Abstract
Gaze avoidance is a hallmark behavioral feature of fragile X syndrome (FXS), but little is known about whether abnormalities in the visual processing of faces, including disrupted autonomic reactivity, may underlie this behavior. Eye tracking was used to record fixations and pupil diameter while adolescents and young adults with FXS and sex- and age-matched typically developing controls passively viewed photographs of faces containing either a calm, happy, or fearful expression, preceded by a scrambled face matched on luminance. Results provide quantitative evidence for significant differences in gaze patterns and increased pupillary reactivity when individuals with FXS passively view static faces. Such abnormalities have significant implications in terms of understanding causes of gaze avoidance observed in individuals with FXS.
Keywords: face processing, fragile X syndrome, FMR1 gene, eye tracking, pupil reactivity
Fragile X syndrome (FXS) is the most common cause of inherited mental impairment (Crawford, 2001) and, to date, the most common known genetic cause of autism (D. Cohen et al., 2005). Although it is widely reported that gaze avoidance is a hallmark behavioral feature of FXS (Bregman, Leckman, & Ort, 1988; I. L. Cohen, Vietze, Sudhalter, Jenkins, & Brown, 1991; Garrett, Menon, MacKenzie, & Reiss, 2004), it remains unknown whether abnormalities in the visual processing of faces, including disrupted autonomic reactivity, may contribute to this observed social deficit.
Difficulty for individuals with FXS to establish and maintain eye-gaze during social interactions has been physiologically associated with markedly increased levels of cortisol or autonomic reactivity, suggesting that gaze aversion may be a coping mechanism that serves to reduce negative arousal (Belser & Sudhalter, 1995; Hall, DeBernardis, & Reiss, 2006; Hessl et al., 2002; Hessl, Glaser, Dyer-Friedman, & Reiss, 2006; Wisbeck et al., 2000). Secretion of cortisol, by means of a cascade of hormones along the hypothalamic-pituitary-adrenal (HPA) axis, involves feedback between several limbic structures, among which the amygdala plays a significant role, and which have been implicated in FXS (Binstock, 1995; Hessl, Rivera, & Reiss, 2004; Paradee et al., 1999).
Studies have shown that pupillary responses are regulated by the autonomic nervous system in part in response to level of emotional arousal (Bradley, Miccoli, Escrig, & Lang, 2008; Ekman, Levenson, & Friesen, 1983; Hess & Polt, 1960; Steinhauer, Siegle, Condray, & Pless, 2004). Pupil dilation has been linked with direct electrical stimulation of the amygdala (Gloor, 1997) and neuroimaging findings have shown that amygdala activity is associated with an observer’s pupil size as well as is sensitive to the pupil size of others (Demos, Kelley, Ryan, Davis, & Whalen, 2008; Harrison, Singer, Rotshtein, Dolan, & Critchley, 2006).
The goals of the current study were to examine number of fixations and gaze duration when individuals with FXS viewed photographs of faces with different emotional content, and to measure face-specific changes in pupil diameter as an index of autonomic arousal.
Methods
Participants
Participants included sixteen individuals with FXS (13 males) and sixteen typically developing (TD) controls (13 males). Individuals with FXS were confirmed to carry the full FMR1 mutation as previously described (Saluto et al., 2005; Tassone et al., 2004). TD participants with no current or past psychiatric diagnoses were recruited from the community. Groups were individually matched on sex and chronological age (t1, 30 = -0.085, p > .05). At the time of testing, 10 individuals with FXS (8 males) were being treated with at least one class of medication; stimulant (n = 3), antipsychotic (n = 4), and anti-depressant (n = 5). One female control participant was being treated with an anti-hypertensive medication.
The Social Communication Questionnaire (SCQ; (Rutter, Bailey, & Lord, 2003) was used to evaluate symptoms of autism spectrum disorder in participants with FXS. Individuals with an SCQ score at or above 15 (n = 11, 9 males), or who raised clinical concerns for ASD (1 male), were referred for a more complete evaluation using the Autism Diagnostic Observation Schedule-Generic (ADOS-G; (Lord, Rutter, DiLavore, & Risi, 2002). Of these twelve, 8 were diagnosed with Autistic Disorder and 2 were diagnosed with Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS). Group characteristics are given in Table 1.
