 gene deletion resulted in a total loss of ACh-stimulated [3H]GABA release from synaptosomes made from the cortex (A), hippocampus (B), striatum (C), and thalamus (D). Animals lacking the α4 nAChR subunit did not exhibit a concentration-dependent response to ACh in any of the four brain regions; n = 2 to 8 for each data point in each graph. Error bars represent S.E.M.
TABLE 1.
Effects of gene deletion on high- and low-sensitivity components of ACh-evoked [3H]GABA release
Rmax and EC50 values were calculated from concentration-response curves as described under Materials and Methods. Each value represents the mean ± S.E.M. as compiled from four to eight individual concentration-effect curves for each genotype. The two components of the concentration-response curve are designated as high-ACh sensitivity (HS) and low-ACh sensitivity (LS).
| Genotype | Rmax (HS) | logEC50 (HS) | Rmax (LS) | logEC50 (LS) |
|---|---|---|---|---|
| μM | μM | |||
| Cortex | ||||
| α4(+/+) | 100.31 ± 11.80 | −0.17 ± 0.098 | 39.78 ± 25.21 | 1.40 ± 0.480 |
| α4(−/−) | ||||
| β2(+/+) | 80.58 ± 29.56 | −0.44 ± 0.381 | 15.06 ± 15.05 | 1.35 ± 0.66 |
| β2(−/−) | ||||
| α5(+/+) | 84.45 ± 13.16 | −0.32 ± 0.171 | 50.10 ± 10.36 | 0.93 ± 0.45 |
| α5(−/−) | 40.15 ± 6.48 | −0.66 ± 0.14 | 56.37 ± 17.85 | 1.31 ± 0.52 |
| Hippocampus | ||||
| α4(+/+) | 81.28 ± 16.13 | −0.34 ± 0.199 | 53.03 ± 16.72 | 2.14 ± 0.28 |
| α4(−/−) | ||||
| β2(+/+) | 77.98 ± 6.46 | −0.44 ± 0.13 | 105.28 ± 39.65 | 2.20 ± 0.23 |
| β2(−/−) | ||||
| α5(+/+) | 69.40 ± 10.92 | −3.42 ± 2.70 | 61.24 ± 7.11 | 1.27 ± 0.53 |
| α5(−/−) | 51.30 ± 14.31 | −1.98 ± 1.43 | 51.23 ± 9.84 | 1.20 ± 0.20 |
| Striatum | ||||
| α4(+/+) | 42.83 ± 4.31 | −1.12 ± 0.15 | 64.98 ± 24.01 | 1.00 ± 0.55 |
| α4(−/−) | ||||
| β2(+/+) | 48.10 ± 8.03 | −1.67 ± 0.62 | 60.35 ± 9.02 | 0.60 ± 0.21 |
| β2(−/−) | ||||
| α5(+/+) | 51.79 ± 6.96 | −0.73 ± 0.18 | 63.17 ± 12.12 | 0.998 ± 0.12 |
| α5(−/−) | 51.18 ± 9.37 | −0.46 ± 0.18 | 41.61 ± 13.68 | 0.82 ± 0.47 |
| Thalamus | ||||
| α4(+/+) | 54.30 ± 10.31 | −2.59 ± 2.10 | 62.98 ± 25.91 | 1.16 ± 0.62 |
| α4(−/−) | ||||
| β2(+/+) | 40.58 ± 8.24 | −2.37 ± 1.29 | 75.72 ± 17.67 | 0.94 ± 0.44 |
