Abstract
This study investigates the rate of preterm birth in babies with congenital brain defects. Autopsy case reports of congenital brain anomalies were obtained from the literature. The control cases were from a large registry, a published report from the Metropolitan Atlanta Congenital Defects Program. From 428 publications, 1168 cases were abstracted that had autopsy studies of congenital brain defects and information on the gestational age at birth. The control data from Atlanta included 7738 infants with significant birth defects of any kind and 264,392 infants without birth defects. In the autopsy cases with brain defects, the mean gestational age was 36.6 weeks, whereas the Atlanta data showed a mean gestational age of 39.3 weeks for infants with no defects and a significantly shorter gestation of 38.1 weeks (p < 0.0001) for infants with defects. In the Atlanta data, the rate of preterm birth was 9.3 % for those with no defects compared to 21.5 % (p < 0.0001) for those with defects. In the autopsy cases with brain defects, the rate of preterm birth was even greater (33.1%, p < 0.0001). In conclusion, these data show an association of brain defects with preterm births.
Keywords: preterm birth, congenital brain defects
The present study is the first to investigate the relative risk of being born preterm for infants who have a variety of congenital brain defects. Numerous studies have shown an association between congenital anomalies of any part of the body and preterm birth (1-18) or low birthweight (19-24). For example, there were three large studies reported in 2001. Rasmussen et al. (14), in studies of 264,392 cases from the Metropolitan Atlanta Congenital Defects Program (MACDP), which is administered by the U.S. Centers for Disease Control and Prevention, reported that preterm infants had a 2.43-fold increased risk of having a significant congenital defect compared to infants born at term. The risk for preterm birth in infants with defects was 21.5%, compared with 9.3% for infants without defects. Shaw et al. (15), in a study of 3,090,514 births from the California Birth Defects Monitoring Program, found a decreasing prevalence of birth defects with increasing gestational age. Holmgren and Hogberg (16), in a study of 66,646 infants from the Swedish Medical Birth Registry, found that significant malformations were more common among infants born at 22-27 wks and 28-31 wks than at 32-36 wks. An earlier large study from the MACDP, by Mili et al. (12), showed an association between malformations and LBW.
An association with preterm birth or LBW has been found in several specific types of defects of the CNS. Creasy (25), in his textbook on maternal-fetal medicine, stated that multiple congenital anomalies and anomalies of the central nervous system are associated with a higher risk for preterm birth, but no references or statistics were offered. Rasmussen et al. (14) and Mili et al. (12) found an increase in preterm birth and LBW, respectively, in some defects affecting the head or CNS (anencephaly, hydrocephaly, microcephaly, encephalocele, spina bifida, microphthalmia, and cleft lip or palate), as well as syndromes that affect the brain (Down syndrome/trisomy 21, trisomy 13, trisomy 18). Infants with anencephaly have long been known to have an abnormal profile of gestational length: there is a high incidence of both preterm and post-term birth (sometimes more than 50 wks gestation), with an overall reduction in mean gestational length (26). In infants with cleft lip or palate, there is also an increased incidence of preterm birth (27). There are similar reports for such associations with defects of other parts of the body such as: reduction limb defects (28); syndactaly (29); simian crease (30); gastroschesis and exophthalmos (31); intestinal atresia (32); congenital diaphragmatic hernia (33); esophageal atresia (34); hypospadias (35); renal agenesis (Potter's syndrome) (36); and congenital heart disease (37).
Investigating the association between congenital brain defects and preterm birth presents some challenges. Ascertainment of brain defects is difficult because they can be hidden within the cranium and may remain undetected until autopsy. Furthermore, the identification and classification of brain defects is not straightforward, as various names and descriptions are found for similar defects. Nevertheless, an extensive search of the neuropathology literature was undertaken in order to accumulate a large number of cases of congenital brain defects in which the GA was reported. This published autopsy data was compared with data from a large registry, the MACDP study by Rasmussen et al. (14), with permission. This may be the best approach at the present time.
Methods
This is a literature review with a comparison of published cases to information in a registry, a very large population-based study by the MACDP. The cases were ascertained according to the presence of brain anomaly, and then gestational age was recorded.
Ascertainment of subjects
Eleven hundred and sixty eight subjects diagnosed with congenital brain defects and having information on GA at birth were ascertained from 428 publications (Supplemental table, online at www.pedresearch.org). Cases from pregnancies with multiple births were included. No subjects were included at less than 20 wks gestation, which is considered abortion rather than delivery. Where there were multiple brain anomalies in a subject, the most common defect was listed first. The defects were as follows (number of cases in brackets): porencephaly (209), polymicrogyria (192), multicystic encephalomalacia (117), pachygyria (112), lissencephaly (108), hydranencephaly (92), microcephaly (82), holoprosencephaly (65), anencephaly (52), hydrocephaly (27), gyral anomaly (25), corpus callosum agenesis (19), arhinencephaly (15), cerebellar anomaly (12), heterotopia (8), optic nerve hypoplasia (8), hemimegalencephaly (5), cerebellum agenesis (4), exencephaly (4), arachnoid cyst (2), encephalocele (2), megalencephaly (2), heterotopic brain (1), massa intermedia absent (1).
