Abstract
Through a survey of the phylogenetic distribution of sialidase among mycoplasmas, we detected activity secreted by the type strains of three of eleven species frequently or first isolated from dogs. The specific activity of washed cells of the type strains of Mycoplasma canis, Mycoplasma cynos, and Mycoplasma molare ranged from 5.2 ± 0.8 × 10-6 to 1.1 ± 0.3 × 10-5 enzymatic units per colony-forming unit (U/CFU). Cells of M. molare strain H542T had twice the specific activity (P < 0.05) of M. canis strain PG14T or M. cynos strain H831T. Significant differences in sialidase activity existed among nine clinical isolates of M. canis, ranging from not detectable to 2.1 ± 0.1 × 10-5 U/CFU. The type strains of other species previously isolated from dogs (Mycoplasma arginini, Mycoplasma bovigenitalium, Mycoplasma edwardii, Mycoplasma felis, Mycoplasma gatae, Mycoplasma maculosum, Mycoplasma opalescens, and Mycoplasma spumans) did not exhibit either secreted or cell-associated sialidase activity. Neither specific nor degenerate PCR primers complementary to the three known mycoplasmal sialidase alleles were able to amplify orthologs in M. canis, M. cynos, or M. molare, further evidence that the secreted sialidase of those species is distinct from the strictly cell-associated sialidases of Mycoplasma alligatoris, Mycoplasma synoviae, and Mycoplasma gallisepticum. This is the first report of a well-known bacterial virulence factor whose expression varies among strains of certain Mycoplasma species that infect dogs.
1. Introduction
Canine mycoplasmosis is associated with multiple species of Mycoplasma. The most firmly established pathogen is Mycoplasma cynos (Røsendal, 1973), which was isolated from fatal infections of puppies, and proven to be a primary respiratory pathogen by experimental infection studies (Rosendal, 1982; Zeugswetter et al., 2007). Mycoplasma canis (Edward, 1955) is best known as an opportunistic pathogen of dogs, and has been recovered from their respiratory, urinary and reproductive tracts. Unlike M. cynos, M. canis often infects other mammalian hosts. For example, M. canis was isolated from the lower respiratory tracts of humans with pneumonia (Armstrong et al., 1971) and both healthy and pneumonic cattle (Ayling et al., 2004), although studies of M. canis strain C3b as a primary pathogen of cattle had equivocal results (ter Laak et al., 1993). Mycoplasma molare (Røsendal, 1974) and several other species isolated from dogs are also potential pathogens (Chalker, 2005).
Sialidase is a virulence factor of diverse microorganisms. It promotes microbial colonization, tissue invasion, and damage to sialylated host molecules, cell surfaces and the extracellular matrix (Corfield, 1992). Although Mycoplasma alligatoris strain A21JP2T, virulent strains of Mycoplasma gallisepticum and Mycoplasma synoviae, and some strains of other avian mycoplasmas produce cell-associated sialidases (Brown et al., 2004; Berčič et al., 2008a; May and Brown, 2008), the distribution of sialidase among other species of Mycoplasma has not been investigated systematically. As part of a broader survey, we detected secreted sialidase activity in three of eleven species isolated from dogs. This is the first report of a well-known bacterial virulence factor occurring in pathogenic species of Mycoplasma that infect dogs.
2. Materials and Methods
2.1 Mycoplasma isolates and culture techniques
The type strains Mycoplasma arginini G230T, Mycoplasma bovigenitalium PG11T, M. canis PG14T, M. cynos H831T, Mycoplasma edwardii PG24T, Mycoplasma felis COT, Mycoplasma gatae CST, Mycoplasma maculosum PG15T, M. molare H542T, Mycoplasma opalescens MH5408T, and Mycoplasma spumans PG13T were obtained from the American Type Culture Collection. Five isolates collected from Shetland sheepdogs with various reproductive disorders, and four isolates from dogs with unknown clinical history, which were obtained during a prior survey (M.B. Brown, unpublished), were identified as M. canis by PCR-RFLP screening (Spergser and Rosengarten, 2007) confirmed by direct 16S rRNA gene sequencing (May et al., 2007).
M. canis, M. cynos, M. edwardii, M. felis, and M. molare were propagated in SP-4 medium containing 0.5% w/v glucose. M. arginini, M. bovigenitalium, M. gatae, M. maculosum, M. opalescens, and M. spumans were propagated in SP-4 medium containing 0.5% w/v glucose plus 0.21% w/v L-arginine. All cultures were incubated at 37 °C under ambient atmospheric conditions. Culture concentrations were determined by inoculating serial 10-fold dilutions in SP-4 broth onto agar. Colonies were counted after 5 days of incubation.