| β2(−/−) | ||||
| α5(+/+) | 41.54 ± 5.23 | −0.33 ± 0.20 | 82.71 ± 22.09 | 1.46 ± 0.21 |
| α5(−/−) | 47.17 ± 12.00 | −2.75 ± 2.13 | 47.10 ± 17.77 | 1.00 ± 0.40 |
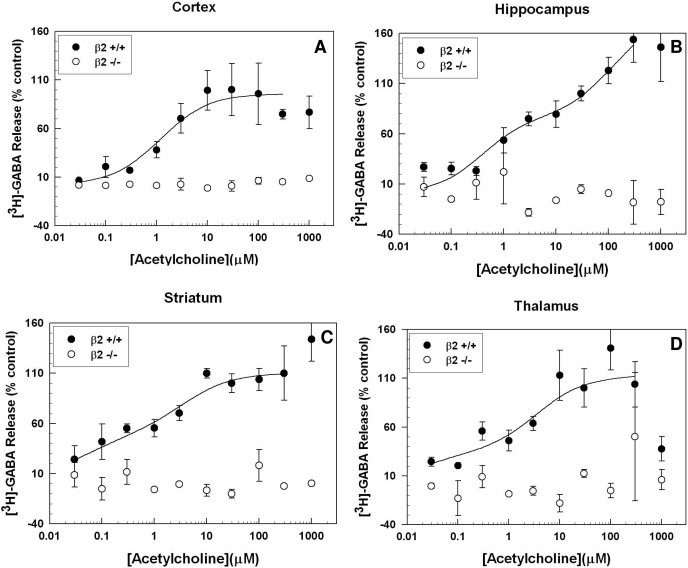
Figure 3 shows that null mutation of Chrnb2, the gene encoding the β2 subunit, also resulted in a total elimination of ACh-stimulated [3H]GABA release. The effects of β2 knockout were statistically significant in all four brain regions. The ACh concentration-effect curves for all four brain regions in wild-type animals were best fit by a two-site model (F test, p < 0.05). The calculated curve-fit parameters for the hippocampus, striatum, cortex, and thalamus are presented in Table 1.
Fig. 3.
Effect of β2 nAChR subunit gene deletion on ACh-evoked [3H]GABA release. ACh-stimulated [3H]GABA release was not detected in synaptosomes made from the cortex (A), hippocampus (B), striatum (C), or thalamus (D) of Chrnb2-null mutant mice. As with α4-null mutants, animals lacking the β2 nAChR subunit did not exhibit a concentration-dependent GABA release response to ACh in any of the four brain regions studied; n = 1 to 3 for each data point in each graph. Error bars represent the S.E.M.
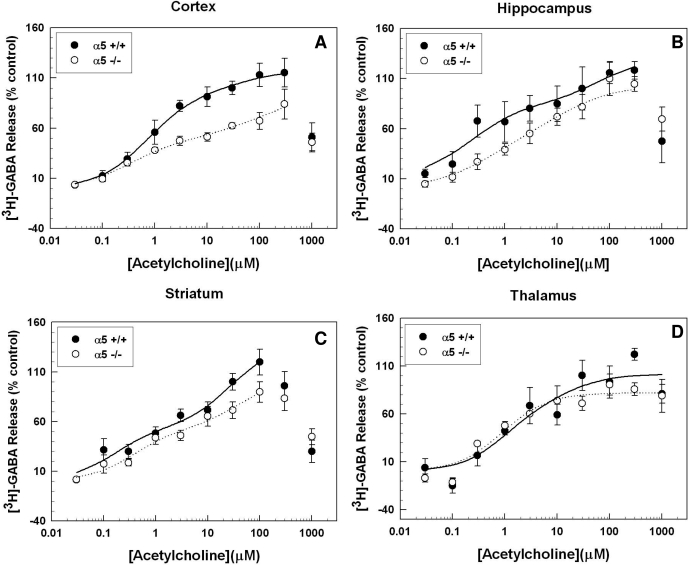
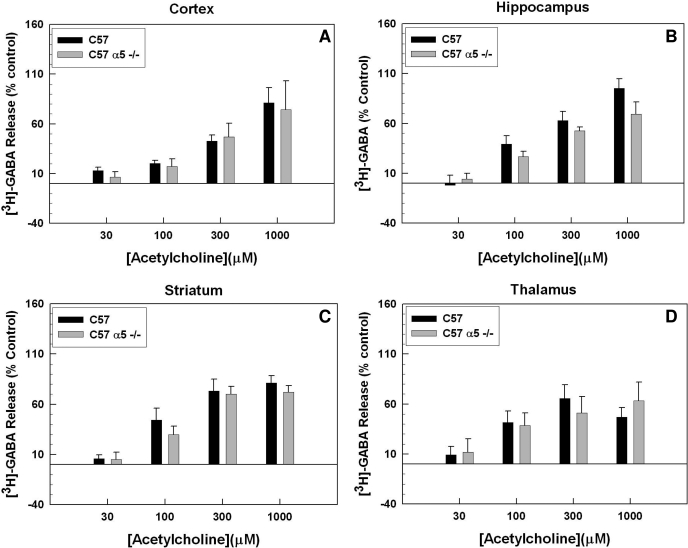
Figure 4 demonstrates that deletion of Chrna5, the gene encoding the α5 subunit, caused robust decreases in ACh-stimulated [3H]GABA release in the cortex (F1,118 = 24.95; p < 0.001). Deletion of the α5 subunit also produced a modest but significant decrease in [3H]GABA release in the striatum and hippocampus (F1,152 = 7.67, p < 0.007; F1,132 = 17.12, p < 0.001, respectively). No observable effect of α5 gene deletion was observed in thalamic synaptosomes (F1,90 = 0.76, p = 0.390). The ACh concentration-effect curves for all four brain regions in both wild-type and knockout animals were best fit by a two-site model (F test, p < 0.05). The calculated curve-fit parameters for the cortex, thalamus, striatum, and hippocampus are presented in Table 1. As noted in the table, α5 gene deletion resulted in no change in EC50 values in the cortex, hippocampus, or striatum. The Rmax