Abstract
Upon the discovery of RANK, RANKL and OPG in the late 1990s, their importance in the maintenance of the skeletal structure and their dramatic role in bone disease were largely unexpected. In recent years the understanding of these proteins, in particular their regulation, has greatly increased. This review aims to bring the interested reader up to date with the latest news and views on the mechanisms controlling bone resorption in normal and pathological conditions.
Keywords: Review, Osteoprotegerin (OPG), Receptor-activator of nuclear factor kappa beta (RANK), Receptor-activator of nuclear factor kappa beta ligand (RANKL), Disease, Structure
Introduction
Bone is a specialised tissue with a complex composite structure that enables it to perform multiple mechanical and metabolic functions. In order to maintain these functions within the body, bone is in a constant state of remodelling. During this process, also known as bone turnover, osteoclasts demineralise and resorb old bone and osteoblasts deposit new bone to maintain a bone mass appropriate to the stresses placed on the skeleton. Any alteration in the process of bone turnover may alter bone mineral density (BMD), bone strength and bone micro architecture. This most commonly occurs when there is an increase in osteoclast activity, leading to increased bone resorption, resulting in diminished bone density (osteopenia). Osteopenia may also occur due to lack of vitamin D (rickets and osteomalacia) or an excess of parathyroid hormone (hyperparathyroidism). Disorders of the skeleton may also involve an increase in BMD (osteosclerosis), either due to defective osteoclast function (osteopetrosis), or as a result of too little parathyroid hormone (hypoparathyroidism).
Molecular biological investigations have led to an increased understanding of the mechanisms and proteins involved in bone resorption. This process is controlled by a system comprised of three key proteins, RANK (receptor-activator of nuclear factor kappa beta), its ligand RANKL (receptor-activator of nuclear factor kappa beta ligand) and a decoy receptor OPG (osteoprotegerin). This system is regulated by many osteotropic hormones and cytokines which either reduce (glucocorticoids, inflammatory cytokines e.g. interleukin-1 (IL-1), parathyroid hormone (PTH), prostaglandin E2 (PGE2), vitamin D3) or increase (transforming growth factor-β (TGF-β) and estrogens) the OPG/RANKL ratio [1].
Structural characteristics of RANK, RANKL and OPG
RANK
RANK was discovered by Anderson et al. [2] by directly sequencing cDNA from a human bone marrow-derived myeloid dendritic cell. Sequencing of the RANK gene showed it to be a type I transmembrane glycoprotein and further gene mapping showed this newly discovered protein to be located on chromosome 18q22.1 [2] and also a member of the tumour necrosis factor receptor (TNFR) family [3]. The expression of RANK has since been found on the surface of a wide variety of cells such as; osteoclast precursors (circulating monocytes) [4], mature osteoclasts [5], dendritic cells [2, 3], mammary gland epithelial cells [6], breast cancer cells [7] and prostate cancer cells [8].
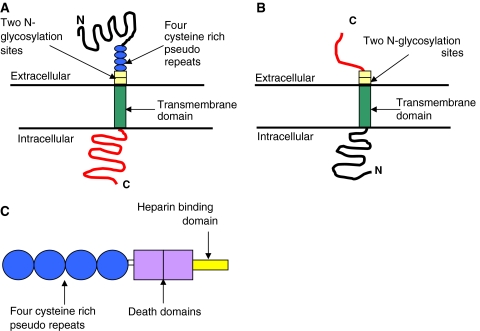
Human RANK (Fig. 1a) consists of 616 amino acids (aa). These aa are divided into a C-terminal cytoplasmic domain of 383 aa [2, 9], an N-terminal extracellular domain of 184 aa, a signal peptide of 28 aa and a transmembrane domain of 21 aa which contains four cysteine rich pseudo repeats and two N-glycosylation sites [2, 9]. The signal peptide binds with a signal recognition particle which determines the destination site for the protein. Once bound, the signal peptide is cleaved and the mature 588 aa protein is expressed on the cell surface as a homotrimer [3, 10, 11].
Fig. 1.
Diagrammatic representations of a RANK, b RANKL and c OPG
RANKL
The cognate ligand for RANK, RANKL, was reported almost simultaneously by four independent research groups [2, 12–14]. RANKL is a tumour necrosis factor (TNF)-related cytokine expressed by various bone cells including osteoblasts and their immature precursors [15], T lymphocytes [16], B lymphocytes [17] and megakaryocytes [18].
RANKL (Fig. 1b) is coded for by a single gene, however, alternative splicing results in the expression of three isoforms. In humans two of these isoforms are type II transmembrane bound glycoproteins of either 317 [11] or 270 aa, the latter differing only by a shorter intracellular domain [19]. The third isoforms of only 243 aa lacks both the transmembrane and cytoplasmic domains and acts as a soluble ligand (sRANKL) [19, 20]. Each of these isoforms is only capable of activating osteoclastogenesis when they associate forming homotrimeric molecules [16, 21].
The human and murine RANKL proteins share 83–87% homology, with the largest murine transmembrane isoform being 316 aa [11]. Sequence analysis of the murine RANKL gene (316 aa) compared to the human RANKL gene (317 aa) showed two potential N-glycosylation sites in the extracellular domain at amino acid fragment positions 197 and 263 [13].
Because of the existence of these splice variants in RANKL it is likely that they are regulated differently and have different functions. Although the exact explanations for various isoforms are unknown, one possibility is that membrane bound RANKL ensures cell–cell contact with osteoclasts and their precursors whereas sRANKL allows for diffusion to activate target cells. In the first case, bone resorption will be tightly localised to the cells expressing RANKL ensuring topographically accurate remodelling. In the second case, resorption will be more generalized perhaps allowing systemic resorption. sRANKL can also be formed by shedding, a process in which RANKL is cleaved from the cell surface membrane by sheddases such as matrix metalloproteinase 14 (MMP-14) [22].
