Abstract
Increased production of nitric oxide (NO) and prostaglandins contribute to development of hypotension during endotoxemia. We have previously demonstrated that endotoxemia-induced increase in NO production suppresses renal cytochrome P450 (CYP) 4A expression and activity, and that selective inhibition of inducible NO synthase (iNOS) with 1,3-PBIT restores renal CYP 4A protein and activity and mean arterial pressure (MAP). By using cyclooxygenase (COX) inhibitor indomethacin, we investigated herein whether prostaglandins, via NO production, inhibit renal CYP 4A1 protein expression and CYP 4A activity and contribute to the endotoxin-induced hypotension. In conscious male Sprague-Dawley rats, endotoxin (10 mg/kg, intraperitoneal (i.p.)) reduced MAP, increased serum nitrite and bicyclo PGE2 levels, renal nitrite production and iNOS protein expression, and decreased renal CYP 4A1 protein expression and CYP 4A activity after 4 h injection. All of the endotoxin-induced changes, except for increase in renal nitrite production, were prevented by indomethacin (5 mg/kg, i.p. 1 h after endotoxin). The effects of indomethacin on the endotoxin-induced decrease in MAP, CYP 4A1 protein expression and CYP 4A activity were minimized by the CYP 4A inhibitor, aminobenzotriazole (50 mg/kg, i.p. 1 h after endotoxin). These data suggest that prostaglandins produced during endotoxemia increase iNOS protein expression and NO synthesis, and decrease CYP 4A protein expression and CYP 4A activity and that inhibition of iNOS or COX restores renal CYP 4A protein level and CYP 4A activity and MAP presumably due to increased production of arachidonic acid metabolites derived from CYP 4A.
Keywords: Blood pressure, Kidney, Nitric oxide, Prostaglandin, CYP 4A
Introduction
Expression of inducible nitric oxide (NO) synthase (iNOS) in many tissues in response to mediators released by endotoxin stimulate generation of NO, which contributes to systemic hypotension, vascular hyporeactivity, multiple organ failure, and high mortality rate that are associated with septic shock. Increased production of prostaglandins by inducible cyclooxygenase (COX-2) has also been shown to contribute to systemic hypotension and related organ damage and decreased survival in animals and humans with sepsis (Ejima and Perrella, 2004; Tslotou et al., 2005). Non-selective COX inhibitors, such as indomethacin, prevent (Ashorbi and Williams, 1995) or do not improve (Tunctan et al., 2000) the lethal effects of endotoxin in animal models of sepsis. The beneficial effects of indomethacin are correlated with decreased levels of nitrite and prostaglandins in biological fluids from endotoxemic rats (Futaki et al., 1997). Indomethacin has also shown to abolish or significantly attenuate the decrease in blood pressure (Fatehi-Hassanabad et al., 1996) or have no significant effect on blood pressure in endotoxemic rats (Pique et al., 1988; Vayssettes-Courchay et al., 2002). Vasoconstriction and increased blood pressure after systemic inhibition of NOS results not only from withdrawal of vasodilator NO, but from increased activity of the vasoconstrictor systems, including sympathetic nervous and renin-angiotensin systems, and production of endothelins and 20-hydroxyeicosatetraenoic acid (20-HETE), an arachidonic acid metabolite derived via cytochrome P450 (CYP) (Fleming, 2001; Roman, 2002; Zatz and Baylis, 1998). The production of kidney CYP-derived arachidonic acid metabolites is altered in diabetes, pregnancy, hepatorenal syndrome, inflammation, and in various models of hypertension, and it is likely that changes in this system contribute to the abnormalities in renal function that are associated with many of these conditions (Fleming, 2001; Roman, 2002; Zhao and Imig, 2003). The co-localization of NOS and CYP 4A isoforms at the same renal sites increases the potential for interaction and this may affect renal function. It has been reported that NO inhibits renal ω-hydroxylase activity and 20-HETE production (Alonso-Galicia et al., 1998; Oyekan et al., 1999; Wang et al., 2003) and that withdrawal of NO leads to increased ω-hydroxylase activity (Wang et al., 2003), expression of CYP 4A protein (Oyekan et al., 1999), and 20-HETE production in the kidney (Escalante et al., 2002; Oyekan et al., 1999; Wang et al., 2003). Inhibition of the endogenous production of 20-HETE has also been shown to contribute to the vasodilator effects of NO on renal and peripheral vascular tone (Alonso-Galicia et al., 1997, 1998).
During endotoxemia, CYP 4A1, -A2, and -A3 mRNAs, as well as hepatic iNOS mRNA (Sewer et al., 1997), are induced in the liver and kidneys of rats (Mitchell et al., 2001; Sewer et al., 1998) in contrast to most other hepatic CYP gene products, which are down-regulated (Renton and Nicholson, 2000; Sewer and Morgan, 1998; Sewer et al., 1996; Takemura et al., 1999). It has been reported that endotoxin-induced down-regulation of hepatic CYP 2C11, 2E1, and 3A2 mRNAs and/or protein expression is not changed by inhibition of iNOS (Sewer et al., 1996). On the other hand, NO produced in endotoxemia is associated with decreased hepatic microsomal total CYP content, CYP 1A1/2, 2B1/2, 2C6, 2C11, 3A1, and 3A2 mRNA, protein expression or activity (Khatsenko and Kikkawa, 1997; Khatsenko et al., 1997; Morgan et al., 2002; Müller et al., 1996; Takemura et al., 1999), which is prevented by NOS inhibitors in rats. Recently, it has been demonstrated that NO produced during pregnancy inhibits CYP 4A expression and 20-HETE synthesis in rat renal micro-vessels and that augmentation of renal microvessel 20-HETE synthesis after NOS inhibition is associated with increased blood pressure, which is prevented by the CYP 4A inhibitor, aminobenzotriazole (Wang et al., 2003).
There is relationship between 20-HETE synthesis by CYP 4A and induction of COX-2 which can convert 20-HETE to its prostaglandin analogs. Metabolism of 20-HETE by COX-2 acts as a brake mechanism that prevents the unopposed action of 20-HETE (Cheng and Harris, 2003). The co-expression of an inducible membrane-associated prostaglandin (PGE2) synthase (Murakami et al., 2000) that acts in concert with COX-2 may favor formation of 20-OH PGE2, a vasodilator prostaglandin analogue of 20-HETE (Carroll et al., 2001). Non-steroidal anti-inflammatory drugs (NSAIDs) including acetylsalicylic acid have been reported to increase CYP 4A activity in rat hepatic microsomes (Okita, 1986). It has also been shown that total CYP content is decreased by ibuprofen and fenbufen, while ibuprofen increases CYP 4A1 protein and CYP 4A activity in rat hepatic microsomes (Rekka et al., 1994). Therefore, it is possible that, during endotoxemia, one or more prostanoids generated via COX-2 stimulate(s) NO production, which in turn reduces the expression and/or activity of CYP 4A and generation of 20-HETE and causes hypotension.