Table 1.
Descriptive characteristics of the fragile X syndrome (FXS) and control groups. (Mean ± SEM)
| FXS (n = 16) | TD (n = 16) | |
|---|---|---|
| Gender (M:F) | 13:3 | 13:3 |
| Chronological Age (years) | 16.99 ± 6.8 | 17.14 ± 6.3 |
| Full Scale IQa | 58.36 ± 9.8 | |
| SCQ | 15.45 ± 7.51 | |
| ADOS Totalb | 10.46 ± 4.07 |
FXS, fragile X Syndrome; TD, Typically Developing; SCQ, Social Communication Questionnaire-Lifetime Version; ADOS, Autism Diagnostic Observation Schedule
Intellectual level was measured using the Weschler Intelligence Scales (Wechsler, 2003), n = 14 (2 participants were not testable due to extremely low functioning).
n = 13
Apparatus and Stimuli
All stimuli were presented on a Tobii 1750 binocular eye tracker monitor. This eye tracking system consists of a high-resolution camera embedded in a 17-inch monitor (1280 × 1024 pixels resolution, 50 Hz sampling rate, average precision of 0.5 degrees of visual angle). There are several benefits of the Tobii 1750 system that make it conducive to testing individuals with developmental disorders, including approximately 20 centimeters of tolerance to head-motion in any direction without requiring any head restraints. Stimuli consisted of sixty colored photographs of adult human faces (equal numbers of males and females, different races and ethnicities) from the NimStim Face Stimulus Set (Tottenham, 2002), each showing a calm, happy, or fearful expression, and sixty scrambled versions of the face images. Since it was critical that pupil responses following the onset of the face stimulus be independent of a pupillary light reflex, each face and its scramble were matched on mean luminance, and equivalence was confirmed using a photometer (Minolta, LS-100, Osaka, Japan). Face images subtended a 12.12 degree by 17.19 degree region (the size of an actual human face) when viewed from a distance of 60 cm, and were presented on a standard 50% grey background.
Procedure
The experimental protocol was approved by the Institutional Review Board at the University of California, Davis, and informed consent was obtained from all participants or their parents. Following a nine-point calibration, participants were told to view the pictures shown. Each trial consisted of a scrambled face for 1 second followed immediately by its matched face for 3 seconds. An inter-trial interval containing a uniform grey screen was shown for 0.5, 1, or 2 seconds. The order of face presentation was randomized.
Analyses
Four area-of-interest (AOI) regions were defined for each face: eyes (including the eyebrows), nose, mouth, and other. Scrambled faces included a single AOI around the ellipse. Measures included number of fixations (where a fixation was defined as any data point within a 30 pixel radius for a minimum duration of 100 milliseconds (ms)) and proportion of looking time to each AOI region.
Each participant’s pupil data, averaged from both eyes, were first filtered to remove any outlier values corresponding to blinks, loss of tracking data, or large changes in head position. Mean pupil diameter was calculated for 250 ms intervals across the trial duration, time-locked to the onset of the face stimulus. In order to determine the presence of a face-specific pupil response, relative change in pupil size was calculated by subtracting the mean pupil size during the scrambled face from the mean pupil size during each interval of the face presentation, and then “standardized” by dividing by the mean pupil size during the scrambled face. A similar pupil change was calculated for the intervals of the scrambled face, relative to the mean pupil size during the inter-trial interval.
Results
Individuals with FXS made significantly fewer fixations to the eye region of all faces (Figure 1). A repeated-measures analysis of variance (RMANOVA) with AOI region, emotion, and group as independent variables and number of fixations as the dependent variable revealed a main effect of AOI region [F(3, 28) = 16.74, p = 0.001, η2 = 0.642]. This effect was qualified by a significant interaction between AOI region and group [F(3, 28) = 5.12, p = 0.006, η2 = 0.354], such that individuals with FXS made fewer fixations to the eye region of faces compared to controls (Figure 2). Individuals with FXS made fewer fixations to the face overall [F(1, 31) = 8.926, p <0.01), but no group difference was found for number of fixations made to the scrambled faces [F(1, 31) = 3.154, p > 0.05], suggesting that the fixation differences observed were specific to faces rather than generalizable to all images.