OPG
OPG was first identified by sequence homology to the TNFR family during a rat intestine cDNA sequencing project [23]. They named the protein because of its protective effects in bone (Latin: os bone, protegere to protect). OPG is a soluble glycoprotein secreted by various mesenchymally derived cells such as osteoblasts [24] and bone marrow stromal cells [25].
Unlike RANK and RANKL, OPG does not have a transmembrane domain or cytoplasmic domain [2]. Composed of 401 aa, human and murine OPG consist of four cysteine rich pseudo repeats located in the N-terminal, two death domains, a heparin binding site located in the C-terminal and a 21 aa signal peptide [2] (Fig. 1c). The four cysteine rich pseudo repeats form an elongated structure and binds to one of the grooves of the active RANKL trimer [20] therefore preventing RANKL/RANK interaction and hence osteoclastogenesis.
This 401 aa structure gives OPG a monomeric molecular weight of 60 kDa which is then assembled at the cys-400 residue in the heparin binding domain to form a 120 kDa disulphide-linked dimer for secretion [26]. Prior to secretion of both the monomeric and dimeric forms of OPG, the signal peptide is cleaved from the N-terminal giving rise to a 380 aa mature OPG protein [2, 23].
The function of RANK, RANKL and OPG
RANK
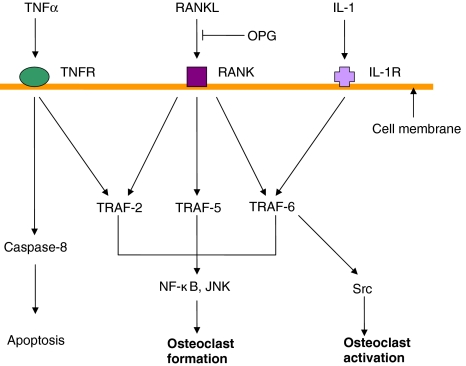
RANK activation by the binding of RANKL initiates an internal signalling cascade via the cytoplasmic adaptor proteins called TRAFs (Fig. 2). RANK has three binding domains for TRAFs, each of which has a different binding affinity for either TRAF 2, 5 or 6 which relay the RANK stimulation signal and activate downstream pathways including NF-кB, c-jun N-terminal kinase (JNK) or Src pathways. TRAF 6 in particular has been shown to be necessary for the differentiation of osteoclastic cells [3] by enhancing Src kinase, essential for osteoclast function [27]. This signalling cascade results in the expression of various genes and thus facilitates the differentiation of monocytes into osteoclasts and also the activation of mature osteoclasts. One of these genes is nuclear factor of activated T cells, calcineurin-dependent 1 (NFATc1) which results in osteoclast differentiation and the expression of the osteoclast marker TRAP (tartrate resistant acid phosphatase) [28].
Fig. 2.
Schematic representation of RANK-RANKL binding signalling pathways, indicating the inhibition of RANK-RANKL binding by OPG
RANKL
The expression of RANKL in human and murine osteoblastic cells is stimulated by various cytokines (IL-1, TNFα and IL-11) [3, 29] and calciotrophic hormones including PTH, 1,25dihydroxyvitamin D3 (1,25D3) and prostaglandin E2 [29]. An increased production of RANKL by osteoblastic cells leads to osteoclast differentiation, activation and survival, which results in increased bone resorption. This, along with the involvement of the decoy receptor OPG is thought to be a key mechanism in the control of bone turnover.
The Wnt signalling pathway contains many proteins involved in embryogenesis, cancer and normal physiological processes and is a growing area of interest concerning the regulation of bone turnover. In brief, when canonical Wnt signalling is activated by one of the 19 secreted Wnts binding to one of 10 frizzled receptors (Fzd), a signalling cascade is initiated which results in the translocation of β-catenin into the nucleus. Once here, β-catenin binds with the transcription factor tcf/lef and initiates target gene transcription. Wnt signalling has been shown to be vital for normal osteoblast function [30–32]. sRANKL has been reported to bind secreted frizzled related protein (sFRP) [33], a soluble Wnt inhibitor produced by osteoblasts, another mechanism by which osteoblasts can inhibit osteoclast formation. This interaction may represent another way in which bone resorption may be controlled at the same time as Wnt signalling is inhibited.
OPG
OPG acts as a decoy receptor by binding with high affinity to RANKL therefore preventing the interaction with RANK [34]. As a consequence of binding to RANKL, OPG acts as an effective inhibitor of osteoclast differentiation, activation and survival and therefore inhibits bone resorption [34].
OPG expression and secretion by osteoblasts/stromal cells are modulated by various metabolic regulators: IL-1, TNF-α and TGF-β increase OPG secretion and it is decreased by various stimulators of bone resorption including PTH, PGE2 and 1,25D3 [15, 25, 29]. Using mouse calvaria treated with indomethacin to block endogenous prostaglandin production, the role of OPG in modulating the adhesion of osteoclasts between the bone surface and the endocranial membrane was investigated [29]. These authors found that OPG inhibited both the release of osteoclasts from the periosteum and adherence of the osteoclasts to the bone surface, therefore inhibiting bone resorption [29].