The control group from the MACDP consisted of 256,654 infants reported as having no significant birth defects (14). A third group for comparison consisted of infants ascertained by the MACDP as having significant congenital defects of any kind. A detailed description of the methods for the MADCP data may be found in Rasmussen et al. (14). Briefly, the MADCP data included only major defects that could adversely affect the infant's health and development, and it was restricted to live-born singleton infants born in the Atlanta area from 1989 through 1995. MADCP cases were ascertained by review of hospital records of newborns and their mothers, vital records, discharge diagnoses, and cytogenetic studies. Pediatric hospital records were also reviewed to ascertain defects and to modify diagnoses after further studies. Defects had to be noted in the first year of life and diagnosed by the sixth birthday. Preterm was defined as 20 to 36 wks gestation, term as 37 to 41 wks, and post-term as 42 to 45 wks. The ascertainment of brain defects was as described above for defects in general. They selected 24 defects for individual analysis and these included the following defects that affect the CNS: anencephaly, spina bifida, Down syndrome, trisomy 13, and trisomy 18.
Statistical analysis
Statistical analyses were performed using the graphing and analysis program Prism (GraphPad Software Inc.). Relative risk (RR) ratios, ORs, and 95% CIs were calculated by Prism, using Fisher's exact test in 2 × 2 contingency tables. The contingency tables were set up so that the two rows (representing the risk factors) were for defects and no defects and the two columns (representing outcomes) were for preterm and term plus post-term. This set up reflects the hypothesis that congenital defects are the risk factors and preterm birth is the outcome. This report focuses on RRs. ORs were always slightly higher. The main analysis included all 1168 autopsy cases. The rates of preterm birth were calculated for defects that occurred in isolation, defects that occurred multiply, and a total. In addition, analyses were performed for each of the ten largest categories of defects.
Results
The rate of preterm birth was 33.1% in the subjects with congenital brain defects. This is higher than the 9.3% rate of preterm births in infants without defects, and 21.5% in infants with serious defects in the MADCP data. Compared to infants without birth defects, the RR for preterm birth in those with brain defects was 3.565 [CI 3.283, 3.872] (p < 0.0001) and the OR was 4.837 [CI 4.279, 5.467] (p < 0.0001). Using the MADCP data, and preterm birth as the outcome, the RR for preterm birth in those with serious defects of any kind was 2.314 (p < 0.0001). In all of these analyses, there was a powerful (p < 0.0001) association between defects and preterm birth. Figure 1 shows the percent of subjects with brain defects, no defects, and defects of any kind, plotted against GA. The curve for brain defects is compared to those for no defects and defects of any kind from the MADCP data. The brain defects curve shows a greater shift toward preterm birth than occurs with the curve for defects of any kind.
Figure 1.
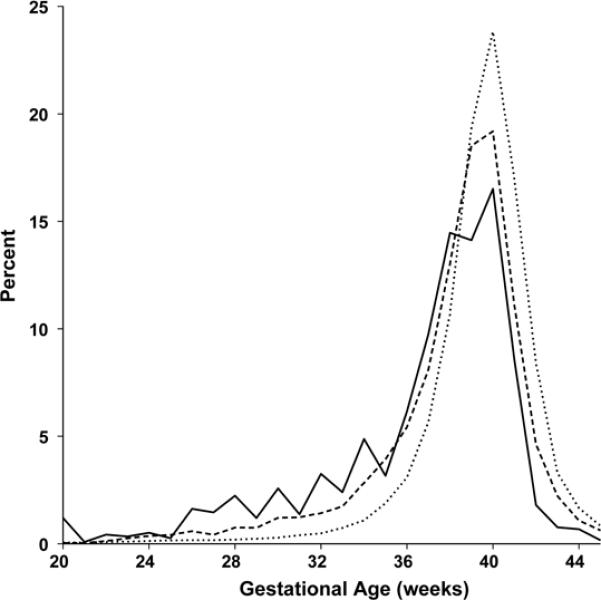
Percent of subjects with and without birth defects by gestational age. The solid line is for brain defects from the autopsy reports; the dashed line is for birth defects from the MACDP registry; and the dotted line is for infants without defects, from the MACDP registry.
The mean GA for subjects with brain defects was 36.6 wks compared to 38.1 wks for infants with birth defects of any kind and 39.3 wks for unaffected infants. Rasmussen et al. (14) reported a statistical significance of P < 0.0001 for the decreased GA age of the infants with defects compared to those unaffected. In Figure 1, a pattern was seen of peaks occurring on even-numbered weeks in the data for infants with brain defects. The curve for the MADCP data on infants with defects showed a more subtle step-wise pattern echoing the saw-tooth pattern in the brain defects curve. This pattern did not occur in the curve for infants without defects. This pattern reflects a bias for estimating the GA as an even number.
