Abstract
Our studies in the RBL-2H3 mast cell line suggest that responses to antigen (Ag) are negatively modulated through upregulation of Src-like adaptor protein (SLAP). Ag stimulation of RBL-2H3 cells leads to increased levels of SLAP (but not SLAP2) transcripts and protein over a period of several hours. The effects of pharmacologic inhibitors indicate that the upregulation of SLAP is dependent on multiple signaling pathways. Knockdown of SLAP with anti-SLAP siRNA is associated with enhanced phosphorylation of Syk, the linker for activation of T cells (LAT), phospholipase Cγ, MAP kinases, and various transcription factors. Production of IL-3 and MCP-1, but not degranulation, is also enhanced. The upregulation of SLAP may thus serve to limit the duration of cytokine production in Ag-stimulated cells.
Keywords: Mast cells, Antigen, Src-like Adaptor Protein, Inhibitory regulator, Signaling, Cytokines
1. Introduction
Stimulation of mast cells via the IgE receptor (FcεRI) results in the activation of phospholipase (PL) C and D as well as the MAP kinases, Erk, JNK, and p38 MAP kinase (Rivera and Gilfillan, 2006; Gilfillan and Tkaczyk, 2006). Activated PLC and PLD, in turn, produce messenger molecules that activate protein kinase (PK) C and mobilize Ca2+ from intracellular and extracellular sources to generate necessary signals for degranulation with the release of preformed inflammatory mediators (Ozawa et al., 1993; Peng and Beaven, 2005). These signals along with the activation of the MAP kinases lead to rapid activation of PLA2, which is essential for production of inflammatory eicosanoids (Hirasawa et al., 1995a; Hirasawa et al., 1995b), and the activation of downstream transcription factors that initiate cytokine gene transcription (Hundley et al., 2004; Qiao et al., 2006). Collectively these events promote the typical hypersensitivity reactions to antigen (Ag).
The proximal components of FcεRI-mediated signaling pathways that lead to the responses noted above include the cytosolic Src kinases Lyn and Fyn, Syk tyrosine kinase, and the transmembrane adaptor proteins linker for activation of T cells (LAT) and non-T-cell activation linker (NTAL) which, when phosphorylated by these kinases, serve as docking sites for the assembly of other adaptor and signaling molecules (Rivera and Gilfillan, 2006; Gilfillan and Tkaczyk, 2006). In addition, the production of phosphatidylinositol 3,4,5-trisphosphate by phosphatidylinositol 3-kinase provides additional nucleation sites for recruitment and activation of other signaling molecules at the plasma membrane. An early signaling event is the phosphorylation of FcεRI by Lyn and recruitment of Syk by phosphorylated FcεRI. Syk, in turn, is essential for downstream phosphorylation of LAT and activation of PLCγ.
Mast cells also possess mechanisms for negatively regulating cell activation (Siraganian, 2003; Molfetta et al., 2007). These mechanisms are most pronounced at supraoptimal concentrations of Ag (Xiao et al., 2005) or on co-ligation of FcεRI with inhibitory receptors that contain the immunoreceptor tyrosine-based inhibitory motif (ITIM) (Ott and Cambier, 2000; Li and Yao, 2004). Inhibitory signals in Ag stimulated cells may be orchestrated through Lyn (Xiao et al., 2005) or the ubiquitin ligases c-Cbl and Cbl-b to induce ubiquitination and degradation of FcεRI subunits (Paolini et al., 2002), Lyn (Kyo et al., 2003; Qu et al., 2004), and Syk (Ota and Samelson, 1997; Paolini et al., 2002). Other mechanisms include the engagement of the inositol phosphatases, phosphatase and tensin homologue deleted on chromosome ten (PTEN) and SH2 domain-containing inositol polyphosphate 5-phosphatase (SHIP) both of which reduce levels of phosphatidylinositol 3,4,5-trisphosphate (Kimura et al., 1997a; Furumoto et al., 2006). The SH2 domain-containing protein tyrosine phosphatases, SHP-1 and SHP-2, are recruited by phosphorylated FcεRI and attenuate mast cell activation by dephosphorylating various components of the signaling pathways (Kimura et al., 1997b). A mechanism that warrants further investigation is the induction of inhibitory regulators such as Dok1 (downstream of tyrosine kinase-1), MAP kinase phosphatase-1 also known as dual specificity protein phosphatase-1 (DUSP1) and Src-like adaptor protein (SLAP). Dok1 has been implicated in the repressive actions of the ITIM-bearing inhibitory receptors, FcγRIIB (Ott et al., 2002; Kepley et al., 2004) and the mast function-associated antigen (MAFA) (Abramson and Pecht, 2002). Dok1 (Hiragun et al., 2005), DUSP1 (Kassel et al., 2001; Jeong et al., 2003), and SLAP (Hiragun et al., 2006; Park and Beaven, 2009) are also upregulated by low concentrations of dexamethasone and, in this manner, contribute to the inhibitory actions of this and other glucocorticoids in mast cells.