OPG has been shown to bind to TNF-related apoptosis inducing ligand (TRAIL) [35] although with less affinity than for RANKL [36]. TRAIL is a 33–34 kDa cell associated ligand which mediates apoptotic cell death particularly in cancer cells. TRAIL binds to specific cell surface receptors which contain cytoplasmic death domains. Following this binding, TRAIL transduces an apoptotic signal [37]. The binding of OPG to TRAIL was found to inhibit TRAIL-induced apoptosis of Jurkat cells in culture [35]. However, the biological significance of the binding of OPG to TRAIL remains unclear. OPG also has a basic heparin binding domain making interactions with heparin and heparin sulphates possible. Heparin sulphates are expressed on the cell surface as heparin sulphate proteoglycans (HSPGs). HSPGs are involved in cell-surface signalling, controlling cell behaviour, actin cytoskeleton regulation, cell adhesion and migration [4].
RANK, RANKL and OPG in disease
There are several inherited human conditions primarily caused by a defect in the osteoclast resulting from a genetic mutation in RANK, RANKL or OPG (summarised in Table 1). There are two ways in which osteoclast activity can be defective. They can be under-active resulting in an excess of bone (osteopetrosis) or they can be over-active resulting in too little bone (osteoporosis and familial expansile osteolysis [FEO]) [38].
Table 1.
A summary of human diseases caused by mutations in the RANK, RANKL and OPG genes
| Gene | Mutation | Disease | Reference |
|---|---|---|---|
| RANK | 18 bp duplication | Familial expansile osteolysis | [39] |
| 27 bp duplication | Early onset Paget’s disease | [39] | |
| 15 bp duplication | Expansile skeletal hyperphosphatasia | [42] | |
| RANKL | Deletion of amino acids 145-177 | Autosomal recessive osteopetrosis | [43] |
| A single nucleotide change (596T-A) in exon 8 of both alleles | Autosomal recessive osteopetrosis | [43] | |
| Deletion of two nucleotides (828_829delCG) | Autosomal recessive osteopetrosis | [43] | |
| OPG | Deletion making OPG inactive | Juvenile Paget’s disease | [44] |
| 20 bp deletion resulting in premature termination of OPG translation | Juvenile Paget’s disease | [47] |
RANK
FEO is an inherited autosomal dominant disease with 100% penetrance resulting from the constitutive activation of RANK due to an 18-base pair tandem duplication. FEO is characterized by deafness which often occurs before the age of 10, early loss of teeth due to the resorption of the cervical region (where a tooth meets the gum), osteolytic lesions from late teenage years until late middle age [39] and increased bone remodelling [38]. Lesions in FEO mostly affect the tibia, ulna, humerus and femur, sparing the axial skeleton such as the skull and pelvis which are common sites for lesions found in Paget’s disease of bone [39].
Another autosomal dominant disease caused by a mutation in RANK is early-onset Paget’s disease [39, 40] which differs from classical Paget’s disease by its early onset and instead of lesions being restricted to one or more bones they are scattered throughout the body. The patients studied with early-onset Paget’s disease all presented with bone pain and/or deformity in their teens and early twenties and showed a 27-base pair duplication in the signal peptide region of the RANK gene causing constitutive activation [39].
RANK mutations in FEO and early-onset Paget’s result in a failure to cleave the signal peptide. This leads to an increase in RANK-mediated activation of NF-кB, stimulation of osteoclast activity and a secondary stimulation of osteoblasts [39]. This stimulation in bone turnover leads to patients with early-onset Paget’s disease to have enlarged and softened bones, particularly those that are long and weight bearing [38].
Expansile skeletal hyperphosphatasia (ESH) is an autosomal dominant disorder characterized by the premature loss of teeth, early-onset deafness, episodic hypocalcaemia, accelerated bone remodelling and widening of long bones causing pain in the finger bones [41]. Mutation screening of patients suffering from ESH revealed a 15-base pair tandem duplication in the signal peptide region of the RANK gene [42]. This mutation has the same affect on RANK as seen in FEO; however, there is not a great increase in osteoclast and osteoblast number and activity [38]. Anti-resorptive therapy with bisphosphonates can be effective in the treatment of FEO, early-onset Paget’s disease and ESH [40], although very recently it has been reported that bisphosphonate toxicity during childhood can impair one remodelling and induce osteopetrosis during later life [41].
RANKL
Autosomal recessive osteopetrosis is a rare genetic bone disease. Bone biopsies taken from four unrelated individuals with this disease showed an absence of osteoclasts and no improvement in bone remodelling following haematopoietic stem cell transplantation, which would be expected to replace the osteoclast population but not the osteoblast [43]. Genetic analysis of the RANKL gene in these four individuals revealed three different mutations. Patient one had an in-frame deletion of amino acids 145–177. The mutation deleted the entire βA strand and half of the AA loop, which are essential for the osteoclastogenic activity of RANKL. The second and third patients had a single nucleotide change (596T-A) in exon 8 of both alleles. The fourth patient had a genomic deletion of two nucleotides (828_829delCG), resulting in a frame shift starting at val277 and a premature stop codon. This mutation was thought to cause the loss of the βF, βG and βH strands of RANKL. These are all important for RANKL trimerisation and therefore the mutation would prevent the activation of osteoclastogenesis [43].
OPG
Mutations in the OPG gene cause a disease called juvenile Paget’s disease, also known as idiopathic hyperphosphatasia [44]. This disease can be fatal if not treated with anti bone resorption drugs [45]. Juvenile Paget’s disease is an autosomal recessive osteopathy which initially presents in infancy or early childhood and is characterized by rapidly remodelling woven bone, osteopenia, fractures, progressive skeletal deformity and increased bone remodelling throughout the skeleton [44, 45]. Individuals with this disease are also typically short in stature [46]. The location and type of gene mutation responsible for this disease was investigated by various groups.