2.2 Measurement of sialidase activity
Sialidase activity of washed cells was quantitated using the fluorogenic substrate 2′-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (MUAN; Sigma-Aldrich, St. Louis MO) and the sialidase inhibitor 2-deoxy-2,3-didehydro-N-acetylneuraminic acid (DANA; Sigma-Aldrich) as previously described (May et al., 2007). The positive control was M. alligatoris, which expresses cell-associated but not secreted sialidase. The negative controls were Mycoplasma crocodyli strain MP145T, which does not express any sialidase (Brown et al., 2004), and fresh culture medium. Secreted sialidase activity was detected qualitatively in late log-phase broth cultures, after the cells were harvested, by diluting the cell-free conditioned supernatant medium 1:1 with 0.35% w/v MUAN, then observing the specimen under shortwave ultraviolet illumination. The positive control was Type VI purified Clostridium perfringens sialidase in cell-free M. crocodyli-conditioned medium, and the negative control was plain cell-free M. crocodyli-conditioned medium.
2.3 PCR amplification of sialidase genes
PCR primers complementary to the cell-associated sialidase gene nanI sequences of M. alligatoris (GenBank AY515695; 5′-TGA CAA AAT GCG CTG AAA AA-3′ and 5′-GCG CCA AAT TTA CAT CCT ACA-3′), M. synoviae (GenBank NC_007294; 5′-TCT CTT CCT TTT TGA GGG CTA-3′ and 5′-GCA AAT CAT CTT AAG AAA AGT CAT T-3′), and M. gallisepticum (GenBank NC_004829; 5′-TCA GAT CAT TAA ACT AGC GCC TAA-3′ and 5′-CGC ATG ATA CGA TAA CGA AAT G-3′), and degenerate primers designed from consensus sequences among those alleles (5′-GA(CT) G(AG)I GGI (ACT)(AT)I (AT)(GC)I TG(AG)-3′ and 5′-CAI (GCT)(AT)I (AGT)(CT)I CCI CC(AG) TC-3′) were used in attempts to detect the sialidase genes in M. canis, M. cynos, and M. molare. Genomic DNA templates were purified using EasyDNA reagents (Invitrogen, Carlsbad CA). The 50-μl reactions included 1 μmol of each primer and 100 ng of template DNA, and consisted of initial template denaturation at 94 °C for 2 min, 30 cycles of denaturation at 94 °C for 20 sec, primer annealing at 48 °C for 20 sec, and extension at 72 °C for 3 min, with a final extension at 72 °C for 10 min. Reactions using degenerate primers were performed with an annealing temperature of 38 °C. The positive control template was M. gallisepticum strain Rlow genomic DNA.
2.4 Statistical analysis
The effect of species on the specific sialidase activity of washed M. canis, M. cynos, and M. molare cells, and the effect of strain within M. canis, were analyzed by analyses of variance (n = 3 independent replications each), and by Fisher's Protected Least Significant Difference test for posthoc comparisons among species or strains when main effects were significant (May and Brown, 2008). P values < 0.05 were considered significant.
3. Results
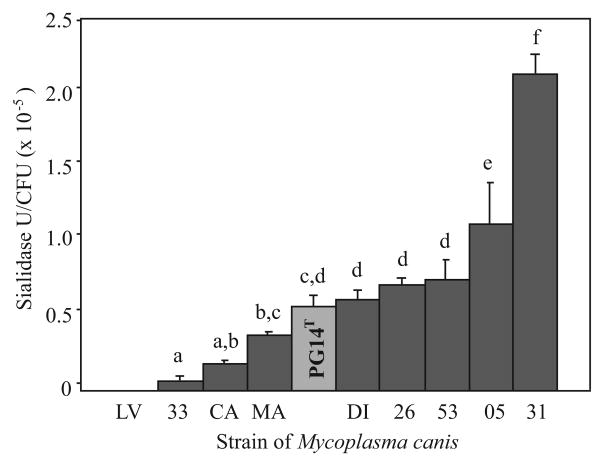
As part of a survey of the genus Mycoplasma, this study sought to detect the presence of sialidase in primary and opportunistic mycoplasmal pathogens that infect dogs. Sialidase activity was evident in washed cells of the type strains of M. canis, M. cynos, and M. molare. The specific activity ranged from 5.2 ± 0.8 × 10-6 to 1.1 ± 0.3 × 10-5 U/CFU (Table 1). M. molare strain H542T had twice as much activity (P < 0.05) as M. canis strain PG14T or M. cynos strain H831T. Nine clinical isolates of M. canis were subsequently examined to sample the extent of intraspecific variation. Washed cells of those isolates ranged in specific activity (P < 0.0001) from 0 (undetectable) for strain LV, to 2.1 ± 0.1 × 10-5 U/CFU for strain 31 (Fig. 1).