for the high-affinity component was significantly reduced in cortex only. The calculated Rmax values for hippocampus and striatum were not significantly different from control, but the ANOVAs performed on the entire concentration-response curves did detect a significant effect of genotype, with a trend toward a decrease in the high-affinity component. Deletion of the α5 subunit did not produce any significant changes in the maximal response or EC50 values for the low-affinity component.
Fig. 4.
Effect of α5 nAChR subunit gene deletion on ACh-evoked [3H]GABA. Chrna5 gene deletion generally resulted in a decrease of ACh-stimulated [3H]GABA release from synaptosomes made from the cortex (A), hippocampus (B), striatum (C), and thalamus (D). The decreased response in the α5-null mutants was readily observable in the cortex, diminished in the striatum and hippocampus, and was not significant in the thalamus; n = 2 to 11 for each data point in each graph. Error bars represent the S.E.M.
High- and low-sensitivity α4β2*-type nAChRs differ in their sensitivity to antagonism by the competitive inhibitor dihydro-β-erythroidine (DHβE) (Marks et al., 1999). At low concentrations (≤ 2 μM), only the high-sensitivity fraction is blocked, allowing for a pharmacological isolation of the receptor responses observed. In synaptosomes from α5-null mutant mice, DHβE(2 μM) blocked the high-sensitivity component of ACh-evoked [3H]GABA; removal of the α5 gene did not alter the low-sensitivity α4β2 responses to ACh (Fig. 5).
Fig. 5.
Effect of α5 nAChR subunit gene deletion on DHβE-resistant nAChR function measured with ACh-evoked [3H]GABA release. ACh-stimulated [3H]GABA release in the presence of 2 μM DHβE from cortex (A), hippocampus (B), striatum (C), and thalamus (D) shows no effect of Chrna5 gene deletion (p > 0.05). Error bars represent S.E.M..
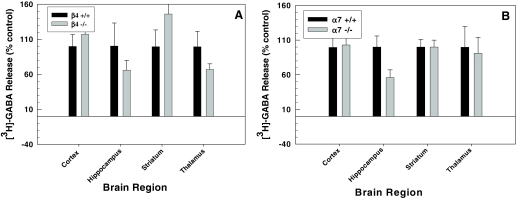
Deletion of either the β4 or α7 subunit did not result in any measurable changes in [3H]GABA release evoked by ACh (30 μM) in any of the four brain regions screened (Fig. 6, A and B, respectively). Thus, no further experimentation proceeded with these animals.