The first group studied nine children from two generations of a family from New Zealand, three of whom displayed typical clinical and radiographical symptoms of juvenile Paget’s disease. Biochemical analysis of the bone turnover markers ALP and N-telopeptide/creatinine ratio showed significantly raised levels in the three affected individuals. PCR analysis of each of the five exons of OPG identified a 3-base pair in-frame deletion of a critical aspartate residue in exon three. OPG was also detected in the plasma of the three affected children suggesting that the mutation does not affect secretion. The activity of the mutant OPG protein was then compared to the normal functional protein. This revealed that normal OPG inhibited bone resorption in mouse calvaria whereas the mutant protein did not. This suggests the mutant OPG with the three base pair deletion is inactive and results in uncontrolled bone remodelling and increased bone turnover [44].
The second group investigated two unrelated Navajo patients with juvenile Paget’s disease. Patient one was a one-year-old male child with bone deformities as a result of the disease. Patient two was a 26-year-old Navajo female who was deaf, severely deformed and incapacitated by the disease. The bone turnover marker, serum alkaline phosphatase (ALP) activity was approximately 10 times above the normal range in both the male and female patient [45] indicating excessive osteoblastic activity. They then analyzed and sequenced the gene for OPG. PCR analysis of genomic DNA from patient one and his parents revealed no OPG gene product. Southern blotting of genomic DNA supported this finding confirming a 100 kilo base deletion that had completely deleted the gene for OPG [45].
A third mutation of OPG in juvenile Paget’s disease was identified [47]. This group studied 10 affected individuals, each of these subjects had long-bone deformities, short stature, raised ALP activity, impaired mobility (7/10 subjects) and enlargement of the skull (6/10 subjects). PCR analysis revealed that three of the 10 individuals had a 3-base pair mutation of OPG. Other affected individuals had a much larger 20-base pair deletion also in exon three of the OPG protein. It is suggested that this mutation results in premature termination of OPG protein translation [47]. As with the 3-base pair deletion [44] and the 100 kb deletion [45] the 20-base pair deletion resulted in increased bone turnover, demonstrating that mutations in the OPG gene can cause autosomal recessive idiopathic hyperphosphatasia [47, 48].
Osseous malignancies
The RANK, RANKL and OPG system is also involved with many other metabolic bone diseases, where their expression is modulated either directly or indirectly by the tumour cell to promote its own survival. Bone metastases are unfortunately a common occurrence in patients with solid tumours such as breast cancer, prostate cancer and lung cancer often resulting in severe pain and pathological fractures amongst other complications [49]. Patients with solid tumours metastatic to bone have been shown to have a severe disruption in the RANK/RANKL/OPG system [50]. In these instances, quality of life and even life expectancy is dramatically reduced.
Breast cancer commonly metastasises to the skeleton causing painful osteolytic lesions [51]. Establishment of the metastatic tumour is gained by influencing the expression ratio of RANKL and OPG to favour bone resorption [51]. It is believed that some genes may predispose the likelihood for breast cancer to metastasise to bone [52].
Bone is the most common and sometimes the only site of metastasis in patients with advanced prostate cancer [53]. Prostatic bone metastases stimulate an increase in both rate of bone remodelling and bone volume [54]. Prostate cancer bone metastases produce RANKL thus enabling them to induce osteolysis through osteoclast activation [55]. Although mostly osteoblastic in nature, prostatic bone metastases can also appear osteolytic in order to promote tumour growth [54].
Lung cancer metastasises to bone in approximately 9–30% of all cases [56]. Lung cancer metastases are generally lytic in nature, although some have been found to also have osteoblastic characteristics [57]. Constitutive expression of the chemokine CCL22 by osteoclasts is thought in some cases to potentially promote the metastasis of lung cancer to bone [58].
Multiple myeloma (MM) is an osteolytic bone disease resulting from excessive bone resorption [59]. Although the exact mechanisms of invasion are unclear, it is believed the tumour invades surrounding bone by up regulating RANKL and down regulating OPG and therefore stimulating local bone resorption [59].
Murine knockout models
RANK
RANK deficient mice (RANK −/−) were generated in order to investigate the direct role of RANK in bone resorption [60]. RANK −/− mice were recognisable by their small-body size, shortened limbs and doming of the skull, which became apparent after weaning at 3 weeks of age. At 6 weeks radiography revealed an osteopetrotic phenotype including shortened long bones and increased radio-density in bone marrow cavities relative to the control mice (RANK +/+). Histological analysis showed disorganized chondrocytes at the growth plates and poorly remodelled osteocartilagenous structures blocking the marrow cavities. Staining for the osteoclast marker TRAP showed positively stained mature osteoclasts in the control mice, yet they were completely absent in the RANK −/− mice, suggesting an explanation for the osteopetrotic phenotype observed. The lack of RANK for RANKL to bind to inhibited osteoclast differentiation and maturation, causing a deficiency of mature osteoclasts at the bone surface [60].
RANKL
The requirement of RANKL for osteoclastogenesis and bone remodelling, along with its function in modulating immune responses was investigated using RANKL −/− mice [5]. Similar to RANK −/− mice, RANKL −/− mice showed a normal rate of growth until weaning at 3 weeks old. After weaning their growth became severely stunted and teeth failed to erupt [34]. The RANKL −/− mice completely lacked TRAP positive stained osteoclasts, in contrast to the large number of TRAP positive cells in the RANKL +/+ control mice [5]. Radiographs showed osteopetrosis was visible from 2 days after birth, with shortened long bones with distinct broadening of the epiphyses. The axial skeleton (skull, vertebrae, sternum, breastbone and ribs) when visualized by X-ray showed a large increase in radio-density, 516 mg cm−3 in RANKL −/− mice compared with 242 mg cm−3 in RANKL +/+ mice [5].