We have previously demonstrated that the endotoxemia-induced increase in NO production primarily via iNOS suppresses renal CYP 4A1/3 protein expression and CYP 4A activity, and inhibition of iNOS with phenylene-1,3-bis [ethane-2-isothiourea] dihydrobromide (1,3-PBIT) restores renal CYP 4A protein and CYP 4A activity and MAP presumably due to increased production of arachidonic acid metabolites derived from CYP 4A (Tunctan et al., 2006b). In this study, we tested the hypothesis that prostaglandins, via NO production, inhibit CYP 4A1 protein expression and CYP 4A activity and contribute to the endotoxin-induced hypotension. Preliminary results have been presented in abstract form (Tunctan et al., 2004).
Materials and Methods
Animals and endotoxic shock model
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 250 to 400 g were fed standard chow (Ralston Purina Co.). All the protocols were approved by the Intitutional Animal Care and Use Committee at the University of Tennessee Health Science Center and were consistent with the Guide for the Care and Use of Laboratory Animals published by the National Health Institute. Endotoxic shock was induced as described by Tunctan et al. (2006b). Briefly, conscious rats received either endotoxin (Escherichia coli lipopolysaccharide (LPS), O111:B4; Sigma Chemical, St. Louis, MO) (10 mg/kg, intraperitoneal (i.p.) sublethal dose) or saline (4 mL/kg, i.p.) at time 0, and systolic and diastolic blood pressure were measured using a plethysmographic non-invasive blood pressure system (XBP1000, Kent Scientific Corporation, Torrington, CT) at 0, 1, 2, 3, and 4 h. Since this system detects systolic and diastolic blood pressure non-invasively from rat tail, mean arterial pressure (MAP) values from each animal were calculated according to the formula: MAP = Diastolic Blood Pressure + 1/3 (Systolic Blood Pressure – Diastolic Blood Pressure). Separate groups of rats were treated with endotoxin alone or in combination with a highly selective iNOS inhibitor 1,3-PBIT (Sigma), a COX inhibitor indomethacin (Sigma) and/or a CYP 4A enzyme inhibitor aminobenzotriazole (Sigma) at 1 h after injection of saline or endotoxin. 1,3-PBIT (10 mg/kg, i.p.) was used at a dose preventing the effects of endotoxin on MAP, nitrite production and CYP 4A protein and activity (Tunctan et al., 2006b; Wang et al., 1999). Dose of indomethacin (5 mg/kg, i.p.) was selected according to our preliminary studies with endotoxin and findings of the previous studies preventing the effects of endotoxin on prostaglandin production (Jaworek et al., 2001). Aminobenzotriazole (50 mg/kg, i.p.) was used at a dose inhibiting renal CYP 4A protein and activity (Su et al., 1998; Tunctan et al., 2006b). Rats were sacrificed 4 h after endotoxin challenge, and the blood and kidneys were collected. Sera were obtained from blood samples by centrifugation at 14,000 g for 15 min at 4°C and stored at -70°C until analyzed for the measurement of nitrite levels. Kidneys were rapidly frozen in liquid nitrogen and ground into powder on ice. The tissue powders (100 mg) were homogenized in 1 mL ice-cold buffer (mmol/L: HEPES 20 [pH 7.5], β-glycerophosphate 20, sodium pyrophosphate 20; sodium orthovanadate 0.2, EDTA 2, sodium fluoride 20, benzamidine 10, dithiothreitol 1, leupeptin 20, and aprotinin 10). Cell debris was removed by centrifugation at 14,000 g for 10 min at 4°C followed by sonication for 15 s on ice with 50 μL ice-cold Tris (50 mmol/L, pH 8.0) and KCl (0.5 M). The samples were centrifuged at 14,000 g for 15 min at 4°C and then supernatants were removed and stored at -70°C until analyzed for the measurement of iNOS, COX, or CYP 4A1 protein levels and activities. The total protein amount was determined by Coomassie blue method using bovine serum albumin as standard.
Immunoblotting for iNOS and CYP 4A1 proteins
Immunoblotting for iNOS and CYP 4A1 proteins were performed according to the method as described by Tunctan et al. (2006b). Briefly, tissue homogenates (100 μg of protein) were subjected to a 10% SDS-polyacrylamide gel electrophoresis and then proteins were transferred to a nitrocellulose membrane. The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline (mmol/L: Tris-HCl 25 [pH 7.4], NaCl 137, KCl 27 and 0.05% Tween 20) and incubated overnight with anti-iNOS monoclonal antibody (Transduction Lab., Lexington, KY) (1:1,000 in 5% milk and 1% BSA) or goat anti-rat CYP 4A1 polyclonal antibody (Gentest, Woburn, MA) (1:500 in 0.5% milk) followed by incubation with anti-mouse IgG antibody for iNOS (Amersham Life Sciences, Arlington Heights, IL) (1:1,000 in 5% milk) or anti-goat IgG antibody for CYP 4A1 (Vector Lab., Burlingame, CA) (1:5,000 in 5% milk) antibodies for 1 h. Lysates of mouse macrophages treated with interferon-γ and LPS (Transduction Lab., Lexington, KY) and rat liver microsomes treated with clofibrate (Gentest, Woburn, MA) were used as positive controls for iNOS and CYP 4A1, respectively. Immunoreactive proteins were detected by chemiluminescence using the ECL kit (Amersham), according to the manufacturer's instructions. Densitometric analysis was performed with NIH image software (ImageJ 1.29). The same membrane was used to determine α-actin expression using a monoclonal antibody against α-actin (Sigma) (1:1,000 in 5% milk) and the content of the latter was used to normalize the expression of iNOS and CYP 4A1 protein in each sample.
Measurement of iNOS and CYP 4A activities
Nitrite production was measured as an index for iNOS activity by diazotization method in the sera and tissue homogenates as described (Tunctan et al., 2006b). Lauric acid hydroxylase activity reflecting CYP 4A activity was measured as the NADPH-dependent formation of [14C]-12-hydroxylauric acid in the tissue homogenates by incubating with [1-14C]-labeled lauric acid as described (Tunctan et al., 2006b).
Measurement of serum bicyclo PGE2 levels
Serum bicyclo PGE2 concentrations were measured as an index for COX activity by ELISA according to the manufacturer's instructions in the bicyclo PGE2 assay kit (Cayman Chemical Co., Ann Arbor, MI).