The inhibitory regulators target specific components of the signaling pathways. Dok1, for example, negatively regulates Ras by activating the Ras GTPase activating protein and thus inhibits the entire Ras/Raf1/Erk/PLA2 pathway in Ag-stimulated cells (Hiragun et al., 2005). DUSP1 is one member of a family of DUSPs that dephosphorylate and inactivate MAP kinases (Lang et al., 2006; Owens and Keyse, 2007). SLAP family members, SLAP (Sosinowski et al., 2000; Tang et al., 1999; Dragone et al., 2006) and SLAP2 (Holland et al., 2001; Pandey et al., 2002; Loreto et al., 2002), interact with components of the T and B cell receptor signaling complexes and thus negatively regulate cellular signaling. The SLAPs possess SH2 and SH3 domains that are homologous to those in Src kinases and binding partners include Src kinase, ZAP70, CD3 ζ, and Cbl (Pandey et al., 1995; Sosinowski et al., 2000; Tang et al., 1999; Holland et al., 2001; Pandey et al., 2002; Loreto et al., 2002). SLAP co-opts c-Cbl to down-regulate expression of T and B cell receptors (Loreto et al., 2002; Dragone et al., 2006; Myers et al., 2006). SLAP and SLAP2 also down-regulate growth factor receptor mediated signaling in fibroblasts in a Src kinase and c-Cbl dependent manner (Pakuts et al., 2007; Sirvent et al., 2008). In dexamethasone-treated mast cells, SLAP negatively regulates Syk (a cognate of ZAP70) and Syk-dependent signaling events such as the phosphorylation of LAT and PLCγ2 as well as the mobilization of Ca2+ ions (Hiragun et al., 2006). As a consequence, increased expression of SLAP, whether induced by dexamethasone or by gene transfection, results in diminished Ag-induced degranulation and release of arachidonic acid (Hiragun et al., 2006).
We have investigated whether or not the inducible inhibitory regulators play a role in regulating responses to Ag itself. Preliminary investigations indicated that Ag stimulation of the RBL-2H3 mast cell line resulted in substantial increases in levels of SLAP mRNA, a small transient increase in DUSP1 mRNA, but no increase in levels of DOK1. Here we have focused on the consequences of the induction of SLAP because it suppresses a primary signaling event in mast cells, namely the activation of Syk (Hiragun et al., 2006). We find that the upregulation of SLAP by Ag not only suppresses the phosphorylation of Syk but also phosphorylation of MAP kinases and downstream transcription factors in addition to cytokine production.
2. Materials and methods
2.1. Reagents and antibodies
Reagents were obtained from the following sources: Media and culture reagents and Trizol from Invitrogen/GIBCO (Carlsbad, CA); dinitrophenyl-human serum albumin (DNP-HSA), Cyclosporin A and mouse anti-DNP IgE, Sigma (St. Louis, MO); polyclonal antibodies against SLAP (C-19), Syk and PLCγ2 from Santa Cruz (Santa Cruz, CA); antibody against Dok1 from Abcam (Cambridge, MA); all other antibodies from Cell Signaling (Danvers, MA); siRNA against SLAP from Dharmacon (Chicago, IL); Bay11-7082, PD98059, SB203580, SP600125, LY294002, Ro318220 from Calbiochem (San Diego, CA ); ELISA kits for TNFα, IL-3, and MCP1 from Biosource (Camarillo, CA); and FITC-conjugated mouse IgE monoclonal isotype control from BD Pharmingen (San Jose, CA).
2.2. Cell culture and transient transfection of siRNAs
RBL-2H3 cells were maintained in minimal essential medium (MEM) supplemented with 15% fetal calf serum, 2 mM L-glutamine, and an antibiotic-antimycotic solution. For transfection with siRNAs, cells were detached with trypsin-EDTA solution and 2 ×106 cells were pelletted and suspended in 100 μl of Nucleofector Solution R (Amaxa, Walkersville, MD). The suspension was mixed with 200 pmoles of ON-Targetplus SMARTpool siRNA against SLAP (Dharmacon, Lafayette, CO) and transfected by electroporation program T-20 (Amaxa, Walkersville, MD). The transfected cells were used after 48 h for each experiment.
2.3. Measurement of degranulation, release of arachidonic acid, and production of cytokines
Transfected cells were plated in 24-well (0.1 × 106 cells/1 ml/well) or 6-well (0.25 × 106 cells/2.5 ml/well) plates for measurement of β-hexosaminidase and cytokines respectively. Cells were incubated for 24 h at 37°C. The culture medium was then replaced with fresh medium containing 50 ng/ml anti-DNP-IgE and incubated for a further 24 h. The cultures were washed twice with glucose saline/PIPES buffer (Choi et al., 2002) and stimulated with 50 ng/ml DNP-HSA in saline/PIPES buffer for 20 min for assay of the granule marker β-hexosaminidase in medium and cells (Ozawa et al., 1993) or for the indicated times in complete growth medium for measurement of cytokines by use of ELISA kits (Qiao et al., 2006). Data were expressed as a percent of cellular β-hexosaminidase that was released into the medium or total cytokine released into medium.
2.4. Western blot analysis
Cells were washed twice with ice-cold phosphate buffered saline (PBS) and lysed in lysis buffer (25 mM Tris-HCl pH7.5, 150 mM NaCl, 1% nonidet P-40, 5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 25 μg/ml leupeptin, 25 μg/ml aprotinin, and 2 μg/ml pepstatin) and left for 30 min on ice. Proteins were separated in 4–12% gradient SDS-PAGE gels and transferred to PVDF membrane. Immunoreactive proteins were visualized by use of horseradish peroxidase-conjugated secondary antibodies and chemiluminescence (Millipore, Billerica, MA).