Figure 1.
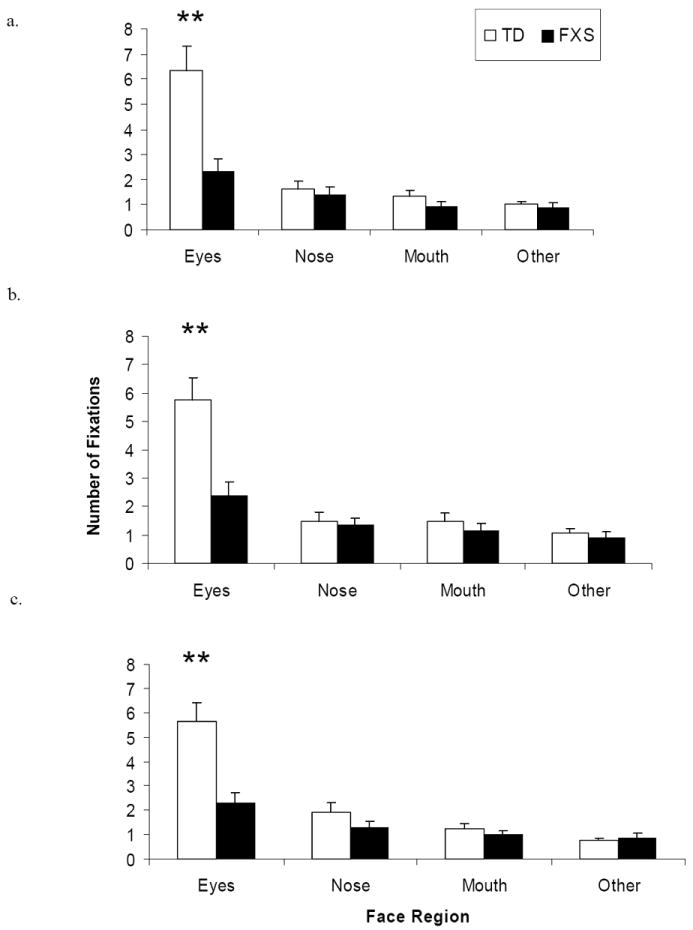
Mean number of fixations to each AOI region by group for (a) calm, (b) happy, and (c) fearful faces. Double asterisk (**) indicates significant difference between pairwise comparisons at the p < .01 level. Error bars represent SEM.
Figure 2.
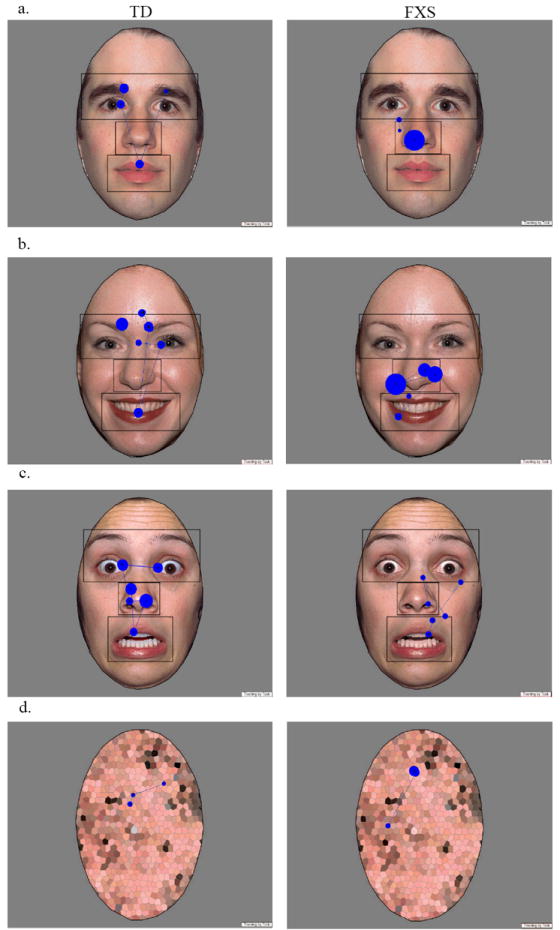
Example fixation plots from one subject in each group (TD on left, FXS on right) for a single (a) calm, (b) happy, (c) fearful, and (d) scrambled face trial. The plot displays a static frame of fixation data for each image. Each fixation is illustrated with a blue circle where the radius represents the length of the fixation.