OPG
OPG and its physiological role as a regulator of normal bone mass was investigated using targeted deletion of the gene in mice (OPG −/−) [50]. They found an increased mortality between birth and 4 weeks of age in OPG −/− mice when compared to the OPG +/+ control mice. The OPG −/− mice also had a higher prevalence of vertebral and bone fractures in the first 2 weeks of life indicating brittle bones and a possibility of low-bone density. OPG −/− mice that survived past weaning were fertile and females successfully carried litters to term. Offspring were also OPG −/− indicating that OPG is not required for normal embryonic development [61].
Radiography showed decreased BMD in OPG −/− mice compared to the OPG +/+ control mice, however, this was only evident from 1 month of age and became more prominent as the mice aged [61]. Histological examination of vertebrae and long bones from OPG −/− mice showed severe osteoporosis with almost a complete lack of trabeculae by 1–2 months of age [61]. OPG −/− mice have been shown to have a high bone turnover rate, disorganized matrix and an impaired attachment of new to old bone in the cement line due to excessive osteoclast activity [62]. Therefore, OPG plays a crucial role in blocking bone resorption. Radiographic investigation of a transgenic increase of OPG in mice revealed clear signs of osteopetrosis immediately following birth, with the severity increasing throughout adolescence and adult life [23].
Conclusion
With the discovery of RANKL came the ability to manufacture osteoclasts in vitro, which has accelerated up the discovery of other factors that affect bone loss and molecular details of differentiation and bone resorption. Further to this came the understanding of RANKL and OPG in diseases where bone is lost. This knowledge has provided new targets for modern therapies, for example re-engineered OPG and anti-RANKL antibodies.
Denusomab (AMG162) is a high affinity anti-RANKL antibody [63] able to prevent RANK/RANKL binding and inhibit bone resorption resulting in an increased bone mass. Denusomab is currently in phase III clinical trials for treatment of osteoporosis [63, 64] and also under investigation for the treatment of osteolytic bone metastases from diseases such as breast cancer (phase II clinical trials) [65], rheumatoid arthritis (phase II clinical trials) [66] and multiple myeloma [67]. Similar attempts have also been made with OPG [68, 69]. AMGN-0007 is a recombinant OPG construct and suppressed bone resorption when administered to MM and breast cancer patients during a phase I clinical trial [68]. A similar result has been observed using a recombinant adeno-associated viral (rAAV) vector in mouse model of osteolytic breast cancer [69].
Treatment of metastases to bone and their mechanisms of action remain elusive. Use of bisphosphonates, a common drug for the treatment of osteoporosis, has proved to be useful in preventing some metastases from breast, prostate and lung cancer by inhibiting the osteoclast [70]. Bisphosphonate treatment also has the added benefit of reducing bone pain caused by the metastases that general analgesics can sometimes fail to relieve [70]. Recent in vitro studies for the prevention/reduction in skeletal metastases include the intravenous administration of RANK-Fc, a chimeric protein that inhibits the RANK/RANKL interaction [57]. Treatment with RANK-Fc reduced osteoclastogenesis, reduced bone tumour volume and inhibited the lytic nature of the lesion [57].
Current publications also tend to discuss the function of these proteins in bone remodelling or they discuss the diseases that revolve around them. Here we have provided an in-depth synopsis of the structures of the RANK, RANKL and OPG proteins including structural diagrams and combined this with an in-depth review of diseases linked to these proteins. We have also reviewed how the osteoblast integrates systemic signals from calciotropic hormones like PTH and 1,25D3, and local signals from growth factors and cytokines to stimulate or inhibit bone resorption.
The discovery of the osteoclast differentiation factor RANKL, its receptor RANK and its decoy receptor OPG, has revolutionised our understanding of bone remodelling. There is currently a large wealth of knowledge already in publication surrounding RANK, RANKL and OPG although many questions remain unanswered.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
H. L. Wright, Phone: +01691-404139, FAX: +01691-404170, Email: HelenL.smith@rjah.nhs.uk
H. S. McCarthy, Email: Helen.mccarthy@rjah.nhs.uk
J. Middleton, Email: Jim.Middleton@rjah.nhs.uk
M. J. Marshall, Email: Michael.Marshall@rjah.nhs.uk
References
- 1.Stejskal D, Bartek J, Pastorkova R, Ruzicka V, Oral I, Horalik D. Osteoprotegerin, RANK, RANKL. Biomed Papers. 2001;145:61–4. doi: 10.5507/bp.2001.013. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose DC, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–9. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 3.Hofbauer LC, Heufelder AE. Osteoprotegerin ligand and osteoprotegerin: new concepts of the pathogenesis and therapy of metabolic bone diseases. Dtsch Med Wochenschr. 2001;126:145–50. doi: 10.1055/s-2001-11050. [DOI] [PubMed] [Google Scholar]