2.5. Measurement of mRNAs
Cell cultures were stimulated with Ag as described above for the assay of cytokines. They were washed twice with PBS and then lysed with 1 ml Trizol. Total RNA was purified from cells of which 1 μg was used for synthesis of cDNA according to the manufacturers’ protocols (Invitrogen, Carlsbad, CA). Transcript levels of Dok1 and SLAP, and 18S rRNA (an internal control to calculate fold induction) were assayed by real-time PCR with the following primers and probes: SLAP, ATCCAGTTTGCAGGAAGGTCCAGA, TAGGATGCGATGCTTTCCCGAAGA, TaqMan® probe FAM-AGAGAACCCACTCAGAGTGGACGAAT –TAMRA; Dok1, TCTTTCAGGCAGTTGAGGCTGCTA, GCACGTGCCCATACAAATCCCAAT; IL-3 TCCTGATGCTCTTCCACC, TCGCAGCTGCAGGAATACAACACT; MCP1, TGGCAAGATGATCCCAAT, AGGTGGTTGTGGAAAAG; TNFα, TTGCCACTTCATACCAGGAGAA, TCACAGAGCAATGACTCCAA.
2.6. Measurement of FcεRIα on cell surface with FITC-labeled IgE
Transfected cells were detached from culture plates 48 h after transfection by repeated pipetting and then washed with Dulbecco’s PBS containing 0.1% sodium azide (FACS buffer). For flow cytometric analysis, 1 μg of FITC-conjugated mouse IgE was incubated with 1 × 106 cells in 100 μl FACS buffer for 1 h at 4°C. Cells were washed with FACS buffer and cell-bound IgE was measured by flow cytometry (Becton Dickinson, FACSCalibur). Preliminary experiments had indicated maximal and saturable binding of the labeled IgE to cells under the conditions described here.
3. Results
3.1. Ag upregulates SLAP through multiple signaling pathways
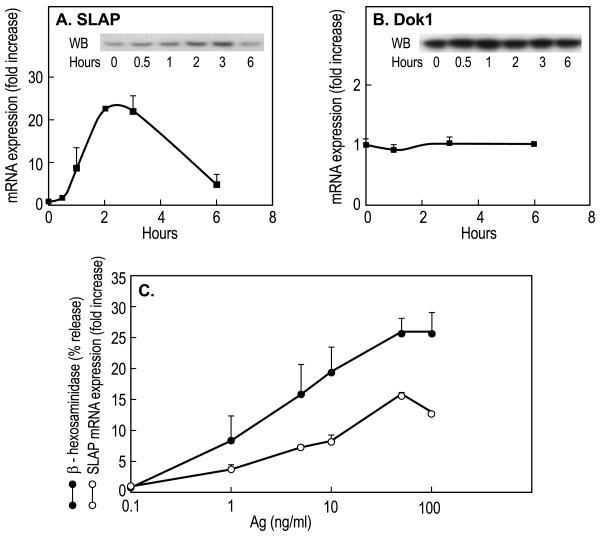
Examination of expression levels of known inhibitory regulators in Ag-stimulated mast cells revealed a more than ten-fold increase in expression of SLAP mRNA (Fig. 1A) whereas the expression of Dok1 was unaffected (Fig. 1B). SLAP mRNA levels reached a maximum within 2 h to 3 h of addition of Ag and then gradually declined towards basal levels but were still elevated at 6 h. The increase in mRNA was accompanied by a substantial increase in SLAP protein reaching a maximum at 3 h (inset, Fig. 1A). The increase in SLAP mRNA was evident with concentrations of Ag that were capable of stimulating degranulation and the concentration-response curves were similar for both responses (Fig. 1C). As in a previous study (Park and Beaven, 2009), SLAP2 mRNA was undetectable in either resting or stimulated cells RBL-2H3 cells when examined with a variety of primers (data not shown).
Fig. 1.
Increased expression of SLAP mRNA and protein in response to Ag-stimulation. RBL-2H3 cells sensitized overnight with 50 ng/ml DNP-specifc IgE were stimulated with 50 ng/ml Ag. Panels A and B, cells were stimulated for the indicated times and relative levels of DOK1 and SLAP mRNA and protein (insets) were determined by real-time PCR and immunoblotting respectively. Error bars show Standard Deviations for PCR determinations. The data indicate relative levels of mRNA (zero hours equals 1) and are from one of two similar experiments. Panel C compares the extent of increase of SLAP protein and degranulation with different concentrations of Ag. SLAP was measured 3 h and degranulation 20 min after addition of Ag. Degranulation was assessed by calculating per cent of cellular β-hexosaminidase (a granule marker) that was released into the medium. Data (mean ± SEM) were from four separate cultures and is representative of two experiments.