Individuals with FXS spent significantly less time looking at the eye region of all faces (Figure 3). A RMANOVA with AOI region, emotion, and group as independent variables and proportion of gaze duration as the dependent variable revealed a main effect of AOI region [F(3,28) = 29.94, p = 0.001, η2 = 0.762] and a significant interaction between AOI region and group [F(3,28) = 3.56, p = 0.028, η2 = 0.274]. Independent t-tests revealed that, across emotion conditions, individuals with FXS spent a smaller proportion of time looking at the eyes [t(1, 30) = 3.28, p = 0.003] and greater proportion of time looking at the nose [t(1, 30) = -2.58, p = 0.015], compared to controls. No group difference was found for gaze duration to the scrambled faces [F(1, 31) = 0.062, p > 0.05].
Figure 3.
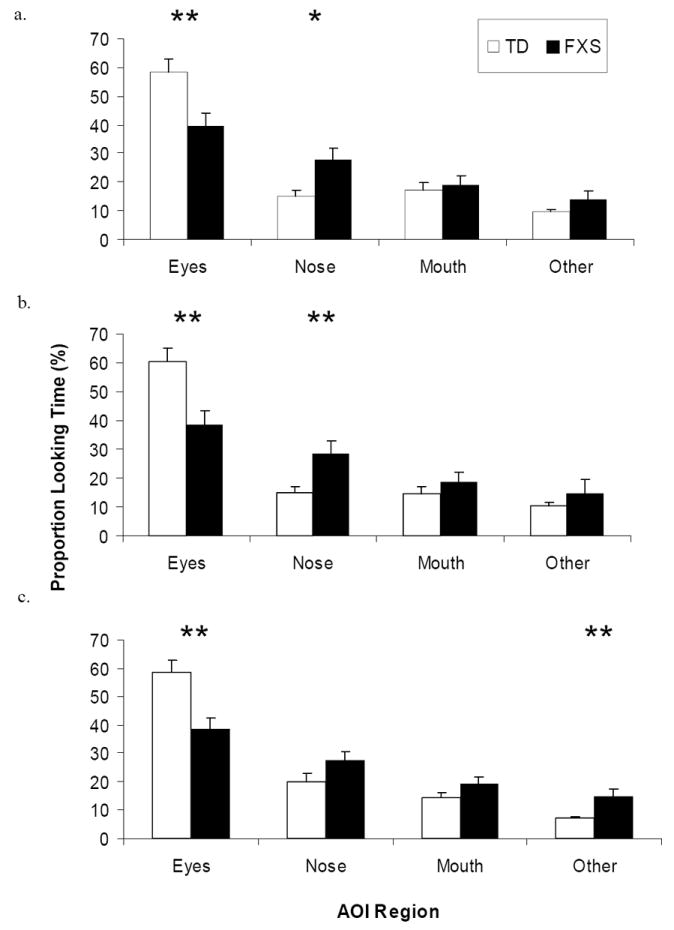
Mean proportion gaze duration to each AOI region by group for (a) calm, (b) happy, and (c) fearful faces. Gaze durations are reported as percentages and error bars represent SEM. Asterisk (*) and double asterisk (**) indicate significant difference between pairwise comparisons at the p < .05 and p < .01 level, respectively.