Data analysis
All data were expressed as means±S.E.M. Data were analyzed by one-way ANOVA followed by Student-Newman-Keuls test for multiple comparisons and Student's t or Mann-Whitney U tests when necessary. p value of < 0.05 was considered to be statistically significant.
Results
Endotoxin-induced fall in MAP is mediated by inhibition of CYP 4A activity by NO and prostaglandins
Injection of endotoxin to rats caused a decrease in MAP (Fig. 1). A COX inhibitor, indomethacin, prevented the endotoxin-induced decrease in MAP, while it alone had no effect on MAP (Fig. 1). Administration of a CYP 4A inhibitor, aminobenzotriazole, diminished the effect of indomethacin in preventing the endotoxin-induced decrease in MAP (Fig. 1). A selective iNOS inhibitor, 1,3-PBIT, potentiated the effect of indomethacin to inhibit the endotoxin-induced decrease in MAP (Fig. 1). This effect of 1,3-PBIT and indomethacin combination to prevent the endotoxin-induced decrease in MAP was also reversed by aminobenzotriazole.
Fig. 1.
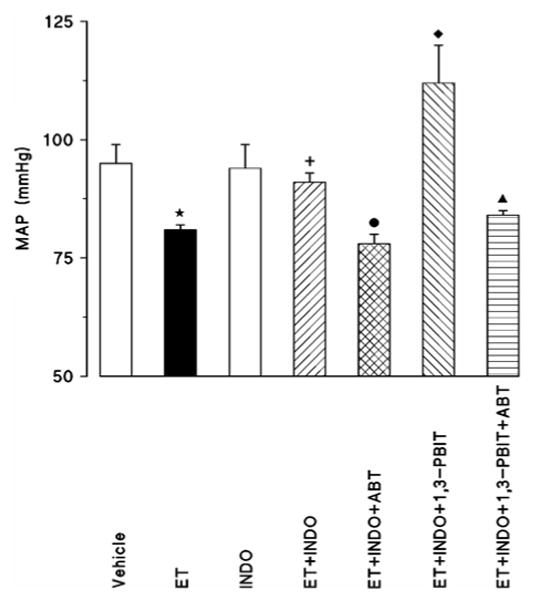
Effect of aminobenzotriazole (ABT) and/or 1,3-PBIT on the indomethacin (INDO)-induced changes in mean arterial pressure (MAP) 4 h after saline (vehicle) or endotoxin (ET) injection in conscious rats. INDO, ABT and/or 1,3-PBIT were given at 1 h after ET injection. Values are expressed as means±S.E.M. from 4-20 rats per treatment group. *ET vs. vehicle (p<0.05). +ET+INDO vs. ET (p<0.05). ●ET+INDO+ABT vs. ET+INDO (p<0.05). ◆ET+INDO+1,3-PBIT vs. ET+INDO (p<0.05). ▲ ET+INDO+1,3-PBIT+ABT vs. ET+INDO+1,3-PBIT (p<0.05).
Aminobenzotriazole minimizes endotoxin-induced rise in serum nitrite level that is decreased by 1,3-PBIT and indomethacin
Indomethacin caused a decrease in the endotoxin-induced rise in serum bicyclo PGE2 levels (control: 125±11; endotoxin: 181±5; indomethacin: 119±13, and endotoxin + indomethacin: 152±12 pg/mL, n=5). Indomethacin also minimized the endotoxin-induced increase in the serum nitrite levels; the basal serum nitrite levels were not affected by indomethacin (Fig. 2A). While serum nitrite levels in the indomethacin-treated endotoxemic rats were not changed by aminobenzotriazole, 1,3-PBIT prevented the effect of indomethacin to reduce nitrite levels (Fig. 2A). On the other hand, the serum nitrite levels induced by indomethacin and 1,3-PBIT in the endotoxin-treated rats were inhibited by aminobenzotriazole (Fig. 2A).
Fig. 2.
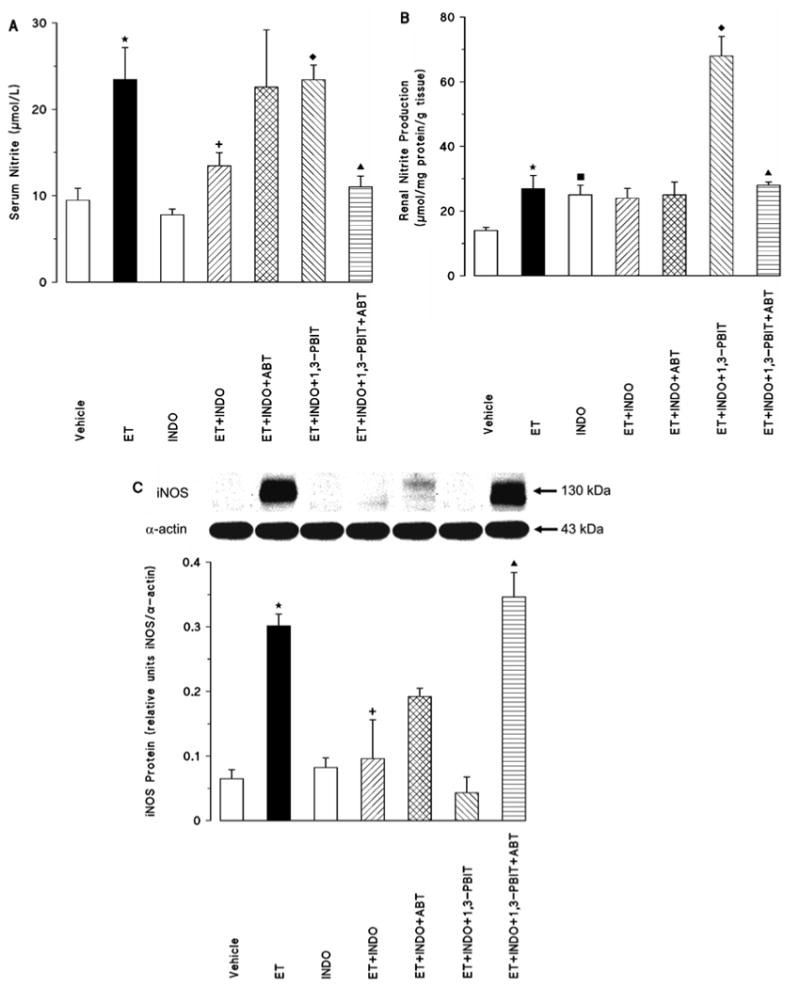
Effect of aminobenzotriazole (ABT) and/or 1,3-PBIT on the indomethacin (INDO)-induced changes in serum nitrite levels (A), renal nitrite production (B) and renal inducible nitric oxide synthase (iNOS) protein expression (C) 4 h after saline (vehicle) or endotoxin (ET) injection in conscious rats. Values are expressed as means±S.E.M. from 3-9 rats per treatment group. *ET vs. vehicle (p<0.05). ■INDO vs. vehicle (p<0.05). +ET+INDO vs. ET (p<0.05). ◆ET+INDO+1,3-PBIT vs. ET+INDO (p<0.05). ▲ET+INDO+1,3-PBIT+ABT vs. ET+INDO+1,3-PBIT (p<0.05).