Fig. 6.
Effects of β4 and α7 subunit null mutation on 30 μM ACh-evoked [3H]GABA release from cortex, hippocampus, striatum, and thalamus. No statistically significant effect of gene deletion was observed.
Discussion
Null mutation of either Chrna4 or Chrnb2 totally eliminated ACh-stimulated [3H]GABA release from every brain region studied. These null mutations also eliminate [3H]-nicotine binding (Picciotto et al., 1995; Marubio et al., 1999) and cytisine-sensitive [3H]epibatidine binding (Marks et al., 2007) throughout the brain. We interpreted the binding results to indicate that α4β2*-type receptors are the major nAChR subtype that binds these ligands with high affinity. By analogy, we conclude that α4β2*-type nAChRs modulate GABA release from the presynaptic nerve terminals in the four brain regions that were studied.
The International Union of Basic and Clinical Pharmacology receptor nomenclature committee (Lukas et al., 1999) recommended that nAChRs should be named by including the Greek letters for known subunits along with an asterisk (*) to designate the potential contribution of other subunits. We found that Chrna5 gene deletion resulted in decreased maximal ACh-evoked [3H]GABA release from cortical, striatal, and hippocampal synaptosomes with differing degrees of effect. This outcome suggests that α4α5β2-type nAChRs play regionally distinct modulatory roles in ACh-evoked GABA release. Chrna7 and Chrnb4 gene deletion did not affect ACh-evoked [3H]GABA release. These observations indicate that receptors which include these subunits are not significant contributors to [3H]GABA release from presynaptic nerve terminals in the four brain regions that were studied.
The finding that both α4 and β2 gene deletion eliminated [3H]GABA release from synaptosomes prepared from all four brain regions examined is not surprising given that the mRNAs for both of these subunits are expressed in high concentrations in these brain regions (Marks et al., 1992). Likewise, it is not surprising that Chrna5 deletion resulted in a change in cortical [3H]GABA release given that single-cell reverse-transcription polymerase chain reaction analyses detected α5 mRNA expression in some but not all cortical GABAergic neurons (Porter et al., 1999). It should be noted, however, that in situ hybridization measures mRNA that is expressed primarily in cell bodies.
Chrna5 gene deletion had a small effect on ACh-stimulated [3H]GABA release from striatal and hippocampal synaptosomes. The in situ hybridization experiments indicate that α4, α5, and β2 mRNAs are all expressed in both of these brain regions. However, the expression pattern for α5 mRNA is limited compared with those observed for α4 and β2 mRNA. These findings may explain why we obtained evidence that suggests both α4β2 and α4α5β2 nAChRs are expressed on GABAergic nerve terminals in three of the four brain regions examined. This correlation between Chrna5 mRNA expression and α5-null mutation effect on ACh-evoked [3H]GABA release could also explain the lack of α5 subunit removal on [3H]GABA release measured from thalamic synaptosomes, because there is no appreciable α5 mRNA expressed in that region. Therefore, regional effects of Chrna5 gene deletion could reflect preferential loss of activity in local GABAergic interneuron populations.
Many studies have used electrophysiological methods to study nicotinic modulation of GABA neuron activity, and many of these studies have focused on the hippocampus (Alkondon and Albuquerque, 1993; Alkondon et al., 1997; Kawai et al., 2002), but nicotinic modulation of GABAergic neurons has also been studied in the cortex (Alkondon et al., 2000), thalamus (Léna and Changeux, 1997), and dopaminerich regions such as the midbrain (Klink et al., 2001), the ventral tegmental area (Mansvelder and McGehee, 2000), and the nucleus accumbens (de Rover et al., 2002). Many of these studies found that pretreatment with the nAChR antagonist DHβE blocked cholinergic activation of GABAergic neurons, leading to the conclusion that α4β2-type nAChRs influence the activity of GABAergic neurons. Electrophysiological analyses of the potential roles of nAChRs containing the α5 subunit in modulating GABAergic function have not been published. Our results suggest that such studies will yield different results depending on the brain region studied.