Figure 2 illustrates the pattern of the RR for birth at various GAs between 20 and 45 wks. Three curves were plotted: i) a straight line at 1.0 for the reference data for infants without defects; ii) the data on brain defects versus that for infants without defects; and iii) the data for infants with defects of any kind. To eliminate distortions from the peaks on even-numbered weeks, the data were calculated in bins of two weeks, i.e., an even week plus the adjacent odd week. This resulted in data at one-week intervals centered on half-weeks. This graph shows a strong tendency for preterm birth in the two groups with congenital defects. The curve for defects of any kind showed an increased RR for preterm birth. The curve for brain defects also showed an increased RRE throughout the preterm period, especially between 25 and 34 wks. The small numbers of cases before 25 wks make that data less reliable. Post-term births, as also shown in Figure 1, were fewer in the two groups with defects.
Figure 2.
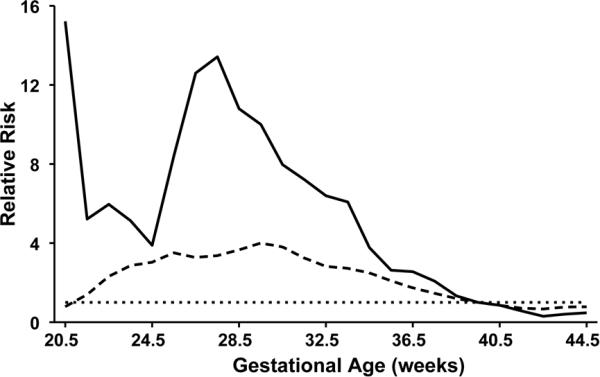
The relative risk for birth at weekly gestational ages. The straight dotted line at 1.0 represents the reference data line for infants without defects (from the MACDP registry). To eliminate distortions that might arise from the saw-tooth pattern of peaks on even-numbered weeks, the data were calculated in bins of two weeks at one-week intervals. The solid line is for brain defects from the autopsy reports, and the dashed line is for birth defects from the MACDP registry.
A further analysis was made of the rates of preterm birth for cases with just one brain anomaly listed, and for the ten most numerous brain defects in this study. The numbers of cases with multiple brain defects was 158, 13.5% of the total. The rate of preterm birth for the 1010 cases with a single brain defect was 32.7%; the rate for total cases (1168, including multiple defects) was 33.1%; and the rate for multiple defects was 36.1%. It appears that having multiple brain defects is associated with a slightly increased rate of preterm birth. This could be interpreted as a dose effect, i.e., more defects leads to a higher rate of preterm birth.
The most common defect, porencephaly, had a preterm birth rate of 30.9% for the 175 cases with porencephaly only, which is slightly less than the 32.7% rate for all cases that had a single brain defect. The other brain defects, in order of highest rate of preterm birth (in cases with only a single defect), were: hydrocephaly (65.2%, 23 cases); anencephaly (57.7%, 52 cases); multicystic encephalomalacia (50.5 %, 107 cases); hydranencephaly (38.5%, 91 cases); holoprosencephaly (33.3%, 63 cases); microcephaly (26.9%, 78 cases); lissencephaly (24.3%, 103 cases); polymicrogyria (17.2%, 145 cases); and pachygyria (8.2%, 98 cases).
Cases with a liveborn twin had a preterm birth rate of 52.1% (48 cases) and those with a dead twin had an even greater rate of 58.7% (63 cases).
Discussion
Limitations of the study
A standard ascertainment process whereby cases are ascertained by review of hospital records, vital records, and discharge diagnoses is quite inadequate because brain defects can be hidden within the cranium. The ascertainment of brain defects from autopsy studies is also difficult. That is the major reason why extensive studies of the association between congenital brain defects and preterm birth have not already been done. Another weakness is that the control data on babies without defects comes from a specific population, whereas the data on babies with brain defects does not. However, the large data sets and the strong statistical correlations (p<0.0001) suggest that the results are meaningful, despite the problems. Standard ascertainments are simply not possible, and this approach may be the best we can do at this time.
Brain defects and preterm birth
This study shows a high risk for preterm birth in babies with brain defects. This suggests that babies with brain defects are predisposed to be born preterm. The reverse, that preterm birth predisposes to congenital brain defects, is not plausible. Although the brains of preterm infants are particularly vulnerable to injury, the brain malformations in this study developed in utero, not at birth. This data on brain defects should not be surprising, in light of the previous studies showing an association between preterm birth and congenital defects of many types.
Can brain defects induce preterm birth?