Table 1.
Sialidase-positive mycoplasmas.
| Mycoplasma Species | Strain | Sialidase Activitya | Host Class | Phylogenetic Cluster | Reference |
|---|---|---|---|---|---|
| M. alligatoris | A21JP2T | 10-9 U/CFU | reptile | M. synoviae | Brown et al., 2004 |
| M. anseris | 1219T | moderate | bird | M. hominis | Berčič et al., 2008a |
| M. canisb,c | PG14T | 10-6 U/CFU | mammal | M. synoviae | current report |
| M. cloacale | 383 | weak | bird | M. hominis | Berčič et al., 2008a |
| M. corogypsi | BV1T | very strong | bird | M. synoviae | Berčič et al., 2008a |
| M. cynos | H831T | 10-6 U/CFU | mammal | M. synoviae | current report |
| M. gallisepticumb,c | R | 10-8 U/CFU | bird | M. pneumoniae | May and Brown, 2008 |
| M. iowae serovar I | 695T | weak | bird | M. muris | Berčič et al., 2008a |
| M. meleagridisc | 17529T | strong | bird | M. bovis | Berčič et al., 2008a |
| M. molare | H542T | 10-5 U/CFU | mammal | M. neurolyticum | current report |
| M. synoviaeb,c | WVU1853T | 10-7 U/CFU | bird | M. synoviae | May et al., 2007 |
Highest reported specific activity (enzymatic units per colony-forming unit) or semi-quantitative estimate for washed cells.
Species with multiple strains documented to exhibit sialidase activity.
Species with some strains documented to lack sialidase activity.
Fig. 1.
Intraspecific variation in sialidase activity of washed Mycoplasma canis cells. Bars depict mean ± standard error of enzyme units (U) per colony-forming unit (CFU) of clinical isolates and the type strain PG14T. Means with a different letter are different (P < 0.05 to P < 0.001) by Fisher's Protected Least Significant Difference test (n = 3 independent replications each). The clinical isolate LV had no activity.
Sialidase activity was also present unexpectedly in cell-free conditioned supernatant broth from cultures of M. cynos H831T, M. molare H542T (Fig. 2A), and all strains of M. canis except LV (Fig. 2B). To investigate whether the secreted activity is conferred by an alternative form of the known mycoplasmal sialidase alleles, conserved and degenerate PCR primers were used in attempts to detect orthologous genes, but no amplification was successful from the genomic DNA of M. cynos, M. molare, or any strain of M. canis. No sialidase activity was detected in the other species examined.
Fig. 2.
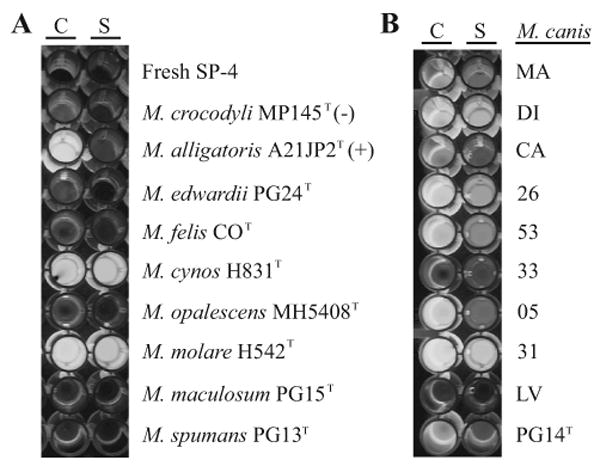
Secreted sialidase activity of canine mycoplasmas. (A) Sialidase was detected by release of fluorescent 4-methylumbelliferone from labeled N-acetylneuraminic acid. Activity was present in washed cells (C) and cell-free conditioned supernatant SP-4 broth (S) from cultures of Mycoplasma cynos H831T and Mycoplasma molare H542T. No activity was detected in the type strains of other species isolated from dogs. Mycoplasma alligatoris A21JP2T was the positive control for strictly cell-associated activity; Mycoplasma crocodyli MP145T and fresh SP-4 broth were negative controls. (B) Sialidase activity was present in Mycoplasma canis PG14T and eight clinical isolates of M. canis. Isolate LV had no activity.
