Abstract
Rho GTPases are essential regulators of signaling networks emanating from many receptors involved in innate or adaptive immunity. The Rho family member RhoA controls cytoskeletal processes as well as the activity of transcription factors such as NF-κB, C/EBP and serum response factor. The multifaceted host cell activation triggered by Toll-like receptors (TLRs) in response to soluble and particulate microbial structures includes rapid stimulation of RhoA activity. RhoA acts downstream of TLR2 in HEK-TLR2 and monocytic THP-1 cells, but the signaling pathway connecting TLR2 and RhoA is still unknown. It is also not clear if RhoA activation is dependent on a certain TLR adapter. Using lung epithelial cells, we demonstrate TLR2- and TLR3-triggered recruitment and activation of RhoA at receptor-proximal cellular compartments. RhoA activity was dependent on TLR-mediated stimulation of Src family kinases. Both Src family kinases and RhoA were required for NF-κB activation, while RhoA was dispensable for type I interferon generation. These results suggest that RhoA plays a role downstream of MyD88-dependent and -independent TLR signaling and acts as a molecular switch downstream of TLR-Src initiated pathways.
Keywords: signal transduction, bacterial, viral, transcription factors, cell activation
Introduction
TLRs recognize conserved microbial and viral ligands and trigger signaling pathways required for mounting a host immune response against infection by these pathogens. In the lung, the pulmonary innate immune system serves as the first line of defense against aerosolized pathogens. A major component of this lung immune response is the airway epithelium, which provides not only the mechanical barrier and interface between the environment and the host, but is also uniquely equipped for sensing and responding to inhaled bacterial and viral organisms. Lung epithelial cells respond to many TLR ligands, although the participation of TLR4 under non-inflammatory conditions seems to be limited due to the intracellular localization and decreased LPS sensitivity of this receptor in airway epithelial cells (1–4). In contrast, TLR2 represents an important functional receptor for bacterial recognition at the lung epithelial cell surface. Key bacterial lung pathogens such as S. aureus, S. pneumonia and P. aeruginosa initiate TLR2-mediated signaling responses (2, 5). Recognition of viral lung pathogens is accomplished by TLR3, located in vesicular endosomal compartments, or by cytosolic receptors such as RIG-I and MDA5 (6, 7). TLR2- and TLR3-initiated signaling differs in its use of adapter molecules, where TLR2 connects to TIRAP/MyD88, while dsRNA-stimulated TLR3 recruits TRIF. Further downstream, both TLR-adapter complexes cause activation of NF-κB-dependent gene transcription and of MAPK/JNK/p38 pathways, although TLR3 mediates additionally type I interferon generation. Thus, receptor-proximal events differ between TLR2 and TLR3, connecting TLR-adapter complexes to separate signaling cascades.
Certain signaling molecules have been tentatively connected to MyD88-dependent and - independent TLR signaling. For example, a PI-3 kinase-Akt pathway is triggered by several TLRs, independently of their adapter usage or cellular localization (8–10). The association between TLR2 and the p85 regulatory subunit of PI-3 kinase requires the tyrosine phosphorylated intracellular TIR domain of TLR2 (8). Inducible TLR tyrosine phosphorylation has been linked to activation of Src family kinases, which seem to be an integral part of the TLR2 and TLR3 signaling complex (11–14). Another component of TLR complexes is the tyrosine kinase Syk, which relays signals downstream of integrin engagement and takes part in ITAM-containing immunoreceptor signal transduction (9, 15, 16). Syk activation seems to be the result of receptor cooperation, in case of TLR2 and TLR4 with the β-glucan receptor Dectin-1 (17), or with CD36 for TLR2/TLR6 (18, 19). Signaling events downstream of TLRs have also been connected to Rho family GTPases. We and others reported a rapid and transient increase in Rac1 and RhoA activity when TLR2 and TLR4 stimulation by soluble PAMPs occurred (8, 20, 21). Rho GTPases are molecular switches that regulate essential cellular processes including actin dynamics, gene transcription and motility. The activity of Rho GTPases is controlled by guanine nucleotide exchange factors (GEFs), which are commonly stimulated by tyrosine phosphorylation and/or phospholipid binding. In the context of TLR signaling, the RacGEF Vav-1 is activated via a TLR9-Src pathway, while the RhoGEF AKAP13 is involved in TLR2 responses (22, 23). Previous studies in TLR2-expressing HEK293 cells indicated that RhoA was not an integral part of the TLR2/IRAK/TRAF6 complex, but was required for PKCζ-dependent NF-κB transactivation (20). Since RhoA, Src and Syk play a role in transmitting signals to NF-κB in several cellular systems, we hypothesized that these proteins could be connected in a TLR-proximal signaling cascade, regulating NF-κB-dependent gene transcription. Thus, biochemical and spatiotemporal detection of active, GTP-bound RhoA in PAMP-stimulated lung epithelial cells was carried out. RhoA activation was observed in distinct cellular compartments and required Src activity. TLR2 and TLR3 stimulation triggered the Src-RhoA pathway to NF-κB-dependent gene transcription, while RhoA was not involved in TLR3/Src-initiated type I interferon generation.
Materials and Methods
Cells and reagents
The immortalized primary human lung epithelial cells (SALE) were a gift from Dr. W. C. Hahn (Harvard Medical School, Boston, MA) and were described previously (24). SABM medium, SAGM SingleQuots and trypsin/EDTA used for SALE cells were purchased from Lonza (Walkersville, MD, USA). L929-ISRE cell line (ISRE- luciferase reporter) was kindly provided by Dr. B. Beutler (The Scripps Research Institute). The synthetic lipopeptide MALP-2 was obtained from Alexis (San Diego, CA), Pam3CSK4 from InvivoGen (San Diego, CA), and Poly IC was purchased from Amersham Biosciences (NJ, USA) and dissolved in pyrogen-free distilled water. dsRNA (a 34 bp sequence from human rhinovirus 16) labeled with Alexa 633 at the 3′ end was synthesized by Invitrogen (Carlsbad, CA). RhoA mAb (26C4), IκBα polyclonal Ab (C-21), TLR2 Ab (H-175) were from Santa Cruz Biotechnology (Santa Cruz, CA); phospho-IRF3 (Ser386) was from IBL-America (Minneapolis, MN, USA); IRF3, phospho-IRF3 (Ser396), phospho-Src (Tyr416), phospho-Syk (Tyr 525-526), phospho-p38, p38, Fyn, Hck, Src, Yes, Lyn Abs were from Cell Signaling Technology (Beverly, MA); Myc (9E10) mAb was from Babco (Richmond, CA), GM130 mAb (BD Pharmingen, USA). AKAP-13 Ab was from Bethyl Laboratories, Inc (TX, USA). Secondary Ab labeled with Alexa 647 was purchased from Invitrogen (Carlsbad, CA). PP2, PP3, Src kinase inhibitor I (SI) and piceatannol were obtained from Calbiochem (La Jolla, CA).