Many of the electrophysiological studies also demonstrated a role for α7-containing nAChRs in the modulation of somatodendritic activities of GABAergic neurons. The lack of an effect of α7 subunit gene deletion on [3H]GABA release from synaptosomal preparations suggests that α7-containing nAChRs present on GABAergic neurons are expressed preferentially in somatodentritic locations, whereas the dominant presynaptic nAChR expressed by GABAergic neurons is the α4β2* type.
It is exceedingly likely that our synaptosomal preparation contains a heterogeneous population of nerve terminals, with contributions to the overall pool being provided by many potentially distinct subregions. We found that null mutation of Chrna5 significantly decreases the calculated Rmax for ACh in cortex. The Rmax for ACh-stimulated GABA release was not significantly altered in striatum and hippocampus, but the ANOVA, which is more sensitive because it analyzes effects over the entire concentration-effect curve, did detect a significant effect of gene deletion. Thus, deleting the α5 subunit produces a more subtle effect in striatum and hippocampus. No differences in agonist potency were observed in any brain region studied. In agreement with a previously published functional assessment of Chrna5 gene deletion using 86Rb+ efflux, it seems that the loss of the α5 subunit results in a preferential decrease in the maximal response of high ACh-sensitivity nAChRs with no subsequent loss of total receptor number (Brown et al., 2007). In addition, isolation of the low-sensitivity α4β2 functional contribution by examining ACh-evoked [3H]GABA release in the presence of 2 μM DHβE (Marks et al., 1999) showed no differences between α5 wild-type and null-mutant animals in any brain region examined. These results are consistent with studies comparing the functional properties of α4β2 and α4α5β2 nAChRs in heterologous expression systems (Kuryatov et al., 2008) and suggest that the α4α5β2-type nAChR expressed on GABAergic nerve terminals is uniquely capable of influencing neurotransmitter release. This enhanced functionality may be due to distinct biophysical properties, such as the enhanced ion (including Ca2+) conductance of α4α5β2 nAChRs compared with high-affinity nAChRs containing α4 and β2 subunits only.
The biphasic responses for α4β2* nAChRs have been described previously in 86Rb+ efflux studies (Marks et al., 1999; Brown et al., 2007) and in electrophysiological measurements of heterologous expression systems (Zwart and Vijverberg, 1998; Moroni et al., 2006). It has been suggested that the high- and low-affinity components represent receptors with different stoichiometries: (α4)2(β2)3 (high affinity), and (α4)3(β2)2 (low affinity) (Zwart and Vijverberg, 1998; Marks et al., 2007). It is possible that the loss of the α5 subunit results in the compensatory formation of receptors with the stoichiometry (α4)2(β2)3 that have an equally high affinity for ACh but are distinctly less efficacious at eliciting neurotransmitter release than the α4α5β2 receptor. This event could explain the selective reduction in the high-sensitivity component of ACh-evoked [3H]GABA release without any significant decrease in low-sensitivity α4β2 nAChR function or loss of receptor number as a whole, which was measured by receptor binding assays in α5-null mutant animals (Brown et al., 2007).
Conclusions
The α4 and β2 nAChR subunits are clearly necessary for nicotinic agonist-stimulated GABA release in all four of the brain regions examined. Chrna5 gene deletion does not result in a total loss of ACh-stimulated GABA release in any brain region. However, it is frequently included along with α4 and β2 subunits, and the absence of the α5 subunit decreases overall nAChR-mediated [3H]GABA release that is restricted to an effect on the high-affinity component of the functional profile. These findings suggest that α4α5β2 nAChRs are important modulators of [3H]GABA release from striatal, hippocampal, and cortical synaptosomes. Furthermore, α5-containing nAChRs are distinct in their regional distribution and functionality. Thus, nAChRs containing the α5 subunit may serve as therapeutic targets with some selectivity for distinct neuronal pathways.