4. Discussion and conclusion
Sialidase was formerly considered to be rare among mycoplasmas (Kahane et al., 1990), but its presence has now been documented in eleven species. All mycoplasmas currently known to produce sialidase either are affiliated with the M. synoviae phylogenetic cluster on the basis of 16S rRNA gene similarity (Johansson and Pettersson, 2002), or they share a common host with one or more sialidase-positive species in that cluster (Table 1). Consistent with that observation, M. canis and M. cynos of the M. synoviae cluster share the secreted form of sialidase with M. molare, a species affiliated with the phylogenetically distant Mycoplasma neurolyticum cluster.
We interpret the activity in washed cells of M. canis, M. cynos and M. molare to reflect newly-synthesized enzyme with the potential to be secreted (Fig. 2), although it seems possible that distinct secreted and cell-associated sialidases occur together in some instances. The finding that sialidase was secreted into the culture medium by nine of the ten strains of M. canis examined is remarkable because the one unnamed M. canis isolate examined by Zakrajšek (2008) had only membrane-associated activity. In a recent abstract, Berčič et al. (2008b) reported that sialidase activity was present in conditioned supernatant medium from a culture of an unspecified Mycoplasma corogypsi strain, another species from birds that is affiliated with the M. synoviae phylogenetic cluster.
The four-fold variation among M. canis strains in specific sialidase activity of washed cells was statistically significant but much less than the 65-fold variation between high- and low-virulence strains of M. synoviae (May et al., 2007). The absence of activity in M. canis isolate LV underscores the necessity to assess multiple strains of each species to characterize the phylogenetic distribution of sialidases. This may also explain the conflict between earlier reports on the occurrence of sialidase in Mycoplasma when only single strains were examined (Roberts, 1967; Glasgow and Hill, 1980), and the equivocal results regarding M. canis strain C3b as a primary pathogen of cattle (ter Laak et al., 1993) if that strain lacked sufficient activity to contribute to virulence.
M. cynos and M. canis are the species most consistently associated with canine mycoplasmosis (Chalker, 2005), but to date no specific virulence factor had been proposed as a basis of their pathogenicity. Dogs inoculated with M. canis strains S6/L42 or SL1/L45 developed urethritis and prostatitis or metritis (Laber and Holzmann, 1977; Holzmann et al., 1979). M. canis strain A56Hzkl, isolated from the pericardium of a dog, colonized the lung, liver and spleen of mice inoculated intraperitoneally, but did not generate lesions (Eberle et al., 1977). Erythrocyte surface desialylation reduced hemadsorption by M. canis strain PG14T (Manchee and Taylor-Robinson, 1969), suggesting that the secreted sialidase might modulate mycoplasmal cytadherence, colonization and transmission (May and Brown, 2008).
In conclusion, we propose that a secreted sialidase is a candidate virulence factor of M. cynos and M. canis. Though M. molare is not a definitive canine pathogen, its ability to secrete sialidase in the urogenital tract may allow it also to act as a primary or co-factor in disease. Further studies will be required to identify the sialidase genes in these mycoplasmas, and to examine a possible link between sialidase expression and pathogenesis during canine mycoplasmosis.
Acknowledgments
Clinical isolates of M. canis were a gift from Dr. Mary B. Brown of the University of Florida. This work was supported by Public Health Service grant 1R01GM076584 from the National Institute of General Medical Sciences (DRB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong D, Yu BH, Yagoda A, Kagnoff MF. Colonization of humans by Mycoplasma canis. J Infect Dis. 1971;124:607–609. doi: 10.1093/infdis/124.6.607. [DOI] [PubMed] [Google Scholar]
- Ayling RD, Bashiruddin SE, Nicholas RAJ. Mycoplasma species and related organisms isolated from ruminants in Britain between 1990 and 2000. Vet Rec. 2004;155:413–416. doi: 10.1136/vr.155.14.413. [DOI] [PubMed] [Google Scholar]