DNA constructs
All plasmids were prepared using endotoxin-free plasmid DNA purification (Qiagen, Valencia, CA). RhoA biosensor (RhoA-CFP-RBD-YFP) was described previously (25). An expression plasmid containing the RhoA biosensor, lentiviral packaging plasmids and pVSV-G were kindly provided by Dr. K. Wong (Univ. of San Francisco, CA, (26)). Myc-RhoA wt, Myc-RhoA T19N were described previously (20). The NF-κB-responsive luciferase reporter (5x NF-κB-Luc) was from Promega (Madison, WI). c-Src K295M was kindly provided by Dr. D. Schlaepfer (UCSD, CA).
RhoA biosensor expressing SALE
Co-transfection of HEK293T cells with RhoA biosensor, packaging plasmid and VSV-G was performed by calcium phosphate method. After 2–3 days, virus-containing supernatants were collected and used for transduction of SALE cells. Cells were incubated with viral supernatant for 6–8h, media was then replaced with fresh SABM media with SAGM SingleQuots. The procedure was repeated 24h later. RhoA biosensor-expressing SALE cells were positively selected by fluorescence-activated cell sorting (98% positive) for low to medium expressers.
Transfection and reporter assays
Transfection of SALE cells was done using Lipofectamine Plus (Invitrogen, Carlsbad, CA). SALE cells were plated in six-well plates at a density of 0.2 × 106/well. 24h later, cells were transiently transfected with 100ng of NF-κB luciferase reporter plasmid alone or co-transfected with RhoA wt (200ng), RhoA T19N (400ng), c-Src K295M (400ng) or empty vector. 18h later (36h for co-transfection) cells were starved for 3h in SABM medium with 0.5% FBS. Stimulation with MALP-2 (10ng/ml), Pam3CSK4 (100 ng/ml) or Poly IC (10–30µg/ml) was for 4h. Cells were lysed in luciferase assay buffer (Promega) and luminescence of lysates was measured in triplicates for each independent assay (n=3). For siRNA treatment Stealth Select Syk RNAi (HSS110401, HSS110402, HSS110403) (Invitrogen, Carlsbad, CA) were tested using Dharmafect 4 (Dharmacon Inc., CO) and HSS110402 RNAi (20nM) was used for Syk knockdown. 48h later 5xNF-κB luc was transfected, followed 24h later by starvation and cell stimulation as described.
Interferon type I production
L929 cells stably expressing an ISRE luciferase reporter construct were kindly provided by Dr. B. Beutler. For measurements of IFN type I production L929-ISRE cells were plated in 96-well plate at a density 5 × 104/well. 24h later media was removed before adding supernatants of SALE cells stimulated with poly IC (100µl/well). After 4h L292-ISRE cells were washed with PBS, lysed in luciferase assay buffer (Promega) and luminescence was measured with a LB 960 Centro Microplate Luminometer in triplicates (n=3 independent experiments).
Rho activation assay
SALE cells were plated in 10 cm dishes (Fisher, USA) at a concentration of 0.8 × 106/ml in SABM media with supplements. 48h later cells were starved in SABM/0.5% FBS overnight followed by RBD pulldown assay as described (20). Stimulation with MALP-2 (10ng/ml) or poly IC (30µg/ml) was performed for the indicated times. For some experiments cells were preincubated with inhibitors 30min before stimulation. Protein samples were analyzed by SDS-PAGE and immunoblotting with anti-RhoA Ab.
Immunoblotting
For the detection of phospho-IRF3 (Ser386) procedure was as described (27). All other immunoblots were performed as described elsewhere (28). Densitometry of immunoblots was performed by using the GS-800 Calibrated Densitometer (BioRad) and Quantity One software (v. 4.5.2). Average density of the protein bands was normalized to the corresponding bands of the loading controls.
Immunoprecipitation
SALE cells stably expressing Myc-RhoA were plated in 10 cm dishes (Fisher, USA) at a concentration of 1 × 106/ml in SABM media with supplements. 48h later cells were starved in SABM/0.5% FBS overnight. After stimulation with MALP-2 (10ng/ml) for 10 min, cells were washed with PBS and lysed (10mM Tris-Cl, 100mM NaCl, 1mM EGTA, 1mM EDTA, 1mMNaF, 20mM Na2P2O7, 1% Triton X-100, 10% glycerol, 0,1% SDS, 0,5% deoxycholate, 2mM orthovanadate, 1µM microcystein, 2µg/ml leupeptin, 2µg/ml aprotinin, 1µg/ml pepstatin, 2mM PMSF). Lysates were cleared by centrifugation and incubated with AKAP-13, TLR2 or rabbit IgG Abs for 1,5 h at 4°C. Incubation with sepharose G-beads was performed for 45 min at 4°C. After 4 washes samples were resuspended in sample buffer, boiled for 5 min and analyzed by SDS-PAGE and immunoblotting.
Sample preparation for confocal microscopy
For FRET experiments SALE/RhoA cells (0.2×105) were seeded on glass coverslips in 35 mm tissue culture dishes (Fisher, CA). Stimulation with MALP-2 (10ng/ml) for 0–10 min or Poly IC (30µg/ml) for 0–30 min in SABM with 0.5% FBS was performed after overnight starvation in the same media. When indicated, Src kinase inhibitor I (SI) (1 µM) was added to cells 30 min before stimulation. Cells were fixed with 4% paraformaldehyde for 15 min at RT and mounted in Pro Long Gold mounting media (Invitrogen, Carlsbad, CA). For co-localization experiments cells were incubated with dsRNA-Alexa 633 (3 µM) for 30 min and then fixed with 4% paraformaldehyde for 15 min at RT and mounted in Pro Long Gold mounting media (Invitrogen, Carlsbad, CA). An average of 50 cells was analyzed per condition, at each time point.
Confocal microscopy and image processing
Imaging was performed with a confocal microscope (2100 Radiance; Bio-Rad Laboratories) using a 60x oil-immersion objective. Intramolecular FRET, as described previously (29), was measured by exciting CFP with a 405 nm laser line and using the sequential scan acquisition mode for the CFP and FRET (YFP) channels; additionally, a YFP image was acquired by excitation with a 488 nm laser line. Three images were acquired in the same order: CFP, FRET and YFP in all experiments. Ratiometric image analysis of FRET was performed using Image Pro 3DS software (Media Cybernetics Inc. v 6.0) and LSM examiner software. The YFP image with high signal-to-noise ratio served to create a binary mask with a value of zero outside and value of one inside the cell using a threshold-based procedure. Subsequently CFP and FRET images were multiplied by the mask. Dividing a FRET masked image by a CFP masked image and multiplying this value by a factor of 1000, a ratio image was obtained. Co-localization analysis was performed using National Institutes of Health (NIH) image analysis software packages Image J (v. 1.33) and LSM examiner software (BioRad-Zeiss LaserSharp 2000 v. 6).
Statistics
When indicated experimental data was analyzed for statistical significance employing the Student’s t-Test for paired samples. Significance is indicated by asterisks and described in figure legends.