A dead embryo or fetus appears to trigger its own abortion, and possibly a defective conceptus can trigger preterm delivery. There is a high incidence of anomalies in aborted embryos and fetuses (38), often affecting the CNS (39,40). Freud (41) concluded that some unknown factor was causing both preterm birth and CP. This is in harmony with the hypothesis of a continuum of reproductive casualty (42-44) whereby pathological factors of pregnancy produce damage from abortion through stillbirth and neonatal death, to survival with brain dysfunctions such as CP, epilepsy, mental deficiency, and learning disorders. Other supporting opinions include: “…early damage to the cerebral motor cortex leads to preterm delivery; that is, the affected fetus triggers its own early delivery (45).” “… birth defects may contribute significantly to the proportion of infants who are delivered before 37 weeks gestation (15).” “The fetus itself may be one of the causes of preterm delivery. If growth-retarded or malformed, the fetus is prone to be born prematurely…(10)”
Congenital brain defects may have some direct interaction with the mother that leads to preterm birth, e.g., through soluble factors or distention of the uterus. Alternatively, some types of brain defects might be emblematic of an underlying condition that affects the mother and leads to preterm birth. The current data show that certain types of defects are most strongly associated with preterm birth, e.g. hydrocephaly (65.2%), anencephaly (57.7%), multicystic encephalomalacia (50.5%), and hydranencephaly (38.5%). Further studies of such defects might lead to clues as to the causes of the preterm birth. Among the causes of preterm birth, some defects might be associated with a greater tendency for medical decisions for early delivery, and some might be associated with greater distention of the uterus, for example from polyhydramnios (excessive amniotic fluid). However, perhaps more interesting and more useful would be any findings of associations with soluble factors that might influence preterm labor.
Polyhydramnios and preterm birth
Polyhydramnios, which causes distention of the uterus, is associated with preterm birth (46-48); with cerebral palsy (11); with malformations (12,17); and particularly with proximal gut obstruction with failure of fetal swallowing (46-48). It has been thought that distention of the uterus may predispose to preterm birth. The distention may be a contributing factor, but it is not that simple. It has been shown that the preterm birth rate does not increase with the severity of the polyhydramnios (49,50). Instead, some additional factor in the cases with polyhydramnios plus malformations appears to be responsible for an increased rate of preterm birth. In cases with polyhydramnios, malformations were associated with preterm births regardless of the amount of amniotic fluid (49,50).
Preterm birth and thromboemboli
Infants with malformations have a higher frequency of IUGR regardless of GA (4,21,51). Furthermore, preterm babies have a significant propensity for IUGR (52). This fact may direct our attention to problems with the placenta. A major feature of the placenta is vascular supply, and many types of malformations of the brain (53,54) and other organs (55-58) have been attributed to disruptions of vascular supply. Studies of the placenta in cases of CP in very LBW infants have shown a significant association with thrombi of chorionic plate vessels (59,60). Thromboemboli can circulate right through the foramen oval of fetal heart to the brain and other organs and may cause infarctions that kill areas of tissue and disrupt normal development. A genetic predisposition to coagulopathy could exacerbate this problem and there is an increased frequency of genetic thrombophilia in women with complications of pregnancy (61). Coagulopathy is also associated with an increased risk for stroke, neural tube defects, spontaneous abortion, IUGR, placental abruption, and limb defects (62). Coagulopathy due to the factor V Leiden mutation is associated with placental thrombosis and CP (63). Lynch et al. (64) have suggested that the factor V Leiden mutation is associated with CP through fetal or neonatal cerebral vascular events, with embolization from the fetal side of the placenta.
Preeclampsia, IUGR, porencephaly, perinatal stroke, and cerebral palsy
Further to the idea that there may be connections between thromboemboli, brain malformations, and preterm birth: Perinatal stroke, an important cause of cerebral palsy, is a type of brain injury caused by thromboembolism or hemorrhage, and it is strongly associated with preeclampsia and IUGR (65). Preeclampsia is thought to result from a vascular defect in the placenta, resulting in reduced uteroplacental blood flow (66), and preeclampsia is associated with thromboembolism (61). Cerebral palsy is strongly associated with preterm birth (67) and CNS malformations (24). Perinatal stroke may also be associated with preterm birth; 18 of the 40 cases in one study were born preterm (68). Porencephaly, the most common cerebral malformation in the current study, is associated with the factor V Leiden mutation (64,69), cerebral palsy (70), and preterm birth (the current study). Hydranencephaly, like porencephaly is a fluid-filled defect in the brain, and it is also thought to be caused by vascular disruption (71). In the current study, more than half of the hydranencephaly cases were born preterm. These numerous strong cross-associations are unlikely to be due to chance, especially since there is a plausible chain of connection.