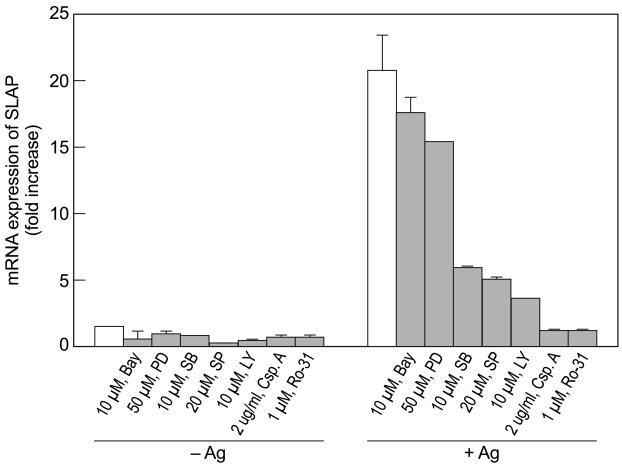
Studies with various inhibitors indicated that production of SLAP mRNA was dependent on p38 MAP kinase, JNK, phosphatidylinositol 3-kinase, calcineurin, and PKC, all of which are activated by Ag and inhibited by respectively, SB202190, SP600125, LY204002, cyclosporin A, and Ro-31 8220 in RBL-2H3 cells (Andrade et al., 2004; Hundley et al., 2004; Qiao et al., 2006). All of these inhibitors suppressed the Ag-induced increase in SLAP mRNA (Fig. 2). The MEK and NFκB inhibitors, PD 98059 and BAY 11-7082, in contrast, had minimal effects on production of SLAP mRNA (Fig. 2) to suggest that the ERK1/2 and NFκB pathways were not essential for transcriptional activation of the SLAP gene. Several transcription factors are activated through JNK, p38 MAP kinase, protein kinase C, and calcineurin by Ag (Razin et al., 1994; Qiao et al., 2006; Tkaczyk et al., 2006). These include components of activator protein-1 (AP-1) complex, ATF2, and NFAT. Therefore, the enhanced production of SLAP transcripts is probably regulated through multiple signaling pathways and transcription factors although ERK and NFκB do not appear to be involved.
Fig. 2.
Suppression of production of SLAP mRNA by inhibitors of signaling pathways. RBL-2H3 cells were exposed to the indicated concentrations of inhibitors for 20 min before addition of 50 ng/ml Ag. SLAP mRNA was determined 2 h after addition of Ag by real time PCR. Data (mean ± SEM) are from one experiment that is representative of two separate experiments. Abbreviations: Bay, Bay 11–7082; PD, PD-98059; SB, SB 202190; SP, SP 600125; LY, LY 294002; Csp.A, cyclosporine; Ro-31, Ro-31 8220.
3.2. Knock down of SLAP with siRNA enhances expression of FcεRI
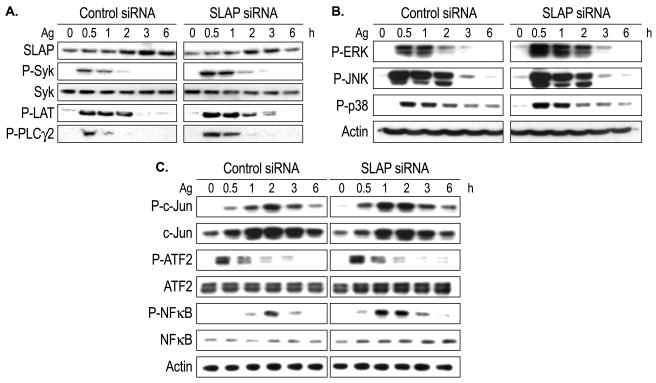
Cells transfected with anti-SLAP siRNA contained ~ 40% less SLAP than control RNA-transfected cells. Following Ag stimulation the difference between the two sets of cells ranged from 40% to 70% for all experiments (see data in Fig. 3A for a typical experiment). As determined by immunoblotting, Ag stimulation resulted in a modest decline in expression of FcεRIγ subunits over the course of six hours (~25% for three experiments) but no consistent difference in rates of decline could be detected between control and siRNA-treated cells (data not shown). However, the capacity of cells to bind FITC-labeled IgE, as determined by flow cytometry, was increased slightly by knock down of SLAP. The average additional binding on anti-SLAP siRNA-treated cells was 14% with values ranging from 9% to 17% in five experiments (data not shown).
Fig. 3.
Effects of anti-SLAP siRNA on phosphorylation of signaling molecules and transcription factors in Ag-stimulated cells. Control and siRNA-treated RBL-2H3 cells were stimulated with 50 ng/ml Ag for the times shown for determination of the extent of phosphorylation of the indicated proteins as determined by PAGE and immunoblotting (see Materials and Methods). The results shown are typical of three or more experiments.
3.3. Effects of SLAP deficiency on signaling events
As noted above, knockdown of SLAP by siRNA diminished SLAP levels in both basal and Ag-stimulated cells (Fig. 3A). The reduction in SLAP was associated with enhanced tyrosine phosphorylation of Syk and its downstream targets LAT and PLCγ2 following Ag stimulation (Fig. 3A). This was also true for the phosphorylation of ERK, JNK, and p38 MAP kinase (Fig. 3B). The enhancement of these early signaling events was reflected in enhanced activation of downstream transcription factors as indicated by increased phosphorylation of c-Jun, ATF2, and NFκB and possibly increased expression of NFB (Fig. 3C). The most notable enhancement of phosphorylation was that of c-Jun and NFκB one to two hours after the addition of Ag.