Results
Responsiveness of SALE cells to TLR ligands
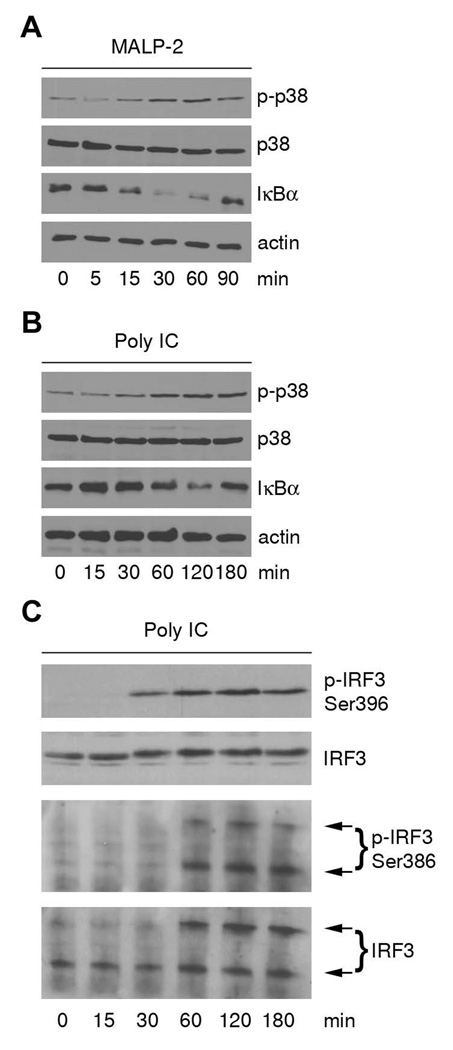
Human airway epithelial (SALE) cells express a variety of TLRs, although they are not very responsive to LPS stimulation (30). Cell surface expression of TLR2 and CD14 as well as intracellular expression of TLR3 was confirmed by flow cytometry (data not shown). TLR2 and TLR3 reside in different cellular compartments and utilize distinct adapters such as TIRAP/MyD88 and TRIF. SALE cells were analyzed for their responsiveness to TLR2 and TLR3 ligands by using diacylated lipopeptide (MALP-2), Pam3CSK4 and dsRNA (poly IC). The specificity of TLR2 and TLR3 ligands was confirmed using TLR2 blocking antibody or chloroquine pretreatment followed by NF-κB-luciferase reporter assays (data not shown). For initial screening of the NF-κB pathway and of MAPK activation, IκBα degradation and p38 phosphorylation by these ligands was assessed. Airway cells did not respond well to the TLR2 ligand Pam3CSK4 (see NF-κB activation, Suppl. Fig. 1A). In contrast, MALP-2-induced degradation of IκBα was detected after 15 min and IκBα was completely resynthesized in 90 min (Fig. 1A). Phosphorylation of p38 by MALP-2 occurred at 30 minutes. A similar pattern was observed with poly IC (Fig. 1B), although the overall kinetic was delayed due to internalization of the TLR ligand and the TLR3 response from the endosomal compartment. It has been previously shown that TLR3 stimulation generates IRF3-dependent type I interferon. Accordingly, poly IC stimulation of SALE cells induced IRF3 phosphorylation at Ser396 and at Ser386. Dimer formation of phospho-IRF3 (S386), a prerequisite for IRF3 transcriptional activity, was observed after 60 min (Fig.1C). Thus, major TLR2 and TLR3 signaling pathways are functional in SALE cells.
FIGURE 1. SALE airway cells contain functional TLR2 and TLR3.
A, IκBα degradation and p38 phosphorylation are induced by MALP-2. SALE cells were stimulated for indicated time points with MALP-2 and lysates were analyzed by immunoblotting. Phospho-p38 and IκBα blots were stripped and reprobed for p38 and actin as loading controls. One representative experiment is shown (n=3). B, IκBα degradation and p38 phosphorylation are induced by poly IC. SALE cells were stimulated for indicated time points with poly IC and lysates were analyzed by immunoblotting. Phospho-p38 and IκBα blots were stripped and reprobed for p38 and actin as loading controls. One representative experiment is shown (n=3). C, IRF3 phosphorylation is induced by poly IC. SALE cells were stimulated for indicated time points with poly IC and lysates were analyzed by immunoblotting. Phospho-IRF3 (S396) and total IRF3 are shown in upper two panels. Phospho-IRF3 (Ser386) was analyzed by native PAGE followed by immunoblotting (panel 3). Blots were stripped and probed for total IRF3 as loading control (panel 4). One representative experiment is shown (n=2).
TLR2 and TLR3 signaling leads to RhoA activity at distinct cellular compartments
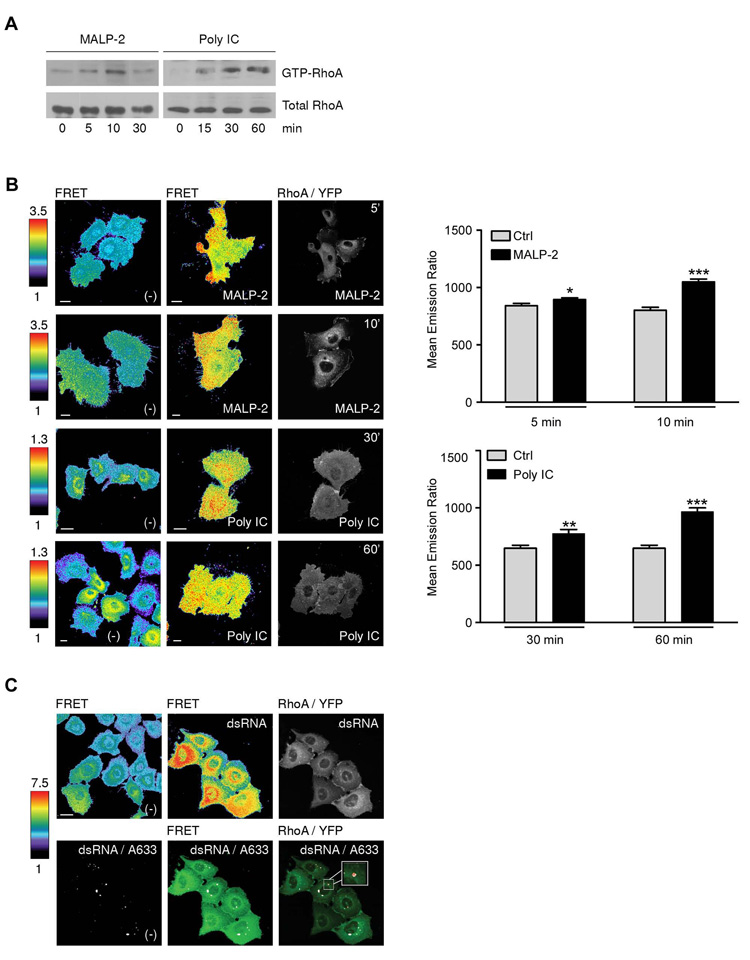
TLR2 stimulation triggers activation of the Rho GTPase RhoA in TLR2-expressing model cell lines and monocytic cells (20). Thus, RhoA activation was determined in SALE cells stimulated either with MALP-2, or for comparison with poly IC. Pulldown assays showed a rapid increase in RhoA activity after 5 min, while poly IC-induced RhoA activation was delayed, as expected for signaling by intracellular TLR3 (Fig. 2A). SALE cells are characterized by large, spreading cell bodies, which is advantageous for visualization of signaling molecule recruitment. To visualize local RhoA activity changes caused by TLR-mediated signals, a single-chain FRET sensor was introduced into SALE cells. A RhoA biosensor, which permits detection of RhoA activation in real time, was lentivirally transduced into SALE cells. These cells, termed SALE/RhoA, were characterized for their RhoA content and screened for their TLR ligand responsiveness. SALE/RhoA cells expressed similar levels of endogenous RhoA and the RhoA biosensor (Suppl. Fig. 1B). Expression of the RhoA biosensor did not disturb TLR signalling, as SALE/RhoA cells responded equally well to TLR ligands as non transduced SALE cells (Suppl. Fig. 1C, D). To study the spatiotemporal dynamics of TLR-triggered RhoA