In conclusion, babies with brain defects have a strong propensity to be born preterm. Either the brain defects themselves or the underlying cause of the defects may be inducing preterm birth. A search for the mechanisms involved in this induction might be facilitated by identifying and focusing on those types of brain defects that are most strongly associated with preterm birth. Because coagulopathy is associated with congenital brain defects, it may also be somehow involved in the induction. Unfortunately, the current study offers no stronger clues for solving the intractable and growing problem of preterm birth. It should also be considered that there might be a number of preterm births that are caused by brain or other defects that are not easily identified.
Acknowledgements
I wish to thank Clara R. Thore, PhD for assistance with the table.
Financial Support: This study was supported by NIH grants CA 113321, NS 20618, and NS 36780, the March of Dimes Birth Defects Foundation (6-FY96-1064), and the Pratt Family Foundation.
Abbreviations
- MACDP
Metropolitan Atlanta Congenital Defects Program
- RR
relative risk
Footnotes
This article contains supplemental material, which can be found online at www.pedresearch.org.
References
- 1.Marden PM, Smith DW, McDonald MJ. Congenital anomalies in the newborn infant, including minor variations. J Pediatr. 1964;64:357–371. doi: 10.1016/s0022-3476(64)80188-8. [DOI] [PubMed] [Google Scholar]
- 2.Yerushalmy J, van den Berg BJ, Erhardt CL, Jacobziner H. Birth weight and gestation as indices of “immaturity.”. Am J Dis Child. 1965;109:43–57. doi: 10.1001/archpedi.1965.02090020045005. [DOI] [PubMed] [Google Scholar]
- 3.Stewart AL, Keay AJ, Smith PG. Congenital malformations; a detailed study of 2500 liveborn infants. Ann Hum Genet. 1969;32:353–360. doi: 10.1111/j.1469-1809.1969.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 4.Mehes K, Mestyan J, Knoch V, Vinceller M. Minor malformations in the neonate. Helv Paediatr Acta. 1973;28:477–483. [PubMed] [Google Scholar]
- 5.Lubchenco LO. The High Risk Infant. WB Saunders; Philadelphia: 1976. p. 217. [Google Scholar]
- 6.Kaltreider DF, Kohl S. Epidemiology of preterm delivery. Clin Obstet Gynecol. 1980;23:17–31. doi: 10.1097/00003081-198003000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Christianson RE, van den Berg BJ, Milkovich L, Oechsli FW. Incidence of congenital anomalies among white and black live births with long-term follow-up. Am J Public Health. 1981;71:1333–1341. doi: 10.2105/ajph.71.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkowitz GS. An epidemiologic study of preterm delivery. Am J Epidemiol. 1981;113:81–92. doi: 10.1093/oxfordjournals.aje.a113068. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman E, Ryan KJ, Monson RR, Schoenbaum SC. Association of maternal hematocrit with premature labor. Am J Obstet Gynecol. 1988;159:107–114. doi: 10.1016/0002-9378(88)90502-9. [DOI] [PubMed] [Google Scholar]
- 10.Hartikainen-Sorri A-L, Sorri M. Occupational and socio-medical factors in preterm birth. Obstet Gynecol. 1989;74:13–16. [PubMed] [Google Scholar]
- 11.Torfs CP, van den Berg BJ, Oechsli FW, Cummins S. Prenatal and prenatal factors in the etiology of cerebral palsy. J Pediatr. 1990;116:615–619. doi: 10.1016/s0022-3476(05)81615-4. [DOI] [PubMed] [Google Scholar]
- 12.Mili F, Edmonds LD, Khoury MJ, McClearn AB. Prevalence of birth defects among low-birth-weight infants. A population study. Am J Dis Child. 1991;145:1313–1318. doi: 10.1001/archpedi.1991.02160110105032. [DOI] [PubMed] [Google Scholar]
- 13.Bhat BV, Babu L. Congenital malformations at birth - a prospective study from south India. Indian J Pediatr. 1998;65:873–881. doi: 10.1007/BF02831352. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen SA, Moore CA, Paulozzi LJ, Rhodenhiser EP. Risk for birth defects among premature infants: A population-based study. J Pediatr. 2001;138:668–673. doi: 10.1067/mpd.2001.112249. [DOI] [PubMed] [Google Scholar]