- 4.Mosheimer BA, Kaneider NC, Feistritzer C, Djanani AM, Sturn DH, Patsch JR, Weidermann CJ. Syndecan-1 is involved in osteoprotegerin-induced chemotaxis in human peripheral blood monocytes. J Clin Endocrinol Metab. 2005;90:2964–71. doi: 10.1210/jc.2004-1895. [DOI] [PubMed] [Google Scholar]
- 5.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 6.Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103:41–50. doi: 10.1016/S0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 7.Kim NS, Kim HJ, Koo BK, Kwon MC, Kim YW, Cho Y, Yokota Y, Penninger JM, Kong YY. Receptor activator of NF-KappaB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol Cell Biol. 2006;26:1002–13. doi: 10.1128/MCB.26.3.1002-1013.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G, Sirkar K, Aprikian A, Potti A, Goltzman D, Rabbani S. Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer. 2006;107:289–98. doi: 10.1002/cncr.21978. [DOI] [PubMed] [Google Scholar]
- 9.Hofbauer LC. Osteoprotegerin ligand and osteoprotegerin: novel implications for osteoclast biology and bone metabolism. Eur J Endocrinol. 1999;141:195–210. doi: 10.1530/eje.0.1410195. [DOI] [PubMed] [Google Scholar]
- 10.Heijne GV. Life and death of a signal peptide. Nature. 1998;396:111–3. doi: 10.1038/24036. [DOI] [PubMed] [Google Scholar]
- 11.Warren G. Sorting signals and cellular membranes. 2nd ed. 1993. pp. 166–72. [DOI] [PMC free article] [PubMed]
- 12.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- 13.Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, Frankel WN, Lee SY, Choi Y. TRANCE Is a novel ligand of the tumor necrosis factor receptor family that activates C-Jun N-terminal kinase in T cells. J Biol Chem. 1997;272:25190–4. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 14.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Goto M, Mochizuki SI, Tsuda E, Morinaga T, Udagawa N, Takahashi N, Suda T, Higashio K. A novel molecular mechanism modulating osteoclast differentiation and function. Bone. 1999;25:109–13. doi: 10.1016/S8756-3282(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 15.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–5. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 16.Drugarin D, Drugarin M, Negru S, Cioaca R. RANKL-RANK/OPG molecular complex-control factors in bone remodeling. Timisora Med J. 2003;53:297–302. [Google Scholar]
- 17.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–30. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bord S, Frith E, Ireland DC, Scott MA, Craig JIO, Compston JE. Synthesis of osteoprotegerin and RANKL by megakaryocytes is modulated by oestrogen. Br J Haematol. 2004;126:244–51. doi: 10.1111/j.1365-2141.2004.05024.x. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda T, Kasai M, Utsuyama M, Hirokawa K. Determination of three isoforms of the receptor activator of nuclear factor-KB ligand and their differential expression in bone and thymus. Endocrinology. 2001;142:1419–26. doi: 10.1210/en.142.4.1419. [DOI] [PubMed] [Google Scholar]
- 20.Sordillo EM, Pearse RN. RANK-Fc: a therapeutic antagonist for RANK-L in myeloma. Cancer. 2003;97:802–12. doi: 10.1002/cncr.11134. [DOI] [PubMed] [Google Scholar]
- 21.Ito S, Hata T. Crystal structure of RANK ligand involved in bone metabolism. Vitam Horm. 2004;67:19–33. doi: 10.1016/S0083-6729(04)67002-6. [DOI] [PubMed] [Google Scholar]
- 22.Hikita A, Kadono Y, Chikuda H, Fukuda A, Wakeyama H, Yasuda H, Nakamura K, Oda H, Miyazaki T, Tanaka S. Identification of an alternatively spliced variant of Ca2+-promoted Ras inactivator as a possible regulator of RANKL shedding. J Biol Chem. 2005;280:41700–6. doi: 10.1074/jbc.M507000200. [DOI] [PubMed] [Google Scholar]
- 23.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 24.Woo KM, Choi Y, Ko S-H, Ko JS, Oh K-O, KK Kim. Osteoprotegerin is present on the membrane of osteoclasts isolated from mouse long bones. Exp Mol Med. 2002;34:347–52. doi: 10.1038/emm.2002.49. [DOI] [PubMed] [Google Scholar]
- 25.Kondo T, Kitazawa R, Maeda S, Kitazawa S. 1 Alpha, 25 Dihydroxyvitamin D3 rapidly regulates the mouse osteoprotegerin gene through dual pathways. J Bone Miner Res. 2004;19:1411–9. doi: 10.1359/JBMR.040604. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi K, Kinosaki M, Goto M, Kobayashi F, Tsuda E, Morinaga T, Higashio K. Characterization of structural domains of human osteoclastogenesis inhibitory factor. J Biol Chem. 1998;273:5117–23. doi: 10.1074/jbc.273.9.5117. [DOI] [PubMed] [Google Scholar]
- 27.Dehm SM, Bonham K. SRC gene expression in human cancer: the role of transcriptional activation. Biochem Cell Biol. 2004;82:263–74. doi: 10.1139/o03-077. [DOI] [PubMed] [Google Scholar]
- 28.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. TRENDS Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien EA, Williams JH, Marshall MJ. Osteoprotegerin is produced when prostaglandin synthesis is inhibited causing osteoclasts to detach from the surface of mouse parietal bone and attach to the endocranial membrane. Bone. 2001;28:208–14. doi: 10.1016/S8756-3282(00)00431-2. [DOI] [PubMed] [Google Scholar]