activation, SALE/RhoA cells were either left unstimulated or stimulated with MALP-2 or poly IC for various time periods. Cells were fixed and analyzed by confocal microscopy for increased FRET. RhoA activation is presented as CFP/FRET emission ratio and is visualized by colorcoding the images with scaling from low (blue) to high (red) FRET, while biosensor localization is shown in RhoA-YFP images. Short stimulation of SALE/RhoA cells with MALP-2 (5 min) increased the emission ratios with areas of the highest intensity close to cell edges. At the same time, enrichment of RhoA was detected at membrane ruffles (Fig. 2B, panel 1). Active RhoA moved from the cell edges to the cytoplasm at later time points (10 min shown). Performance of the RhoA biosensor was validated by co-expression of dominant negative RhoA, which reduced the emission ratio of MALP-2 stimulated, Myc-RhoA DN positive cells (Suppl. Fig. 1E). An increase in FRET was also observed after 15–30 min of poly IC stimulation (Fig. 2B, panel 3), although highest RhoA activity was in this case concentrated in perinuclear, vesicular structures. After 60 min of poly IC stimulation RhoA activity was still maintained. Thus, the imaging time course of RhoA activity correlated well with the biochemical assay. To confirm co-localization of active RhoA with dsRNA-containing vesicles, which are TLR3 signaling compartments (11), SALE/RhoA cells were incubated with labeled dsRNA. After 30 min of stimulation, the labeled TLR3 ligand co-localized with areas of high FRET signal (active RhoA) (Fig. 2C, upper panel) as well as with RhoA-YFP (Fig. 2C, lower panel).
FIGURE 2. TLR2- and TLR3-mediated activation of RhoA.
A, Time-dependent activation of endogenous RhoA by MALP-2 and poly IC in SALE cells. Upper panel shows RhoA-GTP, lower panel total RhoA in lysates (10% of pulldown lysate). One representative experiment is shown (n=3). B, RhoA biosensor activation by MALP-2 and poly IC. Representative FRET ratio images and RhoA-YFP images of SALE/RhoA cells stimulated with MALP-2 for 5 and 10 min or poly IC for 30 and 60 min (scale bar 10 µm). Quantification of whole cell emission ratios is shown; number of cells first panel: n (-) = 59; n (MALP-2, 5min) = 78; second panel: n (-) = 70; n (MALP-2, 10 min) = 70; third panel: n (-) = 29; n (poly IC 30, min) = 19; forth panel: n (-) = 29; n (poly IC, 60min) = 34; error bars represent standard errors; p values ≤ 0,001 (***),≤ 0,01 (**) and ≤ 0,05 (*). C, Co-localization of the RhoA biosensor with dsRNA. Representative FRET ratio images and RhoA-YFP images (upper panel) as well as dsRNA co-localization with FRET and RhoA-YFP (lower panel) in SALE/RhoA cells stimulated with dsRNA/A633 (3 µM) for 30 min. Co-localization is in white; the co-localization coefficient for red to green was 0.83 (FRET) and 0.75 (Rho/YFP) analyzed by Image J (v. 1.33). FRET ratio images in B and C are scaled so that regions of highest RhoA activity are shown in red. Scale bar 10 µm.
RhoA is required for NF-κB activation, but dispensable for type I IFN generation
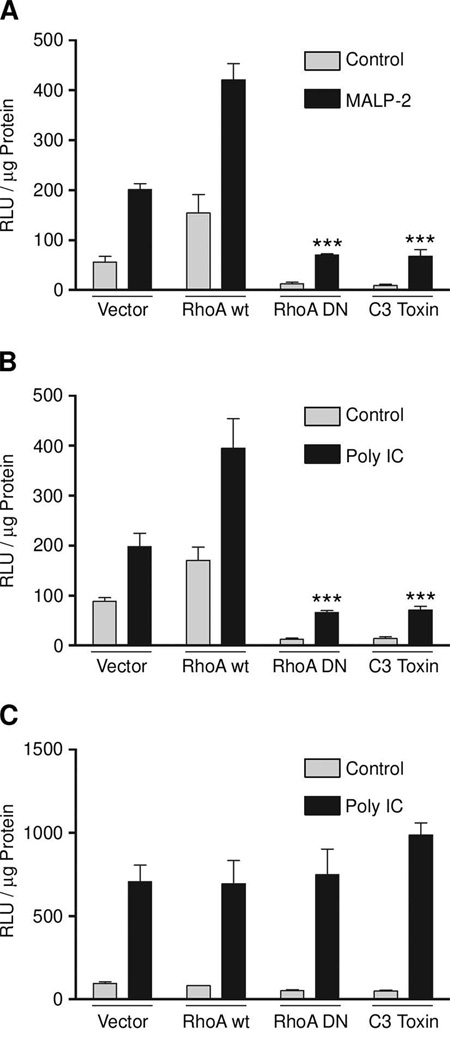
Previous studies in HEK293-TLR2 cells demonstrated a regulatory role for RhoA in TLR2-initiated NF-κB transactivation. This data was verified in SALE cells, which present a more physiologically relevant cell type. As shown in Figure 3 expression of dominant negative RhoA or Clostridium botulinum C3 toxin in SALE cells harboring a NF-κB responsive, luciferase-coupled promoter, inhibited TLR2 NF-κB activation. Overexpression of RhoA wt (approx. 3–6 fold) in these cells augmented NF-κB activity. RhoA signaling was also required for TLR3-mediated NF-κB activation, although production and release of IFNα/β was independent of RhoA (Fig. 3C).
FIGURE 3.
Inhibition of RhoA blocks NF-κB transactivation by TLR2 and TLR3 stimulation. SALE cells were transiently transfected with NF-κB-luc and plasmids as indicated before stimulation with MALP-2 (A) or poly IC (B, C) for 4h. Lysates of (A, B) were analyzed in chemiluminescence and by immunoblot for protein expression (data not shown), supernatants (C) were collected and used to determine type I IFN production. Error bars represent standard deviations; p values are ≤0,001 (***) to stimulated vector control. Data of one representative experiment is shown (n=3).