- 15.Shaw GM, Savitz DA, Nelson V, Thorp JM. Role of structural birth defects in preterm delivery. Paediatr Perinat Epidemiol. 2001;15:106–109. doi: 10.1046/j.1365-3016.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 16.Holmgren PA, Hogberg U. The very preterm infant - a population-based study. Acta Obstet Gynecol Scand. 2001;80:525–531. [PubMed] [Google Scholar]
- 17.Queisser-Luft A, Stolz G, Wiesel A, Schlaefer K. Malformations in newborn: results based on 30940 infants and fetuses from Mainz congenital birth defect monitoring system (1990-1998) Arch Gynecol Obstet. 2002;266:163–167. doi: 10.1007/s00404-001-0265-4. [DOI] [PubMed] [Google Scholar]
- 18.Thong MK, Ho JJ, Khatijah NN. A population-based study of birth defects in Malaysia. Ann Hum Biol. 2005;32:180–187. doi: 10.1080/03014460500075332. [DOI] [PubMed] [Google Scholar]
- 19.van den Berg BJ, Yerushalmy J. The relationship of the rate of intrauterine growth of infants of low birth weight to mortality, morbidity, and congenital anomalies. J Pediatr. 1966;69:531–545. doi: 10.1016/s0022-3476(66)80038-0. [DOI] [PubMed] [Google Scholar]
- 20.Drillien CM. The small-for-date infant: etiology and prognosis. Pediatr Clin North Am. 1970;17:9–24. doi: 10.1016/s0031-3955(16)32372-0. [DOI] [PubMed] [Google Scholar]
- 21.Crichton JU, Dunn HG, McBurney AK, Robertson A-M, Tredger E. Minor congenital defects in children of low birth weight. J Pediatr. 1972;80:830–832. doi: 10.1016/s0022-3476(72)80139-2. [DOI] [PubMed] [Google Scholar]
- 22.Fortune DW, Kitchen WH. Malformations in infants of very low birth weight. Med J Aust. 1977;1:239–242. doi: 10.5694/j.1326-5377.1977.tb130663.x. [DOI] [PubMed] [Google Scholar]
- 23.Riley MM, Halliday JL, Lumley JM. Congenital malformations in Victoria, Australia, 1983-95: An overview of infant characteristics. J Paediatr Child Health. 1998;34:233–240. doi: 10.1046/j.1440-1754.1998.00208.x. [DOI] [PubMed] [Google Scholar]
- 24.Croen LA, Grether JK, Curry CJ, Nelson KB. Congenital abnormalities among children with cerebral palsy: More evidence for prenatal antecedents. J Pediatr. 2001;138:804–810. doi: 10.1067/mpd.2001.114473. [DOI] [PubMed] [Google Scholar]
- 25.Creasy RK. Preterm labor and delivery. In: Creasy RK, Resnik R, editors. Maternal-Fetal Medicine: Principle and Practice. WB Saunders; Philadelphia: 1984. pp. 415–443. [Google Scholar]
- 26.Honnebier WJ, Swaab DF. The influence of anencephaly upon intrauterine growth of fetus and placenta and upon gestation length. J Obstet Gynaecol Br Commonw. 1973;80:577–588. doi: 10.1111/j.1471-0528.1973.tb16030.x. [DOI] [PubMed] [Google Scholar]
- 27.Saxen I. Cleft lip and palate in Finland: Parental histories, course of pregnancy and selected environmental factors. Int J Epidemiol. 1974;3:263–270. doi: 10.1093/ije/3.3.263. [DOI] [PubMed] [Google Scholar]
- 28.Calzolari E, Manservigi D, Garani GP, Cocchi G, Magnani C, Milan M. Limb reduction defects in Emilia Romagna, Italy: epidemiological and genetic study in 173,109 consecutive births. J Med Genet. 1990;27:353–357. doi: 10.1136/jmg.27.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogala EJ, Wynne-Davies R, Littlejohn A, Gormley J. Congenital limb anomalies: frequency and aetiological factors. J Med Genet. 1974;11:221–233. doi: 10.1136/jmg.11.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dar H, Carney FE, Winter ST. Dermatoglyphics and the simian crease in infants of low birth weight. Acta Paediatr Scand. 1971;60:479–481. doi: 10.1111/j.1651-2227.1971.tb06691.x. [DOI] [PubMed] [Google Scholar]
- 31.Byron-Scott R, Haan E, Chan A, Bower C, Scott H, Clark K. A population-based study of abdominal wall defects in South Australia and Western Australia. Paediatr Perinat Epidemiol. 1998;12:136–151. doi: 10.1046/j.1365-3016.1998.00090.x. [DOI] [PubMed] [Google Scholar]
- 32.Cragan JD, Martin ML, Moore CA, Khoury MJ. Descriptive epidemiology of small intestine atresia, Atlanta, Georgia. Teratology. 1993;48:441–450. doi: 10.1002/tera.1420480508. [DOI] [PubMed] [Google Scholar]