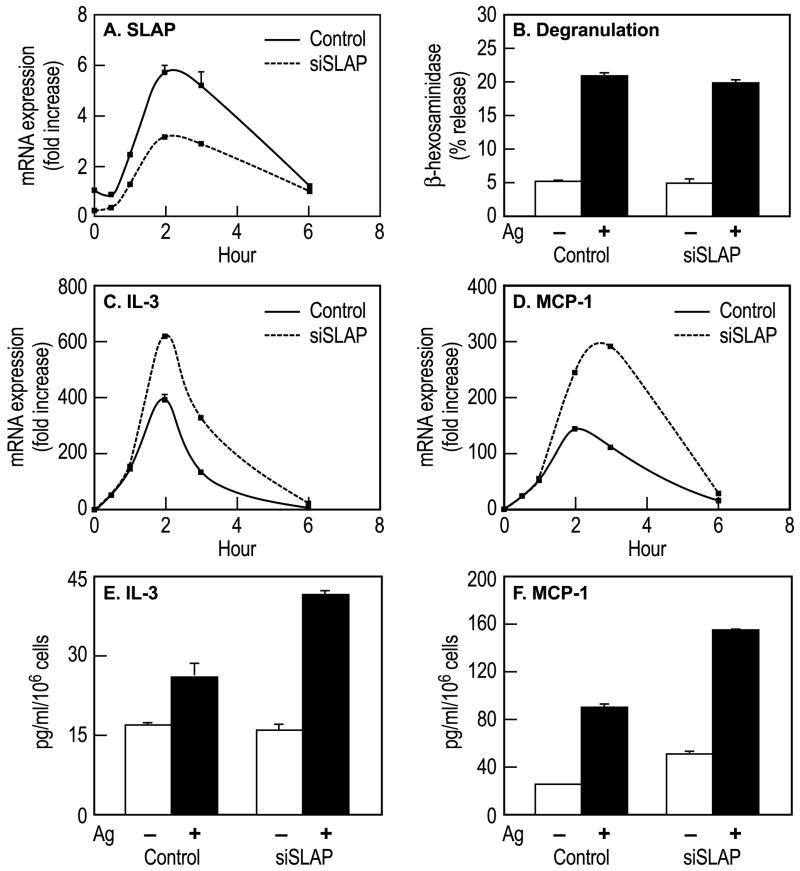
3.4. Effects of knock-down of SLAP on degranulation and cytokine production
The reduction in SLAP mRNA in anti- SLAP siRNA-treated cells (Fig. 4A) had no discernable affect on the release of the granule marker, β-hexosaminidase (Fig. 4B). In contrast, production of IL-3 and monocyte chemoattractant protein-1 (MCP-1) transcripts (Fig. 4C and D) and protein (Fig. 4E and F) were enhanced by knockdown of SLAP. The attenuated increase in SLAP transcripts and enhanced increase in IL-3 and MCP-1 transcripts exhibited an almost reciprocal relationship with the differences being most apparent 2 and 3 h after addition of Ag (Fig. 4A, C, and D). However, Ag stimulated production of TNFα was not significantly enhanced (data not shown) to indicate that the negative effects of SLAP did not extend to all cytokines.
Fig. 4.
Effects of anti-SLAP siRNA on degranulation and production of IL-3 and MCP-1 mRNA and protein. Control and siRNA-treated RBL-2H3 cells were stimulated with 50 ng/ml Ag for the indicated times (Panels A, C–F) or for 20 min (Panel A) for the measurement of SLAP mRNA (Panel A), release of β-hexosaminidase (Panel B), intracellular levels of IL-3 and MCP-1 mRNA (Panels C and D), and IL-3 and MCP-1 released into the medium (Panels E and F). Values in panels A, C, and D indicate fold increase in mRNA relative to the zero time point (equals 1), those in panel B indicate percent release of β-hexosaminidase from cells, and those in panels E and F indicate concentrations of IL-3 or MCP-1 in culture medium. Values are mean ± SEM of data from three cultures in one experiment that is representative of two or more experiments.
4. Discussion
We find that stimulation of RBL-2H3 cells with Ag leads to increased transcription of the SLAP gene (Sla-1) and a substantial increase in SLAP protein (Fig. 1A). The extent of increase in SLAP mRNA is dependent on concentration of Ag (Fig. 1C). At optimal concentrations of Ag the increase (15 to 30 fold) is equal to or greater than that observed in dexamethasone-treated RBL-2H3 cells which typically exhibit about a tenfold increase in SLAP mRNA 12 h after addition of dexamethasone (Hiragun et al., 2006). This upregulation is dependent on activation of several signaling pathways that appear to include PKC, JNK, p38 MAP kinase, and calcineurin (Fig. 2) and presumably on the activation of transcription factors that lie downstream of these enzymes. The increase in SLAP is apparent within 60 min of stimulation and reaches a maximum by two to three hours (Fig. 1A), a time-course that might have little impact on degranulation (Fig. 4B) but sufficiently rapid to reduce production of some cytokines and chemokines at later stages of Ag stimulation (Fig. 4E and F).
An ambiguity is that the reduction of basal levels of SLAP by siRNA in resting RBL-2H3 cells is obviously sufficient to enhance early Syk-dependent signaling events (Fig. 3A) but not degranulation (Fig. 4B). Previous studies have shown that RBL-2H3 cells generate stimulatory signals in excess of that required for degranulation (Maeyama et al., 1986) and it is possible that signals of additional strength would have little impact on degranulation. Also, maximal rates of degranulation occur at concentrations of free Ca2+ (~300 nM) well below those normally observed in optimally stimulated cells (Beaven and Ozawa, 1996). However, the relatively robust phosphorylation of c-Jun and NFκB at one and two hours (Fig. 3C) does apparently result in augmented production of IL-3 and MCP-1 (Fig. 4C to F) in SLAP-deficient cells.