Src family kinase activity is required for TLR2 and TLR3 signaling to NF-κB
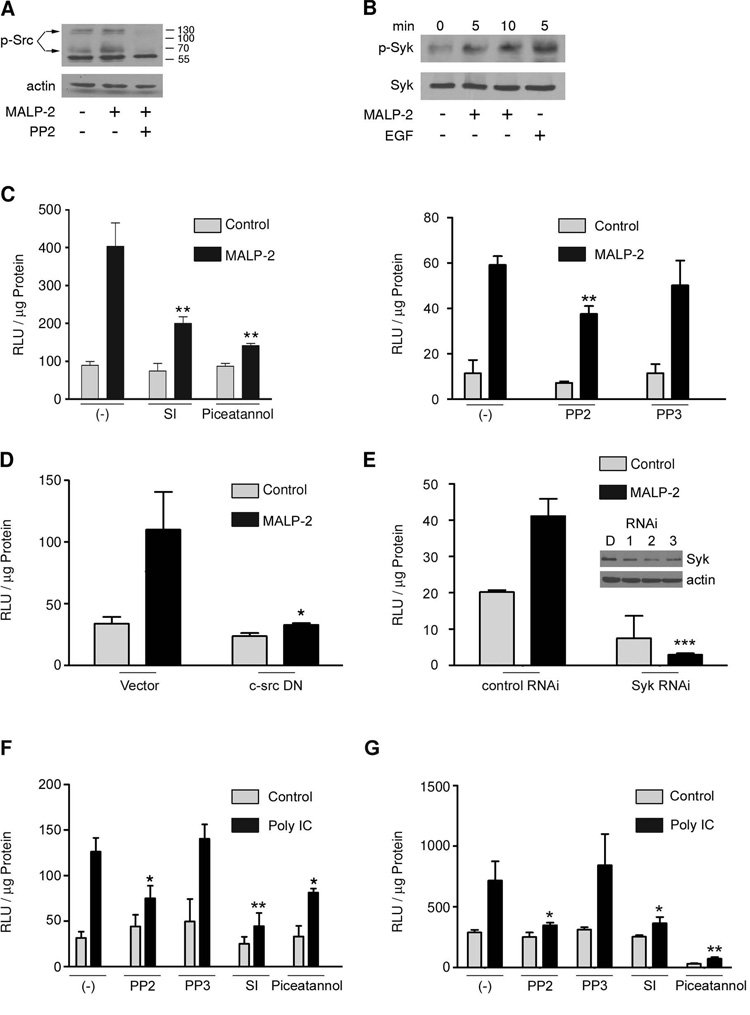
Recent reports placed Src family kinases as receptor-proximal regulators of TLR signaling (11, 14). Similarly, the tyrosine kinase Syk was implicated in TLR pathways (9, 15, 16). When analyzing MALP-2-induced Src kinase activation, several immunoreactive bands were detected using pan-phospho-Src Tyr416 antibody (Fig. 4A). Tyrosine phosphorylation of these proteins was blocked by the Src kinase inhibitor PP2. Immunoblotting revealed that SALE cells express multiple Src family kinases. The tyrosine phosphorylated band at 68kDa may represent c-Src or Yes kinases, the 130kDa band remains unidentified (Suppl. Fig. 2). In addition, rapid activation of Syk by the TLR ligand MALP-2 was detected, using EGF stimulation of SALE cells as positive control (Fig. 4B).
FIGURE 4. Src and Syk mediate TLR2- and TLR3-induced NF-κB-dependent gene transcription.
(A) SALE cells were stimulated with MALP-2 for 10 min with or without preincubation with PP2. Lysates were analyzed for Src activation (phospho-Src Tyr416). Actin served as control. (B) SALE cells were stimulated with MALP-2 or EGF (1 ng/ml) and lysates were analyzed for Syk activation using phospho-Syk Tyr525/526 antibody. Total Syk served as control. (C, F) SALE cells were transiently transfected with NF-κB-luc and preincubated with Src inhibitor 1 (SI, 10 µM), PP2 (10 µM), PP3 (10 µM) or piceatannol (25µM) before incubation with MALP-2 or poly IC for 4h. (D, E) SALE cells were either co-transfected with c-Src K295M (24h) and 5xNF-κB luc (D) or treated with Syk siRNA (# 2, 20nM, 48h) before 5xNF-κB luc transfection (E). Lysates of (C–F) were analyzed for chemiluminescence. Supernatants (G) from (F) were collected to determine type I IFN production. Error bars represent standard deviations; p values are ≤0,01 (**) and ≤0,05 (*) to stimulated control. Data of one representative experiment is shown (n=3).
Next, the involvement of Src family kinases and Syk in TLR2- and TLR3-initiated pathways was probed. Inhibition of Src kinases with PP2 or Src Inhibitor 1 (SI), and of Syk with piceatannol significantly reduced MALP-2 stimulated NF-κB activation (Fig. 4C). These data were confirmed using transfection of dominant negative c-Src or by siRNA knockdown of Syk (Fig. 4 D, E). Similarly, poly IC-stimulated NF-κB activation was abolished by Src kinase inhibition (Fig. 4F), while PP3, an inactive analogue of PP2, did not alter NF-κB-dependent gene transcription. Piceatannol treatment of SALE cells had a modest effect on TLR3 ligand-stimulated NF-κB activation. Both, Src kinase and Syk inhibitors, abolished poly IC-induced IFNα/β production (Fig. 4G). Supernatants derived from unstimulated, piceatannol-treated SALE cells reduced the baseline transcriptional activity in L929-ISRE cells, which could indicate some unspecific effect of this compound.