Individuals with FXS demonstrated significantly greater pupillary reactivity in response to emotional faces, relative to controls (Figure 4). A RMANOVA with interval (12), emotion, and group as independent variables and relative pupil change as the dependent variable resulted in a main effect of interval [F(11, 60) = 4.66, p = 0.001, η2 = 0.134], such that, for all faces combined, there was an increase in pupil size (dilation) across time for both groups. A significant interaction between interval and emotion also was found [F (22, 9) = 3.35, p = 0.033, η2 = 0.891], driven by pupil dilation to happy and fearful faces for both groups. A significant interaction between interval and group [F (11, 60) = 4.72, p = 0.001, η2 = 0.136] showed that individuals with FXS demonstrated greater pupil dilation compared to TD controls. No group difference was found for change in pupil size to scrambled faces [F (1, 31) = 0.722, p > 0.05].
Figure 4.
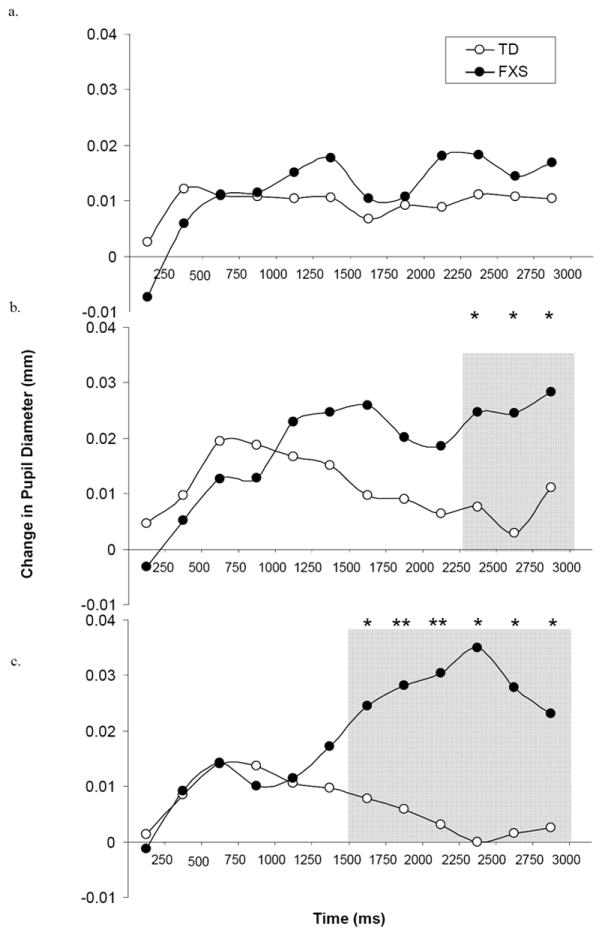
Relative change in pupil diameter (mm) between scrambled face slide to (a) calm, (b) happy, and (c) fearful faces, across 250 ms intervals, by group. Curves are time-locked to the onset of the face stimulus. Gray shaded region with asterisk (*) or double asterisk (**) indicates significant pairwise difference at the p < .05 and p < .01 level, respectively.
An exploratory analysis revealed that, in individuals with FXS, but not controls, pupil dilation in response to the fearful faces was inversely correlated with number of fixations to the eyes of calm [r = -0.550, p = 0.027], happy [r = -0.612, p = 0.012], and fearful [r = -0.641, p = 0.007] faces, as well as proportion of gaze duration to the eyes of calm faces [r = -0.579, p = 0.019]. There were no significant sex differences within either group on any of the above eye tracking measures. However, a lack of difference may also reflect limitations in statistical power as there were only three girls in each group.
Finally, correlational analyses revealed no significant associations between symptoms of autism in individuals with FXS as measured by the SCQ and ADOS and number of fixations, gaze duration, and changes in pupil diameter in response to the faces. This finding is consistent with studies of gaze aversion in individuals with autism (K.M. Dalton, Nacewicz, Alexander, & Davidson, 2007; Klin, Jones, Schultz, Volkmar, & Cohen, 2002; Speer, Cook, McMahon, & Clark, 2007).