Previous studies with dexamethasone and overexpressed SLAP in RBL-2H3 cells demonstrated that SLAP suppressed activation of Syk and Syk-dependent signaling events such as the phosphorylation of LAT and PLCγ2 as well as mobilization of Ca2+ (Hiragun et al., 2006). The present results with siRNA against SLAP imply that SLAP represses not only activation of these signaling events but also the activation of MAP kinases and transcription factors and, as a consequence cytokine production, thus limiting the duration of their production in Ag-stimulated mast cells. For example, repression of induction of SLAP by siRNA enhances phosphorylation of the AP-1 component c-Jun, ATF-2, and NFκB (Fig. 3C) as well as the production of IL-3 and MCP-1 (Fig. 4C to D). These and our previous data (Hiragun et al., 2006) suggest that SLAP can negatively regulate Syk and signaling pathways down to the nuclear and transcriptional levels. A relevant situation is that Syk −/− BMMC exhibit similar defective signaling and physiologic responses to Ag which include impaired activation of Erk2, JNK, Ca2+/calcineurin-dependent dephosphorylation of NFAT, and production of various cytokines (Costello et al., 1996).
The upregulation of SLAP may have other consequences. In addition to negatively regulating cellular signaling, SLAP also interacts with c-Cbl to down-regulate expression of T and B cell receptors (Dragone et al., 2006; Myers et al., 2006) and cortical F-actin assembly in stimulated fibroblasts (Sirvent et al., 2008). Whether the FcεRI signaling complex and F-actin assembly are similarly targeted in a c-Cbl-dependent manner in mast cells will require further study. Our preliminary data (see Results section) indeed suggest that SLAP may negatively regulate surface expression of FcεRI at least in resting cells. Although increased expression of FcεRI in siRNA-treated cells might account for the enhancement of signaling events, this is probably unlikely because RBL-2H3 cells express far more receptors than is required for maximal degranulation (Maeyama et al., 1986).
In conclusion, our data indicate that Ag stimulation of RBL-2H3 cells results in substantial upregulation of the inhibitory regulator SLAP which has significant impact on major signaling events and on delayed functional responses such as the production of IL-3 and MCP-1. The upregulation of SLAP thus provides an another mechanism for suppressing mast cell activity especially at late stages of activation in addition to those mediated through Lyn (Xiao et al., 2005), PTEN, SHIP (Furumoto et al., 2006), SHP1, and SHP2 (Kimura et al., 1997a; Kimura et al., 1997b). Also, SLAP appears to have the same profile of actions in RBL-2H3 cells as that previously described for T cells and B cells.
Acknowledgments
This work was supported by the Intramural Research Program of NHLBI at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramson J, Pecht I. Clustering the mast cell function-associated antigen (MAFA) leads to tyrosine phosphorylation of p62Dok and SHIP and affects RBL-2H3 cell cycle. Immunol Lett. 2002;82:23–28. doi: 10.1016/s0165-2478(02)00013-5. [DOI] [PubMed] [Google Scholar]
- Andrade MV, Hiragun T, Beaven MA. Dexamethasone suppresses antigen-induced activation of phosphatidylinositol 3-kinase and downstream responses in mast cells. J Immunol. 2004;172:7254–7262. doi: 10.4049/jimmunol.172.12.7254. [DOI] [PubMed] [Google Scholar]
- Beaven MA, Ozawa K. Role of calcium, protein kinase C, and MAP kinase in the activation of mast cells. Allergol Internat. 1996;45:73–84. [Google Scholar]
- Choi WS, Kim YM, Combs C, Frohman MA, Beaven MA. Phospholipase D1 and 2 regulate different phases of exocytosis in mast cells. J Immunol. 2002;168:5682–5689. doi: 10.4049/jimmunol.168.11.5682. [DOI] [PubMed] [Google Scholar]
- Costello PS, Turner M, Walters AE, Cunningham CN, Bauer PH, Downward J, Tybulewicz VL. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene. 1996;13:2595–2605. [PubMed] [Google Scholar]
- Dragone LL, Myers MD, White C, Gadwal S, Sosinowski T, Gu H, Weiss A. Src-like adaptor protein (SLAP) regulates B cell receptor levels in a c-Cbl-dependent manner. Proc Natl Acad Sci U S A. 2006;103:18202–18207. doi: 10.1073/pnas.0608965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumoto Y, Brooks S, Olivera A, Takagi Y, Miyagishi M, Taira K, Casellas R, Beaven MA, Gilfillan AM, Rivera J. Cutting Edge: Lentiviral shRNA silencing of PTEN reveals a regulatory role in homeostasis and activation of mast cells. J Immunol. 2006;176:5167–5171. doi: 10.4049/jimmunol.176.9.5167. [DOI] [PubMed] [Google Scholar]
- Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- Hiragun T, Peng Z, Beaven MA. Cutting Edge: Dexamethasone negatively regulates Syk in mast cells by up-regulating Src-like adaptor protein. J Immunol. 2006;177:2047–2050. doi: 10.4049/jimmunol.177.4.2047. [DOI] [PubMed] [Google Scholar]
- Hiragun T, Peng Z, Beaven MA. Dexamethasone up-regulates the inhibitory adaptor protein Dok-1 and suppresses downstream activation of the mitogen-activated protein kinase pathway in antigen-stimulated RBL-2H3 mast cells. Mol Pharmacol. 2005;67:598–603. doi: 10.1124/mol.104.008607. [DOI] [PubMed] [Google Scholar]
- Hirasawa N, Santini F, Beaven MA. Activation of the mitogen-activated protein kinase/cytosolic phospholipase A2 pathway in a rat mast cell line. Indications of different pathways for release of arachidonic acid and secretory granules. J Immunol. 1995a;154:5391–5402. [PubMed] [Google Scholar]
- Hirasawa N, Scharenberg A, Yamamura H, Beaven MA, Kinet JP. A requirement for Syk in the activation of the MAP kinase/phospholipase A2 pathway by FcεR1 is not shared by a G protein-coupled receptor. J Biol Chem. 1995b;270:10960–10967. 10960–10967. doi: 10.1074/jbc.270.18.10960. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Liao XC, Mendenhall MK, Zhou X, Pardo J, Chu P, Spencer C, Fu A, Sheng N, Yu P, Pali E, Nagin A, Shen M, Yu S, Chan E, Wu X, Li C, Woisetschlager M, Aversa G, Kolbinger F, Bennett MK, Molineaux S, Luo Y, Payan DG, Mancebo HS, Wu J. Functional cloning of Src-like adapter protein-2 (SLAP-2), a novel inhibitor of antigen receptor signaling. J Exp Med. 2001;194:1263–1276. doi: 10.1084/jem.194.9.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FcεRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104:2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Na HJ, Hong SH, Kim HM. Inhibition of the stem cell factor-induced migration of mast cells by dexamethasone. Endocrinology. 2003;144:4080–4086. doi: 10.1210/en.2003-0115. [DOI] [PubMed] [Google Scholar]
- Kassel O, Sancono A, Kratzschmar J, Kreft B, Stassen M, Cato AC. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 2001;20:7108–7116. doi: 10.1093/emboj/20.24.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepley CL, Taghavi S, Mackay GA, Zhu D, Morel PA, Zhang K, Ryan JJ, Satin LS, Zhang M, Pandolfi PP, Saxon A. Co-aggregation of FcγRII with FcεRI on human mast cells inhibits antigen-induced secretion and involves SHIP-Grb2-Dok complexes. J Biol Chem. 2004;279:35139–35149. doi: 10.1074/jbc.M404318200. [DOI] [PubMed] [Google Scholar]
- Kimura T, Sakamoto H, Appella E, Siraganian RP. The negative signaling molecule SH2 domain-containing inositol-polyphosphatase (SHIP) binds to the tyrosine-phosphorylated γ subunit of the high affinity IgE receptor. J Biol Chem. 1997a;272:13991–13996. doi: 10.1074/jbc.272.21.13991. [DOI] [PubMed] [Google Scholar]
- Kimura T, Zhang J, Sagawa K, Sakaguchi K, Appella E, Siraganian RP. Syk-independent tyrosine phosphorylation and association of the protein tyrosine phosphatases SHP-1 and SHP-2 with the high affinity IgE receptor. J Immunol. 1997b;159:4426–4434. [PubMed] [Google Scholar]
- Kyo S, Sada K, Qu X, Maeno K, Miah SM, Kawauchi-Kamata K, Yamamura H. Negative regulation of Lyn protein-tyrosine kinase by c-Cbl ubiquitin-protein ligase in FcεRI-mediated mast cell activation. Genes Cells. 2003;8:825–836. doi: 10.1046/j.1365-2443.2003.00679.x. [DOI] [PubMed] [Google Scholar]
- Lang R, Hammer M, Mages J. DUSP meet immunology: dual specificity MAPK phosphatases in control of the inflammatory response. J Immunol. 2006;177:7497–7504. doi: 10.4049/jimmunol.177.11.7497. [DOI] [PubMed] [Google Scholar]
- Li L, Yao Z. Mast cell and immune inhibitory receptors. Cell Mol Immunol. 2004;1:408–415. [PubMed] [Google Scholar]
- Loreto MP, Berry DM, McGlade CJ. Functional cooperation between c-Cbl and Src-like adaptor protein 2 in the negative regulation of T-cell receptor signaling. Mol Cell Biol. 2002;22:4241–4255. doi: 10.1128/MCB.22.12.4241-4255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeyama K, Hohman RJ, Metzger H, Beaven MA. Quantitative relationships between aggregation of IgE receptors, generation of intracellular signals, and histamine secretion in rat basophilic leukemia (2H3) cells. Enhanced responses with heavy water. J Biol Chem. 1986;261:2583–2592. [PubMed] [Google Scholar]
- Molfetta R, Peruzzi G, Santoni A, Paolini R. Negative signals from FcεRI engagement attenuate mast cell functions. Arch Immunol Ther Exp (Warsz) 2007;55:219–229. doi: 10.1007/s00005-007-0028-4. [DOI] [PubMed] [Google Scholar]
- Myers MD, Sosinowski T, Dragone LL, White C, Band H, Gu H, Weiss A. Src-like adaptor protein regulates TCR expression on thymocytes by linking the ubiquitin ligase c-Cbl to the TCR complex. Nat Immunol. 2006;7:57–66. doi: 10.1038/ni1291. [DOI] [PubMed] [Google Scholar]