Src family kinases are upstream of TLR2- and TLR3-induced RhoA activation
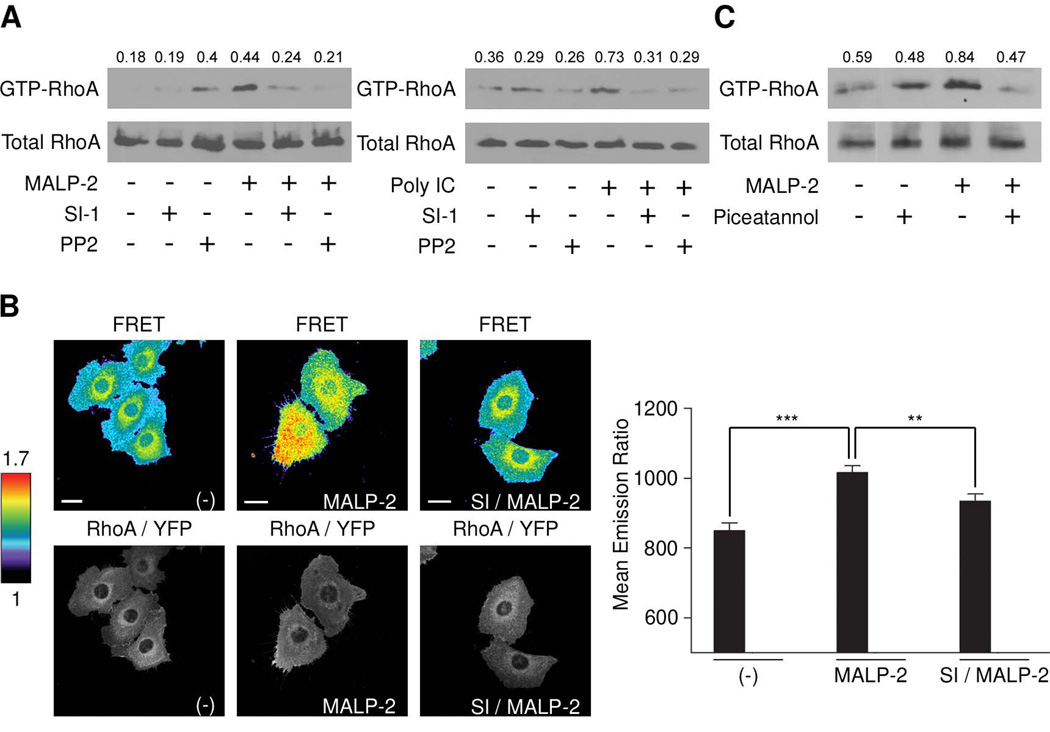
Previous studies indicated that the TLR2-RhoA pathway may diverge from the canonical TLR2-MyD88 pathway (20). Src family kinases have been implicated in TLR-TIR domain phosphorylation, thus providing initial binding sites for association of signaling complexes. Srcmediated phosphorylations are also often prerequisite for GEF and thus GTPase activation, placing Src upstream of GTPases such as RhoA. Preincubation of SALE cells with the Src kinase inhibitors SI and PP2 abolished RhoA activation by MALP-2 and by poly IC when pulldown assays were analyzed (Fig. 5A). The effect of Src kinase inhibitors on RhoA activation was also studied by FRET using biosensor-expressing SALE/RhoA cells. As shown in Figure 5B, MALP-2 stimulated RhoA activity was reduced in the presence of Src kinase inhibitor (SI). Analysis of the whole cell emission ratios revealed a significant FRET reduction upon Src inhibition (Fig. 5B). Overall, this data indicates that Src signaling is essential for transmitting TLR2 and TLR3 signals to RhoA.
FIGURE 5. TLR2 and TLR3-mediated RhoA activation requires Src.
(A) MALP-2 and poly IC-stimulated RhoA activity is inhibited by Src kinase inhibitors. SALE cells were stimulated with MALP-2 for 10 min with or without preincubation with SI or PP2. Lysates were analyzed for RhoA activity by pulldown assay (RhoA-GTP, total RhoA 10% of lysate) and quantitated by densitometry (see Methods). (B) RhoA activity was analyzed by FRET using RhoA biosensor (representative FRET ratio images and RhoA-YFP images of SALE/RhoA cells are shown; scale bar is 10 µm). FRET ratio images are scaled so that regions of highest RhoA activity are shown in red. Quantification of whole cell emission ratios are shown; number of cells analyzed was n (-) = 32; n (MALP-2) = 52; n (SI/MALP-2) = 45; error bars represent standard errors; p values ≤0,001 (***) and ≤0,01 (**). (C) MALP-2-stimulated RhoA activity is inhibited by Syk kinase inhibitor. SALE cells were stimulated with MALP-2 for 10 min with or without piceatannol preincubation. Lysates were used for RBD pulldown assay and total RhoA immunoblotting. Data in A–C are representative of three independent experiments.
Syk kinase is upstream of TLR2- induced RhoA activation
Syk seems to be involved in transmitting TLR2 and TLR3 signals to NF-κB in SALE cells. Analysis of MALP-2-stimulated SALE cells showed that Syk kinase activity was necessary for RhoA activation (Fig. 5C). Although piceatannol treatment of SALE cells increased RhoA activity in unstimulated cells, MALP-2-triggered RhoA activity was decreased. Interestingly, poly IC-mediated RhoA activation was not altered by Syk inhibition (data not shown). The Syk inhibitor piceatannol showed strong autofluorescence, thus precluding imaging studies and confirmation of changes in biosensor FRET in the presence or absence of this compound.
Discussion
Rho GTPases are master regulators of many immunoreceptors ranging from receptors required for differentiation and maturation of immune cells and for pathogen recognition, to receptors involved in pathogen uptake and the subsequent host signalling responses. Several TLRs induce the activation of the Rho family GTPases Rac, Rho and Cdc42. Almost every cell type including professional innate immune cells such as macrophages, neutrophils and dendritic cells responds to TLR stimulation by activating Rho GTPases rapidly (8, 20, 31). Similarly, lung epithelial cells induce Rho GTPase activation when they encounter TLR ligands. It seems apparent that RhoA activation depends on the interaction of a specific TLR ligand with its cognate TLR, independently of the connecting adapters or the cellular compartment where TLR signaling occurs. A RhoA FRET probe was used to examine localized RhoA activity at early time points after initiation of TLR2 or TLR3 signaling. After 3–5 min of treatment with the TLR2 ligand MALP-2 RhoA was rapidly activated at ruffling areas at the cell surface. Later on, RhoA activity was detected in vesicular structures which moved to the perinuclear region and may contain internalized TLR2. To visualize co-localization of TLR3 ligands with RhoA-YFP and with areas of high FRET, which indicate high RhoA activity, a labelled dsRNA ligand was used. Areas of co-localization and FRET were predominantly detected in perinuclear, speckle-like structures. These structures are reminiscent of TRIF speckles which formed in HeLa cells in response to Poly IC stimulation (32). These speckles contained the receptor-interacting protein 1 (RIP1), a signaling molecule connecting TRIF to NF-κB activation, and NF-κB-activating kinase-associated protein 1 (NAP1), which links TRIF to TANK-binding kinase 1 (TBK1) and the IRF3-IFNα/β pathway. Energy transfer from TRIF to NAP1 was not efficient, while RIP1 was tightly associated with TRIF (32). Our data suggest that RhoA is part of this putative speckle signalosome, and that incorporation of RhoA into the TRIF signaling complex is essential for NF-κB activation. On the other hand, RhoA activation occurs also rapidly when TLR2 connected adapter signaling is triggered at the plasma membrane.
Recent reports connect the Src family kinases c-Src, Hck, Lyn, Fyn, Yes and Fgr to TLR3, TLR2, TLR4 and TLR9 signaling in various cell types (5, 11–14, 22, 33, 34). These kinases have been implicated in the tyrosine phosphorylation of the intracellular TLR-TIR domain in order to provide docking sites for signalling molecules, and may initiate activation of downstream targets. Quite likely, some of these targets are GEFs which control positively the GTPase activation cycle. So far, only the GEFs Vav and AKAP13/Lbc have been implicated in TLR signalling (22, 23). Probing RhoA activation by rhothekin RBD-binding assay or by biosensor FRET clearly shows that Src family kinases provide an essential upstream signal for RhoA activity when TLR2 or TLR3 ligands are utilized. The contribution of Syk to the TLR-RhoA pathway was investigated as several reports linked Syk kinase directly to the TLR4 signaling complex (9, 15, 16). TLR2 and TLR3 ligands stimulated Syk activity in lung epithelial cells (see MALP-2, poly IC not shown). Using a Syk inhibitor or Syk siRNA a substantial decrease in MALP-2 stimulated NF-κB activation was observed. Where to place Syk in TLR signalling to RhoA needs to be explored in more detail, as the specificity of the widely used inhibitor piceatannol has been questioned (35).