Discussion
These findings reveal both qualitative and quantitative differences in the way that individuals with FXS visually process static human faces, and to our knowledge, are the first to utilize pupillometry as a measure of autonomic reactivity in individuals with FXS. Individuals with FXS demonstrated fewer fixations and less gaze time to the eye region of faces, and increased pupil reactivity to emotional faces, compared to controls. Importantly, pupil dilation in response to the fearful faces was inversely correlated with number of fixations to the eyes of all faces. This pattern was observed in both males and females with FXS, and was not significantly associated with severity of autism symptomology. Documentation of face processing differences is critical for developing treatment approaches aimed at alleviating the symptoms of social anxiety and has significant potential for use in assessing specific phenotypic outcomes following treatment in clinical research studies (Guastella, Mitchell, & Dadds, 2008).
Research has firmly established that FXS and autism are closely associated. Two to six percent of children with autism have the FMR1 mutation (Reddy, 2005; Wassink, Piven, & Patil, 2001) and approximately 30% of children with FXS are diagnosed with autism and an additional 20-30% have PDDNOS (D. B. J. Bailey, Hatton, Skinner, & Mesibov, 2001; Harris et al., in press; Kaufmann et al., 2004; Rogers, Wehner, & Hagerman, 2001). Researchers have hypothesized that the presence of autism symptoms in FXS exists on a spectrum, such that some individuals with FXS who are also diagnosed with ASD may not be qualitatively different from those without ASD (D. B. Bailey et al., 2004; Lewis et al., 2006).
Our results are in line with research demonstrating that individuals with ASD spend a smaller percentage of time fixating the eyes (Klin et al., 2002; Merin, Young, Ozonoff, & Rogers, 2007; Pelphrey et al., 2002; Spezio, Adolphs, Hurley, & Piven, 2007), and thus support the argument that gaze aversion observed in idiopathic autism and in FXS may reflect similarities in underlying network dysfunctions (Belmonte & Bourgeron, 2006). Several fMRI studies report reduced activity in the fusiform gyrus (FG) when individuals with autism perform face processing tasks (Dalton et al., 2005; Pierce, Haist, Sedaghat, & Courchesne, 2004; Schultz et al., 2000), and fMRI in conjunction with eye tracking has shown that eye-gaze in individuals with autism is positively associated with increased activity and amygdala compared to controls (K. M. Dalton et al., 2005). It has therefore been suggested that gaze aversion may be a result of hyperactivation of the amygdala. On the cellular level, van Kooten et al. (2008) recently reported that the FG in post-mortem brains of patients with autism show significant reduction in neuron density and volume, which could provide a neurobiological explanation for the atypical activation of the FG observed in autism. Neuroimaging studies with individuals with FXS have shown reduced FG activation to forward faces and equal FG activation to angled faces, relative to controls (Garrett et al., 2004). Although our interpretations of a common pathophysiological pathway remain speculative until differences in the underlying neural circuitry can be better identified, they are consistent with clinical and research reports of individuals with FXS, with and without autism, becoming overly emotionally aroused and anxious during social situations and therefore averting gaze to facilitate reduction of arousal.
These results should be viewed in light of a few limitations. Including data from groups of individuals with idiopathic developmental delay or autism would help to specify the interpretations and implications of the findings. While our results establish that individuals with FXS exhibit abnormal fixation patterns when viewing static photographs of faces, suggesting that pictures of faces, particularly eyes, are inherently aversive, using dynamic social stimuli that resemble real-life social situations would extend these results. Future studies are required to investigate these findings as they relate to the function of specific brain areas involved in social behavior and emotion regulation.
Acknowledgments
This work was supported by a grant from the National Institute of Mental Health to DH (MH77554). Thanks are given to the individuals who participated in this study and Alexis L. Schwartz for his assistance in developing specialized data analysis scripts.
Footnotes
Data from this study were presented at the 11th International Fragile X Conference, St. Louis, MO, in July, 2008.