- 33.Dott MM, Wong LY, Rasmussen SA. Population-based study of congenital diaphragmatic hernia: risk factors and survival in metropolitan Atlanta, 1968-1999. Birth Defects Res A Cin Mol Teratol. 2003;67:261–267. doi: 10.1002/bdra.10039. [DOI] [PubMed] [Google Scholar]
- 34.Depaepe A, Dolk H, Lechat MF. The epidemiology of tracheo-oesophageal fistula and oesophageal atresia in Europe. EUROCAT Working Group. Arch Dis Child. 1993;68:743–748. doi: 10.1136/adc.68.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carmichael SL, Shaw GM, Nelson V, Selvin S, Torfs C, Curry CJ. Hypospadias in California. Trends and descriptive epidemiology. Epidemiology. 2003;14:701–706. doi: 10.1097/01.ede.0000091603.43531.d0. [DOI] [PubMed] [Google Scholar]
- 36.Ratten GJ, Beischer NA, Fortune DW. Obstetric complications when the fetus has Potter's syndrome. Am J Obstet Gynecol. 1973;115:890–896. doi: 10.1016/0002-9378(73)90663-7. [DOI] [PubMed] [Google Scholar]
- 37.Tanner K, Sabrine N, Wren C. Cardiovascular malformations among preterm infants. Pediatrics. 2005;116:e833–e838. doi: 10.1542/peds.2005-0397. [DOI] [PubMed] [Google Scholar]
- 38.Colvin ED, Bartholomew RA, Grimes WH, Fish JS. Salvage possibilities in threatened abortion. Am J Obstet Gynecol. 1950;59:1208–1222. doi: 10.1016/0002-9378(50)90291-2. [DOI] [PubMed] [Google Scholar]
- 39.Padget DH. Neuroschisis and human embryonic maldevelopment. New evidence on anencephaly, spina bifida and diverse mammalian defects. J Neuropathol Exp Neurol. 1970;29:192–216. [PubMed] [Google Scholar]
- 40.Creasy MR, Alberman ED. Congenital malformations of the central nervous system in spontaneous abortions. J Med Genet. 1976;13:9–16. doi: 10.1136/jmg.13.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freud S. Die infantile cerebrallahmung. A. Holder; Wein: 1897. [Google Scholar]
- 42.Lilienfeld AM, Parkhurst E. A study of the association of factors of pregnancy and parturition with the development of cerebral palsy; a preliminary report. Am J Hyg. 1951;53:262–282. doi: 10.1093/oxfordjournals.aje.a119453. [DOI] [PubMed] [Google Scholar]
- 43.Lilienfeld AM, Pasamanick B. The association of maternal and fetal factors with the development of cerebral palsy and epilepsy. Am J Obstet Gynecol. 1955;70:93–101. doi: 10.1016/0002-9378(55)90292-1. [DOI] [PubMed] [Google Scholar]
- 44.Nelson KB. The 'continuum of reproductive casualty'. Clin Dev Med. 1968;27:100–109. [Google Scholar]
- 45.Stanley F, Blair E, Alberman E. Cerebral palsies: Epidemiology and causal pathways. (Clinics in Developmental Medicine) Cambridge University Press; Cambridge: 2000. p. 151. [Google Scholar]
- 46.Scott JS, Wilson JH. Hydramnios as an early sign of oesophageal atresia. Lancet. 1957;273:569–572. doi: 10.1016/s0140-6736(57)90060-0. [DOI] [PubMed] [Google Scholar]
- 47.Pierro A, Cozzi F, Colarossi G, Irving IM, Pierce AM, Lister J. Does fetal gut obstruction cause hydramnios and growth retardation? J Pediatr Surg. 1987;22:454–457. doi: 10.1016/s0022-3468(87)80269-5. [DOI] [PubMed] [Google Scholar]
- 48.Cheng W, Mya GH, Saing H. Does the amniotic fluid protein absorption contribute significantly to the fetal weight? J Paediatr Child Health. 1996;32:39–41. doi: 10.1111/j.1440-1754.1996.tb01539.x. [DOI] [PubMed] [Google Scholar]
- 49.Many A, Hill LM, Lazebnik N, Martin JG. The association between polyhydramnios and preterm delivery. Obstet Gynecol. 1995;86:389–391. doi: 10.1016/0029-7844(95)00179-U. [DOI] [PubMed] [Google Scholar]
- 50.Lazebnik N, Many A. The severity of polyhydramnios, estimated fetal weight and preterm delivery are independent risk factors for the presence of congenital malformations. Gynecol Obstet Invest. 1999;48:28–32. doi: 10.1159/000010129. [DOI] [PubMed] [Google Scholar]
- 51.Khoury MJ, Erickson JD, Cordero JF, McCarthy BJ. Congenital malformations and intrauterine growth retardation: A population study. Pediatrics. 1988;82:83–90. [PubMed] [Google Scholar]
- 52.Secher NJ, Hansen PK, Thomsen BL, Keiding N. Growth retardation in preterm infants. Br J Obstet Gynaecol. 1987;94:115–120. doi: 10.1111/j.1471-0528.1987.tb02336.x. [DOI] [PubMed] [Google Scholar]
- 53.Friede RL. Developmental Neuropathology. Springer-Verlag; Berlin: 1989. [Google Scholar]