Indomethacin inhibits the endotoxin-induced increase in iNOS protein expression but not renal nitrite production
Endotoxin-induced decrease in MAP (Fig. 1) and increase in the serum nitrite levels (Fig. 2A) were associated with an increase in renal nitrite production (Fig. 2B) and iNOS protein level (Fig. 2C). Endotoxin-induced increase in the renal nitrite levels was not affected by indomethacin, while it alone increased the basal nitrite production (Fig. 2B). Renal nitrite levels in the indomethacin-treated endotoxemic rats were not changed by aminobenzotriazole, while 1,3-PBIT caused a further increase in the indomethacin-induced levels (Fig. 2B). On the other hand, the effect of 1,3-PBIT and indomethacin in the endotoxin-induced renal nitrite levels was inhibited by aminobenzotriazole (Fig 2B). Indomethacin also minimized the endotoxin-induced increase in the renal iNOS protein content, the basal iNOS protein levels were not affected by indomethacin (Fig. 2C). Renal iNOS protein levels in the indomethacin-treated endotoxemic rats were not changed by aminobenzotriazole and 1,3-PBIT (Fig. 2C). On the other hand, the renal iNOS protein content induced by indomethacin and 1,3-PBIT in the endotoxin-treated rats was increased by aminobenzotriazole (Fig. 2C).
Endotoxemia also decreased renal CYP 4A1 protein content (Fig. 3A) and CYP 4A activity (Fig. 3B). Indomethacin alone markedly caused an increase basal renal CYP 4A1 protein expression (Fig. 3A), but it did not change the basal CYP 4A activity (Fig. 3B). Indomethacin prevented the endotoxin-induced decrease in the renal CYP 4A1 protein content (Fig. 3A) and the CYP 4A activity (Fig. 3B). Aminobenzotriazole diminished the effect of indomethacin to increase the renal CYP 4A1 protein content (Fig. 3A), but not CYP 4A activity (Fig. 3B), in endotoxemic rats (Fig. 3A). Renal CYP 4A1 protein levels (Fig. 3A) and CYP 4A activity (Fig. 3B) in the indomethacin-treated endotoxemic rats were not changed by 1,3-PBIT. On the other hand, the renal CYP 4A1 protein content (Fig. 3A) and CYP 4A activity (Fig. 3B) induced by indomethacin and 1,3-PBIT in the endotoxin-treated rats was decreased by aminobenzotriazole.
Fig. 3.
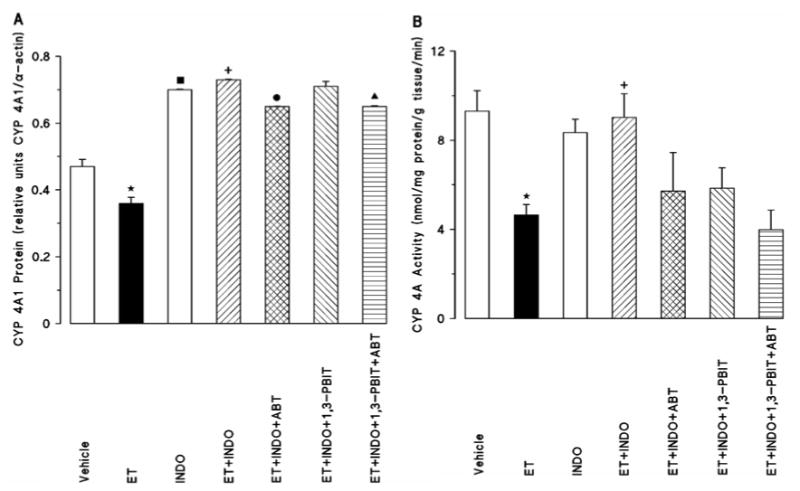
Effect of aminobenzotriazole (ABT) and/or 1,3-PBIT on the indomethacin (INDO)-induced changes in renal cytochrome P450 (CYP) 4A1 protein expression (A) and CYP 4A activity (B) 4 h after saline (vehicle) or endotoxin (ET) injection in conscious rats. Values are expressed as means±S.E.M. from 3-9 rats per treatment group. *ET vs. vehicle (p<0.05). ■INDO vs. vehicle (p<0.05). +ET+INDO vs. ET (p<0.05). ●ET+INDO+ABT vs. ET+INDO (p<0.05). ▲ET+INDO+1,3-PBIT +ABT vs. ET+INDO+1,3-PBIT (p<0.05).
Discussion
This is the first study to provide evidence that prostaglandins produced during endotoxemia increase iNOS protein expression and NO synthesis, and decrease CYP 4A protein expression and activity and that inhibition of iNOS or COX restores renal CYP protein level and activity and MAP presumably due to increased production of arachidonic acid metabolites derived from CYP 4A.
We have previously demonstrated that administration of endotoxin to rats decreases MAP which is associated with an increase in the serum and renal nitrite levels that is prevented by selective inhibitor of iNOS, 1,3-PBIT (Tunctan et al., 2006b). These observations suggest that NO produced mainly by activation of iNOS contributes to the endotoxin-induced hypotension in conscious rats. In the present study, inhibition of COX with indomethacin prevented the endotoxin-induced decrease in MAP, increase in serum nitrite and bicyclo PGE2 levels and renal iNOS protein expression, and decrease in renal CYP 4A1 protein expression and CYP 4A activity. These results demonstrate that increased production of prostaglandins, as well as NO, 4 h after endotoxin injection causes hypotension in conscious rats and possibly COX-2 derived prostaglandins from renal tissue contribute to this effect of endotoxin. These results also indicate that decreased NO production by iNOS mediates the effect of indomethacin to increase MAP, renal CYP 4A1 protein expression, and CYP 4A activity in endotoxemic rats. On the other hand, indomethacin alone increased the renal nitrite production. These results are not surprising, because there are several reports suggesting a direct link between NO and arachidonic acid metabolites (Fleming, 2001; Goodwin et al., 1999; Mollace et al., 2005; Roman, 2002). NOS inhibitors act to increase COX expression and prostaglandin synthesis in response to cytokine stimulation and the addition of NO donors reverses their effects suggesting that NO inhibits COX activity (Amin et al., 1997; Habib et al., 1997; Swierkosz et al., 1995; Tunctan et al., 2006a). NO has also shown to activate COX-1, but inhibit COX-2-derived prostaglandin production in vitro (Clancy et al., 2000). Furthermore, some studies suggest that there is no interaction between NO and prostaglandin synthesis (Boquet et al., 1998; Curtis et al., 1996; Hamilton and Warner, 1998). Similarly, prostaglandins have been shown to inhibit (Shirahase et al., 2000; Tunctan et al., 2006a), increase (Tunctan et al., 2003, 2006b) or not affect (Salvemini et al., 1993) NO production by iNOS in several in vitro and in vivo studies. Based on the results from our previous studies and our present findings, it appears that under basal conditions, prostaglandins produced by COX-1 inhibit NO production by cNOS, while iNOS-derived NO production is increased by prostaglandins under inflammatory conditions.