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grants AA13108, AA13465]; the National Institutes of Health National Cancer Institute [Grant CA089392]; and the National Institutes of Health National Institute on Drug Abuse [Grant DA015663].
ABBREVIATIONS: nAChR, nicotinic acetylcholine receptor; NO-711, 1-[2-[[(diphenylmethylene)imino]oxy]ethyl]-1,2,5,6-tetrahydro-3-pyridinecarboxylic acid hydrochloride; ANOVA, analysis of variance; ACh, acetylcholine; DHβE, dihydro-β-erythroidine.
References
- Alkondon M and Albuquerque EX (1993) Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther 265 1455-1473. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Barbosa CT, and Albuquerque EX (1997) Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther 283 1396-1411. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, and Albuquerque EX (2000) Nicotinic receptor activation in human cerebral cortical interneurons: a mechanism for inhibition and disinhibition of neuronal networks. J Neurosci 20 66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden LA (1996) GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int 29 335-356. [DOI] [PubMed] [Google Scholar]
- Brown RW, Collins AC, Lindstrom JM, and Whiteaker P (2007) Nicotinic alpha5 subunit deletion locally reduces high-affinity agonist activation without altering nicotinic receptor numbers. J Neurochem 103 204-215. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przyblski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, et al. (2003) Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knockout mice. J Neurosci 23 7820-7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Schwartz RD, Paul SM, Pert CB, and Pert A (1985) Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci 5 1307-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, Salminen O, Tritto T, Butt CM, Allen WR, et al. (2003) The beta3 nicotinic receptor subunit: a component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J Neurosci 23 11045-11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rover M, Lodder JC, Kits KS, Schoffelmeer AN, and Brussaard AB (2002) Cholinergic modulation of nucleus accumbens medium spiny neurons. Eur J Neurosci 16 2279-2290. [DOI] [PubMed] [Google Scholar]
- Gahring LC, Persiyanov K, Dunn D, Weiss R, Meyer EL, and Rogers SW (2004) Mouse strain-specific nicotinic acetylcholine receptor expression by inhibitory interneurons and astrocytes in the dorsal hippocampus. J Comp Neurol 468 334-346. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Clementi F, Riganti L, McIntosh JM, Collins AC, Marks MJ, and Whiteaker P (2005) Expression of nigrostriatal α6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by β3 subunit gene deletion. Mol Pharmacol 67 2007-2015. [DOI] [PubMed] [Google Scholar]
- Grady S, Marks MJ, Wonnacott S, and Collins AC (1992) Characterization of nicotinic receptor-mediated [3H]dopamine release from synaptosomes prepared from mouse striatum. J Neurochem 59 848-856. [DOI] [PubMed] [Google Scholar]
- Hancock AA, Bush EN, Stanisic D, Kyncl JJ, and Lin CT (1988) Data normalization before statistical analysis: keeping the horse before the cart. Trends Pharmacol Sci 9 29-32. [DOI] [PubMed] [Google Scholar]
- Kawai H, Zago W, and Berg DK (2002) Nicotinic alpha7 receptor clusters on hippocampal GABAergic neurons: regulation by synaptic activity and neurotrophins. J Neurosci 22 7903-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d'Exaerde A, Zoli M, and Changeux JP (2001) Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 21 1452-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, and Lindstrom J (2008) Roles of accessory subunits in α4β2(*) nicotinic receptors. Mol Pharmacol 74 132-143. [DOI] [PubMed] [Google Scholar]
- Léna C and Changeux JP (1997) Role of Ca2+ ions in nicotinic facilitation of GABA release in mouse thalamus. J Neurosci 17 576-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J, Peng X, Kuryatov A, Lee E, Anand R, Gerzanich V, Wang F, Wells G, and Nelson M (1998) Molecular and antigenic structure of nicotinic acetylcholine receptors. Ann N Y Acad Sci 841 71-86. [DOI] [PubMed] [Google Scholar]