Src family kinases and Syk kinase were required for NF-κB activation and type I IFN production in lung epithelial cells, while inhibition of RhoA activity reduced only NF-κB activation. The IFNβ promoter contains the positive regulatory elements PRD-I, -II, -III and –IV that bind to the transcription factors AP-1, IRF3 and NF-κB. Several studies reported a critical role of NF-κB in poly IC- or virus-induced type I IFN expression (36–38). Our data implicate RhoA as regulator of the NF-κB pathway and dispensable for IFNα/β production. This observation is consistent with studies by Wang and coworkers, who assigned only a minor role to NF-κB transcription factors when analyzing IFNα/β expression in virus-infected MEFs lacking individual members of the NF-κB family (39).
The question still remains how TLR-initiated cellular responses connect via Src/Syk to the Rho GTPase-regulated network. A recent report connects the TLR2-TRAF6 pathway to c-Src (40). Attempts to link RhoA to this pathway were not successful. We also explored the recently described TLR2-AKAP13-NF-κB pathway, as the RhoA-specific guanine nucleotide exchange factor (GEF) AKAP13 may convey TLR2 signals to RhoA. Although constitutive and MALP-2 triggered complex formation of endogenous TLR2 and AKAP13 was observed (Suppl. Fig. 3), the presence of RhoA in this complex could not be confirmed. It seems likely that rapid association and dissociation of RhoA from its GEF takes place. In general, the well-characterized role of Rho GTPases in controlling cytoskeletal remodelling may provide a platform for constant assembly and disassembly of the TLR signalosome. Receptor dimerization, co-receptor usage and involvement of cooperating receptors and integrins may be needed for association of recruited signaling components. During these events Rho GTPases may participate in the dynamic assembly of signaling platforms at specific cellular locations.
Supplementary Material
Acknowledgments
We thank Dr. W. Kiosses for advice and Katrina Schreiber for graphic assistance, and Monica Ruse for critical reading of the manuscript.
Abbreviations used in this paper
- FRET
fluorescence resonance energy transfer
- SALE
small airway lung epithelial cells
- TLR
Toll-like receptor
- MEF
murine embryonic fibroblast
- GEF
guanine nucleotide exchange factor
Footnotes
This work was supported by National Institutes of Health grants AI35947 and GM37696 (to U.G.K.) and GM57464 (to K.M.H.).
Disclosures
The authors have no financial conflict of interest.
References
- 1.Guillot L, Medjane S, Le-Barillec K, Balloy V, Danel C, Chignard M, Si-Tahar M. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J. Biol. Chem. 2004;279:2712–2718. doi: 10.1074/jbc.M305790200. [DOI] [PubMed] [Google Scholar]
- 2.Hippenstiel S, Opitz B, Schmeck B, Suttorp N. Lung epithelium as a sentinel and effector system in pneumonia--molecular mechanisms of pathogen recognition and signal transduction. Respir. Res. 2006;7:97. doi: 10.1186/1465-9921-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer AK, Muehmer M, Mages J, Gueinzius K, Hess C, Heeg K, Bals R, Lang R, Dalpke AH. Differential recognition of TLR-dependent microbial ligands in human bronchial epithelial cells. J. Immunol. 2007;178:3134–3142. doi: 10.4049/jimmunol.178.5.3134. [DOI] [PubMed] [Google Scholar]
- 4.Jia HP, Kline JN, Penisten A, Apicella MA, Gioannini TL, Weiss J, McCray PB., Jr Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L428–L437. doi: 10.1152/ajplung.00377.2003. [DOI] [PubMed] [Google Scholar]
- 5.Chun J, Prince A. Activation of Ca2+dependent signaling by TLR2. J. Immunol. 2006;177:1330–1337. doi: 10.4049/jimmunol.177.2.1330. [DOI] [PubMed] [Google Scholar]
- 6.Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 2005;280:5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto M, Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv. Drug Deliv. Rev. 2008;60:805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat. Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 9.Arndt PG, Suzuki N, Avdi NJ, Malcolm KC, Worthen GS. Lipopolysaccharide-induced c-Jun NH2-terminal kinase activation in human neutrophils: role of phosphatidylinositol 3-Kinase and Syk-mediated pathways. J. Biol. Chem. 2004;279:10883–10891. doi: 10.1074/jbc.M309901200. [DOI] [PubMed] [Google Scholar]
- 10.Schmeck B, Huber S, Moog K, Zahlten J, Hocke AC, Opitz B, Hammerschmidt S, Mitchell TJ, Kracht M, Rosseau S, Suttorp N, Hippenstiel S. Pneumococci induced TLR- and Rac1-dependent NF-kappaB-recruitment to the IL-8 promoter in lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L730–L737. doi: 10.1152/ajplung.00271.2005. [DOI] [PubMed] [Google Scholar]
- 11.Johnsen IB, Nguyen TT, Ringdal M, Tryggestad AM, Bakke O, Lien E, Espevik T, Anthonsen MW. Toll-like receptor 3 associates with c-Src tyrosine kinase on endosomes to initiate antiviral signaling. EMBO J. 2006;25:3335–3346. doi: 10.1038/sj.emboj.7601222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong P, Angelini DJ, Yang S, Xia G, Cross AS, Mann D, Bannerman DD, Vogel SN, Goldblum SE. TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J. Biol. Chem. 2008;283:13437–13449. doi: 10.1074/jbc.M707986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aki D, Mashima R, Saeki K, Minoda Y, Yamauchi M, Yoshimura A. Modulation of TLR signalling by the C-terminal Src kinase (Csk) in macrophages. Genes Cells. 2005;10:357–368. doi: 10.1111/j.1365-2443.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 14.Medvedev AE, Piao W, Shoenfelt J, Rhee SH, Chen H, Basu S, Wahl LM, Fenton MJ, Vogel SN. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J. Biol. Chem. 2007;282:16042–16053. doi: 10.1074/jbc.M606781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulanova M, Asfaha S, Stenton G, Lint A, Schreiber A, Befus D. Involvement of Syk protein tyrosine kinase in LPS-induced responses in macrophages. J. Endotoxin Res. 2007;13:117–125. doi: 10.1177/0968051907079125. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhary A, Fresquez TM, Naranjo MJ. Tyrosine kinase Syk associates with toll-like receptor 4 and regulates signaling in human monocytic cells. Immunol. Cell Biol. 2007;85:249–256. doi: 10.1038/sj.icb7100030. [DOI] [PubMed] [Google Scholar]