- 54.Norman MG, McGillivray BC, Kalousek DK, Hill A, Poskitt KJ. Congenital Malformations of the Brain: Pathological, Embryological, Clinical, Radiological and Genetic Aspects. Oxford University Press; New York: 1995. [Google Scholar]
- 55.Poswillo D. The pathogenesis of the first and second branchial arch syndrome. Oral Surg Oral Med Oral Pathol. 1973;35:302–328. doi: 10.1016/0030-4220(73)90070-4. [DOI] [PubMed] [Google Scholar]
- 56.Hoyme HE, Higginbottom MC, Jones KL. The vascular pathogenesis of gastroschisis: intrauterine interruption of the omphalomesenteric artery. J Pediatr. 1981;98:228–231. doi: 10.1016/s0022-3476(81)80640-3. [DOI] [PubMed] [Google Scholar]
- 57.Robinow M, Schatzman ER, Oberheu K. Peromelia, ipsilateral subclavian atresia, coarctation, and aneurysms of the aorta resulting from intrauterine vascular occlusion. J Pediatr. 1982;101:84–87. doi: 10.1016/s0022-3476(82)80191-1. [DOI] [PubMed] [Google Scholar]
- 58.Soltan HC, Holmes LB. Familial occurrence of malformations possibly attributable to vascular abnormalities. J Pediatr. 1986;108:112–114. doi: 10.1016/s0022-3476(86)80783-1. [DOI] [PubMed] [Google Scholar]
- 59.Kraus FT. Cerebral palsy and thrombi in placental vessels of the fetus: insights from litigation. Hum Pathol. 1997;28:246–248. doi: 10.1016/s0046-8177(97)90114-3. [DOI] [PubMed] [Google Scholar]
- 60.Redline RW, Wilson-Costello D, Borawski E, Faranoff AA, Hack M. Placental lesions associated with neurologic impairment and cerebral palsy in very low-birth-weight infants. Arch Pathol Lab Med. 1998;122:1091–1098. [PubMed] [Google Scholar]
- 61.Kupferminc MJ, Eldor A, Steinman N, Many A, Bar-Am A, Jaffa A, Fait G, Lessing JB. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med. 1999;340:9–13. doi: 10.1056/NEJM199901073400102. [DOI] [PubMed] [Google Scholar]
- 62.Shashi V, Rickheim A, Pettenati MJ. Maternal homozygosity for the common MTHFR mutation as a potential risk factor for offspring with limb defects. Am J Med Genet. 2001;100:25–29. doi: 10.1002/ajmg.1186. [DOI] [PubMed] [Google Scholar]
- 63.Thorarensen O, Ryan S, Hunter J, Younkin DP. Factor V Leiden mutation: an unrecognized cause of hemiplegic cerebral palsy, neonatal stroke, and placental thrombosis. Ann Neurol. 1997;42:372–375. doi: 10.1002/ana.410420316. [DOI] [PubMed] [Google Scholar]
- 64.Lynch JK, Nelson KB, Curry CJ, Grether JK. Cerebrovascular disorders in children with factor V Leiden mutation. J Child Neurol. 2001;16:735–744. doi: 10.1177/088307380101601006. [DOI] [PubMed] [Google Scholar]
- 65.Wu YW, March WM, Croen LA, Grether JK, Escobar GJ, Newman TB. Perinatal stroke in children with motor impairment: A population-based study. Pediatrics. 2004;114:612–619. doi: 10.1542/peds.2004-0385. [DOI] [PubMed] [Google Scholar]
- 66.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–56. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 67.Nelson KB, Ellenberg JH. Antecedents of cerebral palsy. Multivariate analysis of risk. N Engl J Med. 1986;315:81–86. doi: 10.1056/NEJM198607103150202. [DOI] [PubMed] [Google Scholar]
- 68.Govaert P, Mattys E, Zecic A, Roelens F, Oostra A, Vanzieleghem B. Perinatal cortical infarction within middle cerebral artery trunks. Arch Dis Child Fetal Neonatal Ed. 2000;82:F59–F63. doi: 10.1136/fn.82.1.F59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Debus O, Koch HG, Kurlemann G, Strater R, Vielhaber H, Weber P, Nowak-Gottl U. Factor V Leiden and genetic defects of thrombophilia in childhood porencephaly. Arch Dis Child Fetal Neonatal Ed. 1998;78:F121–F124. doi: 10.1136/fn.78.2.f121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ho SS, Kuzniecky RI, Gilliam F, Faught E, Bebin M, Morawetz R. Congenital porencephaly: MR features and relationship to hippocampal sclerosis. AJNR Am J Neuroradiol. 1998;19:135–141. [PMC free article] [PubMed] [Google Scholar]
- 71.Jung JH, Graham JM, Schultz N, Smith DW. Congenital hydranencephaly/porencephaly due to vascular disruption in monozygotic twins. Pediatrics. 1984;73:467–469. [PubMed] [Google Scholar]