References
- Bailey DB, Roberts JE, Hooper SR, Hatton DD, Mirrett PL, Roberts JE, et al. Research on fragile X syndrome and autism: implications for the study of genes, environments, and developmental language disorders. In: Rice ML, Warren SF, editors. Developmental Language Disorders: From Phenotypes to Etiologies. Mahwah, NJ: Lawrence Erlbaum Associates; 2004. pp. 121–150. [Google Scholar]
- Bailey DBJ, Hatton DD, Skinner M, Mesibov GB. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of Autism and Developmental Disorder. 2001;31:165–174. doi: 10.1023/a:1010747131386. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nature Neuroscience. 2006;9(10):121–1225. doi: 10.1038/nn1765. [DOI] [PubMed] [Google Scholar]
- Belser RC, Sudhalter V. Arousal difficulties in males with fragile X syndrome: A preliminary report. Developmental Brain Dysfunction. 1995;8:270–279. [Google Scholar]
- Binstock TC. Fragile X and the amygdala: Cognitive, interpersonal, emotional and neuroendrocrine consideration. Developmental Brain Dysfunction. 1995;8:199–217. [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45(4):602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman JD, Leckman JF, Ort SI. Fragile X syndrome: Genetic predisposition to psychology. Journal of Autism and Developmental Disorders. 1988;18(3):343–354. doi: 10.1007/BF02212191. [DOI] [PubMed] [Google Scholar]
- Cohen D, Pichard N, Tordjman S, Baumann C, Burglen L, Excoffier E, et al. Specific genetic disorders and autism: Clinical contribution towards their identification. Journal of Autism and Developmental Disorders. 2005;35:103–116. doi: 10.1007/s10803-004-1038-2. [DOI] [PubMed] [Google Scholar]
- Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, Brown WT. Effects of age and communication level on eye contact in fragile X males and non-fragile X autistic males. American Journal of Medical Genetics. 1991;38(23):498–502. doi: 10.1002/ajmg.1320380271. [DOI] [PubMed] [Google Scholar]
- Crawford D, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genetics in Medicine. 2001;3(5):359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biological Psychiatry. 2007;61(4):512–520. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos KE, Kelley WM, Ryan SL, Davis FC, Whalen PJ. Human amygdala sensitivity to the pupil size of others. Cerebral Cortex. 2008:bhn034. doi: 10.1093/cercor/bhn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221:1208–1210. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- Garrett AS, Menon V, MacKenzie K, Reiss AL. Here’s looking at you, kid: neural systems underlying face and gaze processing in fragile X syndrome. Archives of General Psychiatry. 2004;61(3):281–288. doi: 10.1001/archpsyc.61.3.281. [DOI] [PubMed] [Google Scholar]
- Gloor P. The Temporal Lobe and Limbic System. New York: Oxford University Press; 1997. [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry. 2008;63(1):3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hall S, DeBernardis M, Reiss A. Social escape behaviors in children with fragile X syndrome. Journal of Autism and Developmental Disorders. 2006;36:935–947. doi: 10.1007/s10803-006-0132-z. [DOI] [PubMed] [Google Scholar]
- Harris SW, Goodlin-Jones B, Nowicki ST, Hessl D, Tassone F, Barabato I, et al. Autism Profiles of Young Males with Fragile X Syndrome. American Journal of Medical Genetics Part A in press. [Google Scholar]
- Harrison NA, Singer T, Rotshtein P, Dolan RJ, Critchley HD. Pupillary contagion: central mechanisms engaged in sadness processing. Social Cognition and Affective Neuroscience. 2006;1:5–17. doi: 10.1093/scan/nsl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess EH, Polt JM. Pupil size as related to interest value of visual stimuli. Science. 1960;132:349–350. doi: 10.1126/science.132.3423.349. [DOI] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Blasey C, Hastie T, Gunnar M, et al. Cortisol and behavior in fragile X syndrome. Psychoneuroendocrinology. 2002;27(7):855–872. doi: 10.1016/s0306-4530(01)00087-7. [DOI] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Reiss AL. Social behavior and cortisol reactivity in children with fragile X syndrome. Journal of Child Psychology and Psychiatry. 2006;47(6):602–610. doi: 10.1111/j.1469-7610.