Among the CYP 4A enzymes, CYP 4A1 has been characterized as the most efficient ω/ω-1 hydroxylase of short- and long-chain fatty acids, including lauric, palmitic, and arachidonic acids (Gibson, 1989; Hardwick, 1991; Nguyen et al., 1999). CYP 4A1 protein expression and CYP 4A activity have been shown to localize in tissues such as liver, lung, kidney, and the vasculature (Bell et al., 1992; Kimura et al., 1989a, 1989b; Persohn et al., 1993). Systemic administration of CYP 4A1 antisense oligodeoxynucleotides to Sprague-Dawley rats significantly decreases renal vascular CYP 4A1 expression and activity, as measured by arachidonic acid conversion to 20-HETE (Wang et al., 1999). It has been reported that ω-hydroxylation product of arachidonic acid, 20-HETE formed by CYP 4A, play an important role in the regulation of blood pressure (Fleming, 2001; Roman, 2002). 20-HETE production is particularly important in the kidney, where it regulates vascular tone and electrolyte excretion (Roman, 2002). Since NO has been shown to inhibit renal ω-hydroxylase activity and 20-HETE production (Alonso-Galicia et al., 1997; Oyekan et al., 1999; Wang et al., 2003) and that withdrawal of NO leads to increased ω-hydroxylase activity (Alonso-Galicia et al., 1997), expression of CYP 4A protein (Oyekan et al., 1999), and 20-HETE production in the kidney (Escalante et al., 2002; Oyekan et al., 1999; Wang et al., 2003), it would appear that CYP 4A1 is most likely the isoform involved in the generation of 20-HETE that restores blood pressure after inhibition of iNOS. We have previously demonstrated that endotoxin decreases CYP 4A1/A3 protein expression and CYP 4A activity in the kidney (Tunctan et al., 2006b). This finding together with our demonstration that iNOS inhibitor 1,3-PBIT and COX inhibitor indomethacin prevented this effect of endotoxin suggests that the decrease in CYP 4A protein expression and CYP 4A activity during endotoxemia is most likely mediated by NO produced by iNOS and prostaglandins produced by COX. NO produced in endotoxemia has also been shown to be associated with decrease in hepatic microsomal total CYP content, CYP 1A1/2, 2B1/2, 2C6, 2C11, 3A1 and 3A2 mRNA, protein expression or activity, which is prevented by NOS inhibitors in rats (Khatsenko and Kikkawa, 1997; Khatsenko et al., 1997; Morgan et al., 2002; Müller et al., 1996; Takemura et al., 1999). Moreover, NSAIDs including acetylsalicylic acid have been reported to increase CYP 4A activity in rat hepatic microsomes (Okita, 1986). It has also been shown that total CYP content is decreased by ibuprofen and fenbufen, while ibuprofen increases CYP 4A1 protein and CYP 4A activity in rat hepatic microsomes (Rekka et al., 1994). Therefore, it can be concluded that prostaglandins produced during endotoxemia decrease CYP 4A protein expression and CYP 4A activity and that inhibition of COX restores renal CYP 4A protein level, CYP 4A activity and MAP presumably due to increased production of arachidonic acid metabolites derived from CYP 4A.
We have previously shown that aminobenzotriazole, a mechanism-based CYP 4A ω-hydroxylase inhibitor that reduces levels of 20-HETE (Su et al., 1998), reverses the effect of 1,3-PBIT (Tunctan et al., 2006b) to minimize endotoxin-induced decrease in MAP. This observation together with our demonstration that indomethacin also abolished the endotoxin-induced decrease in MAP, suggest that during endotoxemia, the decrease in MAP produced by iNOS-derived NO and COX-derived prostaglandins is most likely due to a decrease in the production of the vasocontrictor arachidonic acid metabolites produced via CYP 4A (presumably 20-HETE) as a result of inhibition of CYP 4A ω-hydroxylase activity by NO and prostaglandins. In the present study, aminobenzotriazole also prevented the effects of indomethacin on MAP and CYP 4A1 protein expression and CYP 4A activity. Moreover, inhibition of iNOS prevented the effects of indomethacin to decrease endotoxin-induced rise in serum nitrite levels and to cause a further increase in the indomethacin-induced renal nitrite levels in endotoxemic rats. The effects of COX and iNOS inhibition were also prevented by aminobenzotriazole. Thus, it seems likely that increased production of cNOS-derived NO and 20-HETE might cause to this effect of 1,3-PBIT in endotoxin- and indomethacin-treated rats. It is possible that inhibition of both iNOS and COX enzymes may increase cNOS-derived NO production to maintain physiological functions of constitutively produced NO (Mollace et al., 2005). Then, the increased production of cNOS-derived NO might cause an increase in 20-HETE levels. It seems also likely that 20-HETE might cause an increase in cNOS-derived NO production in endotoxemic rats when iNOS and COX enzymes are inhibited. However, we have previously shown that aminobenzotriazole alone causes a decrease in CYP 4A activity and CYP 4A1/A3 protein level as well as an increase in basal systemic and renal nitrite production without increasing iNOS protein expression (Tunctan et al., 2006b) suggesting an inhibitory effect of 20-HETE on NO production by cNOS. In the present study, aminobenzotriazole markedly increased iNOS protein in endotoxin-, indomethacin- and 1,3-PBIT-treated rats. This could be a compensatory mechanism in response to these effects of aminobenzotriazole on renal 20-HETE levels, and systemic and renal nitrite production when iNOS and COX enzymes are inhibited. Supporting this view are the findings of Wang et al. (2003) who has shown that iNOS-derived NO produced during pregnancy inhibits CYP 4A protein expression and 20-HETE synthesis in rat renal microvessels and that augmentation of renal microvessel 20-HETE synthesis after NOS inhibition is associated with increased blood pressure, which is prevented by aminobenzotriazole. It has also been reported that there are links among CYP 4A activity, 20-HETE synthesis, and induction of COX-2. COX serves as a metabolic pathway for 20-HETE by generating prostaglandin analogs of 20-HETE. Metabolism of 20-HETE by COX-2 acts as a brake mechanism that prevents the unopposed action of 20-HETE (Cheng and Harris, 2003). The co-expression of an inducible membrane-associated PGE2 synthase (Murakami et al., 2000) that acts in concert with COX-2 may favor formation of 20-OH PGE2, a vasodilator prostaglandin analogue of 20-HETE (Carrol et al., 2001). Therefore, it is possible that the decreased production of vasodilator prostaglandins produced by COX-2 from 20-HETE mediate the effect of indomethacin to prevent endotoxin-induced decrease in MAP.