- 30.Glass DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–64. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 31.Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Donofrio G, Bonomini S, Sala R, Mangoni M, Rizzoli V. Production of Wnt inhibitors by myeloma cells: potential effects on canonical Wnt pathway in the bone microenvironment. Cancer Res. 2007;67:7665–74. doi: 10.1158/0008-5472.CAN-06-4666. [DOI] [PubMed] [Google Scholar]
- 32.Hopwood B, Tsykin A, Findlay DM, Fazzalari NL. Microarray gene expression profiling of osteoarthritic bone suggests altered bone remodelling, WNT and transforming growth factor-beta/bone morphogenic protein signalling. Arthritis Res Ther. 2007;9:R100. doi: 10.1186/ar2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hausler KD, Horwood NJ, Chuman Y, Fisher JL, Ellis J, Martin TJ, Rubin JS, Gillespie MT. Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation. J Bone Miner Res. 2004;19:1873–81. doi: 10.1359/JBMR.040807. [DOI] [PubMed] [Google Scholar]
- 34.Kong YY, Boyle WJ, Penninger JM. Osteoprotegerin ligand: a regulator of immune responses and bone physiology. Immunol Today. 2000;21:495–502. doi: 10.1016/S0167-5699(00)01718-7. [DOI] [PubMed] [Google Scholar]
- 35.Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, Dodds RA, James IE, Rosenberg M, Lee JC, Young PR. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–7. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 36.Lam J, Nelson CA, Ross FP, Teitelbaum SL, Fremont DH. Crystal structure of the TRANCE/RANKL cytokine reveals determinants of receptor-ligand specificity. J Clin Invest. 2001;108:971–9. doi: 10.1172/JCI13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gochuico BR, Zhang J, Ma BY, Marshak-Rothstein A, Fine A. TRAIL expression in vascular smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2000;278:1045–51. doi: 10.1152/ajplung.2000.278.5.L1045. [DOI] [PubMed] [Google Scholar]
- 38.Helfrich MH. Osteoclast diseases. Microsc Res Tech. 2003;61:514–32. doi: 10.1002/jemt.10375. [DOI] [PubMed] [Google Scholar]
- 39.Hughes AE, Ralson SH, Marken J, Bell J, MaCPherson H, Wallace RGH, Hul W, Whyte P, Nakatsuka K, Hovy L, Anderson DM. Mutations in the TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nat Genet. 2000;24:45–8. doi: 10.1038/71667. [DOI] [PubMed] [Google Scholar]
- 40.Whyte MP. Paget’s Disease of bone and genetic disorders of RANKL/OPG/RANK/NF-KappaB signalling. Ann N Y Acad Sci. 2006;1068:143–64. doi: 10.1196/annals.1346.016. [DOI] [PubMed] [Google Scholar]
- 41.Whyte MP, McAlister WH, Novack DV, Clements KL, Schoenecker PL, Wenkert D. Bisphosphonate-induced osteopetrosis: novel bone modeling defects, metaphyseal osteopenia, and osteosclerosis fractures after drug exposure ceases. J Bone Miner Res. 2008;23:1698–707. doi: 10.1359/jbmr.080511. [DOI] [PubMed] [Google Scholar]
- 42.Whyte MP, Huhges AE. Expansile skeletal hyperphosphatasia is caused by a 15-base pair tandem duplication in TNFRSF11A encoding RANK and is allelic to familial expansile osteolysis. J Bone Miner Res. 2002;17:26–9. doi: 10.1359/jbmr.2002.17.1.26. [DOI] [PubMed] [Google Scholar]
- 43.Sobacchi C, Frattini A, Guerrini MM, Abinun M, Pangrazio A, Susani L, Bredius R, Mancini G, Cant A, Bishop N, Grabowski P, Fattore AD, Messina C, Errigo G, Coxon FP, Scott DI, Teti A, Rogers MJ, Vezzoni P, Villa A, Helfrich MH. Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat Genet. 2007;39:960–2. doi: 10.1038/ng2076. [DOI] [PubMed] [Google Scholar]
- 44.Cundy T, Hegde M, Naot D, Chong B, King A, Wallace R, Mulley J, Love DR, Seidel J, Fawkner M, Banovic T, Callon KE, Grey AB, Reid IR, Middleton-Hardie CA, Cornish J. A mutation in the gene TNFRSF11B encoding osteoprotegerin causes an idiopathic hyperphosphatasia phenotype. Hum Mol Genet. 2002;11:2119–27. doi: 10.1093/hmg/11.18.2119. [DOI] [PubMed] [Google Scholar]
- 45.Whyte MP, Obrecht SE, Finnegan PM, Jones JL, Podgornik MN, McAlister WH, Mumm S. Osteoprotegerin deficiency and Juvenile Paget’s Disease. N Engl J Med. 2002;347:175–84. doi: 10.1056/NEJMoa013096. [DOI] [PubMed] [Google Scholar]
- 46.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 47.Chong B, Hegde M, Fawkner M, Seidel J, Tuysuz B, Yuksel B, Coker M, Cassinelli H, Tau C, Love D, Cundy T. Mutations in the gene encoding osteoprotegerin cause idiopathic hyperphosphatasia. J Bone Miner Res. 2002;17(suppl 1):S139. doi: 10.1359/jbmr.2003.18.12.2095. [DOI] [PubMed] [Google Scholar]
- 48.Chong B, Hegde M, Fawkner M, Simonet S, Cassinelli H, Coker M, Kanis J, Seidel J, Tau C, Tuysuz B, Yuksel B, Love D. Idiopathic hyperphosphatasia and TNFRSF11B mutations: relationships between phenotype and genotype. J Bone Miner Res. 2003;18:2095–104. doi: 10.1359/jbmr.2003.18.12.2095. [DOI] [PubMed] [Google Scholar]
- 49.Roodman GD. High bone turnover markers predict poor outcome in patients with bone metastasis. J Clin Oncol. 2005;23:4821–2. doi: 10.1200/JCO.2005.02.911. [DOI] [PubMed] [Google Scholar]