- 17.Dennehy KM, Ferwerda G, Faro-Trindade I, Pyz E, Willment JA, Taylor PR, Kerrigan A, Tsoni SV, Gordon S, Meyer-Wentrup F, Adema GJ, Kullberg BJ, Schweighoffer E, Tybulewicz V, Mora-Montes HM, Gow NA, Williams DL, Netea MG, Brown GD. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur. J. Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 19.Stuart LM, Bell SA, Stewart CR, Silver JM, Richard J, Goss JL, Tseng AA, Zhang A, El Khoury JB, Moore KJ. CD36 signals to the actin cytoskeleton and regulates microglial migration via a p130Cas complex. J. Biol. Chem. 2007;282:27392–27401. doi: 10.1074/jbc.M702887200. [DOI] [PubMed] [Google Scholar]
- 20.Teusch N, Lombardo E, Eddleston J, Knaus UG. The low molecular weight GTPase RhoA and atypical protein kinase Czeta are required for TLR2-mediated gene transcription. J. Immunol. 2004;173:507–514. doi: 10.4049/jimmunol.173.1.507. [DOI] [PubMed] [Google Scholar]
- 21.Chen LY, Zuraw BL, Liu FT, Huang S, Pan ZK. IL-1 receptor-associated kinase and low molecular weight GTPase RhoA signal molecules are required for bacterial lipopolysaccharide-induced cytokine gene transcription. J. Immunol. 2002;169:3934–3939. doi: 10.4049/jimmunol.169.7.3934. [DOI] [PubMed] [Google Scholar]
- 22.Stovall SH, Yi AK, Meals EA, Talati AJ, Godambe SA, English BK. Role of vav1- and src-related tyrosine kinases in macrophage activation by CpG DNA. J. Biol. Chem. 2004;279:13809–13816. doi: 10.1074/jbc.M311434200. [DOI] [PubMed] [Google Scholar]
- 23.Shibolet O, Giallourakis C, Rosenberg I, Mueller T, Xavier RJ, Podolsky DK. AKAP13, a RhoA GTPase-specific guanine exchange factor, is a novel regulator of TLR2 signaling. J. Biol. Chem. 2007;282:35308–35317. doi: 10.1074/jbc.M704426200. [DOI] [PubMed] [Google Scholar]
- 24.Lundberg AS, Randell SH, Stewart SA, Elenbaas B, Hartwell KA, Brooks MW, Fleming MD, Olsen JC, Miller SW, Weinberg RA, Hahn WC. Immortalization and transformation of primary human airway epithelial cells by gene transfer. Oncogene. 2002;21:4577–4586. doi: 10.1038/sj.onc.1205550. [DOI] [PubMed] [Google Scholar]
- 25.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 26.Wong K, Pertz O, Hahn K, Bourne H. Neutrophil polarization: spatiotemporal dynamics of RhoA activity support a self-organizing mechanism. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3639–3644. doi: 10.1073/pnas.0600092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori M, Yoneyama M, Ito T, Takahashi K, Inagaki F, Fujita T. Identification of Ser-386 of interferon regulatory factor 3 as critical target for inducible phosphorylation that determines activation. J. Biol. Chem. 2004;279:9698–9702. doi: 10.1074/jbc.M310616200. [DOI] [PubMed] [Google Scholar]
- 28.Lombardo E, Alvarez-Barrientos A, Maroto B, Bosca L, Knaus UG. TLR4-mediated survival of macrophages is MyD88 dependent and requires TNF-alpha autocrine signalling. J. Immunol. 2007;178:3731–3739. doi: 10.4049/jimmunol.178.6.3731. [DOI] [PubMed] [Google Scholar]
- 29.Hodgson L, Pertz O, Hahn KM. Design and optimization of genetically encoded fluorescent biosensors: GTPase biosensors. Methods Cell Biol. 2008;85:63–81. doi: 10.1016/S0091-679X(08)85004-2. [DOI] [PubMed] [Google Scholar]
- 30.Ritter M, Mennerich D, Weith A, Seither P. Characterization of Toll-like receptors in primary lung epithelial cells: strong impact of the TLR3 ligand poly(I:C) on the regulation of Toll-like receptors, adaptor proteins and inflammatory response. J. Inflamm. (Lond) 2005;2:16. doi: 10.1186/1476-9255-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knaus UG, Bamberg A, Bokoch GM. Rac and Rap GTPase activation assays. Methods Mol. Biol. 2007;412:59–67. doi: 10.1007/978-1-59745-467-4_5. [DOI] [PubMed] [Google Scholar]
- 32.Funami K, Sasai M, Ohba Y, Oshiumi H, Seya T, Matsumoto M. Spatiotemporal mobilization of Toll/IL-1 receptor domain-containing adaptor molecule-1 in response to dsRNA. J. Immunol. 2007;179:6867–6872. doi: 10.4049/jimmunol.179.10.6867. [DOI] [PubMed] [Google Scholar]
- 33.Achuthan A, Elsegood C, Masendycz P, Hamilton JA, Scholz GM. CpG DNA enhances macrophage cell spreading by promoting the Src-family kinase-mediated phosphorylation of paxillin. Cell. Signal. 2006;18:2252–2261. doi: 10.1016/j.cellsig.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Kannan S, Audet A, Knittel J, Mullegama S, Gao GF, Wu M. Src kinase Lyn is crucial for Pseudomonas aeruginosa internalization into lung cells. Eur. J. Immunol. 2006;36:1739–1752. doi: 10.1002/eji.200635973. [DOI] [PubMed] [Google Scholar]
- 35.Mocsai A, Zhang H, Jakus Z, Kitaura J, Kawakami T, Lowell CA. G-protein-coupled receptor signaling in Syk-deficient neutrophils and mast cells. Blood. 2003;101:4155–4163. doi: 10.1182/blood-2002-07-2346. [DOI] [PubMed] [Google Scholar]
- 36.Garoufalis E, Kwan I, Lin R, Mustafa A, Pepin N, Roulston A, Lacoste J, Hiscott J. Viral induction of the human beta interferon promoter: modulation of transcription by NF-kappa B/rel proteins and interferon regulatory factors. J. Virol. 1994;68:4707–4715. doi: 10.1128/jvi.68.8.4707-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thanos D, Maniatis T. Identification of the rel family members required for virus induction of the human beta interferon gene. Mol. Cell. Biol. 1995;15:152–164. doi: 10.1128/mcb.15.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merika M, Williams AJ, Chen G, Collins T, Thanos D. Recruitment of CBP/p300 by the IFN beta enhanceosome is required for synergistic activation of transcription. Mol. Cell. 1998;1:277–287. doi: 10.1016/s1097-2765(00)80028-3. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Hussain S, Wang EJ, Li MO, Garcia-Sastre A, Beg AA. Lack of essential role of NF-kappa B p50, RelA, and cRel subunits in virus-induced type 1 IFN expression. J. Immunol. 2007;178:6770–6776. doi: 10.4049/jimmunol.178.11.6770. [DOI] [PubMed] [Google Scholar]
- 40.Lee I-T, Wang S-W, Lee C-W, Chang C-C, Lin C-C, Luo S-F, Yang C-M. Lipoteichoic acid induces HO-1 expression via the TLR2/MyD88/c-Src/NADPH oxidase pathway and Nrf2 in human tracheal smooth muscle cells. J. Immunol. 2008;181:5098–5110. doi: 10.4049/jimmunol.181.7.5098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.