In conclusion, this is the first study to provide evidence that prostaglandins produced during endotoxemia increase iNOS protein expression and NO synthesis, and decrease CYP 4A protein expression and activity and that inhibition of iNOS or COX-2 restores renal CYP protein level and activity and MAP presumably due to increased production of arachidonic acid metabolites derived from CYP 4A. Impairment of cardiovascular and renal function is critically involved in the pathophysiological sequale in septic shock finally resulting in multiorgan failure and death; restoration of these impaired functions should improve therapeutic benefit. Our results suggest that treatment with selective iNOS or COX-2 inhibitors, could restore the cardiovascular function in patients during septic shock. Further characterization of the role of COX-2-derived prostaglandins in the regulation of CYP 4A activity and blood pressure will provide the framework for extension of this work into understanding the role of 20-HETE during endotoxemia. More importantly, further studies with arachidonic acid metabolites generated via CPY4A including stable analogs of 20-HETE in models of endotoxemia could provide a novel approach to treat hypotension in septic shock.
Acknowledgments
This study was supported by USPHS-NIH Grant 19134-31 and the fellowship from TUBITAK-NATO, Turkey, to Dr. B. Tunctan. We gratefully acknowledge Dr. Jean-Hugues Parmentier for helpful suggestions in CYP 4A activity measurement experiments.
Contributor Information
Bahar Tunctan, Department of Pharmacology, Faculty of Pharmacy, Mersin University, Mersin 33169, Turkey.
Fariborz A. Yaghini, Department of Pharmacology, College of Medicine, The University of Tennessee, Center for Health Sciences, Memphis, Tennessee 38163, U.S.A.
Anne Estes, Department of Pharmacology, College of Medicine, The University of Tennessee, Center for Health Sciences, Memphis, Tennessee 38163, U.S.A..
Kafait U. Malik, Department of Pharmacology, College of Medicine, The University of Tennessee, Center for Health Sciences, Memphis, Tennessee 38163, U.S.A.
References
- Alonso-Galicia M, Drummond HA, Reddy KK, Falck JR, Roman RJ. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension. 1997;29:320–325. doi: 10.1161/01.hyp.29.1.320. [DOI] [PubMed] [Google Scholar]
- Alonso-Galicia M, Sun CW, Falck JR, Harder DR, Roman RJ. Contribution of 20-HETE to the vasodilator actions of nitric oxide in renal arteries. Am J Physiol. 1998;275:F370–F378. doi: 10.1152/ajprenal.1998.275.3.F370. [DOI] [PubMed] [Google Scholar]
- Amin AR, Vyas P, Aattur M, Leszczynska-Piziak J, Patel I, Weissmann G, Abramson SW. The mode of action of aspirin-like drugs: effect on inducible nitric oxide synthase. Proc Natl Acad Sci. 1997;92:7926–7930. doi: 10.1073/pnas.92.17.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashorobi RB, Williams PA. Indomethacin and alpha-tocopherol enhanced survival in endotoxic rats. Centr Afr J Med. 1995;41:216–219. [PubMed] [Google Scholar]
- Bell DR, Bars RG, Elcombe CR. Differential tissue-specific expression and induction of cytochrome P450IVA1 and acyl-CoA oxidase. Eur J Biochem. 1992;206:979–986. doi: 10.1111/j.1432-1033.1992.tb17009.x. [DOI] [PubMed] [Google Scholar]
- Boquet M, Cebral E, Motta A, Beron de Astrada M, Gimeno MA. Relationship between mouse uterine contractility, nitric oxide and prostaglandin production in early pregnancy. Prostaglandins Leukot Essent Fatty Acids. 1998;59:163–167. doi: 10.1016/s0952-3278(98)90057-6. [DOI] [PubMed] [Google Scholar]
- Carroll MA, Capparelli MF, Doumand AB, Cheng MK, Jiang H, McGiff JC. Renal vasoactive eicosanoids: interactions between cytochrome P450 and cyclooxygenase metabolites during salt depletion. Am J Hypertens. 2001;14:159A. [Google Scholar]
- Cheng HF, Harris RC. Does cyclooxygenase-2 affect blood pressure? Curr Hypertens Rep. 2003;5:87–92. doi: 10.1007/s11906-003-0016-y. [DOI] [PubMed] [Google Scholar]
- Clancy R, Varenika B, Huang W, Ballou L, Attur M, Amin AR, Abramson SB. Nitric oxide synthase/COX crosstalk: nitric oxide activates COX-1 but inhibits COX-2-derived prostaglandin production. J Immunol. 2000;165:1582–1587. doi: 10.4049/jimmunol.165.3.1582. [DOI] [PubMed] [Google Scholar]
- Curtis JF, Reddy NG, Mason RP, Kalyanaraman B, Eling TE. Nitric oxide: a prostaglandin H synthase 1 and 2 reducing cosubstrate that does not stimulate cyclooxygenase activity or prostaglandin H synthase expression in murine macrophages. Arch Biochem Biophys. 1996;335:369–376. doi: 10.1006/abbi.1996.0518. [DOI] [PubMed] [Google Scholar]
- Ejima K, Perrella MA. Alteration in heme oxygenase-1 and nitric oxide synthase-2 gene expression during endotoxemia in cyclooxygenase-2-deficient mice. Antioxid Redox Signal. 2004;6:850–857. doi: 10.1089/ars.2004.6.850. [DOI] [PubMed] [Google Scholar]
- Escalante BA, McGiff JC, Oyekan AO. Role of cytochrome P-450 arachidonate metabolites in endothelin signalling in rat proximal tubule. Am J Physiol. 2002;282:F144–F150. doi: 10.1152/ajprenal.0064.2001. [DOI] [PubMed] [Google Scholar]
- Fatehi-Hassanabad Z, Muller B, Andriantsitohaina R, Furman BL, Parratt JR, Stoclet JC. Influence of indomethacin on the haemodynamic effects of lipopolysaccharide in rats. Fundam Clin Pharmacol. 1996;10:258–263. doi: 10.1111/j.1472-8206.1996.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Fleming I. Cytochrome p450 and vascular homeostasis. Circ Res. 2001;89:753–762. doi: 10.1161/hh2101.099268. [DOI] [PubMed] [Google Scholar]