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- Hessl D, Rivera SM, Reiss AL. The neuroanatomy and neuroendocrinology of fragile X syndrome. Mental Retardation and Developmental Disabilities Research Review. 2004;10:17–24. doi: 10.1002/mrdd.20004. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, et al. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. American Journal of Medical Genetics Part A. 2004;129A:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Lewis P, Abbeduto L, Murphy M, Richmond E, Giles N, Bruno L, et al. Cognitive, language, and social-cognitive skills of individuals with fragile X syndrome with and without autism. Journal of Intellectual Disability Research. 2006;50(7):532–545. doi: 10.1111/j.1365-2788.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule- Manua. Los Angeles: Western Psychological Services; 2002. [Google Scholar]
- Merin N, Young GS, Ozonoff S, Rogers SJ. Visual Fixation Patterns during Reciprocal Social Interaction Distinguish a Subgroup of 6-Month-Old Infants At-Risk for Autism from Comparison Infants. Journal of Autism and Developmental Disorder. 2007;37(1):108–121. doi: 10.1007/s10803-006-0342-4. [DOI] [PubMed] [Google Scholar]
- Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94(1):185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Neural basis of eye gaze processing deficits in autism. Brain. 2002;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: Findings of fusiform activity and beyond. Brain. 2004;127:2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- Reddy KS. Cytogenetic abnormalities and fragile-X syndrome in Autism Spectrum Disorder. BMC Medical Genetics. 2005;6:3. doi: 10.1186/1471-2350-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Wehner EA, Hagerman RJ. The behavioral phenotype in Fragile X: Symptoms of autism in very young children with Fragile X syndrome, idiopathic autism, and other developmental disorders. Journal of Developmental and Behavioral Pediatrics. 2001;22:409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire (SCQ) Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Saluto A, Brussino A, Tassone F, Arduino C, Cagnoli C, Pappi P, et al. An enhanced polymerase chain reaction assay to detect pre- and full mutation alleles of the fragile X mental retardation 1 gene. Journal of Molecular Diagnostics. 2005;7(5):605–612. doi: 10.1016/S1525-1578(10)60594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Speer LL, Cook AE, McMahon WM, Clark E. Face processing in children with autism:effects of stimulus contents and type. Autism. 2007;11(3):265–277. doi: 10.1177/1362361307076925. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Adolphs R, Hurley RS, Piven J. Abnormal use of facial information in high-functioning autism. Journal of Autism and Developmental Disorder. 2007;37(5):929–939. doi: 10.1007/s10803-006-0232-9. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Siegle GJ, Condray R, Pless M. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. International Journal of Psychophysiology. 2004;52:77–86. doi: 10.1016/j.ijpsycho.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Garcia AD, Khandijian EW, Greco CM, Hagerman PJ. Intranuclear inclusions in neural cells with premutation alleles in fragile X associated tremor/ataxia syndrome. Journal of Medical Genetics. 2004;41:1–3. doi: 10.1136/jmg.2003.012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. MacArthur Foundation Research Network 2002 [Google Scholar]
- van Kooten IAJ, Palmen SJMC, von Cappeln P, Steinbusch HWM, Korr H, Heinsen H, et al. Neurons in the fusiform gyrus are fewer and smaller in autism. Brain. 2008;131:987–999. doi: 10.1093/brain/awn033. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Piven J, Patil SR. Chromosomal abnormalities in a clinic sample of individuals with autistic disorder. Psychiatric Genetics. 2001;11:57–63. doi: 10.1097/00041444-200106000-00001. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. Fourth Edition. San Antonio: Harcourt Assessment, Inc.; 2003. [Google Scholar]
- Wisbeck JM, Huffman LC, Freund L, Gunnar MR, Davis EP, Reiss AL. Cortisol and social stressors in children with fragile X: A pilot study. Journal of Developmental and Behavioral Pediatrics. 2000;21:278–282. doi: 10.1097/00004703-200008000-00004. [DOI] [PubMed] [Google Scholar]