- Berčič RL, Slavec B, Lavrič M, Narat M, Zorman-Rojs O, Dovč P, Benčina D. A survey of avian Mycoplasma species for neuraminidase enzymatic activity. Vet Microbiol. 2008a;130:391–397. doi: 10.1016/j.vetmic.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Berčič RL, Dovč P, Cizelj I, Kalan J, Zakrajšek T, Narat M, Benčina D. Neuraminidase enzymatic activity in mycoplasmas of the Mycoplasma synoviae cluster. 17th International Congress of the International Organization for Mycoplasmology (abstr.).2008b. [Google Scholar]
- Brown DR, Zacher LA, Farmerie WG. Spreading factors of Mycoplasma alligatoris, a flesh-eating mycoplasma. J Bacteriol. 2004;186:3922–3927. doi: 10.1128/JB.186.12.3922-3927.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker VJ. Canine mycoplasmas. Res Vet Sci. 2005;79:1–8. doi: 10.1016/j.rvsc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Corfield T. Bacterial sialidases--roles in pathogenicity and nutrition. Glycobiology. 1992;2:509–521. doi: 10.1093/glycob/2.6.509. [DOI] [PubMed] [Google Scholar]
- Eberle G, Kirchhoff H, Trautwein G. Experimental infection of mice, gerbils, and rats with mycoplasms from canine pericardium and cardiac valve. Zentralbl Bakteriol [Orig A] 1977;239:95–103. [PubMed] [Google Scholar]
- Edward DG. A suggested classification and nomenclature for organisms of the pleuropneumonia group. Int Bull Bacteriol Nomencl Taxon. 1955;5:85–93. [Google Scholar]
- Glasgow LR, Hill RL. Interaction of Mycoplasma gallisepticum with sialyl glycoproteins. Infect Immun. 1980;30:353–361. doi: 10.1128/iai.30.2.353-361.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann A, Laber G, Walzl H. Experimentally induced mycoplasmal infection in the genital tract of the female dog. Theriogenology. 1979;12:355–370. [Google Scholar]
- Johansson KE, Pettersson B. Taxonomy of Mollicutes. In: Razin S, Herrmann R, editors. Molecular Biology and Pathogenicity of Mycoplasmas. Kluwer Academic/Plenum; New York: 2002. pp. 1–30. [Google Scholar]
- Kahane I, Reisch-Saada A, Almagor M, Abeliuck P, Yatziv S. Glycosidase activities of mycoplasmas. Zentralbl Bakteriol [Orig B] 1990;273:300–305. doi: 10.1016/s0934-8840(11)80432-9. [DOI] [PubMed] [Google Scholar]
- Laber G, Holzmann A. Experimentally induced mycoplasmal infection in the genital tract of the male dog. Theriogenology. 1977;7:177–188. [Google Scholar]
- Manchee RJ, Taylor-Robinson D. Utilization of neuraminic acid receptors by mycoplasmas. J Bacteriol. 1969;98:914–919. doi: 10.1128/jb.98.3.914-919.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M, Kleven SH, Brown DR. Sialidase activity in Mycoplasma synoviae. Avian Dis. 2007;51:829–833. doi: 10.1637/7806-120106-REGR.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M, Brown DR. Genetic variation in sialidase and linkage to N-acetylneuraminate catabolism in Mycoplasma synoviae. Microb Path. 2008;45:38–44. doi: 10.1016/j.micpath.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DH. Neuraminidase-like enzyme present in Mycoplasma gallisepticum. Nature. 1967;213:87–88. [Google Scholar]
- Røsendal S. Mycoplasma cynos, a new canine Mycoplasma species. Int J Syst Bacteriol. 1973;23:49–54. [Google Scholar]
- Røsendal S. Mycoplasma molare, a new canine Mycoplasma species. Int J Syst Bacteriol. 1974;24:125–130. [Google Scholar]
- Røsendal S. Canine mycoplasmas: their ecological niche and role in disease. J Am Vet Med Assoc. 1982;180:1212–1214. [PubMed] [Google Scholar]
- Spergser J, Rosengarten R. Identification and differentiation of canine Mycoplasma isolates by 16S-23S rDNA PCR-RFLP. Vet Microbiol. 2007;125:170–174. doi: 10.1016/j.vetmic.2007.04.045. [DOI] [PubMed] [Google Scholar]
- ter Laak EA, van Dijk JE, Noordergraaf JH. Comparison of pathological signs of disease in specific-pathogen-free calves after inoculation of the respiratory tract with Ureaplasma diversum or Mycoplasma canis. J Comp Pathol. 1993;108:121–32. doi: 10.1016/s0021-9975(08)80216-2. [DOI] [PubMed] [Google Scholar]
- Zakrajšek T. Thesis. University of Ljubljana; 2008. Nevraminidazna aktivnost bakterije Mycoplasma canis [Neuraminidase activity of the bacterium Mycoplasma canis] [Google Scholar]
- Zeugswetter F, Weissenböck H, Shibly S, Hassan J, Spergser J. Lethal bronchopneumonia caused by Mycoplasma cynos in a litter of golden retriever puppies. Vet Rec. 2007;161:626–627. doi: 10.1136/vr.161.18.626. [DOI] [PubMed] [Google Scholar]