- Lu Y, Grady S, Marks MJ, Picciotto M, Changeux JP, and Collins AC (1998) Pharmacological characterization of nicotinic receptor-stimulated GABA release from mouse brain synaptosomes. J Pharmacol Exp Ther 287 648-657. [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novère N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, et al. (1999) International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev 51 397-401. [PubMed] [Google Scholar]
- Mansvelder HD and McGehee DS (2000) Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27 349-357. [DOI] [PubMed] [Google Scholar]
- Marks MJ and Collins AC (1982) Characterization of nicotine binding in mouse brain and comparison with the binding of α-bungarotoxin and quinuclidinyl benzilate. Mol Pharmacol 22 554-564. [PubMed] [Google Scholar]
- Marks MJ, Meinerz NM, Drago J, and Collins AC (2007) Gene targeting demonstrates that alpha4 nicotinic acetylcholine receptor subunits contribute to expression of diverse [3H]epibatidine binding sites and components of biphasic 86Rb+ efflux with high and low sensitivity to stimulation by acetylcholine. Neuropharmacology 53 390-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, and Collins AC (1992) Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci 12 2765-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Whiteaker P, Calcaterra J, Stitzel JA, Bullock AE, Grady SR, Picciotto MR, Changeux JP, and Collins AC (1999) Two pharmacologically distinct components of nicotinic receptor-mediated rubidium efflux in mouse brain require the β2 subunit. J Pharmacol Exp Ther 289 1090-1103. [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Léna C, Le Novère N, de Kerchove d'Exaerde A, Huchet M, Damaj MI, and Changeux JP (1999) Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature 398 805-810. [DOI] [PubMed] [Google Scholar]
- Millar NS and Gotti C (2009) Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 56 237-246. [DOI] [PubMed] [Google Scholar]
- Moroni M, Zwart R, Sher E, Cassels BK, and Bermudez I (2006) alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol 70 755-768. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Göldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, and Beaudet AL (1997) Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci 17 9165-9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Léna C, Bessis A, Lallemand Y, Le Novère N, Vincent P, Pich EM, Brûlet P, and Changeux JP (1995) Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature 374 65-67. [DOI] [PubMed] [Google Scholar]
- Porter JT, Cauli B, Tsuzuki K, Lambolez B, Rossier J, and Audinat E (1999) Selective excitation of subtypes of neocortical interneurons by nicotinic receptors. J Neurosci 19 5228-5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF, et al. (2000) Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci 20 6431-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, and De Biasi M (2003) The nicotinic acetylcholine receptor subunit α5 mediates short-term effects of nicotine in vivo. Mol Pharmacol 63 1059-1066. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, and Grady SR (2004) Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol 65 1526-1535. [DOI] [PubMed] [Google Scholar]
- Salminen O, Whiteaker P, Grady SR, Collins AC, McIntosh JM, and Marks MJ (2005) The subunit composition and pharmacology of alpha-Conotoxin MII-binding nicotinic acetylcholine receptors studied by a novel membrane-binding assay. Neuropharmacology 48 696-705. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Cooper JF, Salminen O, Marks MJ, McClure-Begley TD, Brown RW, Collins AC, and Lindstrom JM (2006) Immunolabeling demonstrates the interdependence of mouse brain alpha4 and beta2 nicotinic acetylcholine receptor subunit expression. J Comp Neurol 499 1016-1038. [DOI] [PubMed] [Google Scholar]
- Wonnacott S (1997) Presynaptic nicotinic ACh receptors. Trends Neurosci 20 92-98. [DOI] [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, Patrick JW, Role LW, Beaudet AL, and De Biasi M (1999) Multiorgan autonomic dysfunction in mice lacking the beta2 and the beta4 subunits of neuronal nicotinic acetylcholine receptors. J Neurosci 19 9298-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R and Vijverberg HP (1998) Four pharmacologically distinct subtypes of alpha4beta2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol Pharmacol 54 1124-1131. [PubMed] [Google Scholar]