- 50.Mountzios G, Dimopoulos MA, Bamias A, Papadopoulos G, Kastritis E, Syrigos K, Pavlakis G, Terpos E. Abnormal bone remodeling process is due to an imbalance in the receptor activator of nuclear factor-kappaB ligand (RANKL)/osteoprotegerin (OPG) axis in patients with solid tumors metastatic to the skeleton. Acta Oncol. 2007;46:221–9. doi: 10.1080/02841860600635870. [DOI] [PubMed] [Google Scholar]
- 51.Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10:169–80. doi: 10.1007/s10911-005-5399-8. [DOI] [PubMed] [Google Scholar]
- 52.Klein A, Olendrowitz C, Schmutzler R, Hampl J, Schlag PM, Maass N, Arnold N, Wessel R, Ramser J, Meindl A, Scherneck S, Seitz S. Identification of brain- and bone-specific breast cancer metastasis genes. Cancer Lett. 2008. [Epub ahead of print]. [DOI] [PubMed]
- 53.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 54.Corey E, Brown LG, Kiefer JA, Quinn JE, Pitts TE, Blair JM, Vessella RL. Osteoprotegerin in prostate cancer bone metastasis. Cancer Res. 2005;65:1710–8. doi: 10.1158/0008-5472.CAN-04-2033. [DOI] [PubMed] [Google Scholar]
- 55.Keller ET. The role of osteoclastic activity in prostate cancer skeletal metastases. Drugs Today (Barc) 2002;38:91–102. doi: 10.1358/dot.2002.38.2.820105. [DOI] [PubMed] [Google Scholar]
- 56.Yoshino I, Yohena T, Kitajima M, Ushijima C, Nishioka K, Ichinose Y, Sugimachi K. Survival of non-small cell lung cancer patients with postoperative recurrence at distant organs. Ann Thorac Cardiovasc Surg. 2001;7:204–9. [PubMed] [Google Scholar]
- 57.Feeley BT, Liu NQ, Conduah AH, Krenek L, Roth K, Dougall WC, Huard J, Dubinett S, Lieberman JR. Mixed metastatic lung cancer lesions in bone are inhibited by noggin overexpression and Rank:Fc administration. J Bone Miner Res. 2006;21:1571–80. doi: 10.1359/jbmr.060706. [DOI] [PubMed] [Google Scholar]
- 58.Nakamura ES, Koizumi K, Kobayashi M, Saitoh Y, Arita Y, Nakayama T, Sakurai H, Yoshie O, Saiki I. RANKL-induced CCL22/macrophage-derived chemokine produced from osteoclasts potentially promotes the bone metastasis of lung cancer expressing its receptor CCR4. Clin Exp Metastasis. 2006;23:9–18. doi: 10.1007/s10585-006-9006-1. [DOI] [PubMed] [Google Scholar]
- 59.Giuliani N, Bataille R, Mancini C, Lazzaretti M, Barillé S. Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood. 2001;98:3527–33. doi: 10.1182/blood.V98.13.3527. [DOI] [PubMed] [Google Scholar]
- 60.Dougall WC, Glaccum M, Charrier K, Kathy R, Brasel K, Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrisey PJ, Peschon JJ, Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–24. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–8. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amizuka N, Shimomura J, Li M, Seki Y, Oda K, Henderson JE, Mizuno A, Ozawa H, Maeda T. Defective bone remodelling in osteoprotegerin-deficient mice. J Electron Microsc. 2003;52:503–13. doi: 10.1093/jmicro/52.6.503. [DOI] [PubMed] [Google Scholar]
- 63.McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, Peacock M, Miller PD, Lederman SN, Chesnut CH, Lain D, Kivitz AJ, Holloway DL, Zhang C, Peterson MC, Bekker PJ. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–31. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 64.Brown JP, Prince RL, Deal C, Recker RR, Kiel DP, de Gregorio LH, Hadji P, Hofbauer LC, Alvaro-Gracia JM, Wang H, Austin M, Wagman RB, Newmark R, Libanati C, San Martin J, Bone HG. Comparison of the effect of denosumab and alendronate on bone mineral density and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2008;24:153–61. doi: 10.1359/jbmr.0809010. [DOI] [PubMed] [Google Scholar]
- 65.Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, de Boer R, Berardi R, Gascon P, Tonkin KS, Coleman R, Paterson AH, Peterson MC, Fan M, Kinsey A, Jun S. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25:4431–7. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 66.Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, Van der HD, Zhou L, Tsuji W, Newmark R. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58:1299–309. doi: 10.1002/art.23417. [DOI] [PubMed] [Google Scholar]
- 67.Marathe A, Peterson MC, Mager DE. Integrated cellular bone homeostasis model for denosumab pharmacodynamics in multiple myeloma patients. J Pharmacol Exp Ther. 2008;326:555–62. doi: 10.1124/jpet.108.137703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Body JJ, Greipp P, Coleman RE, Facon T, Geurs F, Fermand JP, Harousseau JL, Lipton A, Mariette X, Williams CD, Nakanishi A, Holloway D, Martin SW, Dunstan CR, Bekker PJ. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003;97:887–92. doi: 10.1002/cncr.11138. [DOI] [PubMed] [Google Scholar]
- 69.Chanda D, Isayeva T, Kumar S, Siegal GP, Szafran AA, Zinn KR, Reddy VV, Ponnazhagan S. Systemic osteoprotegerin gene therapy restores tumor-induced bone loss in a therapeutic model of breast cancer bone metastasis. Mol Ther. 2008;16:871–8. doi: 10.1038/mt.2008.48. [DOI] [PubMed] [Google Scholar]
- 70.Costa L, Lipton A, Coleman RE. Role of bisphosphonates for the management of skeletal complications and bone pain from skeletal metastases. Support Cancer Ther. 2006;3:143–53. doi: 10.3816/SCT.2006.n.012. [DOI] [PubMed] [Google Scholar]