- Futaki N, Takahashi S, Kitagawa T, Yamakawa Y, Tanaka M, Higuchi S. Selective inhibition of cyclooxygenase-2 by NS-398 in endotoxin shock rats in vivo. Inflam Res. 1997;46:496–502. doi: 10.1007/s000110050232. [DOI] [PubMed] [Google Scholar]
- Gibson GG. Comparative aspects of the mammalian cytochrome P450 IV gene family. Xenobiotica. 1989;19:1123–1148. doi: 10.3109/00498258909043166. [DOI] [PubMed] [Google Scholar]
- Goodwin DC, Landino LM, Marnett LJ. Effects of nitric oxide and nitric oxide-derived species on prostaglandin endoperoxide synthase and prostaglandin biosynthesis. FASEB J. 1999;13:1121–1136. doi: 10.1096/fasebj.13.10.1121. [DOI] [PubMed] [Google Scholar]
- Habib A, Bernard C, Lebret M, Creminon C, Esposito B, Tedgui A, Maclouf J. Regulation of the expression of cyclooxygenase-2 by nitric oxide in rat peritoneal macrophages. J Immunol. 1997;158:3845–3851. [PubMed] [Google Scholar]
- Hamilton LC, Warner TD. Interactions between inducible isoforms of nitric oxide synthase and cyclo-oxygenase in vivo: investigations using the selective inhibitors, 1400W and celecoxib. Br J Pharmacol. 1998;125:335–340. doi: 10.1038/sj.bjp.0702077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick JP. CYP4A subfamily: functional analysis by immunohistochemistry and in situ hybridization. Methods Enzymol. 1991;206:273–283. doi: 10.1016/0076-6879(91)06097-m. [DOI] [PubMed] [Google Scholar]
- Jaworek J, Bonior J, Tomaszewska R, Jachimczak B, Kot M, Bielanski W, Pawlik WW, Sendur R, Stachura J, Konturek PC, Konturek SJ. Involvement of cyclo-oxygenase-derived prostaglandin E2 and nitric oxide in the protection of rat pancreas afforded by low dose of lipopolysaccharide. J Physiol Pharmacol. 2001;52:107–126. [PubMed] [Google Scholar]
- Khatsenko OG, Gross SS, Boobis AB. Evidence for nitric oxide participation in down-regulation of CYP2B1/2 gene expression at the pretranslational level. Toxicology Lett. 1997;90:207–216. doi: 10.1016/s0378-4274(96)03857-x. [DOI] [PubMed] [Google Scholar]
- Khatsenko OG, Kikkawa Y. Nitric oxide differentially affects constitutive cytochrome P450 isoforms in rat liver. J Pharmacol Exp Ther. 1997;280:1463–1470. [PubMed] [Google Scholar]
- Kimura S, Hanioka N, Matsunaga E, Gonzalez FJ. The rat clofibrate-inducible CYP4A gene subfamily. I. Complete intron and exon sequence of the CYP4A1 and CYP4A2 genes, unique exon organization, and identification of a conserved 19-bp upstream element. DNA. 1989a;8:503–516. doi: 10.1089/dna.1.1989.8.503. [DOI] [PubMed] [Google Scholar]
- Kimura S, Hardwick JP, Kozak CA, Gonzalez FJ. The rat clofibrate-inducible CYP4A subfamily. II. cDNA sequence of IVA3, mapping of the CYP4A locus to mouse chromosome 4, and coordinate and tissue-specific regulation of the CYP4A genes. DNA. 1989b;8:517–525. doi: 10.1089/dna.1.1989.8.517. [DOI] [PubMed] [Google Scholar]
- Mitchell SR, Sewer MB, Kardar SS, Morgan ET. Characterization of CYP4A induction in rat liver by inflammatroy stimuli: Dependence on sex, strain, and inflammation-evoked hypophagia. Drug Metab Dispos. 2001;29:17–22. [PubMed] [Google Scholar]
- Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57:217–252. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- Morgan ET, Li-Masters T, Cheng PY. Mechanisms of cytochrome P450 regulation by inflammatory mediators. Toxicology. 2002:181–182. 207–210. doi: 10.1016/s0300-483x(02)00283-4. [DOI] [PubMed] [Google Scholar]
- Müller CM, Scierka A, Stiller RL, Kim YM, Cook RD, Lancaster JR, Buffington CW. Nitric oxide mediates hepatic cytochrome P450 dysfunction induced by endotoxin. Anesthesiology. 1996;84:1435–1442. doi: 10.1097/00000542-199606000-00020. [DOI] [PubMed] [Google Scholar]
- Murakami M, Naraba H, Tanioka T, Semmyo N, Nakatani Y, Kojima F, Ikeda T, Fueki M, Ueno A, Oh-ishi S, Kudo I. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem. 2000;275:32783–32792. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- Nguyen X, Wang MH, Reddy KM, Falck JR, Schwartzman ML. Kinetic profile of the rat CYP4A isoforms: arachidonic acid metabolism and isoform-specific inhibitors. Am J Physiol. 1999;276:R1691–R1700. doi: 10.1152/ajpregu.1999.276.6.R1691. [DOI] [PubMed] [Google Scholar]
- Okita R. Effect of salicylic acid on fatty acid ω-hydroxylation in rat liver. Ped Res. 1986;20:1221–1224. doi: 10.1203/00006450-198612000-00003. [DOI] [PubMed] [Google Scholar]
- Oyekan AO, Youseff T, Fulton D, Quilley J, McGiff JC. Renal cytochrome P450 ω-hydroxylase activity are differently modified by nitric oxide and sodium chloride. J Clin Invest. 1999;104:1131–1137. doi: 10.1172/JCI6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persohn E, Thomas H, Waechter F. Immunoelectron microscopic localization of cytochrome P-450 isoenzyme CYP4A1 in liver, ileum and kidney of nafenopin treated male rats. Cell Biol Int. 1993;17:99–103. doi: 10.1006/cbir.1993.1010. [DOI] [PubMed] [Google Scholar]