- Ota Y, Samelson LE. The product of the proto-oncogene c-cbl: A negative regulator of the Syk tyrosine kinase. Science. 1997;276:418–420. doi: 10.1126/science.276.5311.418. [DOI] [PubMed] [Google Scholar]
- Ott VL, Cambier JC. Activating and inhibitory signaling in mast cells: new opportunities for therapeutic intervention? J Allergy Clin Immunol. 2000;106:429–440. doi: 10.1067/mai.2000.109428. [DOI] [PubMed] [Google Scholar]
- Ott VL, Tamir I, Niki M, Pandolfi PP, Cambier JC. Downstream of kinase, p62dok, is a mediator of FcγIIB inhibition of FcεRI signaling. J Immunol. 2002;168:4430–4439. doi: 10.4049/jimmunol.168.9.4430. [DOI] [PubMed] [Google Scholar]
- Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Szallasi Z, Kazanietz MG, Blumberg PM, Mischak H, Mushinski JF, Beaven MA. Ca2+-dependent and Ca2+-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells: Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J Biol Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- Pakuts B, Debonneville C, Liontos LM, Loreto MP, McGlade CJ. The Src-like adaptor protein 2 regulates colony-stimulating factor-1 receptor signaling and down-regulation. J Biol Chem. 2007;282:17953–17963. doi: 10.1074/jbc.M701182200. [DOI] [PubMed] [Google Scholar]
- Pandey A, Duan H, Dixit VM. Characterization of a novel Src-like adapter protein that associates with the Eck receptor tyrosine kinase. J Biol Chem. 1995;270:19201–19204. doi: 10.1074/jbc.270.33.19201. [DOI] [PubMed] [Google Scholar]
- Pandey A, Ibarrola N, Kratchmarova I, Fernandez MM, Constantinescu SN, Ohara O, Sawasdikosol S, Lodish HF, Mann M. A novel Src homology 2 domain-containing molecule, Src-like adapter protein-2 (SLAP-2), which negatively regulates T cell receptor signaling. J Biol Chem. 2002;277:19131–19138. doi: 10.1074/jbc.M110318200. [DOI] [PubMed] [Google Scholar]
- Paolini R, Molfetta R, Beitz LO, Zhang J, Scharenberg AM, Piccoli M, Frati L, Siraganian R, Santoni A. Activation of Syk tyrosine kinase is required for c-Cbl-mediated ubiquitination of FcεRI and Syk in RBL cells. J Biol Chem. 2002;277:36940–36947. doi: 10.1074/jbc.M204948200. [DOI] [PubMed] [Google Scholar]
- Park SK, Beaven MA. Mechanism of upregulation of the inhibitory regulator, src-like adaptor protein (SLAP), by glucocorticoids in mast cells. Mol Immunol. 2009;46:492–497. doi: 10.1016/j.molimm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Beaven MA. An essential role for phospholipase D in the activation of protein kinase C and degranulation in mast cells. J Immunol. 2005;174:5201–5208. doi: 10.4049/jimmunol.174.9.5201. [DOI] [PubMed] [Google Scholar]
- Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcεRI and Toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Sada K, Kyo S, Maeno K, Miah SM, Yamamura H. Negative regulation of FcεRI-mediated mast cell activation by a ubiquitin-protein ligase Cbl-b. Blood. 2004;103:1779–1786. doi: 10.1182/blood-2003-07-2260. [DOI] [PubMed] [Google Scholar]
- Razin E, Szallasi Z, Kazanietz MG, Blumberg PM, Rivera J. Protein kinase C-β and C-ε link the mast cell high-affinity receptor for IgE to the expression of c-fos and c-jun. Proc Natl Acad Sci USA. 1994;91:7722–7726. doi: 10.1073/pnas.91.16.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003;15:639–646. doi: 10.1016/j.coi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Sirvent A, Leroy C, Boureux A, Simon V, Roche S. The Src-like adaptor protein regulates PDGF-induced actin dorsal ruffles in a c-Cbl-dependent manner. Oncogene. 2008;27:3494–3500. doi: 10.1038/sj.onc.1211011. [DOI] [PubMed] [Google Scholar]
- Sosinowski T, Pandey A, Dixit VM, Weiss A. Src-like adaptor protein (SLAP) is a negative regulator of T cell receptor signaling. J Exp Med. 2000;191:463–474. doi: 10.1084/jem.191.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Sawasdikosol S, Chang JH, Burakoff SJ. SLAP, a dimeric adapter protein, plays a functional role in T cell receptor signaling. Proc Natl Acad Sci U S A. 1999;96:9775–9780. doi: 10.1073/pnas.96.17.9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkaczyk C, Jensen BM, Iwaki S, Gilfillan AM. Adaptive and innate immune reactions regulating mast cell activation: from receptor-mediated signaling to responses. Immunol Allergy Clin North Am. 2006;26:427–450. doi: 10.1016/j.iac.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Xiao W, Nishimoto H, Hong H, Kitaura J, Nunomura S, Maeda-Yamamoto M, Kawakami Y, Lowell CA, Ra C, Kawakami T. Positive and negative regulation of mast cell activation by Lyn via the FcεRI. J Immunol. 2005;175:6885–6892. doi: 10.4049/jimmunol.175.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]