- Pique JM, Yonei Y, Whittle BJ, Leung FW, Guth PH. Indomethacin potentiates endotoxin-induced blood flow reduction and histological injury in rat gastric mucosa. Br J Pharmacol. 1988;93:925–931. doi: 10.1111/j.1476-5381.1988.tb11481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekka E, Ayalogu EO, Lewis DFV, Gibson GG, Ioannides C. Induction of hepatic microsomal CYP4A activity and of peroximal b-oxidation by two non-steroidal anti-inflammatory drugs. Arch Toxicol. 1994;68:73–78. doi: 10.1007/s002040050037. [DOI] [PubMed] [Google Scholar]
- Renton KW, Nicholson TE. Hepatic and central nervous system cytochrome P450 are down-regulated during lipopolysaccharide-evoked localized inflammation in brain. J Pharmacol Exp Ther. 2000;294:524–530. [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewer MB, Koop DR, Morgan ET. Differential inductive and suppressive effects of endotoxin and particulate irritants on hepatic and renal cytochrome P-450 expression. J Pharmacol Exp Ther. 1997;280:1445–1454. [PubMed] [Google Scholar]
- Sewer MB, Koop DR, Morgan ET. Endotoxemia in rats is associated with induction of the P4504A subfamily and suppression of several other forms of cytochrome P450. Drug Metab Dispos. 1996;24:401–407. [PubMed] [Google Scholar]
- Sewer MB, Morgan ET. Down-regulation of the expression of three major rat liver cytochrome P450s by endotoxin in vivo occurs independently of nitric oxide production. J Pharmacol Exp Ther. 1998;287:352–358. [PubMed] [Google Scholar]
- Shirahase H, Kanda M, Nakamura S, Tarumi T, Uehara Y, Ichikawa A. Inhibitory effects of PGD2, PGJ2 and 15-deoxy-delta12,14-PGJ2 on iNOS induction in rat mesenteric artery. Life Sci. 2000;66:2173–2182. doi: 10.1016/s0024-3205(00)00544-0. [DOI] [PubMed] [Google Scholar]
- Su P, Kaushal KM, Kroez DL. Inhibition of renal arachidonic acid ω-hydroxylase activity with ABT reduces blood pressure in the SHR. Am J Physiol. 1998;275:R426–R438. doi: 10.1152/ajpregu.1998.275.2.R426. [DOI] [PubMed] [Google Scholar]
- Swierkosz TA, Mitchell JA, Warner TD, Botting RM, Vane JR. Co-induction of nitric oxide synthase and cyclooxygenase: interactions between nitric oxide and prostanoids. Br J Pharmacol. 1995;114:1335–1342. doi: 10.1111/j.1476-5381.1995.tb13353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura S, Minamiyama Y, Imaoka S, Funae Y, Hirohashi K, Inoue M, Kinoshita H. Hepatic cytochrome P450 is directly inactivated by nitric oxide, not by inflammatory cytokines, in the early phase of endotoxemia. J Hepatol. 1999;30:1035–1044. doi: 10.1016/s0168-8278(99)80257-8. [DOI] [PubMed] [Google Scholar]
- Tslotou AG, Sakorafas GH, Anagnostopoulos G, Bramis J. Septic shock; current pathogenetic concepts from a clinical perspective. Med Sci Monit. 2005;11:RA76–RA85. [PubMed] [Google Scholar]
- Tunctan B, Altug S, Uludag O, Abacioglu N. Time-dependent variations in serum nitrite, 6-keto-prostaglandin F1a and thromboxane B2 levels induced by lipopolysaccharide in mice. Biol Rhythm Res. 2000;31:499–514. [Google Scholar]
- Tunctan B, Altug S, Uludag O, Demirkay B, Abacioglu N. Effects of cyclooxygenase inhibitors on nitric oxide production and survival in a mice model of sepsis. Pharmacol Res. 2003;48:37–48. [PubMed] [Google Scholar]
- Tunctan B, Ozveren E, Korkmaz B, Buharalioglu CK, Tamer L, Degirmenci U, Atik U. Nitric oxide reverses endotoxin-induced inflammatory hyperalgesia via inhibition of prostacyclin production in mice. Pharmacol Res. 2006a;53:177–192. doi: 10.1016/j.phrs.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Tunctan B, Yaghini FA, Estes A, Malik KU. Inhibition by nitric oxide and cyclooxygenase of cytochrome P450 4A expression and activity contributes to endotoxin-induced hypotension in rats. Nitric Oxide: Biol Chem. 2006b;14:51–57. doi: 10.1016/j.niox.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Tunctan B, Yaghini FA, Estes A, Malik KU. Prostaglandins inhibit cytochrome P450 4A activity and contribute to endotoxin-induced hypotension in rats via nitric oxide production. FASEB J. 2004;18:A1034–A1035. doi: 10.1007/s12272-001-1238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayssettes-Courchay C, Bouysset F, Verbeuren TJ. Involvement of COX and NOS induction in the sympatho-activation during sepsis. Auton Neurosci. 2002;98:33–36. doi: 10.1016/s1566-0702(02)00027-9. [DOI] [PubMed] [Google Scholar]
- Wang D, Wei J, Hsu K, Jau J, Lieu MW, Chao TJ, Chen HI. Effects of nitric oxide synthase inhibitors on systemic hypotension, cytokines and inducible nitric oxide synthase expression and lung injury following endotoxin administration in rats. J Biomed Sci. 1999;6:28–35. doi: 10.1007/BF02256421. [DOI] [PubMed] [Google Scholar]
- Wang MH, Guan H, Nguyen X, Zand BA, Nasjletti A, Laniado-Schwartzman M. Contribution of cytochrome P-450 4A1 and 4A2 to vascular 20-hydroxyeicosatetraenoic acid synthesis in rat kidneys. Am J Physiol. 1999;276:F246–F253. doi: 10.1152/ajprenal.1999.276.2.F246. [DOI] [PubMed] [Google Scholar]
- Wang MH, Wang J, Chang HH, Zand BA, Jiang M, Nasjletti A, Laniado-Schwartzman M. Regulation of renal CYP4A expression and 20-HETE synthesis by nitric oxide in pregnant rats. Am J Physiol. 2003;285:F295–302. doi: 10.1152/ajprenal.00065.2003. [DOI] [PubMed] [Google Scholar]
- Zatz R, Baylis C. Chronic nitric oxide inhibiton model six years on. Hypertension. 1998;32:958–964. doi: 10.1161/01.hyp.32.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Imig JD. Kidney CYP450 enzymes: Biological actions beyond drug metabolism. Curr Drug Metab. 2003;4:73–84. doi: 10.2174/1389200033336892. [DOI] [PubMed] [Google Scholar]


